Abstract
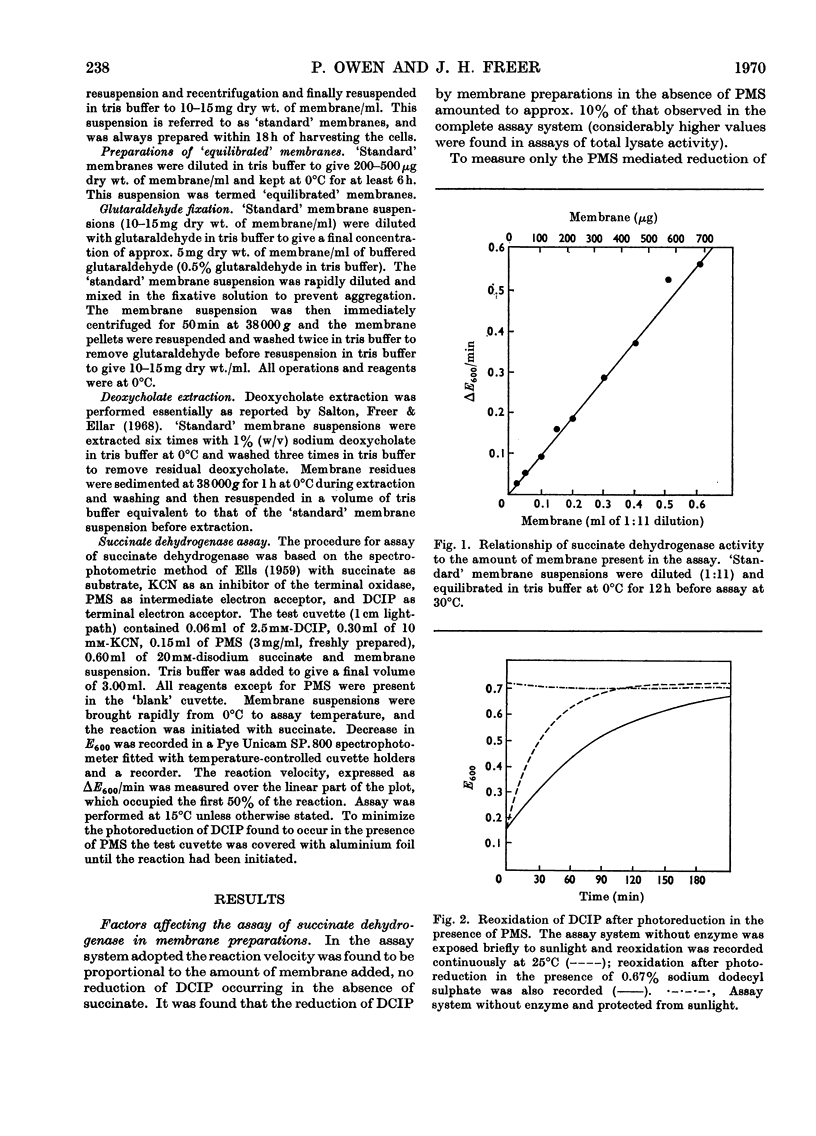
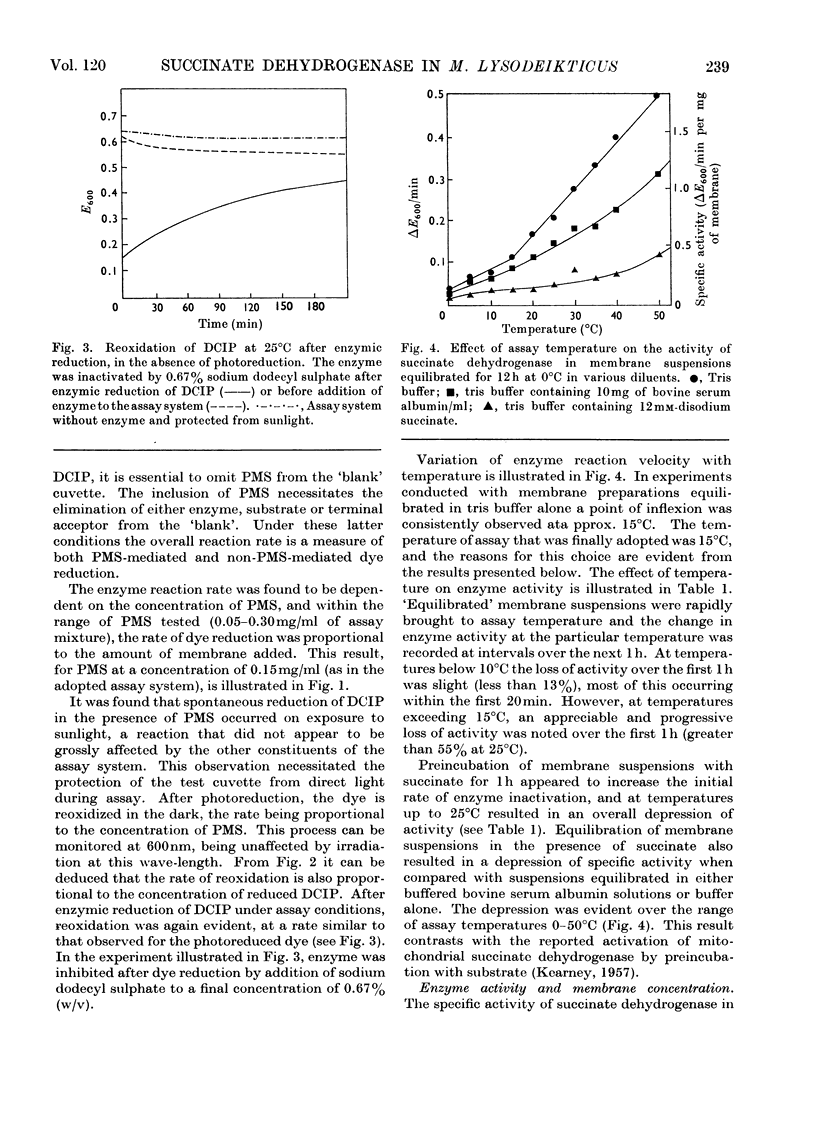
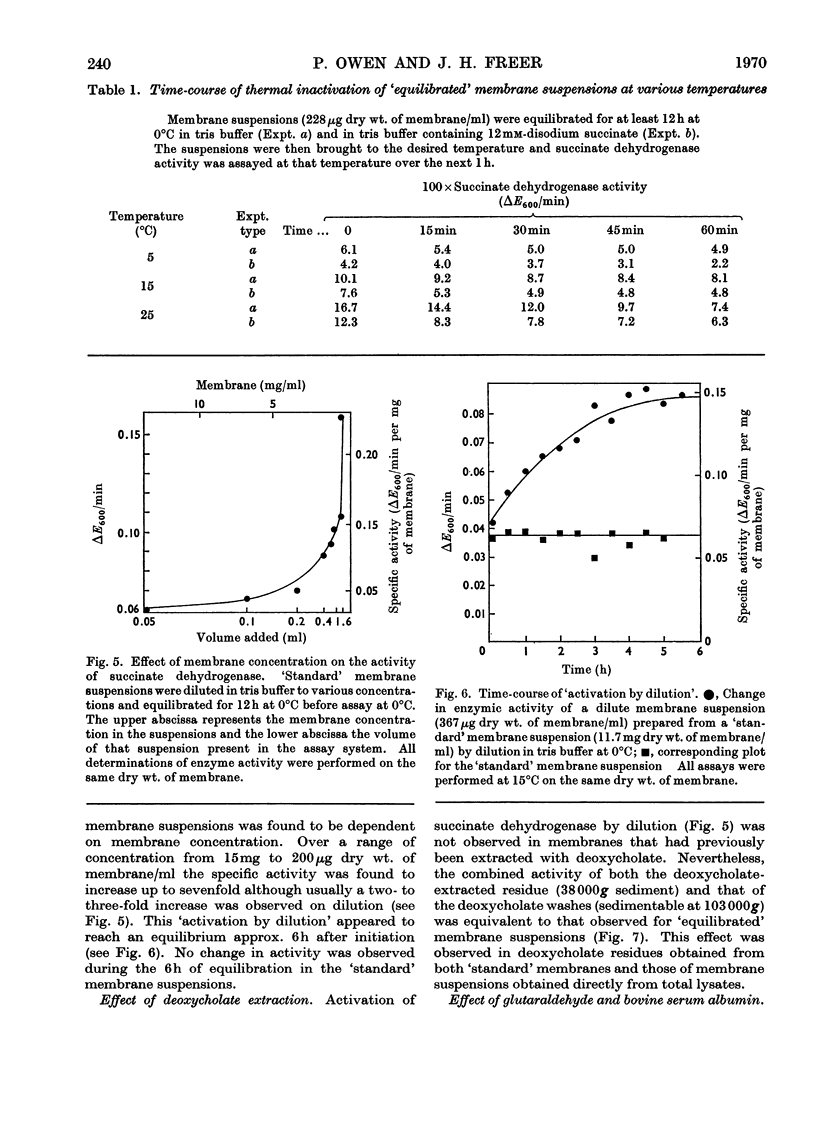
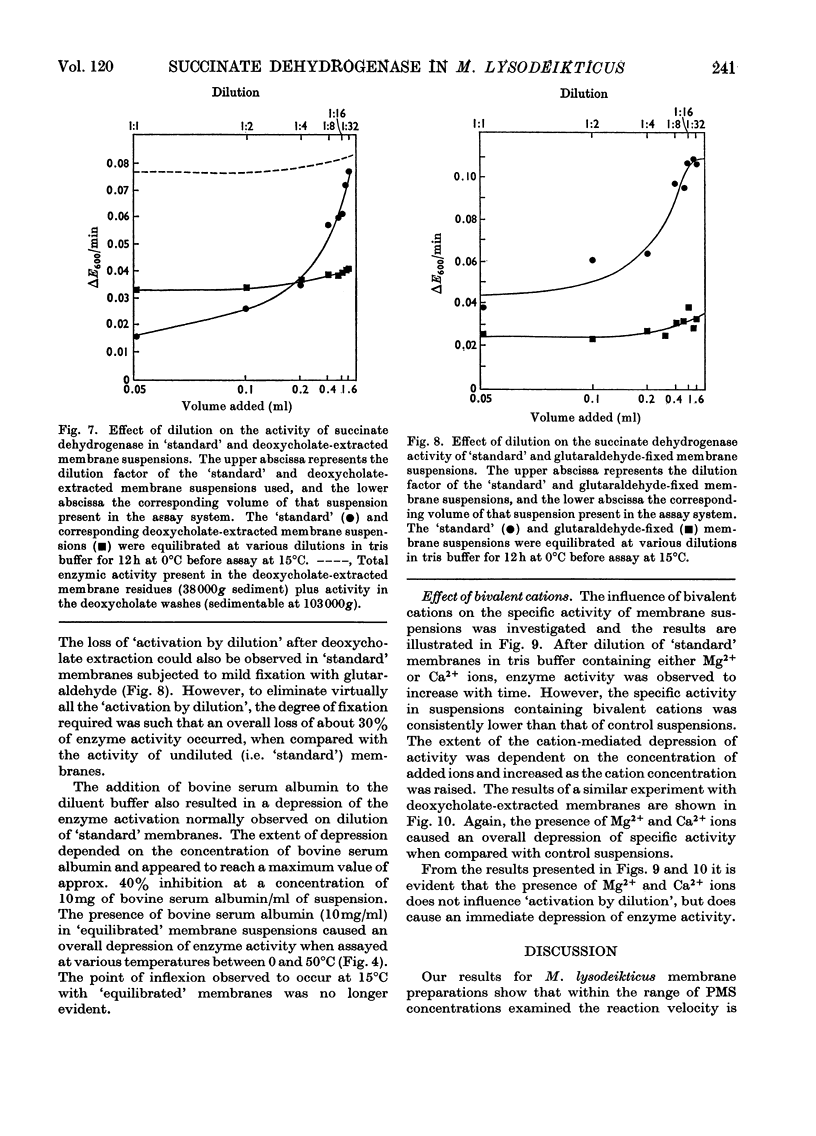
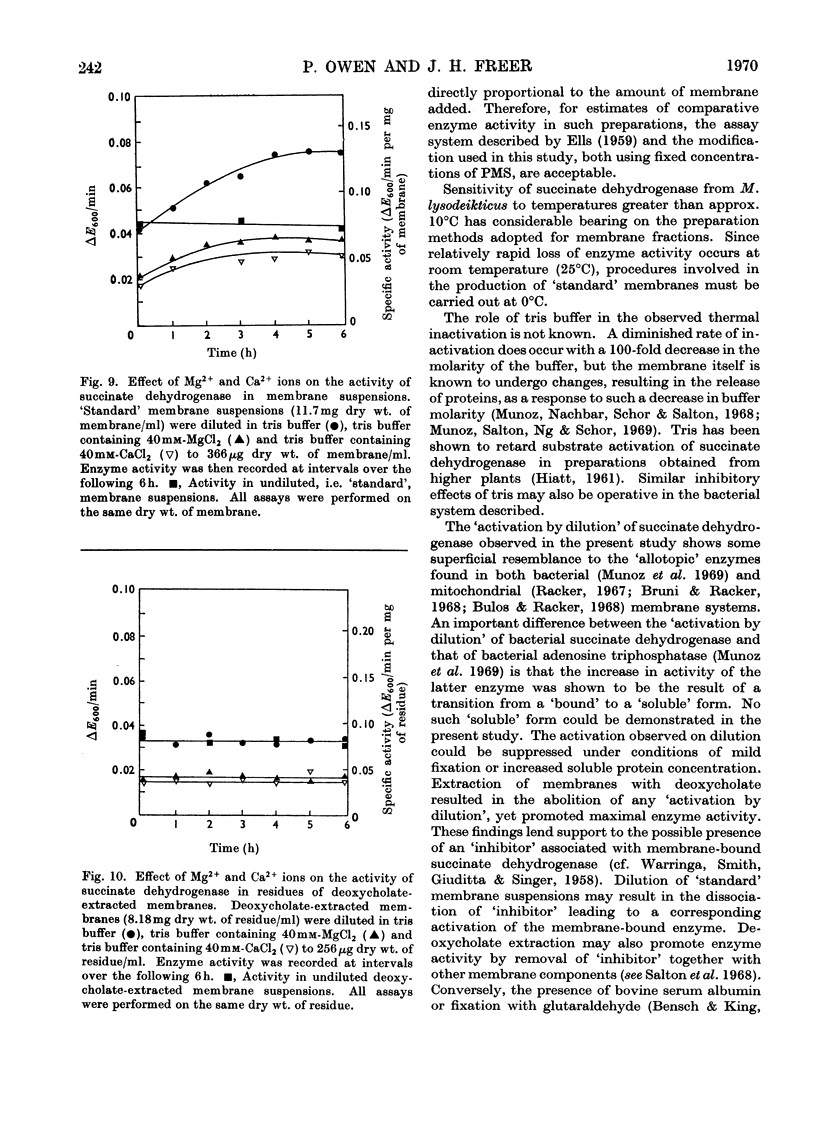
1. Some properties of succinate dehydrogenase [succinate–(acceptor) oxidoreductase, EC 1.3.99.1] in membrane preparations from Micrococcus lysodeikticus (N.C.T.C. 2665) were investigated. 2. In the spectrophotometric assay system adopted the reaction velocity was shown to be proportional to the amount of membrane added. Dichlorophenol-indophenol, reduced photochemically in the presence of phenazine methosulphate, or enzymically by the membrane-bound enzyme, was shown to undergo reoxidation in the dark. 3. The membrane-bound enzyme was found to be inactivated at temperatures above 10°C. 4. The specific activity of membrane-bound succinate dehydrogenase was found to increase between two- and three-fold in diluted membrane preparations equilibrated at 0°C for 6h. Membranes treated with sodium deoxycholate showed no enzyme activation on dilution but displayed maximal activity, all activity being sedimentable at 103000g. The increase in specific activity observed on dilution could be partially inhibited by fixation with glutaraldehyde, or by the presence of bovine serum albumin. 5. The addition of Mg2+ or Ca2+ ions to membrane suspensions caused an overall depression of enzyme activity. 6. The results suggest the presence of an `inhibitor' that affects the expression of membrane bound succinate dehydrogenase activity.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bruni A., Racker E. Resolution and reconstitution of the mitochondrial electron transport system. I. Reconstitution of the succinate-ubiquinone reductase. J Biol Chem. 1968 Mar 10;243(5):962–971. [PubMed] [Google Scholar]
- Bulos B., Racker E. Partial resolution of the enzymes catalyzing oxidative phosphorylation. 18. The masking of adenosine triphosphatase in submitochondrial particles and its reactivation by phospholipids. J Biol Chem. 1968 Jul 25;243(14):3901–3905. [PubMed] [Google Scholar]
- Butler T. F., Smith G. L., Grula E. A. Bacterial cell membranes. I. Reaggregation of membrane subunits from Micrococcus lysodeikticus. Can J Microbiol. 1967 Nov;13(11):1471–1479. doi: 10.1139/m67-195. [DOI] [PubMed] [Google Scholar]
- ELLS H. A. A colorimetric method for the assay of soluble succinic dehydrogenase and pyridinenucleotide-linked dehydrogenases. Arch Biochem Biophys. 1959 Dec;85:561–562. doi: 10.1016/0003-9861(59)90527-2. [DOI] [PubMed] [Google Scholar]
- Ferrandes B., Chaix P., Ryter A. Localisation des cytochromes de Bacillus subtilis dans les structures mésosomiques. C R Acad Sci Hebd Seances Acad Sci D. 1966 Nov 21;263(21):1632–1635. [PubMed] [Google Scholar]
- Ghosh B. K., Murray R. G. Fractionation and characterization of the plasma and mesosome membrane of Listeria monocytogenes. J Bacteriol. 1969 Jan;97(1):426–440. doi: 10.1128/jb.97.1.426-440.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KEARNEY E. B. Studies on succinic dehydrogenase. IV. Activation of the beef heart enzyme. J Biol Chem. 1957 Nov;229(1):363–375. [PubMed] [Google Scholar]
- Muñoz E., Nachbar M. S., Schor M. T., Salton M. R. Adenosinetriphosphatase of Micrococcus lysodeikticus: selective release and relationship to membrane structure. Biochem Biophys Res Commun. 1968 Aug 13;32(3):539–546. doi: 10.1016/0006-291x(68)90696-7. [DOI] [PubMed] [Google Scholar]
- Muñoz E., Salton M. R., Ng M. H., Schor M. T. Membrane adenosine triphosphatase of Micrococcus lysodeikticus. Purification, properties of the "soluble" enzyme and properties of the membrane-bound enzyme. Eur J Biochem. 1969 Feb;7(4):490–501. [PubMed] [Google Scholar]
- Racker E. Resolution and reconstitution of the inner mitochondrial membrane. Fed Proc. 1967 Sep;26(5):1335–1340. [PubMed] [Google Scholar]
- Reaveley D. A., Rogers H. J. Some enzymic activities and chemical properties of the mesosomes and cytoplasmic membranes of Bacillus licheniformis 6346. Biochem J. 1969 Jun;113(1):67–79. doi: 10.1042/bj1130067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reaveley D. A. The isolation and characterisation of cytoplasmic membranes and mesosomes of Bacillus licheniformis 6346. Biochem Biophys Res Commun. 1968 Mar 27;30(6):649–655. doi: 10.1016/0006-291x(68)90562-7. [DOI] [PubMed] [Google Scholar]
- SABATINI D. D., BENSCH K., BARRNETT R. J. Cytochemistry and electron microscopy. The preservation of cellular ultrastructure and enzymatic activity by aldehyde fixation. J Cell Biol. 1963 Apr;17:19–58. doi: 10.1083/jcb.17.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salton M. R., Freer J. H. Composition of the membranes isolated from several Gram-positive bacteria. Biochim Biophys Acta. 1965 Oct 18;107(3):531–538. doi: 10.1016/0304-4165(65)90197-2. [DOI] [PubMed] [Google Scholar]
- Salton M. R., Freer J. H., Ellar D. J. Electron transport components localized in a lipid-depleted sheet isolated from Micrococcus lysodeikticus membranes by deoxycholate extraction. Biochem Biophys Res Commun. 1968 Dec 30;33(6):909–915. doi: 10.1016/0006-291x(68)90398-7. [DOI] [PubMed] [Google Scholar]


