Abstract
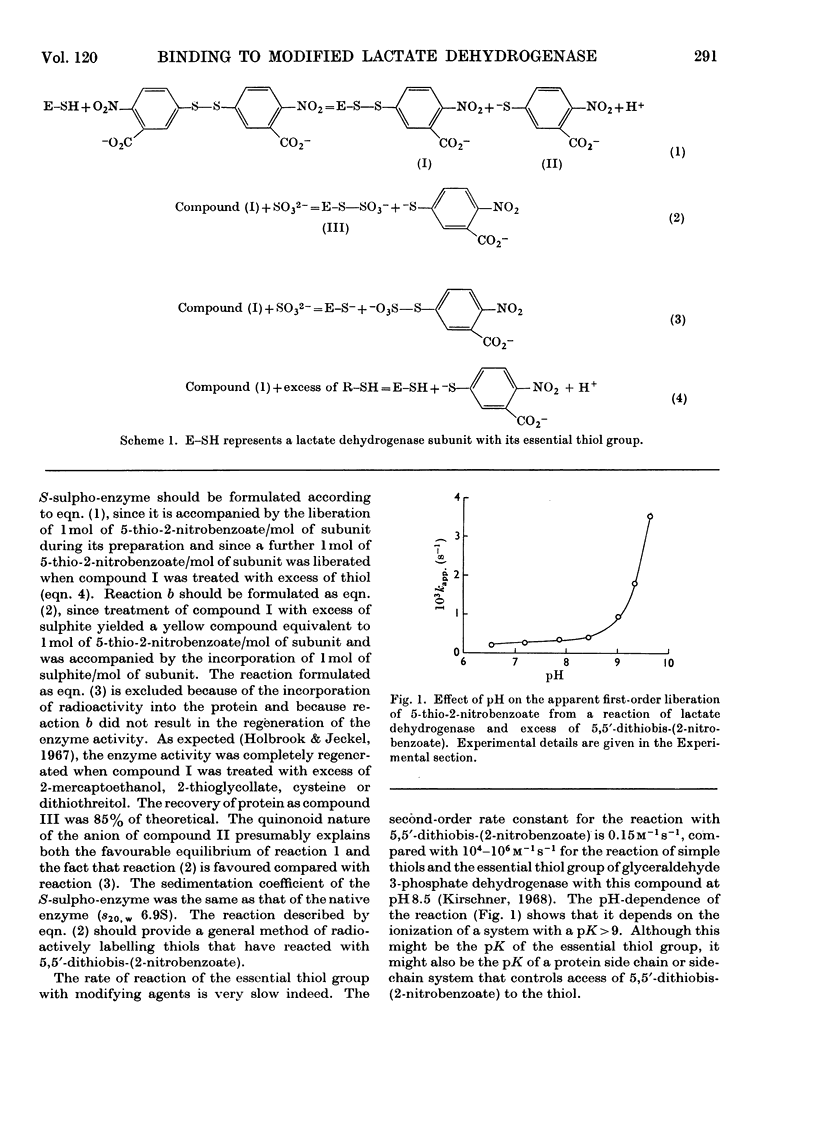
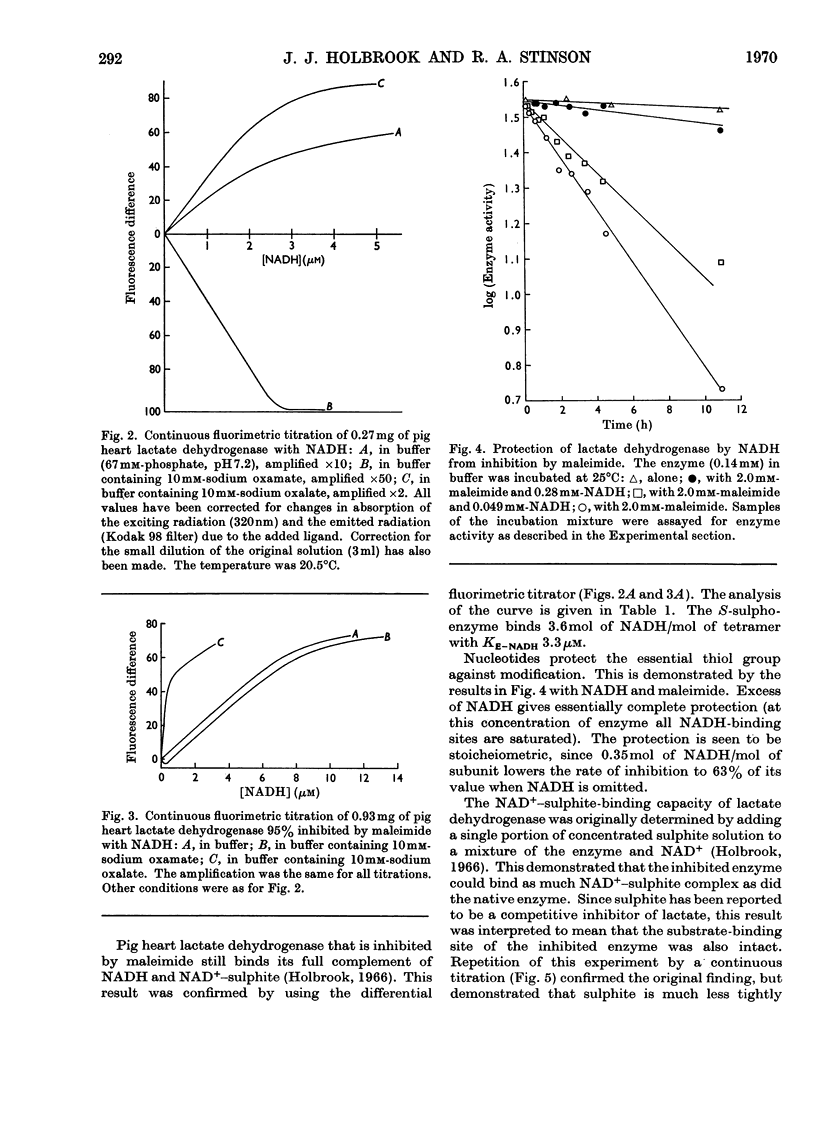
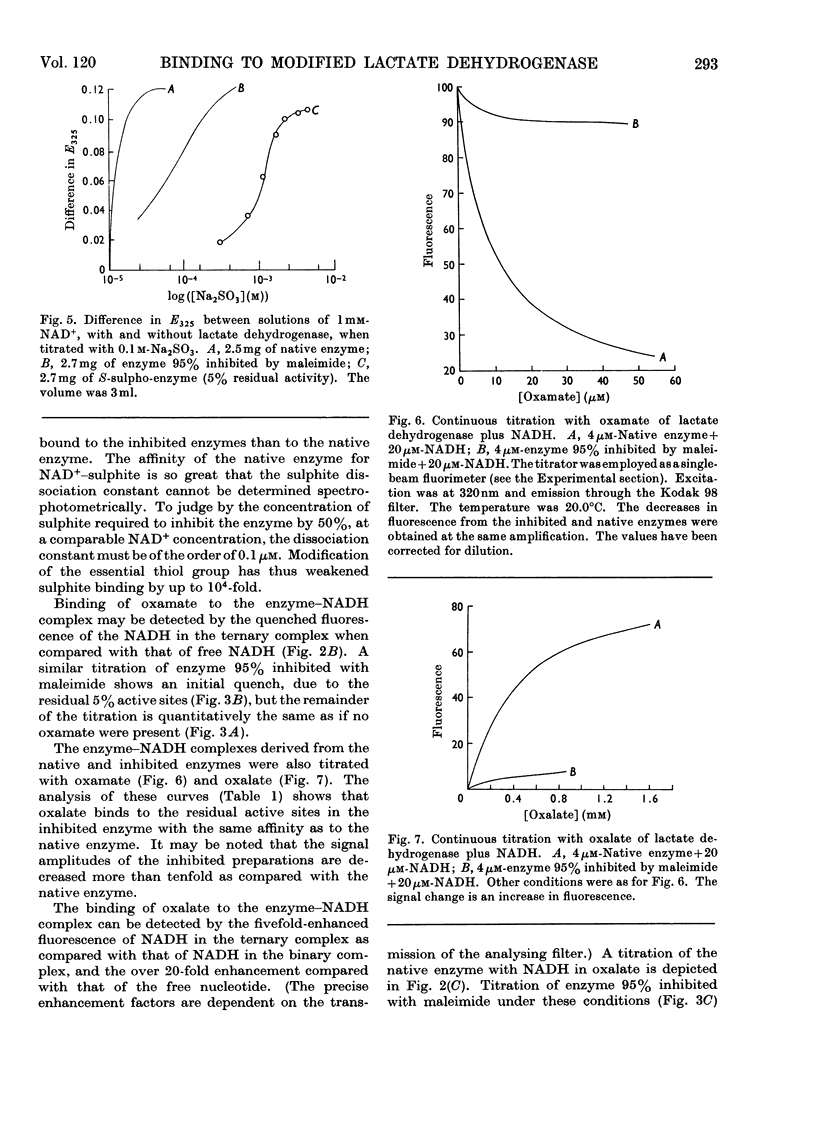
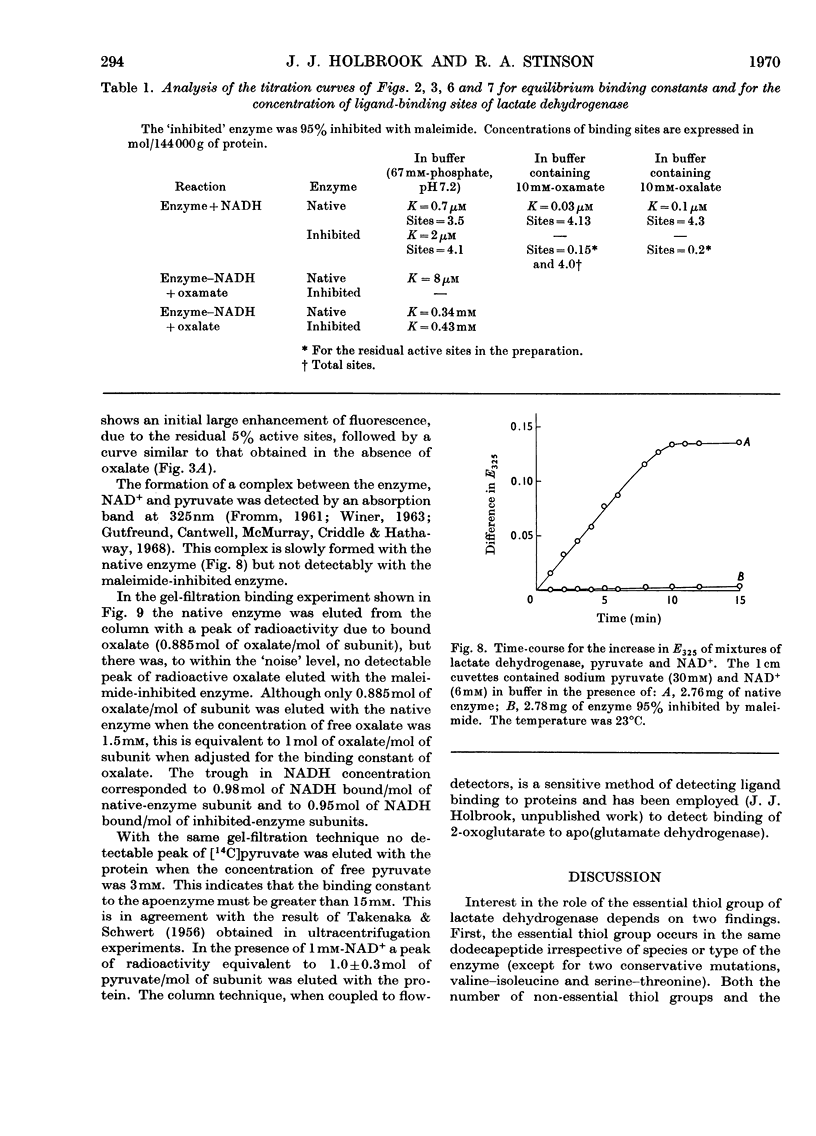
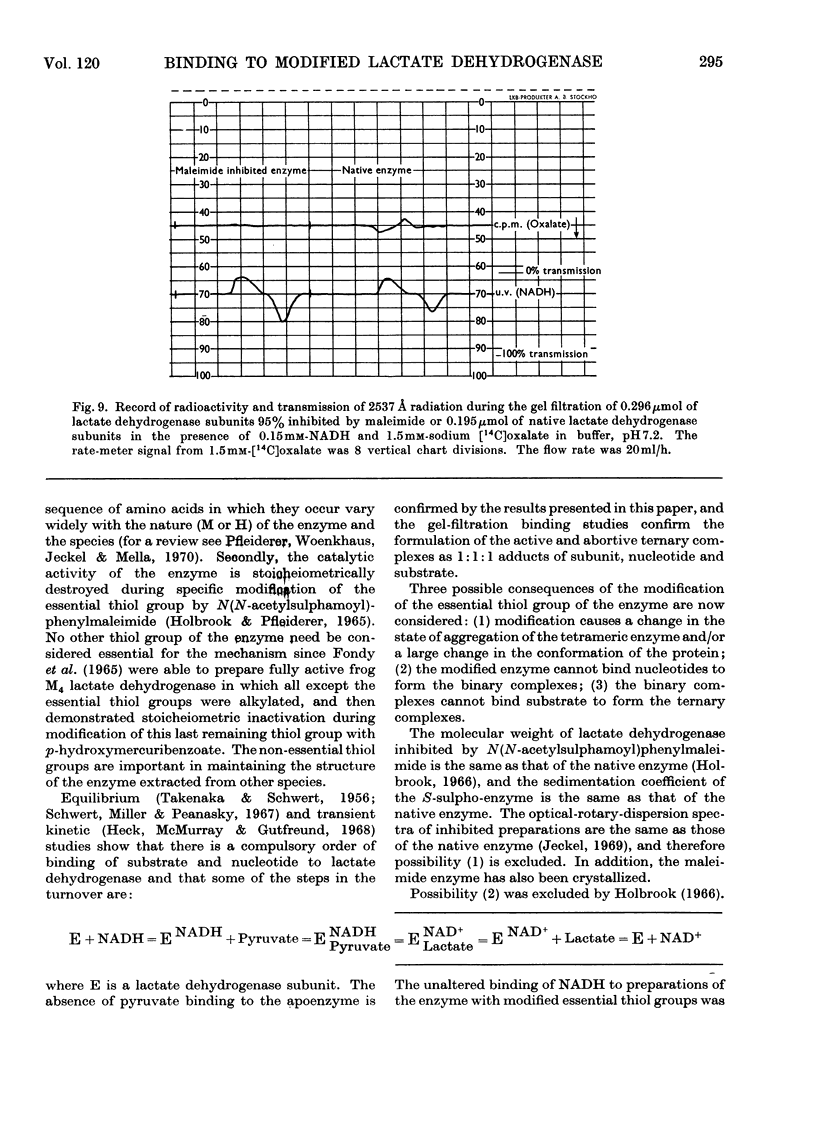
1. The preparation of a derivative of pig heart lactate dehydrogenase in which the essential thiol group has been converted into an S-sulpho group is described. The derivative has unchanged s20,w and is catalytically inactive. 2. The rate of reaction of the essential thiol group is controlled by a system with a pK>9. 3. The essential thiol group is protected by NADH against reaction with maleimide. 4. Lactate dehydrogenase in which the essential thiol group has been converted into an S-sulpho group or alkylated with maleimide still binds one molecule of NADH/subunit but with a three- to four-fold diminished affinity. 5. The inhibited enzymes also bind one molecule of NAD+–sulphite complex/subunit but with affinity decreased 103–104-fold. 6. The inhibited enzymes fail to bind C2 and C3 molecules to give the ternary complexes enzyme–NAD+–pyruvate, enzyme–NADH–oxamate and enzyme–NADH–oxalate. The 1:1:1 stoicheiometry of the last-mentioned complex with the native enzyme was established by gel filtration. 7. Structures that account for these results are discussed.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison W. S. Structure and evolution of triose phosphate and lactate dehydrogenases. Ann N Y Acad Sci. 1968 Jun 14;151(1):180–189. doi: 10.1111/j.1749-6632.1968.tb11888.x. [DOI] [PubMed] [Google Scholar]
- Duncan R. J., Tipton K. F. The oxidation and reductn of glyoxylate by lactic dehydrogenase. Eur J Biochem. 1969 Nov;11(1):58–61. doi: 10.1111/j.1432-1033.1969.tb00738.x. [DOI] [PubMed] [Google Scholar]
- FROMM H. J. Evidence for ternary-complex formation with rabbit-muscle lactic acid dehydrogenase, diphosphopyridine nucleotide and pyruvic acid. Biochim Biophys Acta. 1961 Sep 2;52:199–200. doi: 10.1016/0006-3002(61)90919-2. [DOI] [PubMed] [Google Scholar]
- Fondy T. P., Everse J., Driscoll G. A., Castillo F., Stolzenbach F. E., Kaplan N. O. The comparative enzymology of lactic dehydrogenases. IV. Function of sulfhydryl groups in lactic dehydrogenases and the sequence around the essential group. J Biol Chem. 1965 Nov;240(11):4219–4234. [PubMed] [Google Scholar]
- Gerlach D., Pfleiderer G., Holbrook J. J. Enzymatische Katalyse der Cyanid-Addition an Nicotinamid-Adenin-Dinucleotid. Biochem Z. 1965 Dec 31;343(4):354–359. [PubMed] [Google Scholar]
- Gutfreund H., Cantwell R., McMurray C. H., Criddle R. S., Hathaway G. The kinetics of the reversible inhibition of heart lactate dehydrogenase through the formation of the enzyme-oxidized nicotinamide-adenine dinucleotide-pyruvate compounds. Biochem J. 1968 Feb;106(3):683–687. doi: 10.1042/bj1060683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUMMEL J. P., DREYER W. J. Measurement of protein-binding phenomena by gel filtration. Biochim Biophys Acta. 1962 Oct 8;63:530–532. doi: 10.1016/0006-3002(62)90124-5. [DOI] [PubMed] [Google Scholar]
- Heck H. D., McMurray C. H., Gutfreund H. The resolution of some steps of the reactions of lactate dehydrogenase with its substrates. Biochem J. 1968 Aug;108(5):793–796. doi: 10.1042/bj1080793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holbrook J. J., Jeckel R. Thermal motion as the rate-limiting step in the superprecipitation of actomyosin gels. Arch Biochem Biophys. 1967 Nov;122(2):519–521. doi: 10.1016/0003-9861(67)90229-9. [DOI] [PubMed] [Google Scholar]
- Holbrook J. J., Pfleiderer G., Mella K., Volz M., Leskowac W., Jeckel R. The importance of SH-groups for enzymic activity. 7. The amino acid sequence around the essential SH-group of pig heart lactate dehydrogenase, isoenzyme I. Eur J Biochem. 1967 Jun;1(4):476–481. doi: 10.1111/j.1432-1033.1967.tb00095.x. [DOI] [PubMed] [Google Scholar]
- Holbrook J. J. The importance of SH-groups for enzymic activity. V. The coenzyme-binding capacity of pig heart lactate dehydrogenase, isozyme I, after inhibition by various maleinimides. Biochem Z. 1966 Mar 28;344(2):141–152. [PubMed] [Google Scholar]
- SCHWERT G. W., TAKENAKA Y. Lactic dehydrogenase. III. Mechanism of the reaction. J Biol Chem. 1956 Nov;223(1):157–170. [PubMed] [Google Scholar]
- Schwert G. W., Miller B. R., Peanasky R. J. Lactic dehydrogenase. X. A re-evaluation of the effects of pH upon the kinetics of the reaction. J Biol Chem. 1967 Jul 25;242(14):3245–3252. [PubMed] [Google Scholar]
- WIELAND T., DUESBERG P., PFLEIDERER G., STOCK A., SANN E. [Dehydrogenase-bound conversion products of diphosphopyridine nucleotides]. Arch Biochem Biophys. 1962 Sep;Suppl 1:260–263. [PubMed] [Google Scholar]
- Warren W. A. Catalysis of both oxidation and reduction of glyoxylate by pig heart lactate dehydrogenase isozyme 1. J Biol Chem. 1970 Apr 10;245(7):1675–1681. [PubMed] [Google Scholar]


