Abstract
Background
Primary Bilateral Macronodular Adrenal Hyperplasia (PBMAH) is a rare cause of Cushing’s syndrome due to bilateral adrenocortical macronodules. Germline inactivating variants of the tumor suppressor gene ARMC5 are responsible for 20–25% of apparently sporadic PBMAH cases and 80% of familial presentations. ARMC5 screening is now routinely performed for PBMAH patients and families. Based on literature review and own observation, this study aims to give an overview of both published and unpublished ARMC5 genetic alterations and to compile the available evidence to discriminate pathogenic from benign variants.
Results
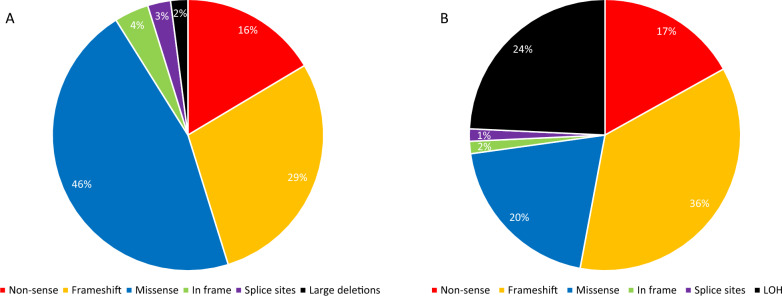
146 different germline variants (110 previously published and 36 novel) are identified, including 46% missense substitutions, 45% truncating variants, 3% affecting splice sites, 4% in-frame variants and 2% large deletions. In addition to the germline events, somatic 16p loss-of-heterozygosity and 104 different somatic events are described. The pathogenicity of ARMC5 variants is established on the basis of their frequency in the general population, in silico predictions, familial segregation and tumor DNA sequencing.
Conclusions
This is the first extensive review of ARMC5 pathogenic variants. It shows that they are spread on the whole coding sequence. This is a valuable resource for genetic investigations of PBMAH and will help the interpretation of new missense substitutions that are continuously identified.
Keywords: ARMC5, Genetics, Adrenal gland, Cushing’s syndrome, Primary Bilateral Macronodular Adrenal Hyperplasia, Cortisol, ACTH
Background
Primary Bilateral Macronodular Adrenal Hyperplasia (PBMAH) is an adrenal cause of cortisol excess (Cushing’s syndrome) due to bilateral adrenocortical macronodules. Even though it is the most frequent cause of bilateral adrenal tumors, PBMAH remains an apparently rare, but probably underdiagnosed disease [1]. Rare syndromic presentations of PBMAH can be observed as part of dominantly inherited diseases such as multiple endocrine neoplasia type 1 (MEN1) [2–4], familial adenomatous polyposis (FAP) [5–7], hereditary leiomyomatosis and renal cell cancer (HLRCC) [8, 9], due to germline pathogenic variants of MEN1 (Menin), APC (Adenomatous Polyposis Coli) and FH (Fumarate Hydratase) genes, respectively, and others [10]. However, PBMAH presents most frequently (probably more than 95%) in an apparent isolated form, and in this presentation, the bilateral nature of adrenal involvement and the reports of familial cases in the early 1990s [11] led to the suspicion of a genetic predisposition. In 2013, ARMC5 (Armadillo repeat containing 5) germline heterozygous inactivating variants have been identified in PBMAH patients treated by adrenalectomy [12]. Adrenal nodules harbor a second somatic hit, whether by loss-of-heterozygosity (LOH) or point variation, leading to a bi-allelic inactivation of the gene, which is a common pattern of tumor suppressor genes. In patients with a germline heterozygous mutation of a given tumor suppressor gene (inherited or de novo), the occurrence of a somatic mutation on the other allele of the same gene in a unique cell (an adrenocortical cell in the case of ARMC5) leads to the loss of both alleles. This is thought to be the starting point of tumorigenesis in this model. At the time of its identification in PBMAH, ARMC5, located at 16p11.2, had never been implicated in human diseases and its function was poorly known. A loss of ARMC5 protein in tumoral tissues with bi-allelic inactivation has been observed [12], driving the development of benign adrenal tumors. Indeed, apoptosis is impaired in cells transiently transfected with ARMC5 mutated expressing vectors, compared to ARMC5 wild-type expressing cells [12, 13]. Furthermore, restoration of the apoptosis process after wild-type ARMC5 re-expression in primary cell cultures from PBMAH patients with ARMC5 variant has been reported [14]. More recent studies support the involvement of ARMC5 in cell cycle regulation [14] and its turn-over processing by the ubiquitin–proteasome system [15, 16]. Mice knockout for Armc5 have been engineered. They brought information about ARMC5 implication in early developmental stages, in particular for adrenal glands [17], and its implication in lymphocytes growth and differentiation [18].
Since the initial 2013 report, ARMC5 germline sequencing is routinely offered in our center to PBMAH patients and to all first-degree relatives of ARMC5 pathogenic variant carriers. Sporadic (i.e. apparently not familial) PBMAH usually presents with mild hypercortisolism [19], but germline ARMC5 pathogenic variants, responsible for around 20 to 25% of PBMAH index-cases, are associated with a more pronounced phenotype than observed in wild-type patients, in terms of cortisol secretion, adrenal morphology and complications of Cushing’s syndrome [13, 20]. ARMC5 variants have also been related to the occurrence of meningioma in some reports [21–27]. A recent study by our group stated that ARMC5 genotyping should be conditional to the clear presence of bilateral adrenal macronodules (regardless of the number of nodules or size of the adrenals), associated with at least a mild autonomous cortisol secretion, defined by a plasma cortisol after overnight 1 mg dexamethasone suppression test (DST) above 50 nmol/L [20].
Results of ARMC5 sequencing have also been reported in patients with different phenotypes of the large spectrum of the disease, sometimes incompletely satisfying PBMAH definitions and also, for academic purpose, in patients presenting with adrenal benign nodules in other contexts (MEN1, primary hyperaldosteronism, adrenal incidentalomas). The germline ARMC5 screening in patients with neuroendocrine tumors (sporadic or associated with MEN1) retrieved germline variants that were at this time classified as likely pathogenic [28]. However, in the light of the most recent data about these variants, all seem to be benign polymorphisms, synonymous variants, or missense variants of uncertain significance, for which the pathogenic nature is unlikely. In the same series, somatic ARMC5 variants and 16p LOH have been identified in adrenal and extra-adrenal tumors from MEN1 patients without germline ARMC5 variant, and sometimes LOH and point variants were observed in the same tissue [28]. Besides, the systematic ARMC5 germline screening in 56 patients with primary aldosteronism identified possibly deleterious variants in 6 unrelated African-American patients [29]. There was no difference regarding the levels of cortisol or aldosterone secretion between variant carriers and wild-type patients, and no second hit has been found in the adrenal tissue of 2 operated variant carriers. Additionally, no ARMC5 variant has been found in an independent series of patients with primary aldosteronism and bilateral adrenal hyperplasia [30], nor in a series of 4 families with familial hyperaldosteronism type II [31]. At present, there is no strong evidence for a direct relation between ARMC5 inactivation and mineralocorticoid excess. Recently, a group reported the results of a systematic germline genetic screening of patients with adrenocortical carcinoma (ACC), including ARMC5 sequencing [32]. Germline variants of ARMC5 were found in 5 of 150 patients with ACC, these variants are likely benign (p.Thr9Met and p.Pro731Arg) or of uncertain significance (p.Ala23del). No malignant evolution of PBMAH has ever been documented in ARMC5 variant carriers, so the causative role of these variants in ACC is very unlikely. To date, we cannot affirm the involvement of ARMC5 in any adrenal condition other than PBMAH.
Numerous ARMC5 variants have been reported at present, including nearly 50% of missense variants, for which the pathogenic nature is often uncertain. This study aims to give an overview of ARMC5 alterations already published and recently identified in our center and to propose clues to discriminate pathogenic variants (mutations) from non-pathogenic variants (benign polymorphisms).
Variants
We performed a systematic literature review for compilation of reported ARMC5 variants by a search of papers on PubMed and MEDLINE databases, reported from inception of the databases and October 1st, 2024, using the term “ARMC5”. Unpublished variants recently identified in PBMAH patients genotyped in our center were also included in the analysis. These variants were identified by Sanger sequencing as previously described [12] or by next generation sequencing using IonTorrent and Illumina technologies (genomic platform of the Cochin Institute and unit of Oncogenetics of the Cochin hospital) after patient written informed consent for genetic analysis and its research use according to the national regulation. Sequence alignment, variant calling, and variant annotation were performed using MOABI Leaves pipeline (APHP). An assessment of variants’ pathogenicity was performed according to the American College of Medical Genetics and Genomics and the Association for Molecular Pathology (ACMG-AMP) guidelines [33]. Variants were classified in one of the five classes given below: class 1: benign variant, class 2: likely benign variant, class 3: variant of uncertain significance (VUS), class 4: likely pathogenic variant, and class 5: pathogenic variant. Assessment of variants implication was mainly performed based on population databases (gnomAD, v2.1.1, https://gnomad.broadinstitute.org/), variant databases (ClinVar), in silico prediction softwares, familial segregation, and tumoral DNA analysis.
Variants identified in germline DNA were differentiated from those identified in DNA tumor samples. The clinical context was briefly described to distinguish variants identified in PBMAH (and with associated meningioma where appropriate) patients from variants identified in other types of adrenal tumors. To highlight recurrent variants affecting one position, we did not take into account the variants cited repeatedly by a same team, with the exception when unrelated patients were reported. Familial presentations of the disease, usually known before genetic screening, were documented when provided. Somatic variants were listed, and associated germline events were specified if known. All the variants affecting coding exonic sequences and splice sites for canonical transcript NM_001105247.1 were collected. HGVS nomenclature was verified using Mutalyzer online tool (https://mutalyzer.nl/). We used Homo sapiens (human) genome assembly GRCh37 (hg19). In silico prediction synthesis was performed for each variant using Varsome (https://varsome.com/).
Germline ARMC5 alterations
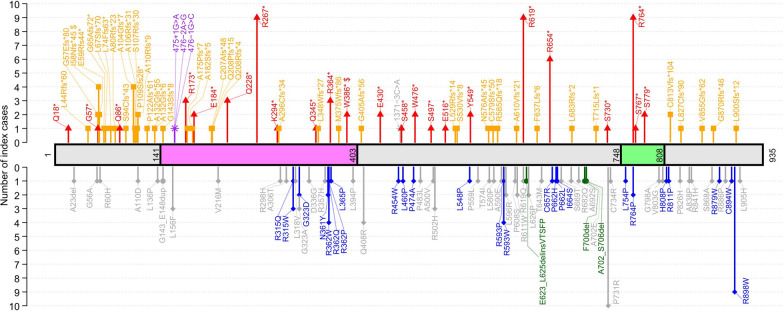
A total of 146 different germline variations of ARMC5 have been observed in 232 unrelated index patients (including 214 patients meeting diagnostic criteria for PBMAH [20], and 18 investigated for other adrenal conditions) (Table 1), including 110 already reported variants and 36 novel variants identified in our center and not published so far. This list excludes the 5’ and 3’ UTR alterations, intronic variants (outside of the canonical sites) and synonymous variants (with an in silico prediction of not affecting splicing). We also describe three very frequent benign polymorphisms: p.(Phe14Tyr), p.(Ile170Val) and p.(Pro507Leu) (minor allele frequency>0.02 in general population according to gnomAD). Among the 146 germline variants, three were large deletions: a complete ARMC5 deletion [12], a deletion of 5’UTR and exons 1 to 3 [34], and a deletion of exons 1 to 5 [35]. Interestingly, large deletions were rare and represented only ~ 2% of reported ARMC5 alterations. The other germline pathogenic variants were 95 single nucleotide variations (SNV) and 48 indel (small insertions or deletions). In total, 67 of the variants were missenses, 42 caused a frameshift, 24 were nonsenses, 4 variants altered the splice sites, 6 were in frame insertions or deletions and 3 were large deletions (Fig. 1 and 2A). These reported genetic alterations affected the entire ARMC5 coding sequence, and most of them were private, without clear mutational hotspot (Fig. 1 and Table 1). Nevertheless, thirty-four protein variants have been identified in at least two unrelated patients. Indeed, p.(Pro731Arg) has been described in 10 unrelated patients; p.(Arg267*), p.(Arg619*), p.(Arg764*) and p.(Arg898Trp) in 9 patients; p.(Arg654*) in 6 patients; p.(Ile58Asnfs*45), p.(Ala104Glyfs*7), p.(Arg362Trp), p.(Gln408Arg) and p.(Arg593Trp) in 4 patients; p.(Arg173*), p.(Gln228*), p.(Arg315Trp), p.(Gly323Ala), p.(Arg364*), p.(Arg502His) and p.(Tyr549*) in 3 patients; p.(Gly57Glufs*80), p.(Glu59Argfs44*), p.(Ala80Argfs*23), p.(Ala110ArgProfs*9), p.(Glu184*), p.(Gly323Asp), p.(Asn361Tyr), p.(Trp386*), p.(Glu430*), p.(Trp476*), p.(Leu596Arg), p.(Arg611Trp), p.(Leu626Pro), p.(Arg764Pro), p.(Ser779*) and p.(Cys813Valfs*104) in 2 patients. It should be noticed that two of these protein variants were encoded by two different genomic variations: p.(Ile58Asnfs*45) due to either c.170dup or c.172dup and p.(Trp386*) due to either c.1157G>A or c.1158G>A (Table 1). Additionally, some amino acids seem to be more frequently affected by different genetic variations: several different amino acid substitutions affect positions 315, 323, 362, 593 and 662; both missense and nonsense variants can occur at position 619 and 764; amino acid positions 132, 143, 208 are subject to different types of frameshift variations; both frameshift and nonsense variants affect position 57.
Table 1.
List of the 146 different germline ARMC5 variants (and 3 benign polymorphisms frequent in the general population), providing the evaluation of their pathogenic nature, based on the criteria according to Richards et al., Genet Med 2015 [33]
| HGVS cDNA NM_001105247.1 |
HGVS Protein | Impact | Classification | ACMG criteria [33] | Somatic 2nd hit | Functional data | Observations | References | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| c.26C>T | p.(Thr9Met) | Missense | Likely benign | BP3 | BP4 | PM2 | ACC | [32] | ||||||||||
| c.41 T>A | p.(Phe14Tyr) | Missense | Benign | BA1 | BP4 | BP3 | BP6 | frequent benign poymorphism | ||||||||||
| c.52C>T | p.(Gln18*) | Nonsense | Pathogenic | PVS1 | PM2 | PP4 | [42] | |||||||||||
| c.68_70del | p.(Ala23del) | In frame deletion | VUS | PM2 | ACC | [32] | ||||||||||||
| c.127_130dup | p.(Leu44Argfs*60) | Frameshift | Pathogenic | PVS1 | PM2 | PP4 | NEW | |||||||||||
| c.170del (= c.165del) | p.(Gly57Glufs*80) | Frameshift | Pathogenic | PVS1 | PM2 | PP4 | [12, 13, 20] | |||||||||||
| c.167G>C | p.(Gly56Ala) | Missense | Likely benign | BP4 | BP6 | PM2 | PP4 | [37] | ||||||||||
| c.169G>T | p.(Gly57*) | Nonsense | Pathogenic | PVS1 | PM2 | PP4 | NEW | |||||||||||
| c.170dup | p.(Ile58Asnfs*45) | Frameshift | Pathogenic | PVS1 | PM2 | PP4 | [14, 24, 81] | |||||||||||
| c.172dup | p.(Ile58Asnfs*45) | Frameshift | Pathogenic | PVS1 | PM2 | PP4 | [24, 36, 44] | |||||||||||
| c.174dup | p.(Glu59Argfs44*) | Frameshift | Pathogenic | PVS1 | PM2 | PP4 | [37, 82] | |||||||||||
| c.179G>A | p.(Arg60His) | Missense | VUS | PM2 | PP4 | NEW | ||||||||||||
| c.194del | p.(Gly65Alafs72*) | Frameshift | Pathogenic | PVS1 | PM2 | PP5 | PS3 | PP4 | yes | [37] | ||||||||
| c.198del | p.(Leu67Serfs*70) | Frameshift | Pathogenic | PVS1 | PM2 | PP4 | NEW | |||||||||||
| c.220_222delinsTT | p.(Leu74Phefs63*) | Frameshift | Pathogenic | PVS1 | PM2 | PP4 | [37] | |||||||||||
| c.237_238insC | p.(Ala80Argfs*23) | Frameshift | Pathogenic | PVS1 | PM2 | PP4 | [20] | |||||||||||
| c.256C>T | p.(Gln86*) | Nonsense | Pathogenic | PVS1 | PS3 | PM2 | PP4 | yes | [12, 13, 20] | |||||||||
| c.281del | p.(Ser94Cysfs*43) | Frameshift | Pathogenic | PVS1 | PM2 | PP4 | no | [44] | ||||||||||
| c.286_310dup | p.(Ala104Glyfs*7) | Frameshift | Pathogenic | PVS1 | PM2 | PP4 | [12, 13, 20] | |||||||||||
| c.315del | p.(Ala106Argfs*31) | Frameshift | Pathogenic | PVS1 | PM2 | PP4 | [13, 20] | |||||||||||
| c.318del | p.(Ser107Argfs*30) | Frameshift | Pathogenic | PVS1 | PM2 | PP4 | [38] | |||||||||||
| c.325_326delinsT | p.(Pro109Serfs28*) | Frameshift | Pathogenic | PVS1 | PM2 | PP4 | [37] | |||||||||||
| c.327dup | p.(Ala110Argfs*9) | Frameshift | Pathogenic | PVS1 | PM2 | PS3 | PP4 | yes | meningioma | [13, 22, 23] | ||||||||
| c.329C>A | (p.Ala110Asp) | Missense | Likely benign | PM2 | BP1 | BP4 | myelolipoma | [83] | ||||||||||
| c.363_373del | p.(Pro122Alafs*61) | Frameshift | Likely pathogenic | PVS1 | PM2 | [84] | ||||||||||||
| c.394dup | p.(Ala132Glyfs*55) | Frameshift | Pathogenic | PVS1 | PM2 | PP4 | NEW | |||||||||||
| c.393_394dup | p.(Ala132Glyfs*6) | Frameshift | Pathogenic | PVS1 | PM2 | PP4 | NEW | |||||||||||
| c.407 T>C | p.(Leu136Pro) | Missense | VUS | PM2 | PP3 | PP4 | meningioma | [27, 44] | ||||||||||
| c.427_454del | p.(Gly143Serfs*8) | Frameshift | pathogenic | PVS1 | PM2 | PP4 | [40] | |||||||||||
| c.423_440dup | p.(Gly143_Glu148dup) | In frame insertion | VUS | PM2 | PP3 | PP4 | PM4 | NEW | ||||||||||
| c.466C>T | p.(Leu156Phe) | Missense | Likely benign | BS1 | BP4 | BP6 | no | [20, 28, 29, 39] | ||||||||||
| c.475 + 1G>A | Splice | Pathogenic | PVS1 | PM2 | PP4 | NEW | ||||||||||||
| c.476-2A>G | Splice | Pathogenic | PVS1 | PM2 | PP4 | [20] | ||||||||||||
| c.476-1G>C | Splice | Pathogenic | PVS1 | PM2 | PS3 | PP4 | yes | meningioma | [14, 24, 27] | |||||||||
| c.508A>G | p.(Ile170Val) | Missense | Benign | BS1 | BP4 | BP6 | frequent benign polymorphism | |||||||||||
| c.517C>T | p.(Arg173*) | Nonsense | Pathogenic | PVS1 | PM2 | PS3 | PP4 | yes | [20, 39, 41, 85, 86] | |||||||||
| c.523del | p.(Ala175Profs*7) | Frameshift | Pathogenic | PVS1 | PM2 | PP4 | [38] | |||||||||||
| c.543dup | p.(Ala182Serfs*5) | Frameshift | Pathogenic | PVS1 | PM2 | PP4 | NEW | |||||||||||
| c.550G>T | p.(Glu184*) | Nonsense | Pathogenic | PVS1 | PM2 | PP4 | [20] | |||||||||||
| c.618del | p.(Cys207Alafs*48) | Frameshift | Pathogenic | PVS1 | PM2 | PP4 | NEW | |||||||||||
| c.622dup | p.(Gln208Profs*15) | Frameshift | Pathogenic | PVS1 | PM2 | PP4 | [38] | |||||||||||
| c.603_622dup | p.(Gln208Argfs*4) | Frameshift | Pathogenic | PVS1 | PM2 | PP4 | NEW | |||||||||||
| c.646G>A | p.(Val216Met) | Missense | VUS | PP4 | NEW | |||||||||||||
| c.682C>T | p.(Gln228*) | Nonsense | Pathogenic | PVS1 | PM2 | PP5 | PS3 | PP4 | yes | [34, 87, 88] | ||||||||
| c.799C>T | p.(Arg267*) | Nonsense | Pathogenic | PVS1 | PM2 | PS3 | PP4 | yes | meningioma | [12–14, 20, 21, 26] | ||||||||
| c.880A>T | p.(Lys294*) | Nonsense | Pathogenic | PVS1 | PM2 | PP4 | NEW | |||||||||||
| c.885_886del | p.(Ala296Cysfs*34) | Frameshift | Pathogenic | PVS1 | PM2 | PP4 | [13] | |||||||||||
| c.893G>A | p.(Arg298His) | Missense | VUS | PM2 | PP4 | NEW | ||||||||||||
| c.916G>A | p.(Ala306Thr) | Missense | Likely benign | BP4 | PP4 | [20] | ||||||||||||
| c.943C>T | p.(Arg315Trp) | Missense | Pathogenic | PM2 | PM5 | PP3 | PP5 | PS3 | PP4 | yes | yes | [13, 20, 21, 89] | ||||||
| c.944G>A | p.(Arg315Gln) | Missense | Likely pathogenic | PM2 | PM5 | PP3 | PP5 | PP4 | [81] | |||||||||
| c.952C>G | p.(Leu318Val) | Missense | Likely benign | PM2 | PP4 | BS3 | no | [24] | ||||||||||
| c.968G>C | p.(Gly323Ala) | Missense | Likely benign | BP4 | BP6 | [28, 29, 44] | ||||||||||||
| c.968G>A | p.(Gly323Asp) | Missense | Likely pathogenic | PM2 | PP4 | PS3 | [44, 82] | |||||||||||
| c.1007A>G | p.(Asp336Gly) | Missense | VUS | PM2 | BP4 | [28] | ||||||||||||
| c.1033C>T | p.(Gln345*) | Nonsense | Pathogenic | PVS1 | PM2 | PP4 | NEW | |||||||||||
| c.1042del | p.(Leu348Trpfs*27) | Frameshift | Pathogenic | PVS1 | PM2 | PP4 | [37] | |||||||||||
| c.1070G>A | p.(Arg357His) | Missense | VUS | PM2 | PP3 | PP4 | NEW | |||||||||||
| c.1081A>T | p.(Asn361Tyr) | Missense | Likely pathogenic | PM2 | PP3 | PS3 | PP4 | yes | [46] | |||||||||
| c.1084C>T | p.(Arg362Trp) | Missense | Pathogenic | PM2 | PM5 | PP3 | PP5 | PS3 | PP4 | yes | meningioma | [27, 37, 39, 41, 44, 81] | ||||||
| c.1085G>A | p.(Arg362Gln) | Missense | Likely pathogenic | PM2 | PM5 | PP3 | PP4 | [40] | ||||||||||
| c.1085G>C | p.(Arg362Pro) | Missense | Likely pathogenic | PM2 | PM5 | PP3 | PP4 | PP1 | [20] | |||||||||
| c.1090C>T | p.(Arg364*) | Nonsense | Pathogenic | PVS1 | PM2 | PP5 | PS3 | PP4 | yes | [37, 39, 81] | ||||||||
| c.1094 T>C | p.(Leu365Pro) | Missense | Pathogenic | PM2 | PM5 | PP3 | PS3 | PP4 | PP1 | yes | meningioma | [24, 27, 44] | ||||||
| c.1123del | p.(Met375Trpfs*86) | Frameshift | Likely pathogenic | PVS1 | PM2 | [34] | ||||||||||||
| c.1157G>A | p.(Trp386*) | Nonsense | Pathogenic | PVS1 | PM2 | PS1 | PP4 | NEW | ||||||||||
| c.1158G>A | p.(Trp386*) | Nonsense | Pathogenic | PVS1 | PM2 | PS1 | PP4 | yes | meningioma | [14, 24, 27] | ||||||||
| c.1181 T>C | p.(Leu394Pro) | Missense | VUS | PM2 | PP3 | PP4 | [24] | |||||||||||
| c.1214del | p.(Gly405Alafs*56) | Frameshift | Pathogenic | PVS1 | PM2 | PP4 | [38] | |||||||||||
| c.1223A>G | p.(Gln408Arg) | Missense | Likely benign | BS2 | BP6 | PS3 | yes | [28, 39, 81] | ||||||||||
| c.1288G>T | p.(Glu430*) | Nonsense | Pathogenic | PVS1 | PM2 | PP4 | [13, 20] | |||||||||||
| c.1360C>T | p.(Arg454Trp) | Missense | Likely pathogenic | PS3 | PP5 | PP4 | [20] | |||||||||||
| c.1371-3C>A | Splice | Likely benign | BS1 | BP4 | [29] | |||||||||||||
| c.1373C>A | p.(Ser458*) | Nonsense | Pathogenic | PVS1 | PM2 | PP4 | NEW | |||||||||||
| c.1379 T>C | p.(Leu460Pro) | Missense | Likely pathogenic | PM2 | PP3 | PS3 | PP4 | PP1 | yes | meningioma | [25] | |||||||
| c.1420C>G | p.(Pro474Ala) | Missense | Likely pathogenic | PM1 | PM2 | PP3 | PP4 | [20] | ||||||||||
| c.1428G>A | p.(Trp476*) | Nonsense | Pathogenic | PVS1 | PM2 | PP4 | [20, 45] | |||||||||||
| c.1448C>T | p.(Pro483Leu) | Missense | Likely benign | BP4 | BP6 | PP4 | [37] | |||||||||||
| c.1490C>A | p.(Ser497*) | Nonsense | Pathogenic | PVS1 | PM2 | PP4 | [20] | |||||||||||
| c.1499C>T | p.(Ala500Val) | Missense | VUS | BP4 | [28] | |||||||||||||
| c.1505G>A | p.(Arg502His) | Missense | Likely benign | BP4 | BP6 | BS3 | no | [23, 90] | ||||||||||
| c.1520C>T | p.(Pro507Leu) | Missense | Benign | BS1 | BP4 | BP6 | frequent benign polymorphism | |||||||||||
| c.1546G>T | p.(Glu516*) | Nonsense | Pathogenic | PVS1 | PM2 | PP4 | NEW | |||||||||||
| c.1586dup | p.(Ser530Valfs*8) | Frameshift | Likely pathogenic | PVS1 | PM2 | [34] | ||||||||||||
| c.1586_1589del | p.(Leu529Argfs*14) | Frameshift | Pathogenic | PVS1 | PM2 | PP4 | [20] | |||||||||||
| c.1643 T>C | p.(Leu548Pro) | Missense | Likely pathogenic | PS3 | PM2 | PP4 | PP3 | yes | yes | [12, 13, 20] | ||||||||
| c.1647C>G | p.(Tyr549*) | Nonsense | Pathogenic | PVS1 | PM2 | PP4 | NEW | |||||||||||
| c.1676C>T | p.(Pro559Leu) | Missense | Likely benign | BP6 | [28] | |||||||||||||
| c.1721C>T | p.(Thr574Ile) | Missense | VUS | PM2 | PP3 | PP4 | NEW | |||||||||||
| c.1726_1753del | p.(Asn576Alafs*45) | Frameshift | Likely pathogenic | PVS1 | PM2 | [91] | ||||||||||||
| c.1736_1739del | p.(Cys579Serfs*50) | Frameshift | Pathogenic | PVS1 | PM2 | PP4 | [39, 81] | |||||||||||
| c.1739 T>C | p.(Leu580Pro) | Missense | VUS | PM2 | PP3 | PP5 | PP4 | [37] | ||||||||||
| c.1754_1755del | p.(Arg585Glnfs*18) | Frameshift | Pathogenic | PVS1 | PM2 | PP4 | [20] | |||||||||||
| c.1769C>A | p.(Ala590Glu) | Missense | VUS | PM2 | PP4 | NEW | ||||||||||||
| c.1777C>T | p.(Arg593Trp) | Missense | Likely pathogenic | PM2 | PP3 | PP5 | PS3 | PP4 | yes | yes | [20, 21, 65] | |||||||
| c.1778G>C | p.(Arg593Pro) | Missense | Likely pathogenic | PM2 | PM5 | PP4 | PP5 | [90] | ||||||||||
| c.1787 T>G | p.(Leu596Arg) | Missense | VUS | PM2 | PP3 | PP4 | [82] | |||||||||||
| c.1822C>T | p.(Pro608Ser) | Missense | VUS | PM2 | BP4 | PP4 | NEW | |||||||||||
| c.1827_1828dup | p.(Ala610Valfs*21) | Frameshift | Pathogenic | PVS1 | PM2 | PP4 | [20, 39] | |||||||||||
| c.1831C>T | p.(Arg611Trp) | Missense | VUS | PM2 | [28] | |||||||||||||
| c.1855C>T | p.(Arg619*) | Nonsense | Pathogenic | PVS1 | PM2 | PS3 | PP4 | yes | [12, 13, 20, 38, 40, 92] | |||||||||
| c.1856G>A | p.(Arg619Gln) | Missense | VUS | PP4 | [90] | |||||||||||||
|
c.1868_1874delins TCACAAGCTTTCC |
p.(Glu623_Leu625delins ValThrSerPhePro) |
In frame indel | Likely pathogenic | PM2 | PM4 | PP3 | PP4 | NEW | ||||||||||
| c.1877 T>C | p.(Leu626Pro) | Missense | VUS | PM2 | PP3 | PP4 | [43] | |||||||||||
| c.1908del | p.(Phe637Leufs*6) | Frameshift | Pathogenic | PVS1 | PM2 | PP4 | NEW | |||||||||||
| c.1928C>T | p.(Thr643Met) | Missense | Likely benign | BS3 | no | [29, 39, 81] | ||||||||||||
| c.1960C>T | p.(Arg654*) | Nonsense | Pathogenic | PVS1 | PM2 | PS3 | PP4 | yes | meningioma | [20, 27, 40, 44, 89] | ||||||||
| c.1969 T>C | p.(Cys657Arg) | Missense | Pathogenic | PM2 | PM5 | PP3 | PP5 | PS3 | PP4 | yes | yes | [13] | ||||||
| c.1985C>A | p.(Pro662His) | Missense | Likely pathogenic | PM2 | PP3 | PS3 | PP4 | yes | NEW | |||||||||
| c.1985C>T | p.(Pro662Leu) | Missense | Likely pathogenic | PM2 | PP3 | PM5 | PP4 | meningioma | [20, 27] | |||||||||
| c.1991 T>G | p.(Ile664Ser) | Missense | Likely pathogenic | PM2 | PP3 | PP5 | PS3 | PP4 | yes | yes | [13, 20] | |||||||
| c.2005 T>A | p.(Ser669Thr) | Missense | VUS | PM2 | BP4 | PP4 | NEW | |||||||||||
| c.2045G>A | p.(Arg682Gln) | Missense | Likely benign | BP1 | BP3 | BP4 | [28] | |||||||||||
| c.2048_2060del | p.(Leu683Argfs*2) | Frameshift | Likely pathogenic | PVS1_S | PM2 | PP4 | NEW | |||||||||||
| c.2074G>T | p.(Ala692Ser) | Missense | VUS | BP4 | PP4 | [20] | ||||||||||||
| c.2097_2099del | p.(Phe700del) | In frame deletion | Likely pathogenic | PM2 | PM4 | PS3 | PP4 | yes | [13] | |||||||||
| c.2105C>A | p.(Ala702Glu) | Missense | VUS | PM2 | PP1 | PP5 | BP4 | [93] | ||||||||||
| c.2104_2118del | p.(Ala702_Ser706del) | In frame deletion | Likely pathogenic | PM2 | PM4 | PS3 | PP4 | yes | [12, 13, 20] | |||||||||
| c.2139del | p.(Thr715Leufs*1) | Frameshift | Likely pathogenic | PVS1_S | PM2 | PP4 | [21] | |||||||||||
| c.2149C>A | p.(Ser730*) | Nonsense | Likely pathogenic | PVS1_S | PM2 | PP4 | [38] | |||||||||||
| c.2192C>G | p.(Pro731Arg) | Missense | Likely benign | BS1 | BP4 | PP5 | ACC | [20, 28, 32, 34, 37, 44] | ||||||||||
| c.2200 T>C | p.(Cys734Arg) | Missense | VUS | PM2 | BP4 | pituitary adenoma | [94] | |||||||||||
| c.2261 T>C | p.(Leu754Pro) | Missense | Pathogenic | PM1 | PM2 | PP3 | PP5 | PS3 | PP4 | yes | yes | [13, 20] | ||||||
| c.2290C>T | p.(Arg764*) | Nonsense | Likely pathogenic | PVS1_S | PM2 | PP5 | PP4 | [13, 20, 37, 38] | ||||||||||
| c.2291G>C | p.(Arg764Pro) | Missense | Likely pathogenic | PM1 | PM2 | PP3 | PP4 | [20] | ||||||||||
| c.2300C>A | p.(Ser767*) | Nonsense | Likely pathogenic | PVS1_S | PM2 | PP4 | [20] | |||||||||||
| c.2336C>G | p.(Ser779*) | Nonsense | Likely pathogenic | PVS1_S | PM2 | PP4 | [24] | |||||||||||
| c.2393G>C | p.(Gly798Ala) | Missense | Likely benign | PM1 | BS1 | BP4 | BP6 | [29] | ||||||||||
| c.2408 T>G | p.(Val803Gly) | Missense | VUS | PM1 | PM2 | PP4 | NEW | |||||||||||
| c.2423A>C | p.(His808Pro) | Missense | Pathogenic | PM1 | PM2 | PP5 | PS3 | PP4 | PP1 | yes | [14, 24] | |||||||
| c.2432G>C | p.(Arg811Pro) | Missense | Likely pathogenic | PM1 | PM2 | PP5 | PS3 | [82] | ||||||||||
| c.2436del | p.(Cys813Valfs*104) | Frameshift | Pathogenic | PVS1_S | PM2 | PS3 | PP4 | yes | [34, 95] | |||||||||
| c.2477C>A | p.(Pro826His) | Missense | VUS | PM2 | BP4 | [29, 81] | ||||||||||||
| c.2479del | p.(Leu827Cysfs*90) | Frameshift | Likely pathogenic | PVS1_S | PM2 | PP4 | [20] | |||||||||||
| c.2512G>C | p.(Ala838Pro) | Missense | VUS | PM2 | PP4 | NEW | ||||||||||||
| c.2522G>A | p.(Arg841His) | Missense | Likely benign | PM2 | PP3 | PP4 | BS3 | no | NEW | |||||||||
| c.2564del | p.(Val855Glyfs*62) | Frameshift | Likely pathogenic | PVS1_S | PM2 | PP4 | [38] | |||||||||||
| c.2602 T>G | p.(Ser868Ala) | Missense | VUS | PM2 | BP4 | PP4 | NEW | |||||||||||
| c.2604_2607del | p.(Gly870Argfs*46) | Frameshift | Likely pathogenic | PVS1_S | PM2 | PP4 | [20] | |||||||||||
| c.2635C>T | p.(Arg879Trp) | Missense | Likely pathogenic | PS3 | PM2 | PP4 | yes | [34] | ||||||||||
| c.2657G>C | p.(Arg886Pro) | Missense | VUS | PM2 | PP4 | NEW | ||||||||||||
| c.2682C>G | p.(Cys894Trp) | Missense | likely pathogenic | PM1 | PM2 | PP3 | PP4 | [20] | ||||||||||
| c.2692C>T | p.(Arg898Trp) | Missense | Likely pathogenic | PS3 | PM2 | PP5 | PP4 | yes | yes | [12, 13, 20, 29, 39, 40, 44] | ||||||||
| c.2697dupG | p.(Leu900Serfs*12) | Frameshift | Likely pathogenic | PVS1_S | PM2 | PP4 | NEW | |||||||||||
| c.2714T>A | p.(Leu905His) | Missense | VUS | PM2 | PP4 | NEW | ||||||||||||
| ARMC5 complete deletion | Large deletion | Pathogenic | PVS1 | PM2 | PP4 | [12] | ||||||||||||
| ARMC5 5’UTR + exons 1–3 deletion | Large deletion | Pathogenic | PVS1 | PM2 | PP4 | [34] | ||||||||||||
| ARMC5 exons 1–5 deletion | Large deletion | Pathogenic | PVS1 | PM2 | PP4 | [35] | ||||||||||||
When available, the presence of a somatic tumoral second hit or functional data is mentioned. VUS, variant of uncertain significance. List of the referred ACMG criteria: BA1: Allele frequency is>5% in Exome Sequencing Project, 1000 Genomes Project, or Exome Aggregation Consortium; BS1: Allele frequency is greater than expected for disorder; BS2: Observed in a healthy adult individual for a recessive (homozygous), dominant (heterozygous), or X-linked (hemizygous) disorder, with full penetrance expected at an early age; BS3: Well-established in vitro or in vivo functional studies show no damaging effect on protein function or splicing; BP1: Missense variant in a gene for which primarily truncating variants are known to cause disease; BP3: In-frame deletions/insertions in a repetitive region without a known function; BP4: Multiple lines of computational evidence suggest no impact on gene or gene product; BP6: Reputable source recently reports variant as benign, but the evidence is not available to the laboratory to perform an independent evaluation; PVS1: Null variant (nonsense, frameshift, canonical ± 1 or 2 splice sites, initiation codon, single or multiexon deletion) in a gene where loss of function is a known mechanism of disease; PS1: Same amino acid change as a previously established pathogenic variant regardless of nucleotide change; PS3: Well-established in vitro or in vivo functional studies supportive of a damaging effect on the gene or gene product; PM1: Located in a mutational hot spot and/or critical and well-established functional domain (e.g., active site of an enzyme) without benign variation; PM2: Absent from controls (or at extremely low frequency if recessive) in Exome Sequencing Project, 1000 Genomes Project, or Exome Aggregation Consortium; PM4: Protein length changes as a result of in-frame deletions/insertions in a non-repeat region or stop-loss variants; PM5: Novel missense change at an amino acid residue where a different missense change determined to be pathogenic has been seen before; PP1: Cosegregation with disease in multiple affected family members in a gene definitively known to cause the disease; PP3: Multiple lines of computational evidence support a deleterious effect on the gene or gene product; PP4: Patient’s phenotype or family history is highly specific for a disease with a single genetic etiology; PP5: Reputable source recently reports variant as pathogenic, but the evidence is not available to the laboratory to perform an independent evaluation
Fig. 1.
Germline ARMC5 variants. ARMC5 variants are shown on a graphical representation of ARMC5 protein from 1 to 935 amino acids (NM_001105247.1 canonical transcript). Both Armadillo and BTB/POZ domains appear respectively as pink and green regions. The class 4 (likely pathogenic) and class 5 (pathogenic) variants are displayed in colors: non-sense (red), frameshift (orange), splice sites (purple), missense (blue) and in frame deletions or insertions (green). The class 2 (likely benign) and class 3 (uncertain significance) variants are represented in grey. Tail size of the lollipops represents the recurrence of each protein alteration from 1 to 10
Fig. 2.
Proportions of protein change categories in germline (panel A) and somatic (panel B) ARMC5 likely pathogenic and pathogenic variants
Assessment of the pathogenic nature of germline missense variants
Differentiation between rare missense benign variants and pathogenic variants could benefit from the analysis of familial segregation of both variant and phenotype. A clear familial PBMAH presentation has been published for 25 families [21–25, 35–43]. Fifteen variations have been identified in familial PBMAH presentations. Five missense variants segregate with PBMAH phenotype in families: p.(Arg362Trp), p.(Leu365Pro), p.(Leu626Pro), p.(His808Pro) and p.(Leu460Pro).
Recent studies of the prevalence of ARMC5 germline variants in patients with other adrenal diseases could help to discriminate benign from pathogenic variants [28, 29, 44]. These data, compared to large scale exome and whole genome DNA sequencing studies performed in the genome of the healthy population from different ethnicities, clearly suggest that some frequent variants play no role in PBMAH development. Indeed, some germline missense variants have been more frequently described in the general population than expected for PBMAH causing variations, arguing for their benignity: p.(Ala306Thr), p.(Thr643Met), with a MAF above 0.0001 in addition to p.(Leu156Phe), p.(Gly323Ala), p.(Gln408Arg), p.(Pro559Leu), p.(Pro731Arg) and p.(Gly798Ala) with a MAF above 0.001.
Given the tumor suppressor nature of ARMC5, the detection of a second pathogenic event altering ARMC5 at the somatic tumoral level (such as truncating variants or loss-of-heterozygosity), leading to a bi-allelic impairment of the gene, strongly supports the hypothesis of the deleterious effect of the missense variant identified in leukocyte DNA. We attributed the ACMG criterion PS3 (“well-established in vitro or in vivo functional studies supportive of a damaging effect on the gene or gene product”) to the germline ARMC5 variants for which one or several second somatic hits have been identified in the adrenal nodules. However, currently, tumoral DNA is not always available because of the frequent use of non-surgical treatment options in PBMAH, such as long-term medical treatment with steroidogenesis inhibitors. Furthermore, copy-number variations (CNV) may be underestimated when using Sanger sequencing method for tumoral DNA.
The occurrence of a second somatic ARMC5 alteration is strong support for the pathogenic nature of some germline missense ARMC5 variants: p.(Arg315Trp), p.(Gly323Asp), p.(Asn361Tyr), p.(Arg362Trp), p.(Leu365Pro), p.(Leu460Pro), p.(Leu548Pro), p.(Arg593Trp), p.(Cys657Arg), p.(Pro662His), p.(Ile664Ser), p.(Leu754Pro), p.(Arg764Pro), p.(His808Pro), p.(Arg811Pro), p.(Arg879Trp) and p.(Arg898Trp). On the other hand, no second somatic ARMC5 alteration has been found after extensive search in patients harboring germline likely benign variants: p.(Leu318Val), p.(Arg502His), p.(Thr643Met) and p.(Arg841His). Similarly, some variants have been identified in non-PBMAH patients: p.(Ala110Asp), p.(Ala500Val), p.(Arg502His), p.(Pro559Leu), p.(Arg611Trp), and p.(Arg682Gln).
Functional studies assessing the loss of apoptotic effect of ARMC5 protein variants, as well as their failed interaction with cullin 3 or substrate proteins, has allowed to assume their pathogenic nature: p.(Arg315Trp), p.(Leu548Pro), p.(Arg593Trp), p.(Cys657Arg), p.(Ile664Ser), p.(Leu754Pro) and p.(Arg898Trp).
At present, no somatic or functional studies have been reported to confirm the pathogenicity of the following 26 missense variants of uncertain significance: p.(Arg60His), p.(Leu136Pro), p.(Val216Met), p.(Arg298His), p.(Arg362Gln), p.(Asp336Gly), p.(Arg357His), p.(Leu394Pro), p.(Thr574Ile), p.(Leu580Pro), p.(Ala590Glu), p.(Arg593Pro), p.(Leu596Arg), p.(Pro608Ser), p.(Arg619Gln), p.(Leu626Pro), p.(Ser669Thr), p.(Ala692Ser), p.(Ala702Glu), p.(Cys734Arg), p.(Val803Gly), p.(Pro826His), p.(Ala838Pro), p.(Ser868Ala), p.(Arg886Pro) and p.(Leu905His); nor of these four likely pathogenic missense variants: p.(Arg362Pro), p.(Pro474Ala), p.(Pro662Leu) and p.(Cys894Trp).
Somatic ARMC5 alterations
In PBMAH, DNA sequencing of adrenal nodules reveals different somatic ARMC5 alterations for a single patient with a single germline ARMC5 event, in accordance with the Knudson hypothesis for a tumor suppressor gene (Table 2).
Table 2.
List of the 104 different somatic tumoral ARMC5 variants, found in PBMAH tissues (or meningioma, when mentioned) and the germline events with which they have been found associated
| HGVS cDNA NM_001105247.1 |
HGVS Protein | Impact | Germline associated variant | Observations | References |
|---|---|---|---|---|---|
| cnLOH | Deletion |
p.(Gln86*), p.(Arg267*), p.(Asn361Tyr), p.(Arg364*), p.(Leu365Pro), p.(Trp386*), p.(Gln408Arg), p.(Trp476*), p.(Leu548Pro), p.(Ile664Ser), p.(Arg764*), p.(Arg879Trp), p.(Arg898Trp), none |
[12–14, 24, 26, 28, 34, 37, 44–46] | ||
| cnLOH | Deletion | p.(Arg267*) | Found in meningioma | [26] | |
| Exons 4–6 deletion | Deletion | none | [34] | ||
| c.91A>T | p.(Lys31*) | Nonsense | p.(Ala296Cysfs*34) | [12, 13] | |
| c.118del | p.(Leu40*) | Nonsense | large deletion | [12, 13] | |
| c.170dupG | p.(Ile58Asnfs*45) | Frameshift | p.(Ala110Argfs*9) | [22] | |
| c.172del | p.(Ile58Serfs*79) | Frameshift | p.(Arg764*) | [46] | |
| c.205_322del | p.(Pro69Alafs*29) | Frameshift | p.(Gln228*) | [87] | |
| c.210_297del | p.(Ala72Leufs*36) | Frameshift | p.(Ala702_Ser706del) | [12, 13] | |
| c.226C>T | p.(Arg76*) | Nonsense | p.(Ala104Glyfs*7) | [12, 13] | |
| c.231_265del | p.(Ala78Argfs*13) | Frameshift | p.(Cys813Valfs*104) | [34] | |
| c.239C>G | p.(Ala80Gly) | Missense | p.(Ala702_Ser706del) | [12, 13] | |
| c.243_289del | p.(Ser82Valfs*5) | Frameshift | p.(Ala702_Ser706del) | [12, 13] | |
| c.247_256del | p.(Ala83Argfs*51) | Frameshift | p.(His808Pro) | [24] | |
| c.247G>C | p.(Ala83Pro) | Missense | p.(Trp476*) | [45] | |
| c.249_270del | p.(Ser85Profs*45) | Frameshift | p.(Arg315Trp) | [89] | |
| c.261_264del | p.(Gly88Profs*48) | Frameshift | p.(Arg267*) | [46] | |
| c.267del | p.(Gly90Alafs*47) | Frameshift | p.(Gln228*) | [87] | |
| c.276_288del | p.(Pro93Argfs*40) | Frameshift | p.(Arg619*) | [12, 13] | |
| c.279del | p.(Ser94Argfs*43) | Frameshift | p.(Arg654*) | [89] | |
| c.283_286del | p.(Ser95Profs*41) | Frameshift | p.(Arg811Pro) | [82] | |
| c.283_289del | p.(Ser95Argfs*40) | Frameshift | p.(Gln228*) | [87] | |
| c.284C>A | p.(Ser95*) | Nonsense | p.(Arg619*) | [92] | |
| c.290_294del | p.(Ala97Glyfs*4) | Frameshift | p.(Ser779*) | [24] | |
| c.294del | p.(Gly99Glufs*38) | Frameshift | p.(Arg619*) | [92] | |
| c.295G>T | p.(Gly99*) | Nonsense | p.(Asn361Tyr) | [46] | |
| c.306_318del | p.(Pro103Argfs*30) | Frameshift | p.(Leu548Pro) | [46] | |
| c.306_342del | p.(Pro103Argfs*22) | Frameshift | p.(Ala110Argfs*9) | [22] | |
| c.310del | p.(Ala104Profs*33) | Frameshift | p.(Gln228*) | [87] | |
| c.311del | p.(Ala106Argfs*31) | Frameshift | p.(Ala110Argfs*9) | [22] | |
| c.316dup | p.(Ala106Glyfs*13) | Frameshift | p.(Ala110Argfs*9) | [22] | |
| c.319_320del | p.(Ser107Glyfs*11) | Frameshift | p.(Arg898Trp) | [12, 13] | |
| c.325_326delinsT | p.(Pro109Serfs*28) | Frameshift | p.(Gly65Alafs72*) | [37] | |
| c.327del | p.(Ala110Profs*27) | Frameshift | p.(Glu430*), p.(Trp476*) | [12, 13, 45] | |
| c.346del | p.(Ser116Argfs*21) | Frameshift | p.(Trp476*) | [45] | |
| c.347_357del | p.(Ser116Tyrfs*67) | Frameshift | p.(Gln228*) | [87] | |
| c.415T>C | p.(Cys139Arg) | Missense | p.(Arg267*) | [12, 13] | |
| c.420T>A | p.(Cys140*) | Nonsense | p.(Arg267*) | [46] | |
| c.430del | p.(Ala144Argfs*16) | Frameshift | p.(Arg764*) | [46] | |
| c.435C>A | p.(Cys145*) | Nonsense | p.(Arg619*) | [92] | |
| c.456_475 + 5del | Splice | p.(Arg267*) | [12, 13] | ||
| c.476-1G>A | Splice | p.(Trp476*) | [45] | ||
| c.608del | p.(Ser203Thrfs*2) | Frameshift | p.(Trp476*) | [45] | |
| c.617_845del | p.(Ala206Aspfs*22) | Frameshift | p.(Arg267*) | [12, 13] | |
| c.658del | p.(Leu220Serfs*35) | Frameshift | p.(Leu754Pro) | [13] | |
| c.671C>A | p.(Ala224Glu) | Missense | p.(Arg315Trp) | [89] | |
| c.682C>T | p.(Gln228*) | Nonsense | none | [34] | |
| c.696del | p.(Leu233Trpfs*22) | Frameshift | p.(Arg267*) | [46] | |
| c.703C>T | p.(Gln235*) | Nonsense | p.(Cys657Arg), p.(Arg173*) | [13, 86] | |
| c.789_808del | p.(Glu264Profs*5) | Frameshift | p.(Trp476*) | [45] | |
| c.807C>A | p.(Cys269*) | Nonsense | p.(Trp476*) | [45] | |
| c.913del | p.(Leu305Serfs*9) | Frameshift | p.(Asn361Tyr) | [46] | |
| c.943C>T | p.(Arg315Trp) | Missense | p.(Leu548Pro) | [12, 13] | |
| c.992T>C | p.(Leu331Pro) | Missense | p.(Gly57Glufs*80) | [12, 13] | |
| c.1033C>T | p.(Gln345*) | Nonsense | p.(Trp476*) | [45] | |
| c.1039_1049del | p.(Pro347Glyfs*8) | Frameshift | p.(Arg267*) | [46] | |
| c.1042del | p.(Leu348Trpfs*27) | Frameshift | p.(Arg764*) | [37] | |
| c.1059_1080del | p.(Cys353*) | Nonsense | p.(Trp476*) | [45] | |
| c.1059C>A | p.(Cys353*) | Nonsense | p.(Trp476*) | [45] | |
| c.1084C>T | p.(Arg362Trp) | Missense | p.(Ala110Argfs*9) | [22] | |
| c.1085G>T | p.(Arg362Leu) | Missense | p.(Phe700del) | [12, 13] | |
| c.1158G>A | p.(Trp386*) | Nonsense | p.(Arg267*) | [46] | |
| c.1174del | p.(Ala392Leufs*69) | Frameshift | p.(Arg764*) | [46] | |
| c.1222C>T | p.(Gln408*) | Nonsense | p.(Arg267*) | [46] | |
| c.1297G>T | p.(Glu433*) | Nonsense | p.(Ala110Argfs*9) | [22] | |
| c.1330del | p.(Thr444Profs*17) | Frameshift | p.(Arg593Trp) | [21] | |
| c.1369A>T | p.(Arg457Trp) | Missense | p.(Gln228*) | [88] | |
| c.1474del | p.(Ala492Profs*52) | Frameshift | p.(Cys657Arg) | [13] | |
| c.1507_1508del | p.(Thr503Profs*34) | Frameshift | p.(Ala110Argfs*9) | Found in meningioma | [22] |
| c.1549G>A | p.(Glu517Lys) | Missense | p.(Arg654*) | [89] | |
| c.1572_1607del | p.(Ala525_Pro536del) | In frame | p.(Arg619*) | [92] | |
| c.1671_1678dup | p.(Gly560Alafs*73) | Frameshift | p.(Arg267*) | [46] | |
| c.1712C>G | p.(Ser571*) | Nonsense | p.(Arg173*) | [86] | |
| c.1746del | p.(Phe583Serfs*47) | Frameshift | p.(Gln86*) | [12, 13] | |
| c.1751T>A | p.(Val584Glu) | Missense | p.(Trp476*) | [45] | |
| c.1777C>T | p.(Arg593Trp) | Missense | p.(Arg267*) | [46] | |
| c.1843C>G | p.(His615Asp) | Missense | p.(Phe14Tyr) | [28] | |
| c.1851del | p.(His518Thrfs*12) | Frameshift | p.(Arg764*) | [38] | |
| c.1855C>T | p.(Arg619*) | Nonsense | p.(Arg267*) | [12, 13, 46] | |
| c.1864G>A | p.(Gly622Arg) | Missense | p.(Arg764*) | [46] | |
| c.1913G>A | p.(Gly638Glu) | Missense | p.(Arg267*) | [46] | |
| c.1960C>T | p.(Arg654*) | Nonsense | p.(Ala110Argfs*9) | [22] | |
| c.1971C>G | p.(Cys657Trp) | Missense | p.(Leu365Pro) | [24] | |
| c.1982T>A | p.(Leu661Gln) | Missense | p.(Arg267*) | [46] | |
| c.2011del | p.(Trp671Glyfs*18) | Frameshift | p.(Arg267*) | [46] | |
| c.2025del | p.(Leu676Trpfs*13) | Frameshift | p.(Gly323Asp) | [82] | |
| c.2029G>T | p.(Glu677*) | Nonsense | p.(Asn361Tyr) | [46] | |
| c.2053_2055del | p.(Leu685del) | In frame | c.476-1G>C | [14] | |
| c.2113del | p.(Leu705Phefs*12) | Frameshift | p.(Gly57Glufs*80) | [13] | |
| c.2116dup | p.(Ser706Phefs*32) | Frameshift | p.(Asn361Tyr) | [46] | |
| c.2123del | p.(Leu708Profs*9) | Frameshift | p.(Ala296Cysfs*34) | [13] | |
| c.2207A>C | p.(Tyr736Ser) | Missense | p.(Arg898Trp) | [12, 13] | |
| c.2228C>T | p.(Ala743Val) | Missense | p.(Trp476*) | [45] | |
| c.2302G>C | p.(Ala768Pro) | Missense | p.(Pro507Leu) | [28] | |
| c.2405C>G | p.(Pro802Arg) | Missense | p.(Trp476*) | [45] | |
| c.2444del | p.(Ala815Leufs*102) | Frameshift | p.(Trp476*) | [45] | |
| c.2486G>A | p.(Gly829Asp) | Missense | none | [34] | |
| c.2522G>A | p.(Arg841His) | Missense | p.(Arg315Trp) | [89] | |
| c.2525T>A | p.(Phe842Tyr) | Missense | p.(Arg654*) | [89] | |
| c.2542G>T | p.(Glu848*) | Nonsense | p.(Arg619*) | [92] | |
| c.2599G>T | p.(Glu867*) | Nonsense | p.(Arg619*) | [38] | |
| c.2611G>T | p.(Glu871*) | Nonsense | p.(Asn361Tyr) | [46] | |
| c.2647C>T | p.(His883Tyr) | Missense | p.(Phe14Tyr) | [28] | |
| c.2666T>A | p.(Leu889Gln) | Missense | p.(Gln408Arg) | [28] | |
| c.2734G>A | p.(Glu912Lys) | Missense | p.(Arg267*) | [46] | |
| c.2755del | p.(Ala919Leufs*6) | Frameshift | p.(Arg267*) | [46] |
cnLOH, copy-neutral loss of heterozygosity
Initially, ARMC5 locus was first identified by the evidence of 16p LOH in PBMAH [12]. Somatic 16p copy neutral LOH has been reported in several studies in adrenocortical nodules from PBMAH patients [12–14, 24, 26, 34, 37, 44–46]. Apart from 16p LOH, numerous other somatic ARMC5 genetic alterations have been observed. In total, 104 different somatic events (Table 2) have been described: LOH, 51 SNV (27 missense variants, 23 nonsense variants, and 2 SNV affecting splice sites), 51 indels (49 frameshift deletions or insertions, 2 in frame deletions) and 1 large deletion of exons 4–6) (Table 2 and Fig. 2B). Interestingly, 12 of these somatic variants (p.(Ile58Asnfs*45), p.(Pro109Serfs*28), p.(Gln228*), p.(Arg315Trp), p.(Gln345*), p.(Leu348Trpfs*27), p.(Arg362Trp), p.(Trp386*), p.(Arg593Trp), p.(Arg619*), p.(Arg654*) and p.(Arg841His)) have also been described as first germline events (Tables 1 and 2).
Taking into account the tumor suppressor gene model of ARMC5, extra-adrenal tumoral DNA has been analyzed for bi-allelic ARMC5 alterations. Besides PBMAH, ARMC5 alterations affecting both alleles have been implicated in the development of meningiomas: p.(Thr503Profs*34) somatic event added to the germline already known p.(Ala110Argfs*9) variant in a first patient [22]; tumoral loss of heterozygosity (LOH) along with the germline p.(Arg267*) variant in another patient [26]. In the current literature, besides these two cases with demonstrated somatic ARMC5 alterations, meningiomas have been reported in 10 other ARMC5 patients [21, 23–25, 27] and in two Korean sisters reported before the identification of ARMC5 [47]. Meningioma is, at present, the only other tumor type besides PBMAH, for which molecular studies suggest a causative role for ARMC5. Somatic tumoral ARMC5 alterations were not identified in breast, parathyroid, thyroid tumors or pancreatic NET from PBMAH patients with a germline ARMC5 pathogenic variant [22, 38].
Genotype/phenotype correlation
A more pronounced phenotype has been reported in patients with PBMAH and germline ARMC5 pathogenic variant than in wild-type patients [13, 20, 48], with regard to the intensity of Cushing’s syndrome according to the usual endocrine and clinical markers of cortisol excess: elevated 24-h urinary free cortisol, morning plasma cortisol after 1 mg DST, and midnight plasma cortisol, associated with a more suppressed ACTH. Consequently, PBMAH patients present more often with diabetes and high blood pressure requiring the use of antihypertensive medications. Their adrenal glands appear larger than in wild-type patients, and harbor more numerous nodules. Therefore, ARMC5-mutated patients are more frequently treated than wild-type patients, whether by adrenalectomy or by steroidogenesis inhibitors in order to control Cushing’s syndrome. A recent study from our group reported the largest series of patients with PBMAH to date, including a broad range of phenotypes, from bilateral adrenal incidentaloma without any clinical evidence of endogenous cortisol excess to massively enlarged adrenals associated with severe hypercortisolism and showed that, additionally to the bilateral adrenal nodules, ARMC5-mutated patients constantly present with a cortisol dysregulation, at least with a mild autonomous cortisol secretion [20]. In keeping with these observations, the study stated that ARMC5 genotyping should be offered to all index patients presenting clear bilateral adrenal macronodules associated with at least a mild autonomous cortisol secretion defined by a morning plasma cortisol after 1 mg DST above 50 nmol/L as previously proposed [48, 49] and to all first-degree relatives of ARMC5 pathogenic variant carriers [20]. For index-cases, the negative predictive value of these simple criteria is 100%, meaning that no ARMC5-mutated patient should be missed, while the positive predictive value is close to 20% [20] which is higher than various genetic screening for other types of endocrine tumors (pituitary, parathyroid). Furthermore, additional genetic screening should be considered in specific situations. Indeed, in 2021, two teams have identified KDM1A germline pathogenic variants as the molecular cause of hereditary PBMAH associated with food-dependent Cushing’s syndrome [50, 51], a very rare presentation of PBMAH due to the illegitimate expression of the glucose-dependent insulinotropic polypeptide receptor (GIPR) in adrenocortical cells, reinforcing the idea that PBMAH is a genetic disease [52]. KDM1A genotyping should then be proposed to all patients with a suspicion of food-dependent cortisol secretion (i.e., low fasting plasma cortisol, increasing after meals or oral glucose test) [1, 53–55]. In the case of associated clinical features suggesting a syndromic presentation such as MEN1, FAP or HLRCC, a complementary genotyping of MEN1, APC or FH genes should be considered [1]. However, with the increasing use of multigenic panel in routine practice one should consider to include all these gene in a PBMAH panel.
In most of the papers describing the clinical presentation of ARMC5 mutated and wild-type PBMAH patients, ethnic origin is not mentioned. But PBMAH due to ARMC5 germline alteration is probably spread all over the world, since there are many reports from North and South America, Europe, Middle East and Asia; and in our own experience in patients from African ancestry.
If ARMC5-mutated patients harbor a distinct phenotype compared to wild-type PBMAH patients, we can also speculate that truncating variants could be more deleterious than missense variants and associated with a more severe form of the disease. Accordingly, 56% of patients with truncating ARMC5 variants are primarily referred to clinical departments in front of overt signs of Cushing’s syndrome, versus only 21% of patients with non-truncating pathogenic variants, for which the incidental diagnosis in abdominal imaging is far more frequent [20]. This could be explained, at least in part, by the significantly higher values of midnight plasma cortisol in patients with truncating variants (nonsense and frameshift with premature stop codon) compared to non-truncating missense variants, witnessing for a perceptible increased cortisol output in these patients [20]. However, the other biological parameters are not statistically different, maybe due to the limited number of patients with ARMC5 variant. Likewise, this approach was used to compare variants affecting the different domains of the ARMC5 protein, but no significant difference could be detected [20]. These observations raise pending questions about the pathogenic mechanisms of ARMC5 proteins altered by amino acids substitutions, which should be addressed in further basic and clinical investigations. ARMC5 protein has not been successfully crystallized at this point, but this could be a major help for the interpretation of missense variants.
The penetrance of PBMAH in ARMC5 pathogenic variant carriers seems high but incomplete in the reported familial cases [23, 24] but remains largely unknown at present, mostly because of the rarity and the late onset of the disease. Similarly, the affected relatives are often younger than the index case and appear with a more attenuated disease compared to the index case. However, the mechanisms causing this phenotypic variability have not been studied to date and would need extensive familial investigations.
PBMAH is often associated with aberrant response of cortisol secretion to various stimuli, through the illegitimate expression of G-protein coupled receptors in adrenocortical cells, such as GIP receptor associated with food-dependent Cushing’s syndrome [56, 57], LH/hCG receptor associated with post-menopausal Cushing’s syndrome [58], beta-adrenergic receptors associated with a cortisol response to upright posture, stress or sport [59], vasopressin receptors associated with cortisol response to upright posture and vasopressin agonists [60], serotonin receptors [61], and other less common receptors. Illegitimate expression of beta-adrenergic or vasopressin receptors has already been described in ARMC5 variant carriers [12, 13, 23]. In contrast, illegitimate expression of the GIP receptor has never been reported in ARMC5 patients [13]. This was suggestive of a potential different molecular predisposition, which was later elucidated in 2021 with the identification of KDM1A as the genetic cause of PBMAH associated with food-dependent Cushing’s syndrome [50, 51].
The presence of a germline ARMC5 variant is strongly correlated with typical histopathological patterns of PBMAH subtype 1 according to Violon and colleagues [62], associating large coalescent yellow nodules composed of 70–90% of clear cells, 10–30% of compact cells and < 10% of oncocytic cells, with the presence of round fibrous septa within the macronodules. Residual internodular adrenal is unfrequently observed. Immunohistochemistry usually shows strong positivity for HSD3B2 in clear cells and CYP17A1 in compact cells.
ARMC5 function: in vitro data and animal models
ARMC5 inactivation results in apparently contradictory effects: an impaired apoptosis and a decreased steroidogenesis. Indeed, on one hand, the pro-apoptotic effect of wild-type ARMC5 transfected in adrenocortical carcinoma cell line H295R is lost when the cells are transfected with the ARMC5 mutants [12, 13], likely to explain the adrenal tumorigenesis. On the other hand, ARMC5 silencing by siRNA or shRNA in H295R leads to a decreased steroidogenesis associated with a reduced expression of steroidogenic enzymes, as observed in PBMAH primary cell cultures from ARMC5 mutated patients [12, 14]. However, the effects of ARMC5 mutants’ overexpression on steroidogenesis have not been assessed. Altogether, despite the altered steroidogenesis, the uncontrolled tumorigenesis resulting from the impaired apoptosis is likely to explain a global excessive cortisol output by the adrenals [63].
ARMC5 protein comprises a C-terminal BTB (Broad-Complex, Tramtrack and Bric-à-Brac) domain, which allows the interaction with the E3-ubiquitine ligase cullin 3, leading to ARMC5 ubiquitination and further degradation by the proteasome. Our group recently showed that an ARMC5 missense variant affecting its BTB domain results in a loss of the interaction with cullin 3 and then in a stabilization of ARMC5 protein [15]. Moreover, it has just been demonstrated that ARMC5 also regulates the degradation of other proteins [64–68]. Indeed, ARMC5 acts as an adaptor for Cullin 3 complex by recruiting protein substrates including RPB1 [65, 67], SREBF [66, 68] and NRF1 [64]. Interestingly, ARMC5 p.R593W variant located in the intermediate region fails to interact with RPB1 protein, leading to the accumulation of RPB1 protein in mutated PBMAH tissues [65].
Two Armc5 deficient murine models have been generated: the complete knock-out Armc5-/- is lethal in most cases between days 6.5 and 8.5 of embryonic development [17] but the rare living pups are smaller than the wild-type ones and the aged mice develop a non-nodular adrenal hyperplasia along with high corticosterone levels [18] and have a higher risk of neural tube defects [67]. The heterozygous knock-out mice Armc5 ± show a normal development and present with transient low corticosterone levels at 1 year which could result from an impaired steroidogenesis, followed by a normalization and even an increased corticosteronemia at 18 months in one third of cases, this could be consecutive to a raise of ACTH levels in response to the initial low corticosteronemia [17], without adrenal nodule nor hyperplasia, in accordance with the two-hit model of ARMC5-driven adrenocortical tumorigenesis, which cannot result from haploinsufficiency.
Discussion
The pathogenic nature of truncating ARMC5 variants is rarely a matter of debate but the assessment of germline missense variants is far more delicate and rests on a beam of arguments comprising their frequency in general population, in silico predictions, the identification of somatic ARMC5 variants in the tumoral DNA from operated patients, familial segregation, and in vitro studies for some patients. In our experience, considering that functional in vitro studies of ARMC5 function are difficult to standardize for routine diagnosis, the occurrence of a second somatic hit in the adrenal nodules is strong evidence for the pathogenic nature of a germline missense variant. It is not clear whether or not this feature covers the definition of the PS3 ACMG criterion (“well-established in vitro or in vivo functional studies supportive of a damaging effect on the gene or gene product”), but we assume that this should be considered for tumor-suppressor genes with a proven two-hit inactivation model that are usually not found as pure somatic alterations in sporadic tumors.
These criteria and their interpretation are framed by the five classes of the ACMG guidelines [33], which have been primarily designed for classical Mendelian diseases and genes. But the field is constantly evolving, thanks notably to genome-wide association studies, revealing new insights in the high complexity of the relation between genetics and diseases [69], which may not be entirely reflected by the five ACMG classes. This led many researchers to suggest amendments to the classification, which will be still subject to continuous refinements. For instance, the distinction between disease-causing and disease-predisposing genes has been recently proposed, based on the observations in chronic pancreatitis [70].
The available data are not sufficient to classify some missense variants, notably because many PBMAH patients do not undergo adrenal surgery and thus, tumor samples are now rarely available for somatic genotyping. Functional studies, so far, are limited to the demonstration of the loss of the apoptotic effect of the wild-type ARMC5 by some missense variants [12, 13] and loss of ARMC5 interaction with cullin-3 or its protein substrates by missense variants in the BTB domain or in the intermediate domain [15, 65]. The other missense alterations might also lead to a loss of ARMC5 function but functional studies need to demonstrate this hypothesis. A better understanding of ARMC5 physiological role is required to develop systematic functional studies of ARMC5 variants to corroborate the loss of function induced by the genetic alterations observed in PBMAH patients.
Until recently, all the somatic pathogenic variants identified were observed in patients having also a germline ARMC5 variant. This is different from other tumor suppressor genes, which can be found mutated only in tumor DNA as a pure somatic alteration in sporadic diseases. This suggests that ARMC5 alterations, with at least haploinsufficiency, need to be present early in adrenal development to promote the onset of PBMAH. However, a recent study reported 5 complete gene deletions, 1 partial deletion (exons 4–6) and 2 SNV affecting ARMC5 at the somatic level in 6 PBMAH patients without germline ARMC5 variation [34]. These very interesting findings would need to be corroborated in further studies. Conversely, no concomitant CTNNB1, PRKACA, PRKAR1A or GNAS somatic variant (classically met in cortisol-producing adenomas [71–79]) with a germline ARMC5 alteration has been yet reported to our knowledge. The occurrence of different second somatic alterations in multiple adrenal nodules of the same patient is also a quite unique feature of the tumor suppressor gene ARMC5. It should be noted also that bi-allelic germline inactivation of ARMC5 – whether by homozygosity or compound heterozygosity – has not been reported so far, suggesting that it might be lethal during embryonic development, as observed in murine models.
ARMC5 germline and somatic alterations have been associated with the occurrence of meningiomas in several patients, including familial cases. But the real incidence of meningiomas in ARMC5 mutated patients remains unknown and needs to be extensively investigated, since no systematic brain imaging in these patients have been reported to date. Apart from meningiomas, there is no clear association between ARMC5 genetic alterations and sporadic nor familial other tumor type. This suggests that despite a rather ubiquitous expression of ARMC5 [80], its inactivation can promote tumorigenesis in only few tissues.
ARMC5 variants account for around 20% of PBMAH index cases, and KDM1A for less than 5%. Therefore, 75% or more of PBMAH index cases have no identified molecular cause at present, which is a challenging field of future research.
Conclusion
This work is the first extensive analysis of all the ARMC5 genetic alterations reported so far in the literature with an addition of 36 unpublished variants identified in our center. A total of 238 different ARMC5 alterations are reported here: 146 identified on germline DNA and 104 on tumoral DNA (12 reported both as germline and somatic alterations). ARMC5 pathogenic variants are spread through the entire coding sequence of the gene. There is no clear hotspot region although some amino acids can be more frequently altered.
The present study is an important source of information and provides a list of variants, classified upon their pathogenic nature, which could help clinicians and geneticists to discriminate pathogenic from benign variants.
This list of variants would benefit from additional data from the various centers worldwide performing ARMC5 genotyping routinely, and could result in the creation of a public and evolving database, as it has already been developed for TP53 (https://tp53.isb-cgc.org/), compiling the whole ARMC5 germline and somatic variants, as well as the available experimental data and anonymized clinical information of variant carriers, both index and related cases, allowing to increase and share the knowledge about the genotype/phenotype correlation of this challenging disease.
Acknowledgements
We thank the members of the genetic department of Cochin hospital and the genomic facility of Cochin Institute for sequencing, all the members of the Genomics and Signaling of Endocrine Tumors team of Cochin Institute for their help in these studies.
Author contributions
LB and AV designed and analyzed the work, interpreted the data, and drafted the manuscript. IC, FV, AJ, AB, PV, SE, KP, PK, MCV, AT, GR, CLR, UD, MR, MCF, CAS contributed to data acquisition and substantially revised the manuscript. AC and EP analyzed the work, interpreted the data, and substantially revised the manuscript. BR and JB conceptualized, designed and analyzed the work, interpreted the data, and substantially revised the manuscript.
Funding
LB is recipient of research fellowships from the Cancer Research for Personalized Medicine (CARPEM) and the Fondation ARC pour la Recherche contre le Cancer; JB laboratory is supported by the Agence Nationale pour la Recherche grant ANR-18-CE14-0008–01 and the Fondation pour la Recherche Médicale (EQU201903007854). JB, UD and MR clinical departments are part of the European Reference Network on Rare Endocrine Conditions (Endo-ERN) – Project ID No 739572. MR is supported by grants of the ElseKröner-Fresenius Stiftung (2012_A103 and 2015_A228) and the Deutsche Forschuungsgemeinschaft (DFG, German Research Foundation, Projektnummer: 314061271-TRR 205).
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Declarations
Ethics approval and consent to participate
Written informed consent was obtained from all patients for which a new ARMC5 variant is reported in this work. The study was approved by the local ethic committee of Ile de France I, Paris.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Lucas Bouys and Anna Vaczlavik contributed equally.
References
- 1.Bertherat J, Bourdeau I, Bouys L, Chasseloup F, Kamenický P, Lacroix A. Clinical, pathophysiologic, genetic, and therapeutic progress in primary bilateral macronodular adrenal hyperplasia. Endocr Rev. 2023;44(4):567–628. [DOI] [PubMed] [Google Scholar]
- 2.Burgess JR, Harle RA, Tucker P, Parameswaran V, Davies P, Greenaway TM, et al. Adrenal lesions in a large kindred with multiple endocrine neoplasia type 1. Arch Surg. 1996;131(7):699–702. [DOI] [PubMed] [Google Scholar]
- 3.Gatta-Cherifi B, Chabre O, Murat A, Niccoli P, Cardot-Bauters C, Rohmer V, et al. Adrenal involvement in MEN1. Analysis of 715 cases from the Groupe d’etude des Tumeurs Endocrines database. Eur J Endocrinol. 2012;166(2):269–79. [DOI] [PubMed] [Google Scholar]
- 4.Chavoshi V, Tamehri Zadeh SS, Khalili S, Rabbani A, Matini SAH, Mohsenifar Z, et al. Long delay in diagnosis of a case with MEN1 due to concomitant presence of AIMAH with insulinoma: a case report and literature review. BMC Endocr Disord. 2022;22(1):108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hsiao HP, Kirschner LS, Bourdeau I, Keil MF, Boikos SA, Verma S, et al. Clinical and genetic heterogeneity, overlap with other tumor syndromes, and atypical glucocorticoid hormone secretion in adrenocorticotropin-independent macronodular adrenal hyperplasia compared with other adrenocortical tumors. J Clin Endocrinol Metab. 2009;94(8):2930–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yamakita N, Murai T, Ito Y, Miura K, Ikeda T, Miyamoto K, et al. Adrenocorticotropin-independent macronodular adrenocortical hyperplasia associated with multiple colon adenomas/carcinomas which showed a point mutation in the APC gene. Intern Med. 1997;36(8):536–42. [DOI] [PubMed] [Google Scholar]
- 7.Gaujoux S, Pinson S, Gimenez-Roqueplo AP, Amar L, Ragazzon B, Launay P, et al. Inactivation of the APC gene is constant in adrenocortical tumors from patients with familial adenomatous polyposis but not frequent in sporadic adrenocortical cancers. Clin Cancer Res. 2010;16(21):5133–41. [DOI] [PubMed] [Google Scholar]
- 8.Matyakhina L, Freedman RJ, Bourdeau I, Wei MH, Stergiopoulos SG, Chidakel A, et al. Hereditary leiomyomatosis associated with bilateral, massive, macronodular adrenocortical disease and atypical cushing syndrome: a clinical and molecular genetic investigation. J Clin Endocrinol Metab. 2005;90(6):3773–9. [DOI] [PubMed] [Google Scholar]
- 9.Shuch B, Ricketts CJ, Vocke CD, Valera VA, Chen CC, Gautam R, et al. Adrenal nodular hyperplasia in hereditary leiomyomatosis and renal cell cancer. J Urol. 2013;189(2):430–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Charchar HLS, Fragoso MCBV. An overview of the heterogeneous causes of cushing syndrome resulting from primary macronodular adrenal hyperplasia (PMAH). J Endocr Soc. 2022;6(5):bvac041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Findlay JC, Sheeler LR, Engeland WC, Aron DC. Familial adrenocorticotropin-independent Cushing’s syndrome with bilateral macronodular adrenal hyperplasia. J Clin Endocrinol Metab. 1993;76(1):189–91. [DOI] [PubMed] [Google Scholar]
- 12.Assié G, Libé R, Espiard S, Rizk-Rabin M, Guimier A, Luscap W, et al. ARMC5 mutations in macronodular adrenal hyperplasia with Cushing’s syndrome. N Engl J Med. 2013;369(22):2105–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Espiard S, Drougat L, Libé R, Assié G, Perlemoine K, Guignat L, et al. ARMC5 mutations in a large cohort of primary macronodular adrenal hyperplasia: clinical and functional consequences. J Clin Endocrinol Metab. 2015;100(6):E926-935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cavalcante IP, Nishi M, Zerbini MCN, Almeida MQ, Brondani VB, Botelho MLA de A, et al. The role of ARMC5 in human cell cultures from nodules of primary macronodular adrenocortical hyperplasia (PMAH). Mol Cell Endocrinol. 2018;460:36–46. [DOI] [PubMed]
- 15.Cavalcante IP, Vaczlavik A, Drougat L, Lotfi CFP, Perlemoine K, Ribes C, et al. Cullin 3 targets the tumor suppressor gene ARMC5 for ubiquitination and degradation. Endocr Relat Cancer. 2020;27(4):221–30. [DOI] [PubMed] [Google Scholar]
- 16.Yan G, Liu N, Tian J, Fu Y, Wei W, Zou J, et al. Deubiquitylation and stabilization of ARMC5 by ubiquitin-specific processing protease 7 (USP7) are critical for RCC proliferation. J Cell Mol Med. 2021;25(6):3149–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Berthon A, Faucz FR, Espiard S, Drougat L, Bertherat J, Stratakis CA. Age-dependent effects of Armc5 haploinsufficiency on adrenocortical function. Hum Mol Genet. 2017;26(18):3495–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hu Y, Lao L, Mao J, Jin W, Luo H, Charpentier T, et al. Armc5 deletion causes developmental defects and compromises T-cell immune responses. Nat Commun. 2017;8:13834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bouys L, Chiodini I, Arlt W, Reincke M, Bertherat J. Update on primary bilateral macronodular adrenal hyperplasia (PBMAH). Endocrine. 2021;71(3):595–603. [DOI] [PubMed] [Google Scholar]
- 20.Bouys L, Vaczlavik A, Jouinot A, Vaduva P, Espiard S, Assié G, et al. Identification of predictive criteria for pathogenic variants of primary bilateral macronodular adrenal hyperplasia (PBMAH) gene ARMC5 in 352 unselected patients. Eur J Endocrinol. 2022;187(1):123–34. [DOI] [PubMed] [Google Scholar]
- 21.Gagliardi L, Schreiber AW, Hahn CN, Feng J, Cranston T, Boon H, et al. ARMC5 mutations are common in familial bilateral macronodular adrenal hyperplasia. J Clin Endocrinol Metab. 2014;99(9):E1784-1792. [DOI] [PubMed] [Google Scholar]
- 22.Elbelt U, Trovato A, Kloth M, Gentz E, Finke R, Spranger J, et al. Molecular and clinical evidence for an ARMC5 tumor syndrome: concurrent inactivating germline and somatic mutations are associated with both primary macronodular adrenal hyperplasia and meningioma. J Clin Endocrinol Metab. 2015;100(1):E119-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bourdeau I, Oble S, Magne F, Lévesque I, Cáceres-Gorriti KY, Nolet S, et al. ARMC5 mutations in a large French-Canadian family with cortisol-secreting β-adrenergic/vasopressin responsive bilateral macronodular adrenal hyperplasia. Eur J Endocrinol. 2016;174(1):85–96. [DOI] [PubMed] [Google Scholar]
- 24.Alencar GA, Lerario AM, Nishi MY, Mariani BM de P, Almeida MQ, Tremblay J, et al. ARMC5 mutations are a frequent cause of primary macronodular adrenal Hyperplasia. J Clin Endocrinol Metab. 2014;99(8):E1501–1509. [DOI] [PubMed]
- 25.Ferreira MJ, Pedro J, Salazar D, Costa C, Aragão Rodrigues J, Costa MM, et al. ARMC5 primary bilateral macronodular adrenal hyperplasia associated with a meningioma: a family report. Case Rep Endocrinol. 2020;2020:8848151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jojima T, Kogai T, Iijima T, Kato K, Sagara M, Kezuka A, et al. Genetic alteration of ARMC5 in a patient diagnosed with meningioma and primary macronodular adrenal hyperplasia: a case report. Eur J Endocrinol. 2020;183(6):K7-12. [DOI] [PubMed] [Google Scholar]
- 27.Salame AAM, Charchar HLS, de Oliveira Dourado JP, Mendonca B, Alencar GA, de Araújo LJT, et al. Neuroradiological features of patients with bilateral macronodular adrenocortical disease and meningiomas associated or not with genetic variants of ARMC5- a case series. J Neurooncol. 2024;168(3):405–13. [DOI] [PubMed] [Google Scholar]
- 28.Damjanovic SS, Antic JA, Elezovic-Kovacevic VI, Dundjerovic DM, Milicevic IT, Beleslin-Cokic BB, et al. ARMC5 alterations in patients with sporadic neuroendocrine tumors and multiple endocrine neoplasia Type 1 (MEN1). J Clin Endocrinol Metab. 2020;105(12):e4531-4542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zilbermint M, Xekouki P, Faucz FR, Berthon A, Gkourogianni A, Schernthaner-Reiter MH, et al. Primary aldosteronism and ARMC5 variants. J Clin Endocrinol Metab. 2015;100(6):E900-909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mulatero P, Schiavi F, Williams TA, Monticone S, Barbon G, Opocher G, et al. ARMC5 mutation analysis in patients with primary aldosteronism and bilateral adrenal lesions. J Hum Hypertens. 2016;30(6):374–8. [DOI] [PubMed] [Google Scholar]
- 31.De Sousa SMC, Stowasser M, Feng J, Schreiber AW, Wang P, Hahn CN, et al. ARMC5 is not implicated in familial hyperaldosteronism type II (FH-II). J Hum Hypertens. 2017;31(12):857–9. [DOI] [PubMed] [Google Scholar]
- 32.Scatolini M, Grisanti S, Tomaiuolo P, Grosso E, Basile V, Cosentini D, et al. Germline NGS targeted analysis in adult patients with sporadic adrenocortical carcinoma. Eur J Cancer. 2024;205: 114088. [DOI] [PubMed] [Google Scholar]
- 33.Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17(5):405–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morelli V, Elli FM, Frigerio S, Vena W, Palmieri S, Lucca C, et al. Prevalence and clinical features of armadillo repeat-containing 5 mutations carriers in a single center cohort of patients with bilateral adrenal incidentalomas. Eur J Endocrinol. 2023;189(2):242–51. [DOI] [PubMed] [Google Scholar]
- 35.Suzuki S, Tatsuno I, Oohara E, Nakayama A, Komai E, Shiga A, et al. Germline deletion of armc5 in familial primary macronodular adrenal hyperplasia. Endocr Pract. 2015;21(10):1152–60. [DOI] [PubMed] [Google Scholar]
- 36.Rego T, Fonseca F, Espiard S, Perlemoine K, Bertherat J, Agapito A. ARMC5 mutation in a Portuguese family with primary bilateral macronodular adrenal hyperplasia (PBMAH). Endocrinol Diabetes Metab Case Rep. 2017;2017:16–0135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Albiger NM, Regazzo D, Rubin B, Ferrara AM, Rizzati S, Taschin E, et al. A multicenter experience on the prevalence of ARMC5 mutations in patients with primary bilateral macronodular adrenal hyperplasia: from genetic characterization to clinical phenotype. Endocrine. 2017;55(3):959–68. [DOI] [PubMed] [Google Scholar]
- 38.Yu L, Zhang J, Guo X, Chen X, He Z, He Q. ARMC5 mutations in familial and sporadic primary bilateral macronodular adrenal hyperplasia. PLoS ONE. 2018;13(1): e0191602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hannah-Shmouni F, Berthon A, Faucz FR, Briceno JM, Maria AG, Demidowich A, et al. Mass spectrometry-based steroid profiling in primary bilateral macronodular adrenocortical hyperplasia. Endocr Relat Cancer. 2020;27(7):403–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kyo C, Usui T, Kosugi R, Torii M, Yonemoto T, Ogawa T, et al. ARMC5 alterations in primary macronodular adrenal hyperplasia (PMAH) and the clinical state of variant carriers. J Endocr Soc. 2019;3(10):1837–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wurth R, Kamilaris C, Nilubol N, Sadowski SM, Berthon A, Quezado MM, et al. Inhibin A as a tumor marker for primary bilateral macronodular adrenal hyperplasia. Endocrinol Diabetes Metab Case Rep. 2020;2020:20-0006, EDM200006. [DOI] [PMC free article] [PubMed]
- 42.Zhang F, Lin X, Yu X. Primary macronodular adrenal hyperplasia (PMAH) can be generated by a new ARMC5 germline variant (c.52C>T (p.Gln18X)). Endocr J. 2020;67(12):1179–86. [DOI] [PubMed] [Google Scholar]
- 43.Piñar-Gutiérrez A, Mangas-Cruz MÁ, de Lara-Rodríguez I, Remón-Ruiz P, Del Can-Sánchez D, Tous Castillo M, et al. Familial bilateral macronodular adrenal hyperplasia due to a novel ARMC 5 germline mutation: clinical status and possible association with other neoplasms. Endocrinol Diabetes Nutr (Engl Ed). 2024;71(3):119–23. [DOI] [PubMed] [Google Scholar]
- 44.Mariani BM de P, Nishi MY, Wanichi IQ, Brondani VB, Lacombe AMF, Charchar H, et al. Allelic Variants of ARMC5 in Patients With Adrenal Incidentalomas and in Patients With Cushing’s Syndrome Associated With Bilateral Adrenal Nodules. Front Endocrinol (Lausanne). 2020;11:36. [DOI] [PMC free article] [PubMed]
- 45.Correa R, Zilbermint M, Berthon A, Espiard S, Batsis M, Papadakis GZ, et al. The ARMC5 gene shows extensive genetic variance in primary macronodular adrenocortical hyperplasia. Eur J Endocrinol. 2015;173(4):435–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Violon F, Bouys L, Vaduva P, Chansavang A, Vaquier L, Letourneur F, et al. Somatic molecular heterogeneity in bilateral macronodular adrenocortical disease (BMAD) differs among the pathological subgroups. Endocr Pathol. 2024 [DOI] [PubMed]
- 47.Lee S, Hwang R, Lee J, Rhee Y, Kim DJ, Chung UI, et al. Ectopic expression of vasopressin V1b and V2 receptors in the adrenal glands of familial ACTH-independent macronodular adrenal hyperplasia. Clin Endocrinol (Oxf). 2005;63(6):625–30. [DOI] [PubMed] [Google Scholar]
- 48.Faucz FR, Zilbermint M, Lodish MB, Szarek E, Trivellin G, Sinaii N, et al. Macronodular adrenal hyperplasia due to mutations in an armadillo repeat containing 5 (ARMC5) gene: a clinical and genetic investigation. J Clin Endocrinol Metab. 2014;99(6):E1113-1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lacroix A. Heredity and cortisol regulation in bilateral macronodular adrenal hyperplasia. N Engl J Med. 2013;369(22):2147–9. [DOI] [PubMed] [Google Scholar]
- 50.Vaczlavik A, Bouys L, Violon F, Giannone G, Jouinot A, Armignacco R, et al. KDM1A inactivation causes hereditary food-dependent Cushing syndrome. Genet Med. 2022;24(2):374–83. [DOI] [PubMed] [Google Scholar]
- 51.Chasseloup F, Bourdeau I, Tabarin A, Regazzo D, Dumontet C, Ladurelle N, et al. Loss of KDM1A in GIP-dependent primary bilateral macronodular adrenal hyperplasia with Cushing’s syndrome: a multicentre, retrospective, cohort study. Lancet Diabetes Endocrinol. 2021;9(12):813–24. [DOI] [PubMed] [Google Scholar]
- 52.Cavalcante IP, Berthon A, Fragoso MC, Reincke M, Stratakis CA, Ragazzon B, et al. Primary bilateral macronodular adrenal hyperplasia: definitely a genetic disease. Nat Rev Endocrinol. 2022;18(11):699–711. [DOI] [PubMed] [Google Scholar]
- 53.Lacroix A. Extensive expertise in endocrinology: glucose-dependent insulinotropic peptide-dependent Cushing’s syndrome. Eur J Endocrinol. 2023;188(3):R56-72. [DOI] [PubMed] [Google Scholar]
- 54.Bouys L, Bertherat J. From the first case reports to KDM1A identification: 35 years of food (GIP)-dependent Cushing’s syndrome. Exp Clin Endocrinol Diabetes. 2024;132:697. [DOI] [PubMed] [Google Scholar]
- 55.Bouys L, Violon F, Louiset E, Sibony M, Lefebvre H, Bertherat J. Bilateral Adrenocortical Nodular Disease and Cushing’s Syndrome. J Clin Endocrinol Metab. 2024;dgae419. [DOI] [PubMed]
- 56.Reznik Y, Allali-Zerah V, Chayvialle JA, Leroyer R, Leymarie P, Travert G, et al. Food-dependent Cushing’s syndrome mediated by aberrant adrenal sensitivity to gastric inhibitory polypeptide. N Engl J Med. 1992;327(14):981–6. [DOI] [PubMed] [Google Scholar]
- 57.Lacroix A, Bolté E, Tremblay J, Dupré J, Poitras P, Fournier H, et al. Gastric inhibitory polypeptide-dependent cortisol hypersecretion—A new cause of Cushing’s syndrome. N Engl J Med. 1992;327(14):974–80. [DOI] [PubMed] [Google Scholar]
- 58.Lacroix A, Hamet P, Boutin JM. Leuprolide acetate therapy in luteinizing hormone—dependent Cushing’s syndrome. N Engl J Med. 1999;341(21):1577–81. [DOI] [PubMed] [Google Scholar]
- 59.Lacroix A, Tremblay J, Rousseau G, Bouvier M, Hamet P. Propranolol therapy for ectopic beta-adrenergic receptors in adrenal Cushing’s syndrome. N Engl J Med. 1997;337(20):1429–34. [DOI] [PubMed] [Google Scholar]
- 60.Lacroix A, Tremblay J, Touyz RM, Deng LY, Lariviere R, Cusson JR, et al. Abnormal adrenal and vascular responses to vasopressin mediated by a V1-vasopressin receptor in a patient with adrenocorticotropin-independent macronodular adrenal hyperplasia, Cushing’s syndrome, and orthostatic hypotension. J Clin Endocrinol Metab. 1997;82(8):2414–22. [DOI] [PubMed] [Google Scholar]
- 61.Cartier D, Lihrmann I, Parmentier F, Bastard C, Bertherat J, Caron P, et al. Overexpression of serotonin4 receptors in cisapride-responsive adrenocorticotropin-independent bilateral macronodular adrenal hyperplasia causing Cushing’s syndrome. J Clin Endocrinol Metab. 2003;88(1):248–54. [DOI] [PubMed] [Google Scholar]
- 62.Violon F, Bouys L, Berthon A, Ragazzon B, Barat M, Perlemoine K, et al. Impact of morphology in the genotype and phenotype correlation of bilateral macronodular adrenocortical disease (BMAD): a series of clinicopathologically well-characterized 35 cases. Endocr Pathol. 2023;34(2):179–99. [DOI] [PubMed] [Google Scholar]
- 63.Vaduva P, Bonnet F, Bertherat J. Molecular basis of primary aldosteronism and adrenal cushing syndrome. J Endocr Soc. 2020;4(9):bvaa075. [DOI] [PMC free article] [PubMed]
- 64.Cavalcante IP, Rizk-Rabin M, Ribes C, Perlemoine K, Hantel C, Berthon A, et al. Tumor suppressor gene ARMC5 controls adrenal redox state through NRF1 turnover. Endocr Relat Cancer. 2022;29(11):615–24. [DOI] [PubMed] [Google Scholar]
- 65.Lao L, Bourdeau I, Gagliardi L, He X, Shi W, Hao B, et al. ARMC5 is part of an RPB1-specific ubiquitin ligase implicated in adrenal hyperplasia. Nucleic Acids Res. 2022;50(11):6343–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Okuno Y, Fukuhara A, Otsuki M, Shimomura I. ARMC5-CUL3 E3 ligase targets full-length SREBF in adrenocortical tumors. JCI Insight. 2022;7(16): e151390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Luo H, Lao L, Au KS, Northrup H, He X, Forget D, et al. ARMC5 controls the degradation of most Pol II subunits, and ARMC5 mutation increases neural tube defect risks in mice and humans. Genome Biol. 2024;25(1):19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Okuno Y, Fukuhara A, Shimomura I. The role of oxidative stress, glucocorticoid receptor and ARMC5 in lipid metabolism. Endocr J. 2024;71:1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Heyne HO, Karjalainen J, Karczewski KJ, Lemmelä SM, Zhou W, Finn G, et al. Mono- and biallelic variant effects on disease at biobank scale. Nature. 2023;613(7944):519–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Masson E, Zou WB, Génin E, Cooper DN, Le Gac G, Fichou Y, et al. Expanding ACMG variant classification guidelines into a general framework. Hum Genomics. 2022;16(1):31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bonnet S, Gaujoux S, Launay P, Baudry C, Chokri I, Ragazzon B, et al. Wnt/β-catenin pathway activation in adrenocortical adenomas is frequently due to somatic CTNNB1-activating mutations, which are associated with larger and nonsecreting tumors: a study in cortisol-secreting and -nonsecreting tumors. J Clin Endocrinol Metab. 2011;96(2):E419-426. [DOI] [PubMed] [Google Scholar]
- 72.Gaujoux S, Tissier F, Groussin L, Libé R, Ragazzon B, Launay P, et al. Wnt/beta-catenin and 3’,5’-cyclic adenosine 5’-monophosphate/protein kinase A signaling pathways alterations and somatic beta-catenin gene mutations in the progression of adrenocortical tumors. J Clin Endocrinol Metab. 2008;93(10):4135–40. [DOI] [PubMed] [Google Scholar]
- 73.Tissier F, Cavard C, Groussin L, Perlemoine K, Fumey G, Hagneré AM, et al. Mutations of beta-catenin in adrenocortical tumors: activation of the Wnt signaling pathway is a frequent event in both benign and malignant adrenocortical tumors. Cancer Res. 2005;65(17):7622–7. [DOI] [PubMed] [Google Scholar]
- 74.Beuschlein F, Fassnacht M, Assié G, Calebiro D, Stratakis CA, Osswald A, et al. Constitutive activation of PKA catalytic subunit in adrenal Cushing’s syndrome. N Engl J Med. 2014;370(11):1019–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cao Y, He M, Gao Z, Peng Y, Li Y, Li L, et al. Activating hotspot L205R mutation in PRKACA and adrenal Cushing’s syndrome. Science. 2014;344(6186):913–7. [DOI] [PubMed] [Google Scholar]
- 76.Di Dalmazi G, Kisker C, Calebiro D, Mannelli M, Canu L, Arnaldi G, et al. Novel somatic mutations in the catalytic subunit of the protein kinase A as a cause of adrenal Cushing’s syndrome: a European multicentric study. J Clin Endocrinol Metab. 2014;99(10):E2093-2100. [DOI] [PubMed] [Google Scholar]
- 77.Bertherat J, Groussin L, Sandrini F, Matyakhina L, Bei T, Stergiopoulos S, et al. Molecular and functional analysis of PRKAR1A and its locus (17q22-24) in sporadic adrenocortical tumors: 17q losses, somatic mutations, and protein kinase A expression and activity. Cancer Res. 2003;63(17):5308–19. [PubMed] [Google Scholar]
- 78.Kobayashi H, Usui T, Fukata J, Yoshimasa T, Oki Y, Nakao K. Mutation analysis of Gsalpha, adrenocorticotropin receptor and p53 genes in Japanese patients with adrenocortical neoplasms: including a case of Gsalpha mutation. Endocr J. 2000;47(4):461–6. [DOI] [PubMed] [Google Scholar]
- 79.Ronchi CL, Di Dalmazi G, Faillot S, Sbiera S, Assié G, Weigand I, et al. Genetic landscape of sporadic unilateral adrenocortical adenomas without PRKACA p.Leu206Arg mutation. J Clin Endocrinol Metab. 2016;101(9):3526–38. [DOI] [PubMed] [Google Scholar]
- 80.Berthon A, Faucz F, Bertherat J, Stratakis CA. Analysis of ARMC5 expression in human tissues. Mol Cell Endocrinol. 2017;441:140–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wurth R, Tirosh A, Kamilaris CDC, Camacho J, Faucz FR, Maria AG, et al. Volumetric modeling of adrenal gland size in primary bilateral macronodular adrenocortical hyperplasia. J Endocr Soc. 2021;5(1):bvaa162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Giacché M, Panarotto A, Mori L, Poliani PL, Lanzi R, Lena MS, et al. New pathogenic variants in ARMC5 gene in a series of Italian patients affected by primary bilateral macronodular adrenocortical hyperplasia (PBMAH). Mol Genet Genomic Med. 2023;11(4): e2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Naser N, Nava V, Khosla S, Nylen E. Allelic variant of armadillo repeat containing protein 5 (ARMC5) in myelolipoma mimicking pheochromocytoma: a case report. Cureus. 2023;15(1): e34454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang W, Wei F. A novel pathogenic variant of ARMC5 in a patient with primary bilateral macronodular adrenal hyperplasia: a case report. BMC Endocr Disord. 2022;22(1):211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Liu Q, Tong D, Xu J, Yang X, Yi Y, Zhang D, et al. A novel germline ARMC5 mutation in a patient with bilateral macronodular adrenal hyperplasia: a case report. BMC Med Genet. 2018;19(1):49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Berthon A, Hannah-Shmouni F, Maria AG, Faucz FR, Stratakis CA. High expression of adrenal P450 aromatase (CYP19A1) in association with ARMC5-primary bilateral macronodular adrenocortical hyperplasia. J Steroid Biochem Mol Biol. 2019;191: 105316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jin P, Janjua MU, Zhang Q, Dong CS, Yang Y, Mo ZH. Extensive ARMC5 genetic variance in primary bilateral macronodular adrenal hyperplasia that started with exophthalmos: a case report. J Med Case Rep. 2018;12(1):13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.He WT, Wang X, Song W, Song XD, Lu YJ, Lv YK, et al. A novel nonsense mutation in ARMC5 causes primary bilateral macronodular adrenocortical hyperplasia. BMC Med Genom. 2021;14(1):126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhang Q, Cui L, Gao JP, Yan WH, Jin N, Chen K, et al. Whole-genome sequencing revealed armadillo repeat containing 5 (ARMC5) mutation in a Chinese family with ACTH-independent macronodular adrenal hyperplasia. Endocr J. 2018;65(3):269–79. [DOI] [PubMed] [Google Scholar]
- 90.Emms H, Tsirou I, Cranston T, Tsagarakis S, Grossman AB. Do patients with incidentally discovered bilateral adrenal nodules represent an early form of ARMC5-mediated bilateral macronodular hyperplasia? Endocrine. 2016;53(3):801–8. [DOI] [PubMed] [Google Scholar]
- 91.Hella Z, Tőke J, Patócs A, Varga Z, Dabasi G, Kovács GL, et al. Macronodular adrenal hyperplasia causing Cushing’s syndrome due to ARMC5 gene mutation. Orv Hetil. 2023;164(32):1271–7. [DOI] [PubMed] [Google Scholar]
- 92.Tang P, Zhang J, Peng S, Yan X, Wang Y, Wang S, et al. Primary bilateral macronodular adrenocortical hyperplasia (PBMAH) patient with ARMC5 mutations. BMC Endocr Disord. 2023;23(1):77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Eghbali M, Cheraghi S, Samanian S, Rad I, Meghdadi J, Akbari H, et al. A novel ARMC5 germline variant in primary macronodular adrenal hyperplasia using whole-exome sequencing. Diagnostics (Basel). 2022;12(12):3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Khosla S, Alsarraf F, Nylen ES. Pituitary macroadenoma with macronodular adrenal hyperplasia and novel armadillo repeat-containing protein 5 (ARMC5) mutation. JCEM Case Rep. 2024;2(2):luad138. [DOI] [PMC free article] [PubMed]
- 95.Vena W, Morelli V, Carrabba M, Elli F, Fabio G, Muller I, et al. Case report: a novel ARMC5 germline mutation in a patient with primary bilateral macronodular adrenal hyperplasia and hypogammaglobulinemia. Front Genet. 2022;13: 834067. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.