Abstract
1. The Widnell & Tata (1966) assay method for Mg2+-activated DNA-dependent RNA polymerase was used for initial-velocity determinations of rat liver nuclear RNA polymerase. One unit (U) of RNA polymerase was defined as that amount of enzyme required for 1 mmol of [3H]GMP incorporation/min at 37°C. 2. Colony fed rats were found to have a mean RNA polymerase activity of 65.9μU/mg of DNA and 18h-starved rats had a mean activity of 53.2μU/mg of DNA. Longer periods of starvation did not significantly decrease RNA polymerase activity further. 3. Rats that had been starved for 18h were used for all feeding experiments. Complete and tryptophan-deficient amino acid mixtures were given by stomach tube and the animals were killed 15–120min later. The response of RNA polymerase to the feeding with the complete amino acid mixture was rapid and almost linear over the first hour of feeding, resulting in a doubling of activity. The activity was still elevated above the starvation value at 120min after feeding. The tryptophan-deficient amino acid mixture produced a much less vigorous response about 45min after the feeding, and the activity had returned to the starvation value by 120min after the feeding. 4. The response of RNA polymerase to the feeding with the complete amino acid mixture was shown to occur within a period of less than 5min to about 10min after the feeding. 5. Pretreatment of the animals with puromycin or cycloheximide was found to abolish the 15min RNA polymerase response to the feeding with the complete amino acid mixture, but the activity of the controls was unaffected. 6. The characteristics of the RNA polymerase from 18h-starved animals and animals fed with the complete or incomplete amino acid mixtures for 1h were examined. The effects of Mg2+ ions, pH, actinomycin D and nucleoside triphosphate omissions were determined. The [Mg2+]– and pH–activity profiles of the RNA polymerase from the animal fed with the complete mixture appeared to differ from those of the enzyme from the other groups, but this difference is probably not significant. 7. [5-3H]Orotic acid incorporation by rat liver nuclei in vivo was shown to be affected by the amino acid mixtures in a similar manner to the RNA polymerase. 8. The tryptophan concentrations of plasma and liver were determined up to 120 min after feeding with the amino acid mixtures. Feeding with the complete mixture produced a rapid increase in free tryptophan concentrations in both plasma and liver, but feeding with the incomplete mixture did not alter the plasma concentration. The liver tryptophan concentration increased at about 45min after feeding with the tryptophan-deficient diet. 9. There was a good correlation between the liver tryptophan concentration and RNA polymerase activity in all groups of animals. 10. It was concluded that the rat liver nucleus responded to an increase in amino acid supply by increased synthesis of RNA as a result of synthesis of RNA polymerase de novo. The correlation of tryptophan concentration and RNA polymerase activity appears to reflect the general amino acid concentration required to support hepatic protein synthesis and to produce new RNA polymerase. This new polymerase appears to differ from the basal RNA polymerase by its rapid synthesis and destruction, which may be a means of regulating RNA synthesis by the amino acid concentration in the liver.
Full text
PDF


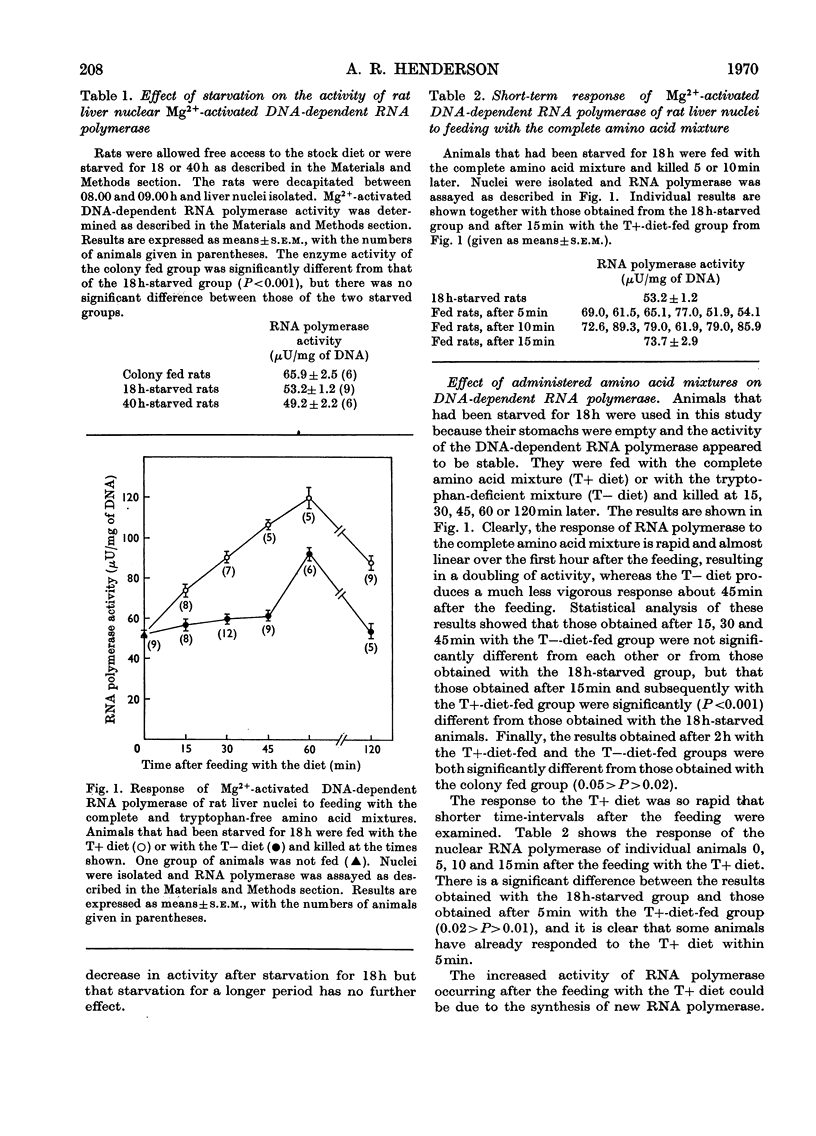


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
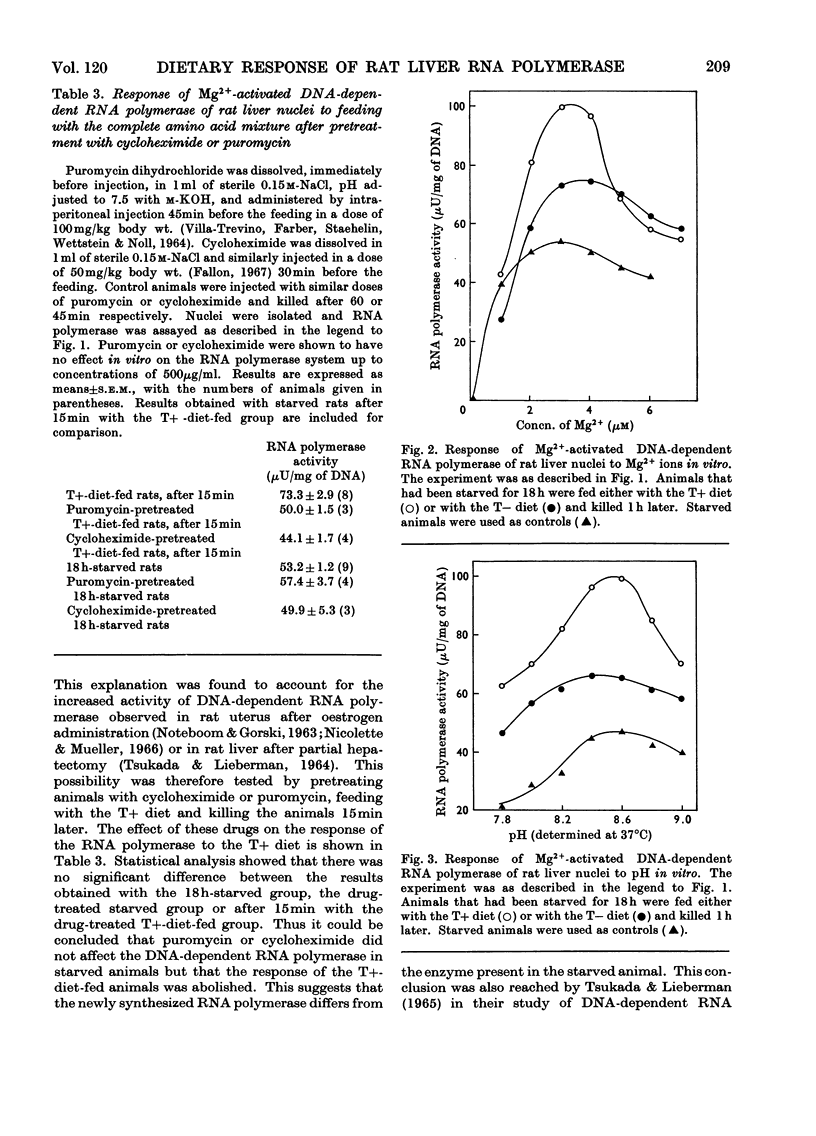
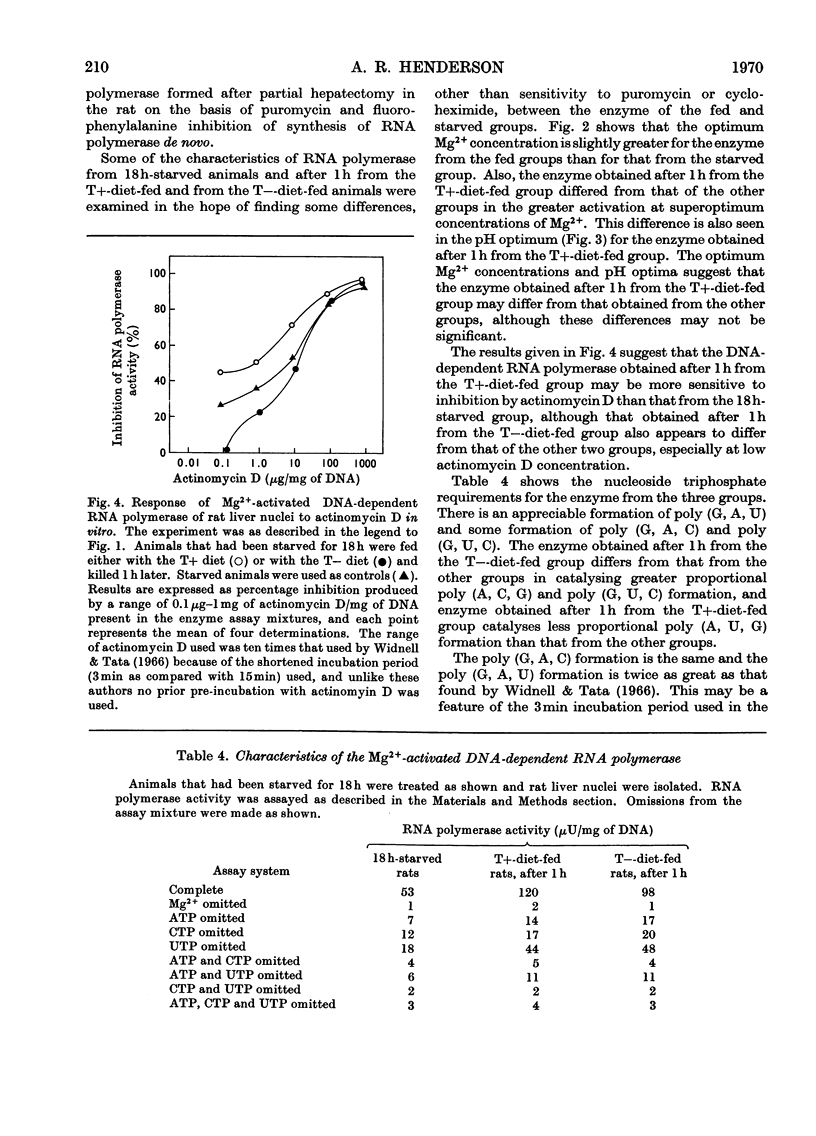
- Allen R. E., Raines P. L., Regen D. M. Regulatory significance of transfer RNA charging levels. I. Measurements of charging levels in livers of chow-fed rats, fasting rats, and rats fed balanced or imbalanced mixtures of amino acids. Biochim Biophys Acta. 1969 Oct 22;190(2):323–336. doi: 10.1016/0005-2787(69)90083-5. [DOI] [PubMed] [Google Scholar]
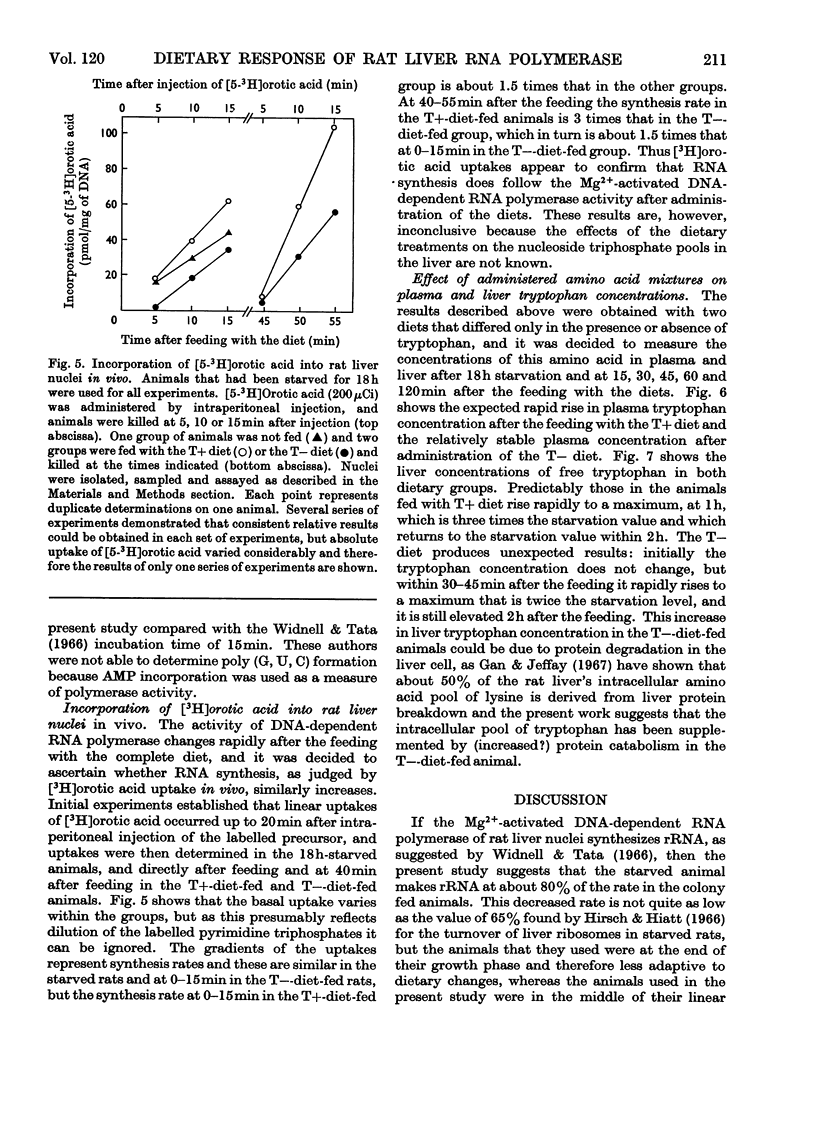
- BERNHARD W., HAGUENAU F., GAUTIER A., OBERLING C. La structure submicroscopique des elements basophiles cytoplasmiques dans le foie, le pancreas et les glandes salivaires; étude de coupes ultrafines au microscope électronique. Z Zellforsch Mikrosk Anat. 1952;37(3):281–300. [PubMed] [Google Scholar]
- Baliga B. S., Pronczuk A. W., Munro H. N. Regulation of polysome aggregation in a cell-free system through amino acid supply. J Mol Biol. 1968 Jul 14;34(2):199–218. doi: 10.1016/0022-2836(68)90247-7. [DOI] [PubMed] [Google Scholar]
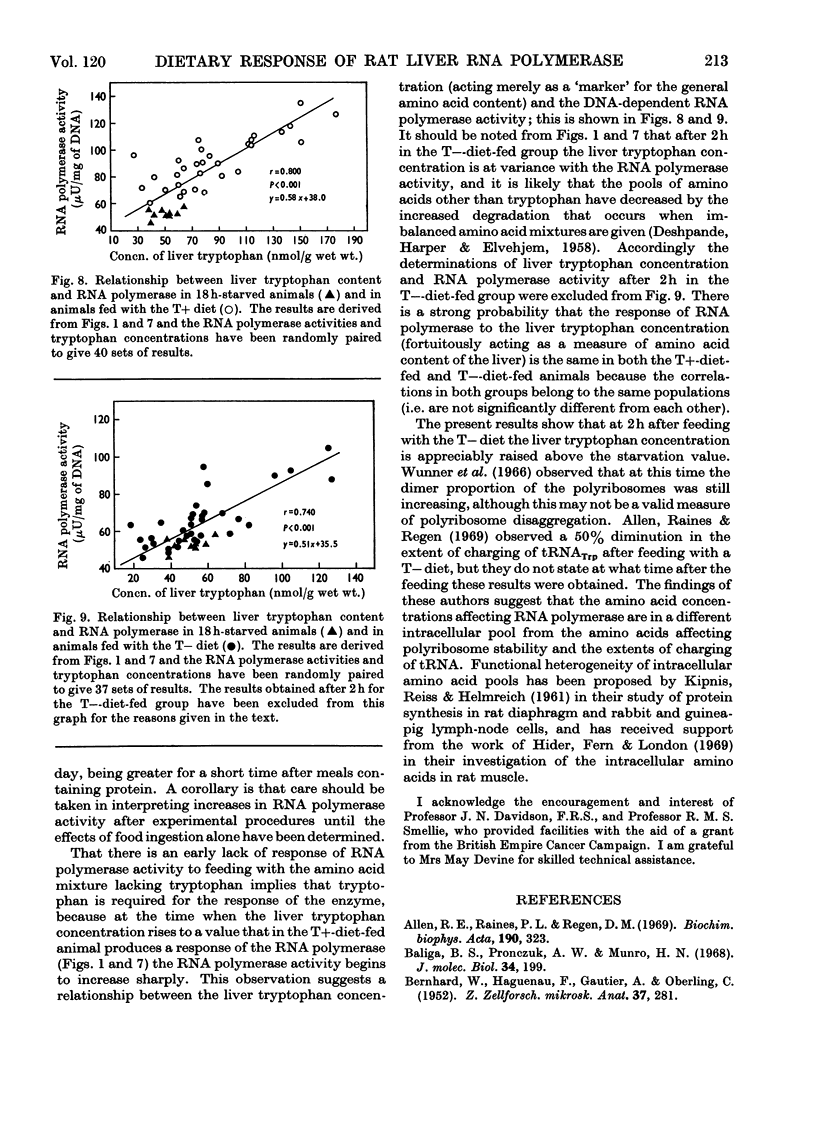
- CERIOTTI G. A microchemical determination of desoxyribonucleic acid. J Biol Chem. 1952 Sep;198(1):297–303. [PubMed] [Google Scholar]
- CERIOTTI G. Determination of nucleic acids in animal tissues. J Biol Chem. 1955 May;214(1):59–70. [PubMed] [Google Scholar]
- DESHPANDE P. D., HARPER A. E., ELVEHJEM C. A. Amino acid imbalance and nitrogen retention. J Biol Chem. 1958 Jan;230(1):335–342. [PubMed] [Google Scholar]
- Denckla W. D., Dewey H. K. The determination of tryptophan in plasma, liver, and urine. J Lab Clin Med. 1967 Jan;69(1):160–169. [PubMed] [Google Scholar]
- FLECK A., MUNRO H. N. The precision of ultraviolet absorption measurements in the Schmidt-Thannhauser procedure for nucleic acid estimation. Biochim Biophys Acta. 1962 May 14;55:571–583. doi: 10.1016/0006-3002(62)90836-3. [DOI] [PubMed] [Google Scholar]
- Fallon H. J. Regulatory phenomena in mammalian serine metabolism. Adv Enzyme Regul. 1967;5:107–120. doi: 10.1016/0065-2571(67)90012-x. [DOI] [PubMed] [Google Scholar]
- Gan J. C., Jeffay H. Origins and metabolism of the intracellular amino acid pools in rat liver and muscle. Biochim Biophys Acta. 1967 Nov 28;148(2):448–459. doi: 10.1016/0304-4165(67)90141-9. [DOI] [PubMed] [Google Scholar]
- HERBERG R. J. Phosphorescence in liquid scintillation counting of proteins. Science. 1958 Jul 25;128(3317):199–200. doi: 10.1126/science.128.3317.199. [DOI] [PubMed] [Google Scholar]
- Hider R. C., Fern E. B., London D. R. Relationship between intracellular amino acids and protein synthesis in the extensor digitorum longus muscle of rats. Biochem J. 1969 Sep;114(2):171–178. doi: 10.1042/bj1140171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch C. A., Hiatt H. H. Turnover of liver ribosomes in fed and in fasted rats. J Biol Chem. 1966 Dec 25;241(24):5936–5940. [PubMed] [Google Scholar]
- KAMMEN H. O., KLEMPERER H. G., CANELLAKIS E. S. The binding of nucleoside di- and triphosphates by a rat-liver component. Biochim Biophys Acta. 1961 Jul 22;51:175–177. doi: 10.1016/0006-3002(61)91032-0. [DOI] [PubMed] [Google Scholar]
- KIPNIS D. M., REISS E., HELMREICH E. Functional heterogeneity of the intracellular amino acid pool in mammalian cells. Biochim Biophys Acta. 1961 Aug 19;51:519–524. doi: 10.1016/0006-3002(61)90608-4. [DOI] [PubMed] [Google Scholar]
- Kosterlitz H. W. The effects of changes in dietary protein on the composition and structure of the liver cell. J Physiol. 1947 Jun 2;106(2):194–210.1. [PMC free article] [PubMed] [Google Scholar]
- Muramatsu M., Hodnett J. L., Steele W. J., Busch H. Synthesis of 28-S RNA in the nucleolus. Biochim Biophys Acta. 1966 Jul 20;123(1):116–125. doi: 10.1016/0005-2787(66)90164-x. [DOI] [PubMed] [Google Scholar]
- NOTEBOOM W. D., GORSKI J. AN EARLY EFFECT OF ESTROGEN ON PROTEIN SYNTHESIS. Proc Natl Acad Sci U S A. 1963 Aug;50:250–255. doi: 10.1073/pnas.50.2.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolette J. A., Mueller G. C. In vitro regulation of RNA polymerase in estrogen-treated uteri. Biochem Biophys Res Commun. 1966 Sep 22;24(6):851–857. doi: 10.1016/0006-291x(66)90326-3. [DOI] [PubMed] [Google Scholar]
- Penman S., Smith I., Holtzman E. Ribosomal RNA synthesis and processing in a particulate site in the HeLa cell nucleus. Science. 1966 Nov 11;154(3750):786–789. doi: 10.1126/science.154.3750.786. [DOI] [PubMed] [Google Scholar]
- Roeder R. G., Rutter W. J. Multiple forms of DNA-dependent RNA polymerase in eukaryotic organisms. Nature. 1969 Oct 18;224(5216):234–237. doi: 10.1038/224234a0. [DOI] [PubMed] [Google Scholar]
- STENRAM U. Nucleolar size in the liver of rats fed diets deficient in essential amino acids. Acta Pathol Microbiol Scand. 1956;38(5):364–374. [PubMed] [Google Scholar]
- STENRAM U. Nucleolar size in the liver of rats fed on high and nonprotein diets after starvation. Acta Anat (Basel) 1956;26(4):352–360. doi: 10.1159/000141108. [DOI] [PubMed] [Google Scholar]
- Siebert G., Villalobos J., Jr, Ro T. S., Steele W. J., Lindenmayer G., Adams H., Busch H. Enzymatic studies on isolated nucleoli of rat liver. J Biol Chem. 1966 Jan 10;241(1):71–78. [PubMed] [Google Scholar]
- TSUKADA K., LIEBERMAN I. LIVER NUCLEAR RIBONUCLEIC ACID POLYMERASE FORMED AFTER PARTIAL HEPATECTOMY. J Biol Chem. 1965 Apr;240:1731–1736. [PubMed] [Google Scholar]
- TSUKADA K., LIEBERMAN I. SYNTHESIS OF RIBONUCLEIC ACID BY LIVER NUCLEAR AND NUCLEOLAR PREPARATIONS AFTER PARTIAL HEPATECTOMY. J Biol Chem. 1964 Sep;239:2952–2956. [PubMed] [Google Scholar]
- Tata J. R., Widnell C. C. Ribonucleic acid synthesis during the early action of thyroid hormones. Biochem J. 1966 Feb;98(2):604–620. doi: 10.1042/bj0980604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VILLA-TREVINO S., FARBER E., STAEHELIN T., WETTSTEIN F. O., NOLL H. BREAKDOWN AND REASSEMBLY OF RAT LIVER ERGOSOMES AFTER ADMINISTRATION OF ETHIONINE OR PUROMYCIN. J Biol Chem. 1964 Nov;239:3826–3833. [PubMed] [Google Scholar]
- Widnell C. C., Tata J. R. Studies on the stimulation by ammonium sulphate of the DNA-dependent RNA polymerase of isolated rat-liver nuclei. Biochim Biophys Acta. 1966 Sep;123(3):478–492. doi: 10.1016/0005-2787(66)90216-4. [DOI] [PubMed] [Google Scholar]
- Wunner W. H., Bell J., Munro H. N. The effect of feeding with a tryptophan-free amino acid mixture on rat-liver polysomes and ribosomal ribonucleic acid. Biochem J. 1966 Nov;101(2):417–428. doi: 10.1042/bj1010417. [DOI] [PMC free article] [PubMed] [Google Scholar]


