Abstract
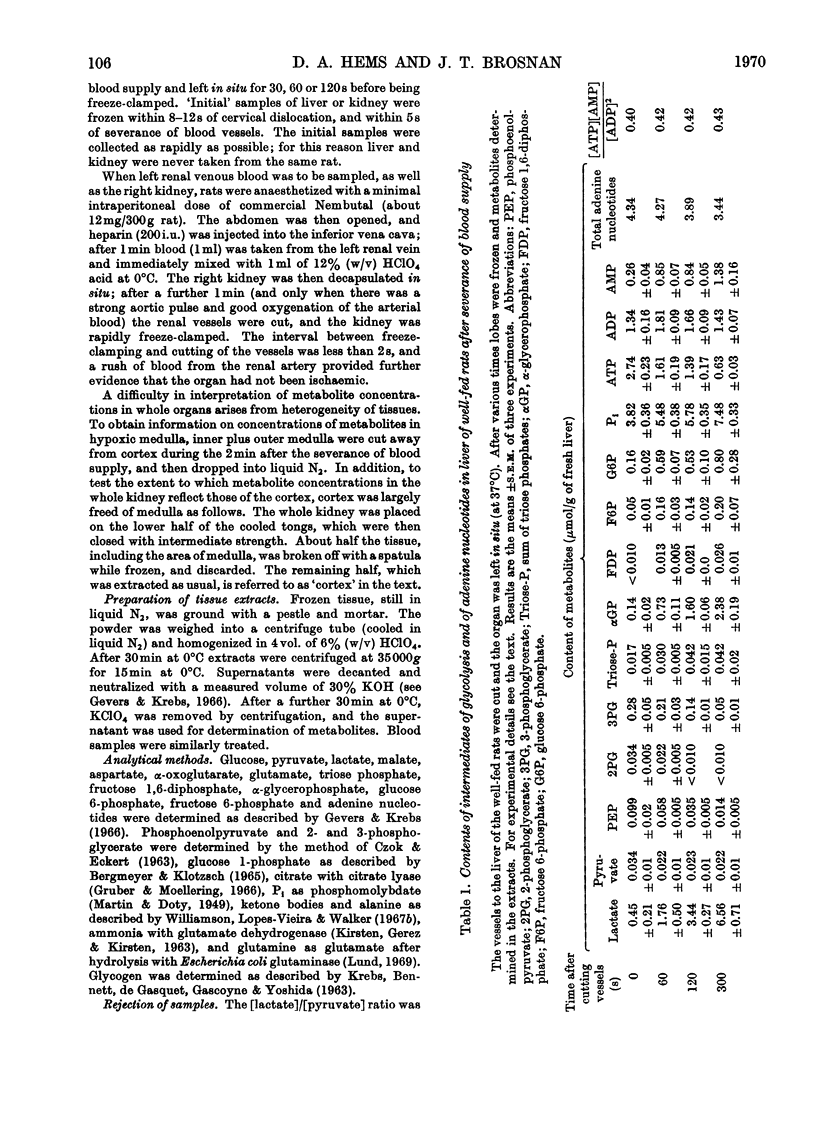
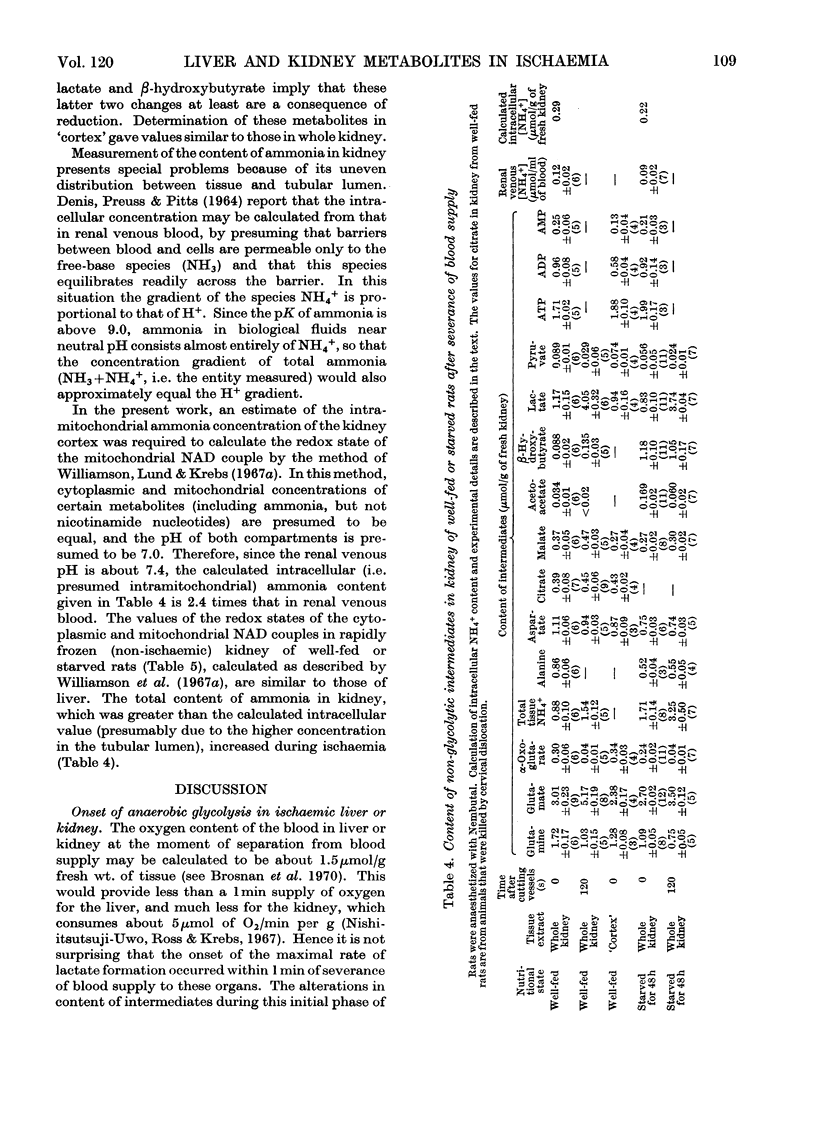
1. The time-course of changes in content of intermediates of glycolysis in rat liver and kidney cortex after severance of blood supply was investigated. 2. The decline in content of ATP was more rapid in kidney (1.7–0.5μmol/g in 30s) than in liver (2.7–1.6μmol/g in 60s). In both tissues AMP and Pi accumulated. 3. Net formation of lactate was 1.7μmol/g during the second minute of ischaemia in liver from well-fed rats, 1.1μmol/g in liver from 48h-starved rats, and about 1.0μmol/g during the first 30s of ischaemia in kidney. Net formation of α-glycerophosphate was rapid, especially in liver. 4. In kidney the concentration of β-hydroxybutyrate rose, but that of α-oxoglutarate and acetoacetate decreased. 5. In both organs the concentrations of fructose diphosphate and triose phosphates increased during ischaemia and those of other phosphorylated C3 intermediates decreased. 6. The concentration of the hexose 6-phosphates rose rapidly during the first minute of ischaemia in liver, but decreased during renal ischaemia. 7. In kidney the content of glutamine fell after 2min of ischaemia, and that of ammonia and glutamate rose. 8. The redox states of the cytoplasmic and mitochondrial NAD couple in kidney cortex were similar to those in liver. 9. The regulatory role of glycogen phosphorylase, pyruvate kinase and phosphofructokinase is discussed in relation to the observed changes in the concentrations of the glycolytic intermediates.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARTLEY W., SOBRINHO-SIMOES M., NOTTON B. M., MONTESI G. The anaerobic metabolism of citrate in rat liver. Biochem J. 1959 Jan;71(1):26–32. doi: 10.1042/bj0710026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brock D. J. Purification and properties of sheep liver phosphofructokinase. Biochem J. 1969 Jun;113(2):235–242. doi: 10.1042/bj1130235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosnan J. T., Krebs H. A., Williamson D. H. Effects of ischaemia on metabolite concentrations in rat liver. Biochem J. 1970 Mar;117(1):91–96. doi: 10.1042/bj1170091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DENIS G., PREUSS H., PITTS R. THE PNH3 OF RENAL TUBULAR CELLS. J Clin Invest. 1964 Apr;43:571–582. doi: 10.1172/JCI104942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans E. A. The metabolism of pyruvate in pigeon liver. Biochem J. 1940 Jun;34(6):829–837. doi: 10.1042/bj0340829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaja G., Ragnotti G., Cajone F., Bernelli-Zazzera A. Further studies on the stimulation of glycolysis by previous aerobiosis. Biochem J. 1967 Nov;105(2):647–654. doi: 10.1042/bj1050647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gevers W., Krebs H. A. The effects of adenine nucleotides on carbohydrate metabolism in pigeon-liver homogenates. Biochem J. 1966 Mar;98(3):720–735. doi: 10.1042/bj0980720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIRSTEN E., GEREZ C., KIRSTEN R. [An enzymatic microdetermination method for ammonia, specifically for extracts of animal tissues and fluids. Determination of NH4 ions in blood]. Biochem Z. 1963;337:312–319. [PubMed] [Google Scholar]
- KREBS H. A., BENNETT D. A., DE GASQUET P., GASQUET P., GASCOYNE T., YOSHIDA T. Renal gluconeogenesis. The effect of diet on the gluconeogenic capacity of rat-kidney-cortex slices. Biochem J. 1963 Jan;86:22–27. doi: 10.1042/bj0860022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraupp O., Niessner H., Ploszczanski B., Adler-Kastner L., Springer A., Chirikdjian J. J. A comparison of the effects of hexobendine with those of anoxia on the concentration of myocardial metabolites in vivo. Eur J Pharmacol. 1967 Mar;1(2):140–152. doi: 10.1016/0014-2999(67)90050-7. [DOI] [PubMed] [Google Scholar]
- Krebs H. A., Eggleston L. V. Biological synthesis of oxaloacetic acid from pyruvic acid and carbon dioxide. Biochem J. 1940 Nov;34(10-11):1383–1395. doi: 10.1042/bj0341383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs H. A., Eggleston L. V., Kleinzeller A., Smyth D. H. The fate of oxaloacetate in animal tissues. Biochem J. 1940 Sep;34(8-9):1234–1240. doi: 10.1042/bj0341234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs H. A., Johnson W. A. Metabolism of ketonic acids in animal tissues. Biochem J. 1937 Apr;31(4):645–660. doi: 10.1042/bj0310645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., PASSONNEAU J. V., HASSELBERGER F. X., SCHULZ D. W. EFFECT OF ISCHEMIA ON KNOWN SUBSTRATES AND COFACTORS OF THE GLYCOLYTIC PATHWAY IN BRAIN. J Biol Chem. 1964 Jan;239:18–30. [PubMed] [Google Scholar]
- MENDICINO J., VASARHELY F. RENAL D-FRUCTOSE 1,6-DIPHOSPHATASE. J Biol Chem. 1963 Nov;238:3528–3534. [PubMed] [Google Scholar]
- Maddaiah V. T., Madsen N. B. Kinetics of purified liver phosphorylase. J Biol Chem. 1966 Sep 10;241(17):3873–3881. [PubMed] [Google Scholar]
- Needleman P., Passonneau J. V., Lowry O. H. Distribution of glucose and related metabolites in rat kidney. Am J Physiol. 1968 Sep;215(3):655–659. doi: 10.1152/ajplegacy.1968.215.3.655. [DOI] [PubMed] [Google Scholar]
- Nishiitsutsuji-Uwo J. M., Ross B. D., Krebs H. A. Metabolic activities of the isolated perfused rat kidney. Biochem J. 1967 Jun;103(3):852–862. doi: 10.1042/bj1030852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozengurt E., Jiménez de Asúa L., Carminatti H. Some kinetic properties of liver pyruvate kinase (type L). II. Effect of pH on its allosteric behavior. J Biol Chem. 1969 Jun 25;244(12):3142–3147. [PubMed] [Google Scholar]
- Start C., Newsholme E. A. The effects of starvation and alloxan-diabetes on the contents of citrate and other metabolic intermediates in rat liver. Biochem J. 1968 Apr;107(3):411–415. doi: 10.1042/bj1070411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T., Sue F., Morimura H. Feed-forward activation and feed-back inhibition of pyruvate kinase type L of rat liver. Biochem Biophys Res Commun. 1967 Nov 17;29(3):444–449. doi: 10.1016/0006-291x(67)90477-9. [DOI] [PubMed] [Google Scholar]
- UNDERWOOD A. H., NEWSHOLME E. A. PROPERTIES OF PHOSPHOFRUCTOKINASE FROM RAT LIVER AND THEIR RELATION TO THE CONTROL OF GLYCOLYSIS AND GLUCONEOGENESIS. Biochem J. 1965 Jun;95:868–875. doi: 10.1042/bj0950868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UNDERWOOD A. H., NEWSHOLME E. A. SOME PROPERTIES OF FRUCTOSE 1,6-DIPHOSPHATASE OF RAT LIVER AND THEIR RELATION TO THE CONTROL OF GLUCONEOGENESIS. Biochem J. 1965 Jun;95:767–774. doi: 10.1042/bj0950767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Underwood A. H., Newsholme E. A. Control of glycolysis and gluconeogenesis in rat kidney cortex slices. Biochem J. 1967 Jul;104(1):300–305. doi: 10.1042/bj1040300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Underwood A. H., Newsholme E. A. Some properties of phosphofructokinase from kidney cortex and their relation to glucose metabolism. Biochem J. 1967 Jul;104(1):296–299. doi: 10.1042/bj1040296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOLLENBERGER A., RISTAU O., SCHOFFA G. [A simple technic for extremely rapid freezing of large pieces of tissue]. Pflugers Arch Gesamte Physiol Menschen Tiere. 1960;270:399–412. [PubMed] [Google Scholar]
- WU R. RATE-LIMITING FACTORS IN GLYCOLYSIS AND INORGANIC ORTHOPHOSPHATE TRANSPORT IN RAT LIVER AND KIDNEY SLICES. J Biol Chem. 1965 Jun;240:2373–2381. [PubMed] [Google Scholar]
- Williamson D. H., Lopes-Vieira O., Walker B. Concentrations of free glucogenic amino acids in livers of rats subjected to various metabolic stresses. Biochem J. 1967 Aug;104(2):497–502. doi: 10.1042/bj1040497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson D. H., Lund P., Krebs H. A. The redox state of free nicotinamide-adenine dinucleotide in the cytoplasm and mitochondria of rat liver. Biochem J. 1967 May;103(2):514–527. doi: 10.1042/bj1030514. [DOI] [PMC free article] [PubMed] [Google Scholar]


