Abstract
Induction by low temperature is a common feature of the lip19 subfamily members of the basic region leucine zipper gene family in plants. Here, we characterize two tobacco (Nicotiana tabacum) genes, tbzF and tbz17, belonging to the lip19 subfamily, whose gene products, TBZF and TBZ17, show 73% identity and are located in nuclei. They preferentially bind to DNA fragments spanning A-box/G-box and C-box/G-box hybrid motifs and show transactivation activity in cobombarded tobacco BY-2 cells, indicating they function as transcriptional activators. Transcripts of tbzF were detected at a high level in senescing leaves and flowers. In contrast, tbz17 transcripts could be shown to accumulate in aged leaves but not in flowers. In situ hybridization analysis revealed transcripts of tbzF and tbz17 to be predominantly located in guard cells and vascular tissues of senescing leaves. These results suggest that TBZF and TBZ17 are both involved in controlling gene transcription related to functions of guard cells in senescing leaves and that TBZF bifunctionally acts in floral development.
The control of transcription is mediated through recognition of cis elements by transcription factors (Meshi and Iwabuchi, 1995; Yanagisawa, 1998), classified into several groups on the basis of structural motifs. One of the major families of transcription factors is constituted by the basic region Leu zipper (bZIP) proteins (Landschulz et al., 1988; Hurst, 1994). Genome analysis of Arabidopsis resulted in an estimate that this organism contains almost 100 bZIP-encoding genes (Riechmann and Ratcliffe, 2000).
Within the bZIP gene family in plants, the lip19 subfamily consists of rice lip19 (Aguan et al., 1993), maize mlip15 (Kusano et al., 1995) and OBF1 (Singh et al., 1990), radish rlip (Ito et al., 1999), 910 and 911 from snapdragon (Antirrhinum majus; Martínez-García et al., 1998), and tobacco (Nicotiana tabacum) tbz17 (Kusano et al., 1998). These are all characterized by up-regulation of transcript levels upon exposure to low temperature. The snapdragon genes, 910 and 911, are expressed in flowers, predominantly in vascular tissues, carpels, and anthers (Martínez-García et al., 1998). OBF1 of maize was first identified as a gene encoding a binding factor to the ocs element found in the promoter region of the octopine synthase gene of the Agrobacterium tumefaciens T-DNA (Singh et al., 1990) and demonstrates a gradient in transcript levels in developing leaves. In the basal portions, which contain dividing and differentiating cells, the OBF1 transcript level is 40- to 50-fold higher than in apical portions, where the cells are fully differentiated. This indicates developmental control. We have found that levels of mlip15 transcripts in maize increase with leaf aging (Berberich et al., 1999), indicating regulation not only by environmental factors but also by developmental cues. In order to examine whether this feature is generalized in other plant species, particularly in dicots, we analyzed two tobacco genes: tbz17, a counterpart of lip19, and tbzF, a second tobacco member of the lip19 subfamily.
In this report, we describe that levels of tbzF and tbz17 transcripts are up-regulated in senescing leaves and that their transcripts are restricted to stomatal guard cells. Molecular features of the products of these genes are also presented.
RESULTS
Isolation of tbzF
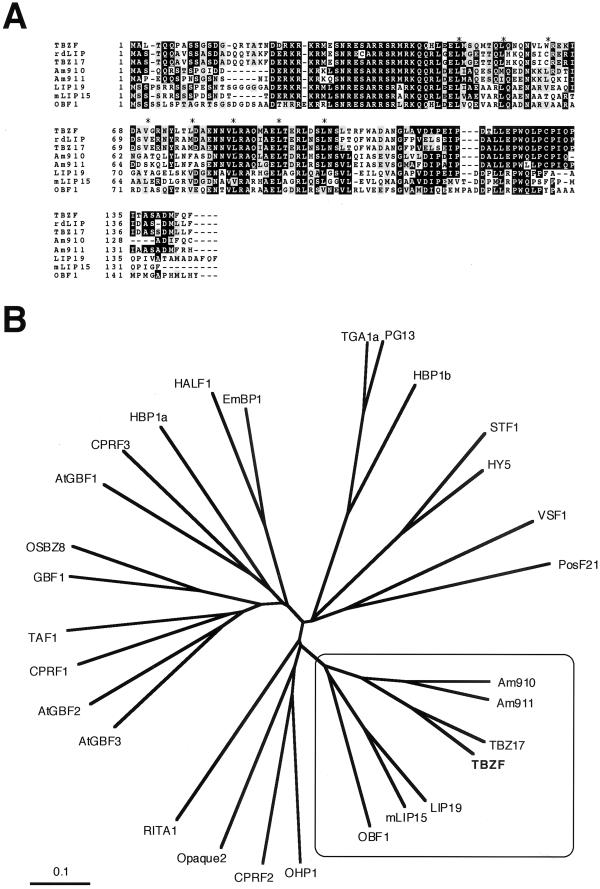
During the course of investigation of transcript accumulation of tbz17 with developmental growth, slightly larger transcripts were detected on northern hybridization using the tbz17 cDNA as a probe. These larger transcripts were observed in a fraction of flowers (data not shown), suggesting the presence of another tbz17 homolog in tobacco. For identification, a tobacco cDNA library derived from flowers was screened with the tbz17 probe. All positive clones obtained originated from a gene distinct from tbz17 and designated as tbzF (tobacco bzip protein in flower). The nearly full-length tbzF cDNA was 1,099 bp and contained an open reading frame (ORF) of 435 bp encoding a 144-amino acid protein (termed TBZF) with a relative molecular mass of 16.6 kD. Pairwise identities between TBZF and other lip19 subfamily members were found to be 73% (TBZ17), 72% (rdLIP), 60% (Am910), 57% (Am911), 52% (LIP19), and 46% (mLIP15) (Fig. 1A). Construction of a phylogenic tree indicated TBZF belongs to the lip19 subfamily (Fig. 1B), characterized by small size, OBF1 being the largest at 151 amino acids, with eight to nine heptad Leu repeats (Fig. 1A).
Figure 1.
A, Amino acid sequence alignment of TBZF and LIP19 subfamily proteins. Identical residues are highlighted in black and similar residues in gray. Dashes were introduced to maximize the alignment. A basic region and the positions of heptad Leu residues are indicated by hooked line and asterisks, respectively. TBZF (this paper), rdLIP (accession no. AB005187; Ito et al., 1999), TBZ17 (accession no. D63951; Kusano et al., 1998), Am910 (accession no. T17108), Am911 (accession no. T17110; Martínez-García et al., 1998), LIP19 (accession no. X57325; Aguan et al., 1993), mLIP15 (accession no. D26563; Kusano et al., 1995), and OBF1 (accession no. X62745; Singh et al., 1990) are used for multi-alignment. B, Phylogenic tree of plant bZIP proteins. Trees were built using Clustal X program (Thompson et al., 1997) and visualized by a TreeView program (Page, 1996). Accession nos. are as follows: AtGBF1–3 (accession nos. X63894–X63896), CPRF1–3 (accession nos. X58575–X58577), EmBP1 (accession no. P25032), GBF1 (accession no. U10270), HALF1 (accession no. D64051; 1996), HBP1a (accession no. X56781), HBP1b (accession no. X56782), HY5 (accession no. AB005456), OHP1 (accession no. L00623), Opaque 2 (accession nos. X15544 and M29411), OSBZ8 (accession no. U42208), PG13 (accession no. M62855), PosF21 (accession no. X61031), RITA1 (accession no. L34551), STF1 (accession no. L28003), TAF1 (accession no. X60363), TGA1a (accession no. S05452), TGA1b (accession no. S05453), and VSF1 (accession no. X73635). LIP19 subfamily was squared and TBZF was highlighted by bold type. The bar corresponds to 0.1 Jukes-Cantor substitutions per nucleotide.
Nuclear Localization
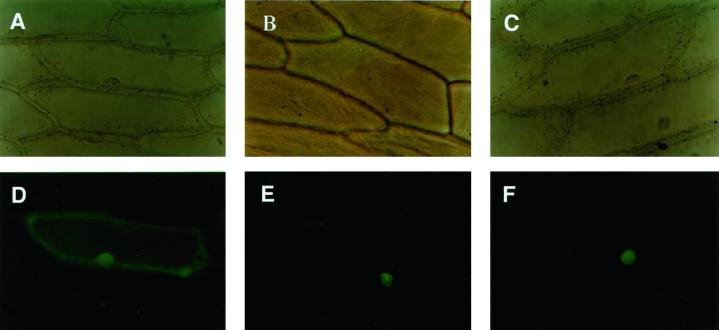
To examine the cellular localization of TBZF and TBZ17 proteins, cauliflower mosaic virus (CaMV) 35S promoter tbzF::GFP and CaMV 35S promoter tbz17::GFP genes were constructed and biolistically bombarded into onion (Allium cepa) epidermal cells. The fluorescent signals derived from these constructs were observed exclusively in nuclei (Fig. 2).
Figure 2.
Nuclear localization of TBZF and TBZ17 in onion epidermal cells. Onion bulbs were bombarded with gold particles coated with pGFP2 (A and D), pTBZF::GFP (B and E), and pTBZ17::GFP (C and F) plasmids. The proteins were transiently expressed and individual cells are observed by epifluorescence (D–F) and corresponding differential interference contrast imaging (A–C).
DNA Binding Specificity and Transcriptional Activation
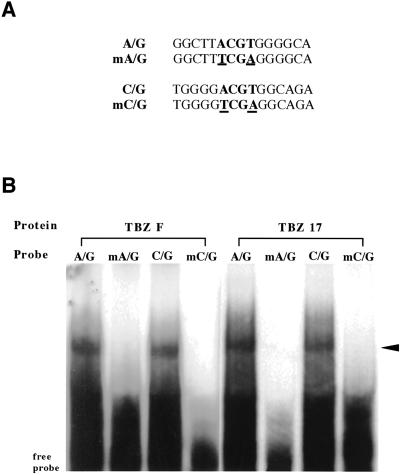
To determine the target DNA sequences of TBZF and TBZ17 homodimers in vitro, random binding site selection assay was performed (Kosugi and Ohashi, 2000). With repeated selection assay, the target sequences were enriched. TBZF and TBZ17 preferred sequences contained an A/G-hybrid box (5′-TACGTG-3′) and a C/G-hybrid box (5′-GACGTG-3′) (data not shown). Using one of each of the enriched A/G- and C/G-hybrid box sequences, electrophoretic mobility shift assays were performed. TBZF and TBZ17 bound to the A/G- and C/G-hybrid box sequences, but not to their mutated sequences (Fig. 3).
Figure 3.
TBZF and TBZ17 preferentially bind to DNA sequences containing A/G- and C/G-hybrid motifs. A, A/G- and C/G-hybrid motifs and their mutated motif sequences (mA/G and mC/G) used for electrophoretic mobility shift assay (EMSA). The mutated nucleotides are underlined. The sequences used were 60 bp, tandemly repeated four times. B, EMSA of the recombinant TBZF and TBZ17 proteins. The positions of the DNA-protein complexes are indicated by the arrowhead.
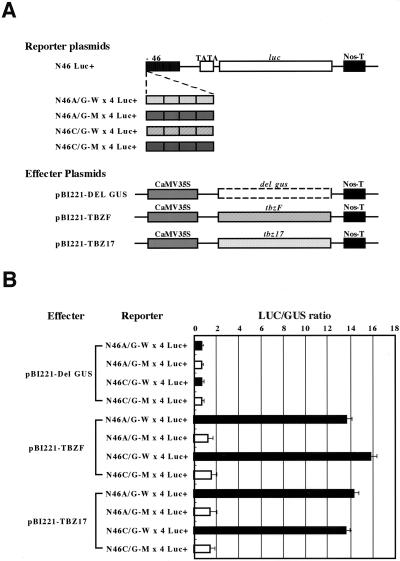
When these A/G- and C/G-hybrid box sequences were fused to the CaMV −46 minimal promoter driving the luc (luciferase) reporter gene and cobombarded with TBZF- and TBZ17-producing effector plasmids into tobacco BY-2 cells, expression of the reporter genes was enhanced approximately 14- to 16-fold (Fig. 4). Three versions of truncated TBZF derivatives, including those with a deletion of 10 amino acids in the C terminus, demonstrated diminished transactivation activity (data not shown). In the case of mutated TBZ17, which lacks the amino-terminal half (TBZ17-Δ78), activation activity of about 90% of the full-length protein was still retained. Further truncation to Δ99 or a 10-amino acid truncation at the C terminus reduced the activity to about 50% of the control (data not shown). Almost identical results were obtained with a yeast system (data not shown). These results indicate that TBZF and TBZ17 proteins efficiently bind A/G- and C/G-hybrid sequences and that they are transcriptional activators, for which their most carboxy-distal 10 amino acids are essential, although the C-terminal alone is insufficient for transactivation.
Figure 4.
TBZF and TBZ17 act as transcriptional activators in tobacco BY-2 cells. A, Schematic drawing of a basal reporter plasmid N46 Luc+ (see “Materials and Methods”) and its four different derivatives with A/G-hybrid and C/G-hybrid cis motifs and their respective mutated motifs shown in Figure 3. In N46 Luc+, the luciferase gene (luc) is placed downstream of the CaMV −46 minimal promoter (−46). B, Transactivation activity assays of TBZF and TBZ17. Effector, reporter, and reference plasmids were cobombarded into BY-2 cells by a particle delivery system (PDS-1000 He, Bio-Rad, Hercules, CA). After 18 h of incubation at 26°C, LUC (luciferase) and GUS activities were determined. LUC/GUS ratio obtained by a combination of pBI221-Del GUS and N46A/G-W ×4 Luc+ was set as 1.0 and the relative LUC/GUS values obtained from three independent experiments in duplicate assays were calculated with ses.
Stress and Hormonal Responses
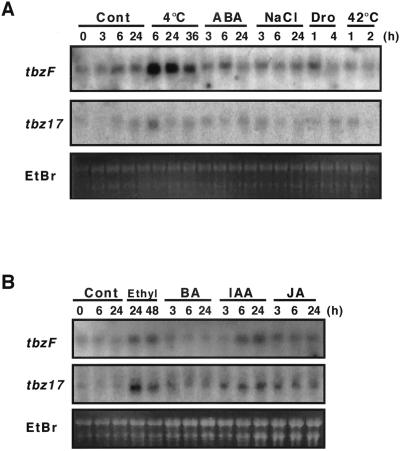
Rice lip19 was first identified as a low-temperature-responsive gene (Aguan et al., 1993). To address the responses of tbz genes to abiotic stress, northern hybridization analysis was performed. The tbzF transcripts were profoundly up-regulated by 4°C exposure for 6 h (Fig. 5), but heat shock had no effect. Drying for 1 h up-regulated tbzF expression, but high salt did not induce transcripts. In contrast, tbz17 was mildly up-regulated by low temperature, whereas other treatments were without obvious influence (Fig. 5A). Investigation of effects of phytohormones demonstrated tbzF and tbz17 transcripts to be up-regulated by ethylene, IAA, and JA, but not by BA (Fig. 5B).
Figure 5.
Response of tbzF and tbz17 genes in tobacco plants subjected to several abiotic stresses and hormonal treatments. Tobacco seedlings were subjected to abiotic stresses and hormone treatment as described in “Materials and Methods.” A, Control (Cont), 4°C, abscisic acid (ABA), 0.2 m NaCl treated (NaCl), air dried (Dro), and heat shocked (42°C). B, Ethylene treated (Ethyl), benzyladenine (BA), indole-3-acetic acid (IAA), and jasmonic acid (JA).
Transcript Accumulation in Senescing Leaves and Flowers
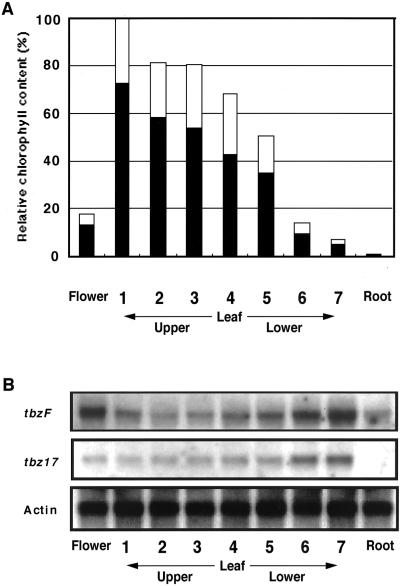
Developmental change in tbzF and tbz17 transcript levels was analyzed in matured tobacco plants bearing flower buds. In flowers, the tbzF mRNA expression was high, whereas that of tbz17 was low. In roots, tbzF transcripts were detected at low levels, whereas tbz17 transcripts were absent (Fig. 6). In leaves, the levels of tbzF and tbz17 transcripts were inversely proportional to the chlorophyll content (Fig. 6). The results indicate both tbzF and tbz17 to be novel senescense-associated genes.
Figure 6.
Tissue-specific expression of tbzF and tbz17 in a mature tobacco plant. Tobacco plant was grown in a greenhouse. Various tissues, including seven different positions of leaves, young flower buds, and roots of a tobacco plant, were harvested at approximately 12 am on a sunny day and kept at −80°C until use. A, Chlorophyll a (black bar) and b (white bar) contents of various tissues were measured as described in “Materials and Methods.” B, Total RNAs were also extracted from the same tissues and the abundance of tbzF and tbz17 transcripts were estimated by northern hybridization using the respective 3′-untranslated regions. The same filter was subsequently hybridized with the tobacco actin cDNA (actin; Thangavelu et al., 1993) to ensure equal sample loading.
Accumulation of tbzF Transcripts in Floral Organs
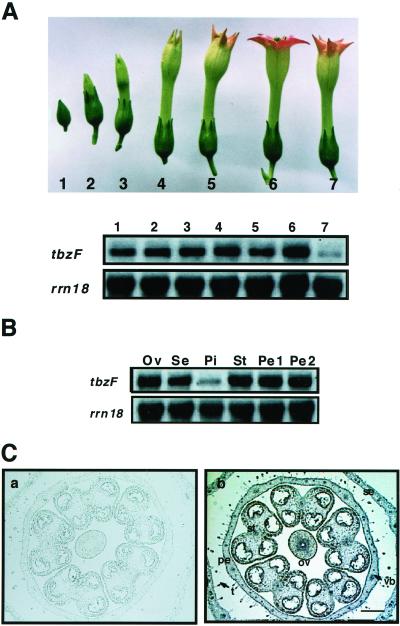
Monitoring of tbzF transcripts during flower development by northern analysis revealed a constant presence until the stage 6 of full expansion of petals, but just after petal shrinkage (stage 7) levels rapidly decreased (Fig. 7A). Transcripts of tbzF were ubiquitously detected in all flower organs except pistils (Fig. 7B). In situ RNA hybridization clearly showed that the temporal and spatial accumulation of tbzF transcripts were mainly in sepals, petals, stamens, ovaries, and pistils (Fig. 7C). Some signals were also detected in the vascular tissues of the sepals and the stems of the young inflorescence.
Figure 7.
Abundance of tbzF-transcripts along with flower development (A), in floral organs (B), and tbzF expression in flower determined by in situ hybridization (C). B, Ov, ovule; Se, sepal; Pi, pistil; St, stamen; Pe1, pink part of petal; Pe2, lower white part of petal. A and B, The same filters were subsequently hybridized with the tobacco rrn18 cDNA (rrn18; Ganal and Hemleben 1986) to ensure equal sample loading. C, Horizontal sections of flower bud at third stage were hybridized with tbzF sense (a) and antisense (b) probes. C, Bar = 20 μm.
Predominance of Transcripts in Guard Cells
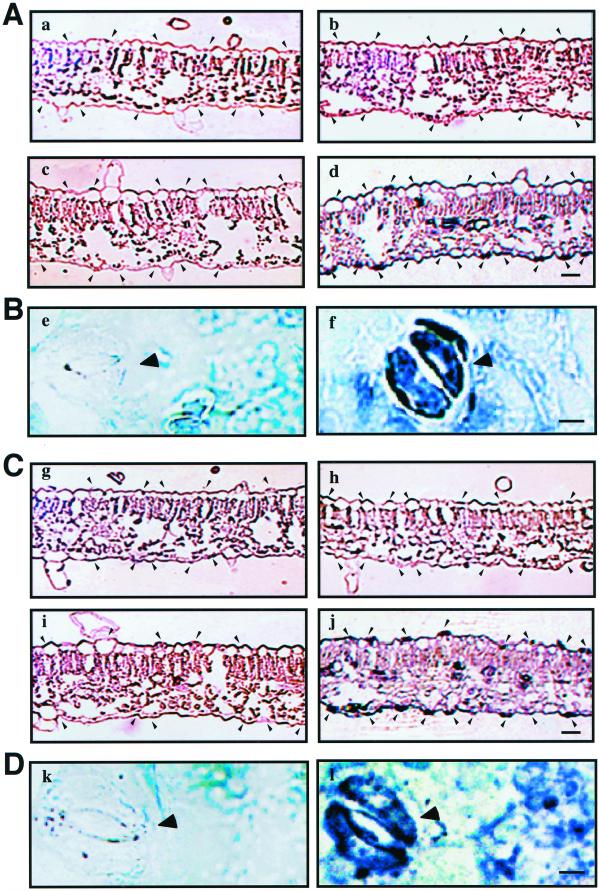
To test spatial expression of tbzF and tbz17 in senescing leaves, in situ RNA hybridization was performed. With antisense probes, very intense signals were observed for both tbzF and tbz17 in guard cells of senescing leaves, whereas only background levels of signals were detected in mesophyll cells. Their transcripts were detected in both abaxial and adaxial stomatal guard cells (Fig. 8, c, d, i, and j), and also within the epidermal cell layer of mature leaves, although signals were much weaker. Consistent with the northern results (Fig. 6), very low signals were detected in young leaves (Fig. 8, a, b, g, and h). We further confirmed the guard cell-specific expression of tbzF and tbz17 using horizontal cross sections of mature leaves (Fig. 8, e, f, k, and l).
Figure 8.
tbzF and tbz17 expression in leaves determined by in situ hybridization. Vertical sections of young leaves (a, b, g, and h) and senescing leaves (c, d, i, and j) and horizontal sections of senescing leaves (e, f, k, and l) of tobacco plants were hybridized with gene-specific sense (a, c, e, g, i, and k) and antisense (b, d, f, h, j, and l) probes of tbzF (A and B) and tbz17 (C and D) genes. A and C, Bar = 10 μm; B and D, bar = 50 μm.
DISCUSSION
Most members of the lip19 subfamily are characterized by low temperature induction, and transcripts of tbzF and tbz17 also accumulate at low temperature. In addition to this stress response, expression was found to be positively controlled with leaf development, abundance inversely correlating with the total chlorophyll content in leaves (Fig. 6). It should be emphasized that these bZIP genes are senescence-associated (Smart, 1994) and that our study revealed transcripts of tbzF and tbz17 to be predominantly expressed in guard cells of senescing leaves. To our knowledge, this is the first report of such preferential expression of bZIP-type transcription factor-encoding genes. Arabidopsis TGA6, encoding a bZIP protein of the TGA (TGACG sequence-specific binding protein) family, is expressed in aging cotyledons, vascular tissue and trichomes of senescing rosette leaves, in lateral roots, and in mature pollen grains, but not in guard cells (Xiang et al., 1997). In the case of tbzF, predominant expression in vascular tissues and trichomes in petals and sepals of flowers was also observed.
Martínez-García et al. (1998) pointed out that lip19 subfamily members have a conserved upstream ORF (uORF) in their 5′ leader sequences and suggested a common mode of posttranscriptional control. This feature was also observed in both tbzF and tbz17 cDNAs. tbzF and tbz17 contain uORFs of 25 and 28 amino acids, respectively, and the uORFs show high identity to those of other lip19 subfamily members.
From our results, TBZF, the product of a novel bZIP gene from tobacco, binds to hybrid sequences of A-box/G-box and C-box/G-box motifs and can function as a transcriptional activator. TBZ17, the product of the previously isolated tobacco member of the lip19 subfamily, shows 73% identity to TBZF and has a similar DNA binding specificity and potential for transactivation. Snapdragon bZIP proteins, 910 and 911, prefer DNA sequences containing a hybrid C-box/G-box motif (Martínez-García et al., 1998), and the products of the lip19 subfamily may bind primarily to hybrid sequences of C-box/G-box and/or A-box/G-box motifs when they take a homodimer form. As a worldwide effort, DNA sequences of the Arabidopsis genome have been completely determined. A search for TBZF- and TBZ17-recognizing sequences on Arabidopsis chromosomes II and IV reveals that there are approximately 700 sites on each, under the condition of monitoring regions within 1 kb 5′ upstream of each gene. Therefore, it is difficult to predict target genes for TBZ proteins only through genomic sequence information. However, another cue could be obtained from their specific expression in guard cells.
Stomatal guard cells are highly specialized and differentiated plant cells that play a critical role in metabolism by modulating gas exchange in photosynthetic tissues. They respond to various endogenous and exogenous signals such as light, humidity, CO2 concentration, and hormones (Assmann, 1993), and a sophisticated gene network is likely to be essential to control the metabolism of the stomatal complex, although our knowledge is as yet quite limited (Müller-Röber et al., 1998). With the aim of obtaining insights into this system, construction of a guard cell-specific cDNA library and massive DNA sequencing of the expressed clones have been started (Kopka et al., 1997; Kwak et al., 1997). Genes expressed in guard cells can be classified into at least four groups. The first encodes signal transduction components, such as AAPK (ABA-activated Ser-Thr protein kinase; Li et al., 2000) and RHA1 (Ypt/Rab-type small G protein; Terryn et al., 1993). The second encodes channel components like KAT1 and KST1 (inward-rectifying K+ channel; Müller-Röber et al., 1995), PMA4 (H+-ATPase; Moriau et al., 1993), and HAAP (aquaporin; Sarda et al., 1997). The third group comprises structural protein genes like StGCPRP (Pro-rich protein; Menke et al., 2000), and GRP1 (Gly-rich protein; Smart et al., 2000), whereas the last includes defense-related genes such as kin1 and cor6.6 (Kurkela and Frank, 1990; Gilmour et al., 1992), the ABA-responsive CdeT6–19 (Taylor et al., 1995), an acidic chitinase gene (Samac and Shah, 1991), a lipid transfer protein gene (Smart et al., 2000), and a kind of dehydrin gene, tas14 (Parra et al., 1996). Among the four, the last group seems to provide the most likely candidates as targets of TBZF or TBZ17 because kin1 and cor6.6 are also responsive to low temperature (Kurkela et al., 1990; Gilmour et al., 1992). Furthermore, transgenic plants with kin1 and cor6.6 promoter-β-glucuronidase (GUS) constructs demonstrate strong GUS activity in guard cells, pollen, and trichomes, which increased with leaf age (Wang and Cutler, 1995). The expression pattern of tbzF and tbz17 described here is strikingly similar to those of Arabidopsis kin1 and cor6.6 genes. Whereas induction of the latter genes by cold and drought has been explained by an interaction between a DRE (dehydration-responsive element)/C-repeat element and DREBP(DRE binding protein)/CBF1 (C-repeat/DRE binding factor 1) protein (Stockinger et al., 1997; Liu et al., 1998), cooperation between these regulatory factors and TBZ proteins in regulation of kin1 and cor6.6 gene expression is possible.
What could be the function of tbzF/tbz17 in guard cells of senescing leaves? One of the distinct features of guard cells is that they are the only cell type in the leaf epidermal cell layer that contains chloroplasts and are therefore photosynthetically active. It could be possible that tbzF/tbz17 expression is associated with the loss of photosynthetic activity in these specialized cells during leaf senescence. On the other hand, stomata of senescing leaves remain operable well after the mesophyll cells of the leaf have turned yellow (Willmer and Fricker, 1996). It is also probable that TBZF/TBZ17 may play a role in senescence retardation in guard cells enabling these cells to respond to environmental and endogenous stimuli, although the rest of the leaf tissue is reprogrammed for degradation. The products of tbzF and tbz17 may activate unidentified genes that function to retain cellular activity in senescing and cold-stressed guard cells and/or vascular tissues of flowers, although it remains to be clarified whether their expression is up-regulated in guard cells upon exposure to low temperature.
MATERIALS AND METHODS
Plant Materials and Various Treatments
Tobacco (Nicotiana tabacum L. cv Xanthi) seeds were surface sterilized for 10 min in a 1% (v/v) NaClO solution containing 0.1% (v/v) Tween 20, washed several times in sterile water, and placed onto 0.5% (w/v) agar containing one-half-strength Murashige and Skoog medium in plastic containers (Sigma, St. Louis). Plants were grown to their 10th leaf stage in continuous white light (90 μE) at 25°C unless described otherwise. Plants were then transferred into a compound soil mixture of metromix/vermiculite/perlite (2:1:1) in a growth chamber (TOMY, Tokyo) with a 16-h-light/8-h-dark cycle and a temperature of 25°C. Stress and hormone application was with tobacco seedlings. For treatments directed to roots, the agar was carefully removed and the plants were transferred to liquid one-half-strength Murashige and Skoog medium and maintained for another 2 d under the same conditions before the start of any treatment. In the case of hormone treatments, ABA (20 μm), BA (20 μm), JA (20 μm), or IAA (20 μm) was added to the one-half-strength Murashige and Skoog medium. For salt treatment, NaCl was added from a stock solution to the medium to give a 0.2 m final concentration. For ethylene treatment, plants were exposed to 10 μL L−1 ethylene gas in special glass containers. At each time point, second and third leaves from the tops of two plants were harvested, frozen in liquid nitrogen, and stored at −80°C until use.
Isolation and Characterization of a tbzF cDNA Clone
A tobacco cDNA library from young flower buds was screened with the entire coding region of tbz17 cDNA as a probe under standard hybridization conditions as described earlier (Sambrook et al., 1989). Nucleotide sequences were determined by the dideoxy chain termination method (Sanger et al., 1977) and assembled and analyzed with the SDC-GENETYX genetic information processing program (Lipman and Pearson, 1985; Software Development Co., Tokyo). Comparison with sequences in non-redundant databases was achieved with the BLAST program on network servers (Altschul et al., 1990). The nucleotide sequence reported in this paper has been submitted to EMBL, GenBank, and DDBJ under accession no. AB032478.
Construction of Green Fluorescent Protein (GFP) Fusion Plasmids and Fluorescence Microscopy
Entire coding region fragments for tbzF and tbz17, sandwiched with XbaI and KpnI sites, were subcloned into pGFP2 (provided by Nam-Hai Chua and Pius Spielhofer, Rockefeller University, NY), resulting in pTBZF::GFP and pTBZ17::GFP, respectively. These plasmids encode fusion proteins with truncated TBZF and TBZ17 at the N-terminal portion and GFP at the C-terminal portion. Onion bulbs cut into 9 cm2 were biolistically bombarded, as described previously (Hara et al., 2000), with gold particles (Bio-Rad) coated with the plasmids pGFP2, pTBZF::GFP, or pTBZ17::GFP. After 6-h incubation at room temperature in complete darkness, the epidermal cell layers were viewed using a microscope (PROVIS AX70, Olympus, Tokyo) equipped with a fluorescence module.
EMSA
The following oligonucleotides, A/G-hybrid (60 mer, 5′-GGCTTACGTGGGGCA-3′ ×4), C/G-hybrid (60 mer, 5′-TGGGGACGTGGCAGA-3′ ×4), mutated A/G-hybrid (60 mer, 5′-GGCTTTCGAGGGGCA-3′ ×4), mutated C/G-hybrid (60 mer, 5′-TGGGGTCGAGGCAGA-3′ ×4), and their complementary oligonucleotides were synthesized, phosphorylated with [γ-32P]ATP and T4 polynucleotide kinase, annealed, and used as probes in EMSA. EMSA was performed as described (Kusano et al., 1995) using glutathione S-transferase-fused TBZF and TBZ17 proteins produced in Escherichia coli JM109.
Assay for Transactivation Activity
Plasmid N46 Luc+, in which CaMV 35S −46 minimal promoter was placed upstream of the luc reporter gene, was constructed as follows: two primers (5′-GGGAAGCTTAAGCTCGAGATCTCGCAAGACCCTTCCTCTATATAAGGAAGTTCAT-3′ and 5′-CGTACCATGGTCTAGACAGCGTGTCCTCTCCAAATGGAAATGAACTTCCTTATATAG-3′) were annealed and filled in with dNTPs and Klenow fragment, then digested with HindIII and NcoI. The resulting HindIII-NcoI fragment was subcloned into the respective sites of 221-Luc+ (Matsuo et al., 2001) to create N46 Luc+. Four reporter plasmids, A/G-W ×4 Luc+ and C/G-W ×4 Luc+ and their mutated derivatives, mA/G-M ×4 Luc+ and mC/G-M ×4 Luc+, were constructed as follows. The primer pairs of 60 bp described above were phosphorylated, annealed, and cloned into HincII site of pBlueScript II SK−, and the respective recombinant plasmids were digested with HindIII and XhoI. The resulting HindIII-XhoI fragments were subcloned into the N46-Luc+ vector. GUS gene of the pBI221 (CLONTECH Laboratories, Palo Alto, CA) was replaced with the XbaI-SacI DNA fragments covering TBZF- and TBZ17-coding regions, resulting in pBI221-TBZF and pBI221-TBZ17. In case of pBI221-DEL GUS, the XbaI-SacI fragment derived from the multicloning sites of the pBlueScript II SK− was inserted instead of GUS gene of the pBI221. The reference plasmid pRTL2-GUS (Restrepo et al., 1990), which contains a GUS gene under a control of CaMV 35S dual promoter, was used to normalize for the efficiency of bombardment.
Tobacco BY-2 cells were maintained as described (Nagata et al., 1981). Five-day-old BY-2 cells (2 mL) after subculture were spread on 3% (w/v) agar plates and briefly allowed to dry to remove excess liquid. After bombardment, cells were harvested with 2 mL of liquid medium, transferred to a flask, and then incubated at 26°C with gentle agitation. Protein concentrations were measured using a Protein Assay kit (Bio-Rad) and luciferase activity with a PicaGene kit (Toyo Ink Co. Ltd., Tokyo). The GUS assay was carried out using an AURORA GUS assay kit (ICN Pharmaceuticals, CA) and chemical luminescence was measured with a luminometer (Lumat LB9507, Berthold, Bad Wildbad, Germany).
Chlorophyll Assays
Chlorophyll a and b content was measured spectrophotometrically by the method of Lichtenthaler (1987). Chlorophylls were extracted with acetone, and absorbance was recorded at 664 nm and 647 nm.
RNA Gel Blot Analysis
Total RNA was isolated according to the method of Nagy et al. (1988) or the acid guanidinium thiocyanate-phenol-chloroform method of Chomczynski and Sacchi (1987), and 20-μg aliquots were separated by electrophoresis in formaldehyde-1.2% (w/v) agarose gels and blotted onto Hybond N membranes (Amersham-Pharmacia Biotech, Uppsala) in 20× SSC. Hybridization was carried out as described previously (Berberich et al., 1999).
In Situ RNA Hybridization
In situ hybridization was performed using digoxigenin-labeled sense or antisense RNA produced from 3′-untranslated regions of tbzF and tbz17 cDNAs. RNA probes were synthesized by in vitro transcription with digoxigenin-UTP and T7 or SP6 RNA polymerase, according to the manufacturer's instructions (Boehringer Mannheim, Basel), and were subjected to partial alkaline hydrolysis for 20 min at 60°C (Jackson, 1991). Hybridization and immunological detection of the hybridized probes were performed according to the methods of Kouchi and Hata (1993).
ACKNOWLEDGMENTS
We wish to thank Yoshiharu Yamamoto, Minami Matsui, James Carrington, Nam-Hai Chua, and Pius Spielhofer for supplying the plasmids, and Tsukaho Hattori and Kazuyuki Hiratsuka for helpful comments and discussion. We also thank Malcolm Moore for critical reading of this manuscript.
Footnotes
This work was supported in part by the Japan Society for Promotion of Science (grant nos. JSPS–RFTF96L00602 and JSPS–RFTF 00L01604) and by the Deutsche Forschungsgemeinschaft (grant no. Be 2183/2–1).
LITERATURE CITED
- Aguan K, Sugawara K, Suzuki N, Kusano T. Low-temperature-dependent expression of a rice gene encoding a protein with a leucine-zipper motif. Mol Gen Genet. 1993;240:1–8. doi: 10.1007/BF00276876. [DOI] [PubMed] [Google Scholar]
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Assmann SM. Signal transduction in guard cells. Annu Rev Cell Biol. 1993;9:345–375. doi: 10.1146/annurev.cb.09.110193.002021. [DOI] [PubMed] [Google Scholar]
- Berberich T, Sano H, Kusano T. Involvement of a MAP kinase, ZmMPK5, in senescence and recovery from low-temperature-stress in maize. Mol Gen Genet. 1999;262:534–542. doi: 10.1007/s004380051115. [DOI] [PubMed] [Google Scholar]
- Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Ganal M, Hemleben V. Comparison of the ribosomal RNA genes in four closely related Cucurbitaceae. Plant Syst Evol. 1986;154:63–77. [Google Scholar]
- Gilmour SJ, Artus NN, Thomashow MF. cDNA sequence analysis and expression of two cold-regulated genes of Arabidopsis thaliana. Plant Mol Biol. 1992;18:13–21. doi: 10.1007/BF00018452. [DOI] [PubMed] [Google Scholar]
- Hara K, Yagi M, Kusano T, Sano H. Rapid systemic accumulation of transcripts encoding a tobacco WRKY transcription factor upon wounding. Mol Gen Genet. 2000;263:30–37. doi: 10.1007/pl00008673. [DOI] [PubMed] [Google Scholar]
- Hurst H. Transcription factors: I. bZIP proteins. Protein Profiles. 1994;1:123–168. [PubMed] [Google Scholar]
- Ito K, Kusano T, Tsutsumi K. Cold-inducible bZIP protein gene in radish root regulated by calcium- and cycloheximide-mediated signals. Plant Sci. 1999;142:57–66. [Google Scholar]
- Jackson DP. In situ hybridization in plants. In: Bowles DJ, Gurr SJ, McPhereson M, editors. Molecular Plant Pathology: A Practical Approach. Oxford: IRL Press; 1991. pp. 163–174. [Google Scholar]
- Kopka J, Provart NJ, Mueller-Röber B. Potato guard cells respond to drying soil by a complex change in the expression of genes regulated to carbon metabolism and turgor regulation. Plant J. 1997;11:871–882. doi: 10.1046/j.1365-313x.1997.11040871.x. [DOI] [PubMed] [Google Scholar]
- Kosugi S, Ohashi Y. Cloning and DNA-binding properties of a tobacco ethylene-insensitive3 (EIN3) homolog. Nucleic Acids Res. 2000;28:960–967. doi: 10.1093/nar/28.4.960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouchi H, Hata S. Isolation and characterization of novel nodulin cDNAs representing genes expressed at early stages of soybean nodule development. Mol Gen Genet. 1993;238:106–119. doi: 10.1007/BF00279537. [DOI] [PubMed] [Google Scholar]
- Kurkela S, Frank M. Cloning and characterization of a cold- and ABA-inducible Arabidopsis gene. Plant Mol Biol. 1990;15:137–144. doi: 10.1007/BF00017731. [DOI] [PubMed] [Google Scholar]
- Kusano T, Berberich T, Harada M, Suzuki N, Sugawara K. A maize DNA binding factor with bZIP motif is induced by low temperature. Mol Gen Genet. 1995;248:1–8. doi: 10.1007/BF02423445. [DOI] [PubMed] [Google Scholar]
- Kusano T, Sugawara K, Harada M, Berberich T. Molecular cloning and partial characterization of a tobacco cDNA encoding a small bZIP protein. Biochim Biophys Acta. 1998;1395:171–175. doi: 10.1016/s0167-4781(97)00161-9. [DOI] [PubMed] [Google Scholar]
- Kwak JM, Kim S, Hong SW, Nam HG. Evaluation of 515 expressed sequence tags obtained from guard cells of Brassica campestris. Planta. 1997;202:9–17. doi: 10.1007/s004250050097. [DOI] [PubMed] [Google Scholar]
- Landschulz WH, Johnson PF, McKnight SL. The leucine zipper: a hypothetical structure common to a new class of DNA binding proteins. Science. 1988;240:1759–1763. doi: 10.1126/science.3289117. [DOI] [PubMed] [Google Scholar]
- Li J, Wang X-Q, Watson MB, Assmann SM. Regulation of abscisic acid-induced stomatal closure and anion channels by guard cell AAPK kinase. Science. 2000;287:300–303. doi: 10.1126/science.287.5451.300. [DOI] [PubMed] [Google Scholar]
- Lichtenthaler HK. Chlorophylls and carotenoids: Pigment of photosynthetic biomembranes. Methods Enzymol. 1987;148:350–382. [Google Scholar]
- Lipman DJ, Pearson WR. Rapid and sensitive protein similarity searches. Science. 1985;227:1435–1441. doi: 10.1126/science.2983426. [DOI] [PubMed] [Google Scholar]
- Liu Q, Kasuga M, Sakuma Y, Abe H, Miura S, Yamaguchi-Shinozaki K, Shinozaki K. The transcription factors, DREB1 and DREB2, with an EREBP/AP2 DNA binding domain separate two cellular signal transduction pathways in drought- and low-temperature-responsive gene expression, respectively, in Arabidopsis. Plant Cell. 1998;10:1391–1406. doi: 10.1105/tpc.10.8.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-García JF, Moyano E, Alcocer MJC, Martin C. Two bZIP proteins from Antirrhinum flowers preferentially bind a hybrid C-box/G-box motif and help to define a new sub-family of bZIP transcription factors. Plant J. 1998;13:489–505. doi: 10.1046/j.1365-313x.1998.00050.x. [DOI] [PubMed] [Google Scholar]
- Matsuo N, Minami M, Maeda T, Hiratsuka K. Dual luciferase assay for monitoring transient gene expression in higher plants. Plant Biotech. 2001;18:71–75. [Google Scholar]
- Menke U, Renault N, Mueller-Röber B. StGCPRP, a potato gene strongly expressed in stomatal guard cell, defines a novel type of repetitive proline-rich proteins. Plant Physiol. 2000;122:677–686. doi: 10.1104/pp.122.3.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meshi T, Iwabuchi M. Plant transcription factors. Plant Cell Physiol. 1995;36:1405–1420. [PubMed] [Google Scholar]
- Moriau L, Bogaerts P, Jonniaux JL, Boutry M. Identification and characterization of a second plasma membrane H(+)-ATPase gene subfamily in Nicotiana plumbaginifolia. Plant Mol Biol. 1993;21:955–963. doi: 10.1007/BF00023594. [DOI] [PubMed] [Google Scholar]
- Müller-Röber B, Ehrhardt T, Plesch G. Molecular features of stomatal guard cells. J Exp Bot. 1998;49:293–304. [Google Scholar]
- Müller-Röber B, Ellenberg J, Provart N, Willmitzer L, Busch H, Becker D, Dietrich P, Hoth S, Hedrich R. Cloning and electrophysiological analysis of KST1, an inward rectifying K+ channel expressed in potato guard cells. EMBO J. 1995;14:2409–2416. doi: 10.1002/j.1460-2075.1995.tb07238.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata T, Okada K, Takebe I, Matsui C. Delivery of tobacco mosaic virus RNA into plant protoplasts mediated by reverse-phase evaporation vesicles (liposomes) Mol Gen Genet. 1981;184:161–165. [Google Scholar]
- Nagy F, Kay SA, Chua NH. Analysis of gene expression in transgenic plants. In: Gelvin SB, Schilperoort RA, editors. Plant Molecular Biology Manual, B4. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1988. pp. 1–29. [Google Scholar]
- Page RDM. TREEVIEW: an application to display phylogenic trees on personal computers. Comput Appl Biosci. 1996;12:357–358. doi: 10.1093/bioinformatics/12.4.357. [DOI] [PubMed] [Google Scholar]
- Parra MM, del Pozo O, Luna R, Godoy JA, Pintor-Toro JA. Structure of the dehydrin tas14 gene of tomato and its developmental and environmental regulation in transgenic tobacco. Plant Mol Biol. 1996;32:453–460. doi: 10.1007/BF00019097. [DOI] [PubMed] [Google Scholar]
- Restrepo MA, Freed DD, Carrington JC. Nuclear transport of plant potyviral proteins. Plant Cell. 1990;2:987–998. doi: 10.1105/tpc.2.10.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riechmann JL, Ratcliffe OJ. A genomic perspective on plant transcription factors. Curr Opin Plant Biol. 2000;3:423–434. doi: 10.1016/s1369-5266(00)00107-2. [DOI] [PubMed] [Google Scholar]
- Samac DA, Shah DM. Developmental and pathogen-induced activation of the Arabidopsis acidic chitinase promoter. Plant Cell. 1991;3:1063–1072. doi: 10.1105/tpc.3.10.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. Ed 2. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- Sanger F, Nicklen S, Coulson AR. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarda X, Tousch D, Ferrare K, Legrand E, Dupuis JM, Casse-Delbart F, Lamaze T. Two TIP-like genes encoding aquaporins are expressed in sunflower guard cells. Plant J. 1997;12:1103–1111. doi: 10.1046/j.1365-313x.1997.12051103.x. [DOI] [PubMed] [Google Scholar]
- Singh K, Dennis ES, Ellis JG, Llewellyn DJ, Tokuhisa JG, Wahleithner JA, Peacock WJ. OCSBF-1, a maize ocs enhancer binding factor: isolation and expression during development. Plant Cell. 1990;2:891–903. doi: 10.1105/tpc.2.9.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smart CM. Gene expression during leaf senescence. New Physiol. 1994;126:419–448. doi: 10.1111/j.1469-8137.1994.tb04243.x. [DOI] [PubMed] [Google Scholar]
- Smart LB, Cameron KD, Bennett AB. Isolation of genes predominantly expressed in guard cells and epidermal cells of Nicotiana glauca. Plant Mol Biol. 2000;42:857–869. doi: 10.1023/a:1006480107407. [DOI] [PubMed] [Google Scholar]
- Stockinger EJ, Gilmour SJ, Thomashow MF. Arabidopsis thaliana CBF1 encodes an AP2 domain-containing transcription activator that binds to the C-repeat/DRE, a cis-acting DNA regulatory element that stimulates transcription in response to low temperature and water deficit. Proc Natl Acad Sci USA. 1997;94:1035–1040. doi: 10.1073/pnas.94.3.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JE, Renwick KF, Webb AAR, McAinsh MR, Furini A, Bartels D, Quatrano RS, Marcotte WR, Jr, Hetherington AM. ABA-regulated promoter activity in stomatal guard cells. Plant J. 1995;7:129–134. doi: 10.1046/j.1365-313x.1995.07010129.x. [DOI] [PubMed] [Google Scholar]
- Terryn N, Arias MB, Engler G, Tiré C, Villarroel, Montagu MV, Inzé D. rha1, a gene encoding a small GTP binding protein from Arabidopsis, is expressed primarily in developing guard cells. Plant Cell. 1993;5:1761–1769. doi: 10.1105/tpc.5.12.1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thangavelu M, Belostotsky D, Bevan MW, Flavell RB, Rogers HJ, Lonsdale DM. Partial characterization of the Nicotiana tabacum actin gene family: evidence for pollen-specific expression of one of the gene family members. Mol Gen Genet. 1993;240:290–295. doi: 10.1007/BF00277069. [DOI] [PubMed] [Google Scholar]
- Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG. The CLUSTAL X windows interface: flexible strategies for multiple alignment aided by quality analysis tools. Nucleic Acids Res. 1997;25:4876–4882. doi: 10.1093/nar/25.24.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Cutler AJ. Promoters from kin1 and cor6.6, two Arabidopsis thaliana low-temperature- and ABA-inducible genes: direct strong β-glucuronidase expression in guard cells, pollen and young developing seeds. Plant Mol Biol. 1995;28:619–634. doi: 10.1007/BF00021188. [DOI] [PubMed] [Google Scholar]
- Willmer C, Fricker M. Stomata: Topics in Plant Functional Biology. Ed 2. London: Chapman & Hall; 1996. [Google Scholar]
- Xiang C, Miao Z, Lam E. DNA-binding properties, genomic organization and expression pattern of TGA6, a new member of the TGA family of bZIP transcription factors in Arabidopsis thaliana. Plant Mol Biol. 1997;34:403–415. doi: 10.1023/a:1005873500238. [DOI] [PubMed] [Google Scholar]
- Yanagisawa S. Transcription factors in plants: physiological functions and regulation of expression. J Plant Res. 1998;111:363–371. [Google Scholar]