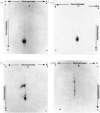
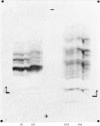
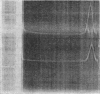
Abstract
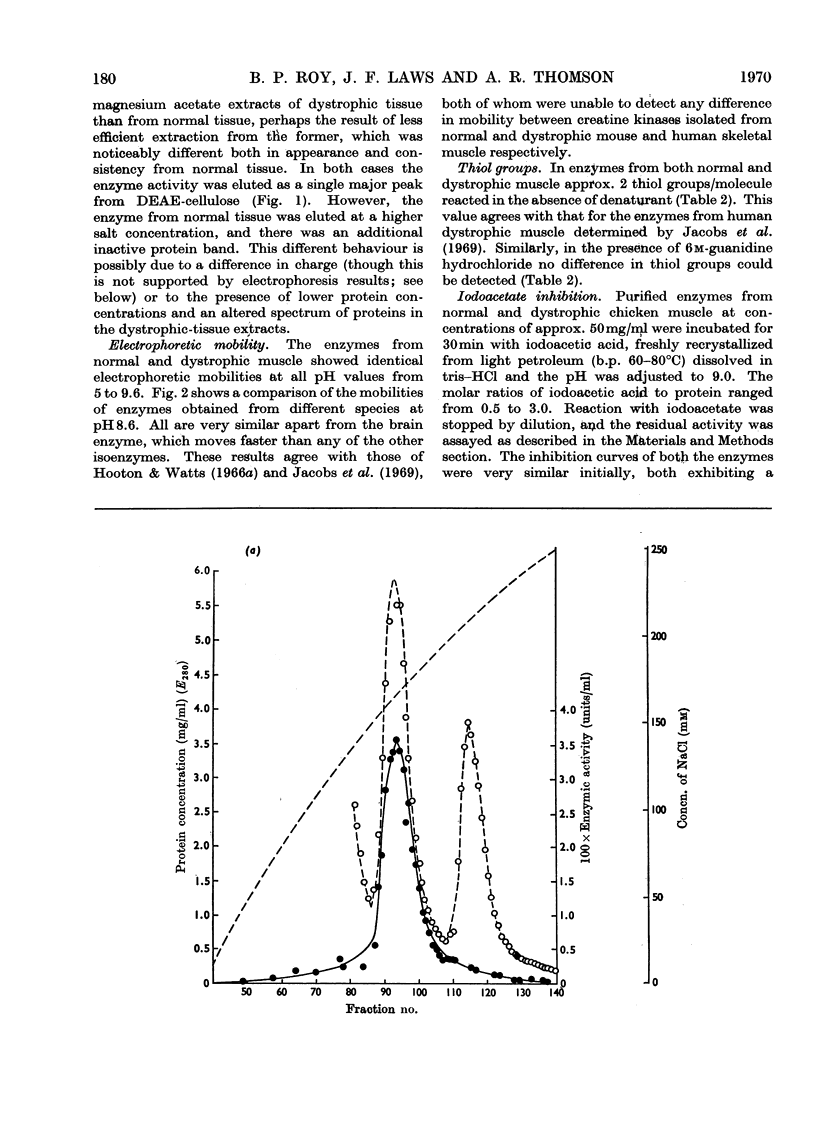
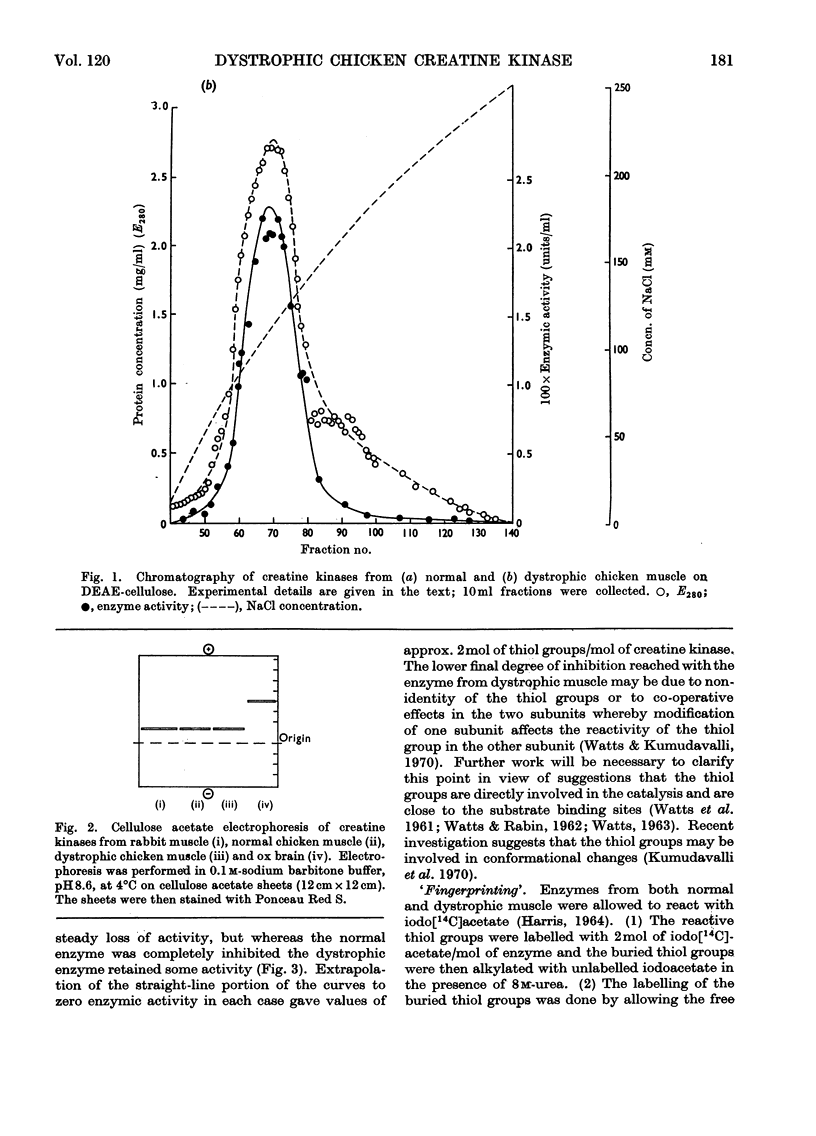
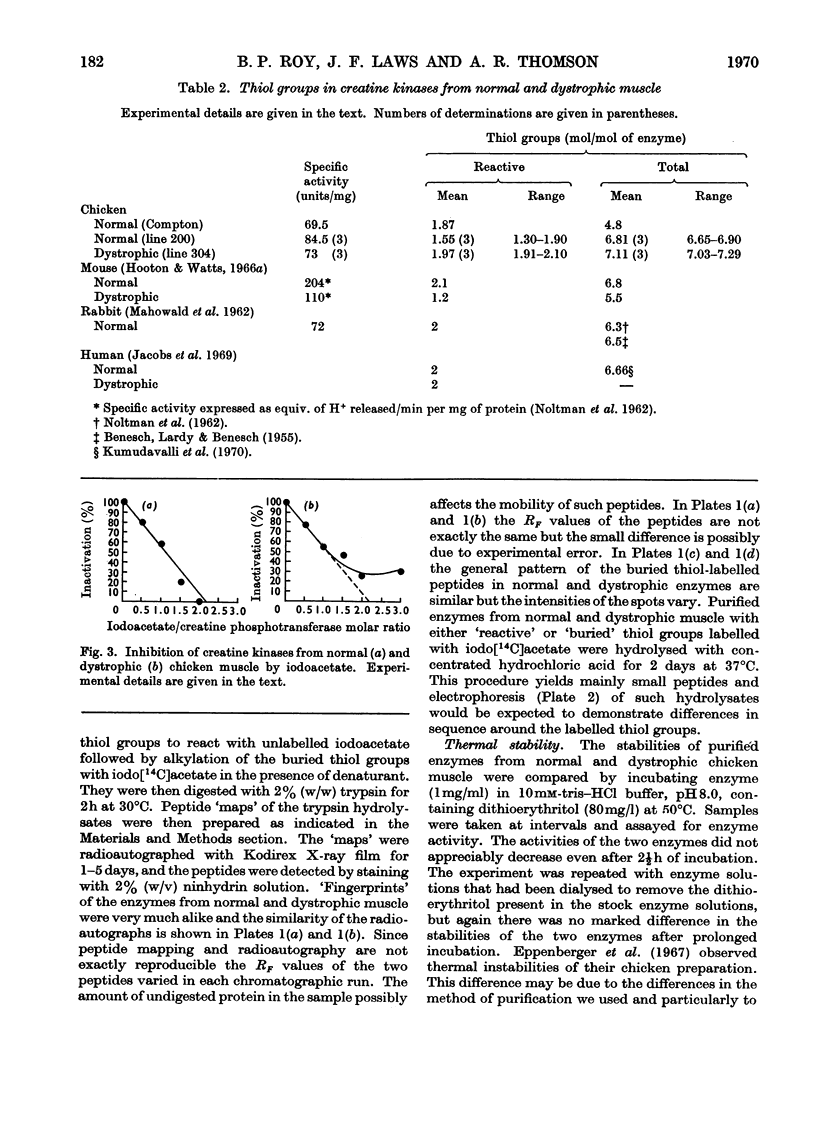
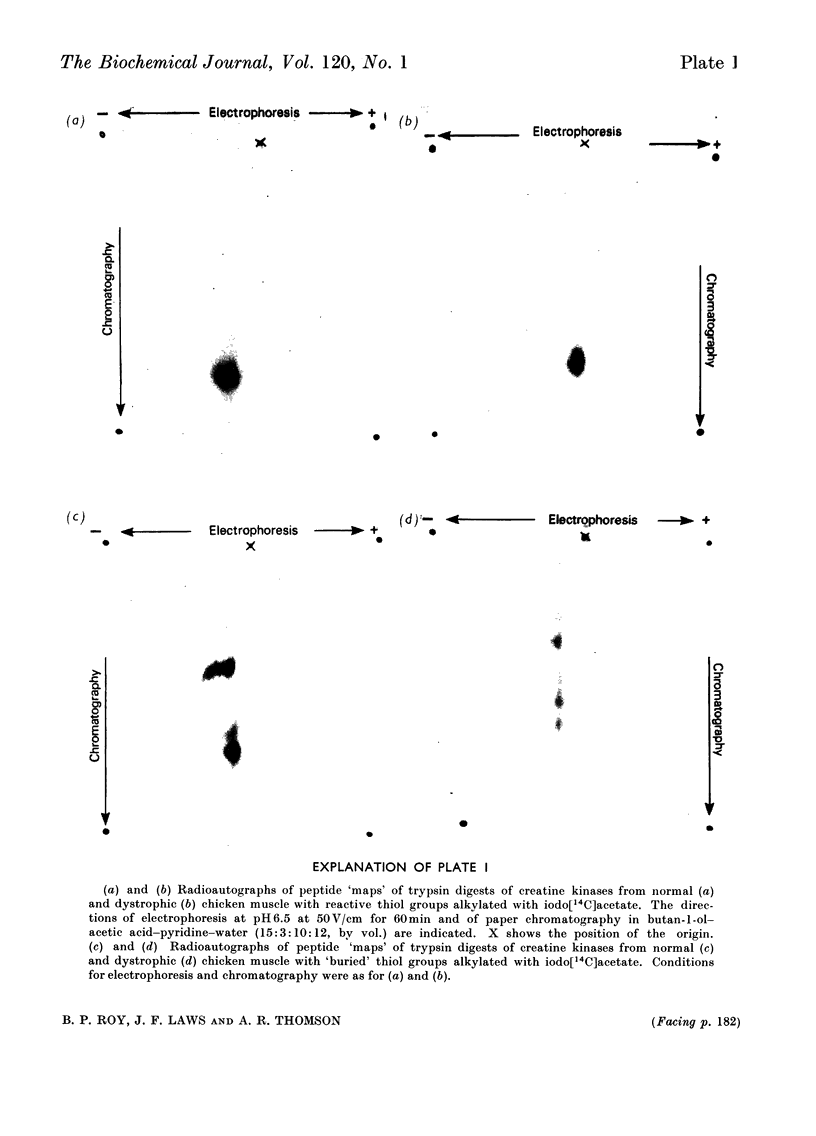
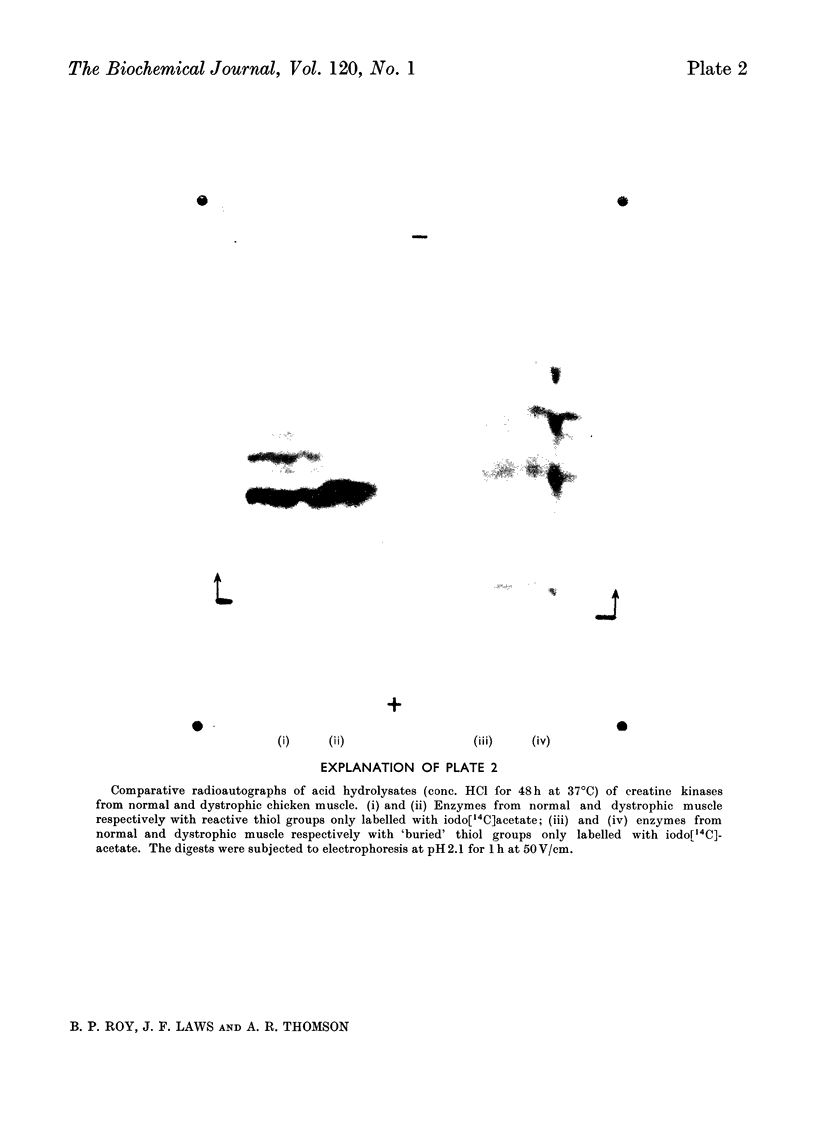
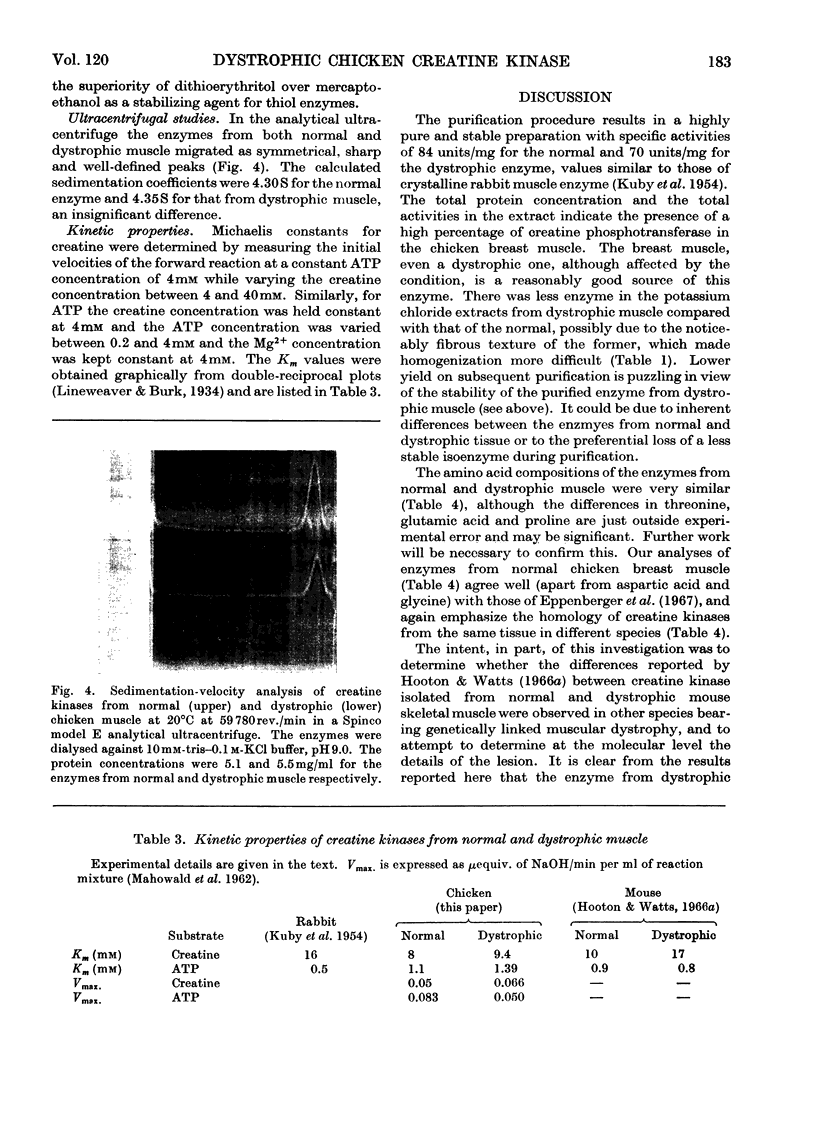
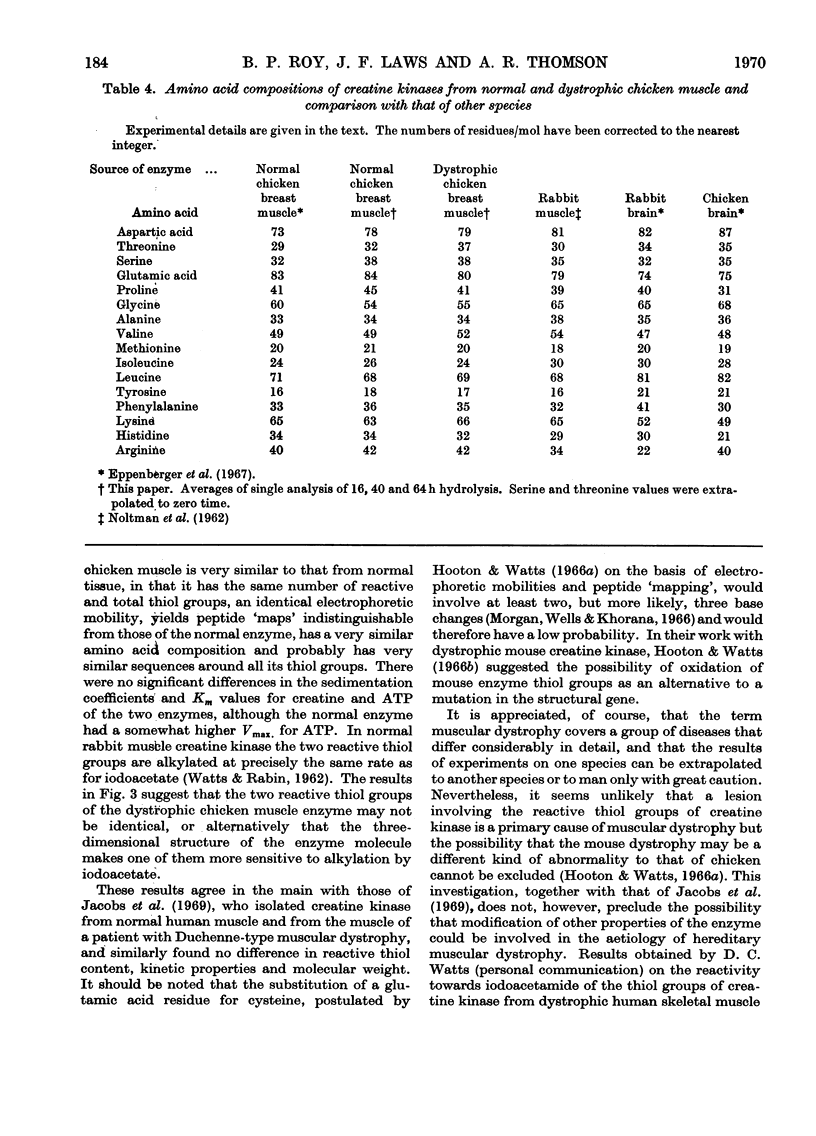
1. The purification of creatine kinase from normal and genetically dystrophic chicken breast muscle is described. Enzyme recovery was significantly lower from dystrophic muscle. 2. Both enzymes had the same number of reactive and total thiol groups and had similar specific activities and similar amino acid compositions. 3. No significant differences were observed in sedimentation, electrophoretic or kinetic properties. 4. Peptide `maps' showed no significant differences, and electrophoresis of partial acid hydrolysates of the labelled enzymes suggested that corresponding amino acid sequences around all the thiol groups were very similar. 5. The enzymes showed identical temperature stabilities. 6. No significant differences between the enzymes from normal and dystrophic muscle were observed.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMBLER R. P. THE AMINO ACID SEQUENCE OF PSEUDOMONAS CYTOCHROME C-551. Biochem J. 1963 Nov;89:349–378. doi: 10.1042/bj0890349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BENESCH R. E., LARDY H. A., BENESCH R. The sulfhydryl groups of crystalline proteins. I. Some albumins, enzymes, and hemoglobins. J Biol Chem. 1955 Oct;216(2):663–676. [PubMed] [Google Scholar]
- DAWSON D. M., KAPLAN N. O. FACTORS INFLUENCING THE CONCENTRATION OF ENZYMES IN VARIOUS MUSCLES. J Biol Chem. 1965 Jul;240:3215–3221. [PubMed] [Google Scholar]
- EPPENBERGER H. M., EPPENBERGER M., RICHTERICH R., AEBI H. THE ONTOGENY OF CREATINE KINASE ISOZYMES. Dev Biol. 1964 Aug;10:1–16. doi: 10.1016/0012-1606(64)90002-8. [DOI] [PubMed] [Google Scholar]
- Eppenberger H. M., Dawson D. M., Kaplan N. O. The comparative enzymology of creatine kinases. I. Isolation and characterization from chicken and rabbit tissues. J Biol Chem. 1967 Jan 25;242(2):204–209. [PubMed] [Google Scholar]
- HARRIS I. STRUCTURE AND CATALYTIC ACTIVITY OF ALCOHOL DEHYDROGENASES. Nature. 1964 Jul 4;203:30–34. doi: 10.1038/203030a0. [DOI] [PubMed] [Google Scholar]
- Hooton B. T., Watts D. C. Adenosine 5 -triphosphate--creatine phosphotransferase from dystrophic mouse skeletal muscle. A genetic lesion associated with the catalytic-site thiol group. Biochem J. 1966 Sep;100(3):637–646. doi: 10.1042/bj1000637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooton B. T., Watts D. C. Levels of protein and non-protein sulphydryl groups in the skeletal muscle of normal and dystrophic Bar Harbor mice. Clin Chim Acta. 1967 Apr;16(1):173–176. doi: 10.1016/0009-8981(67)90286-0. [DOI] [PubMed] [Google Scholar]
- KUBY S. A., NODA L., LARDY H. A. Adenosinetriphosphate-creatine transphosphorylase. I. Isolation of the crystalline enzyme from rabbit muscle. J Biol Chem. 1954 Jul;209(1):191–201. [PubMed] [Google Scholar]
- Kendrick-Jones J., Perry S. V. Enzymatic adaptation to contractile activity in skeletal muscle. Nature. 1965 Dec 11;208(5015):1068–1070. doi: 10.1038/2081068a0. [DOI] [PubMed] [Google Scholar]
- Kumudavalli I., Moreland B. H., Watts D. C. Properties and reaction with iodoacetamide of adenosine 5'-triphosphate-creatine phosphotransferase from human skeletal muscle. Further evidence about the role of the essential thiol group in relation to the mechanism of action. Biochem J. 1970 Apr;117(3):513–523. doi: 10.1042/bj1170513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michelson A. M., Russell E. S., Harman P. J. Dystrophia Muscularis: A HEREDITARY PRIMARY MYOPATHY IN THE HOUSE MOUSE. Proc Natl Acad Sci U S A. 1955 Dec 15;41(12):1079–1084. doi: 10.1073/pnas.41.12.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan A. R., Wells R. D., Khorana H. G. Studies on polynucleotides, lix. Further codon assignments from amino Acid incorporations directed by ribopolynucleotides containing repeating trinucleotide sequences. Proc Natl Acad Sci U S A. 1966 Dec;56(6):1899–1906. doi: 10.1073/pnas.56.6.1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigdon R. H. Hereditary myopathy in the white Pekin duck. Ann N Y Acad Sci. 1966 Sep 9;138(1):28–48. doi: 10.1111/j.1749-6632.1966.tb41152.x. [DOI] [PubMed] [Google Scholar]
- WATTS D. C., RABIN B. R. A study of the 'reactive' sulphydryl groups of adenosine 5'-triphosphate-creatine phosphotransferase. Biochem J. 1962 Dec;85:507–516. doi: 10.1042/bj0850507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WATTS D. C. STUDIES ON THE MECHANISM OF ACTION OF ADENOSINE 5'-TRIPHOSPHATE-CREATINE PHOSPHOTRANSFERASE. INHIBITION BY MANGANESE IONS AND BY P-NITROPHENYL ACETATE. Biochem J. 1963 Nov;89:220–229. doi: 10.1042/bj0890220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOOD T. Adenosine triphosphate-creatine phosphotransferase from ox brain: purification and isolation. Biochem J. 1963 Jun;87:453–462. doi: 10.1042/bj0870453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts D. C., Kumudavalli I. Further evidence for the role of the essential thiol groups in adenosine triphosphate-creatine phosphotransferase from a comparison of the human and rabbit enzymes. Biochem J. 1970 Jun;118(2):22P–23P. doi: 10.1042/bj1180022pb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts D. C., Rabin B. R., Crook E. M. The number of catalytic sites in creatine phosphokinase as determined by a study of its reactive sulphydryl groups. Biochem J. 1962 Mar;82(3):412–417. doi: 10.1042/bj0820412. [DOI] [PMC free article] [PubMed] [Google Scholar]