Abstract
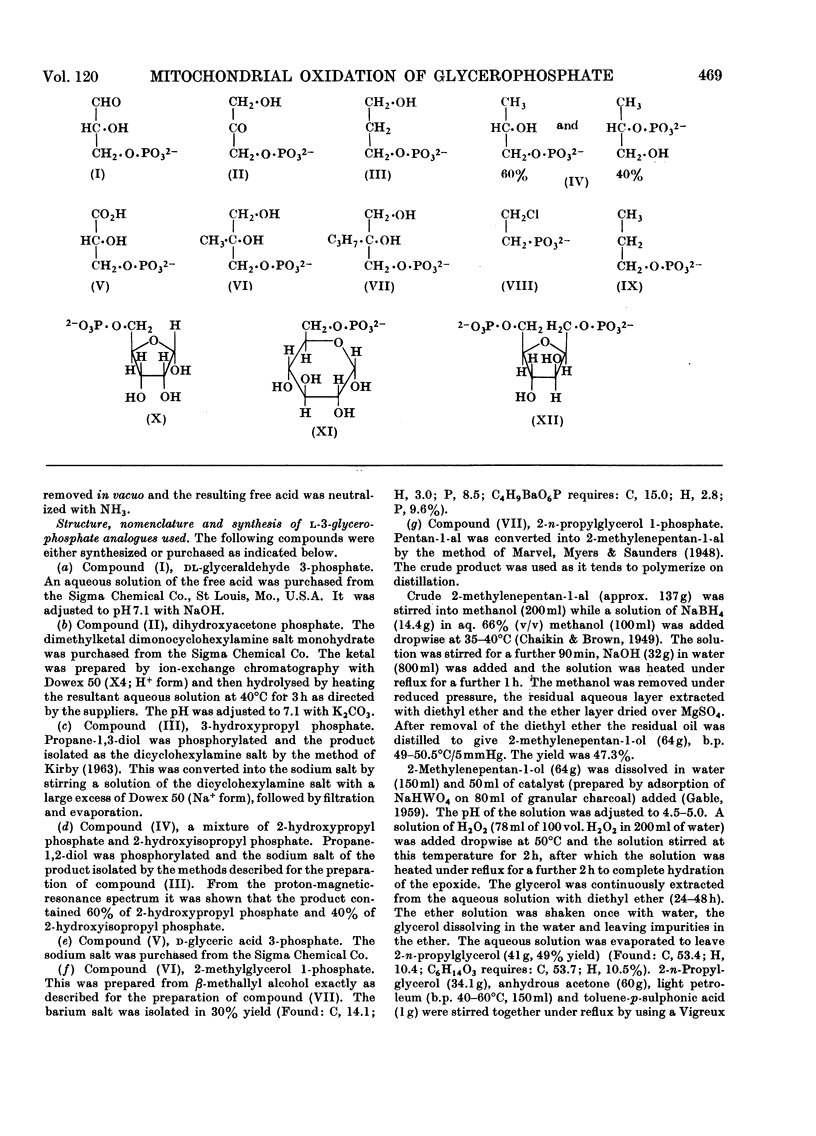
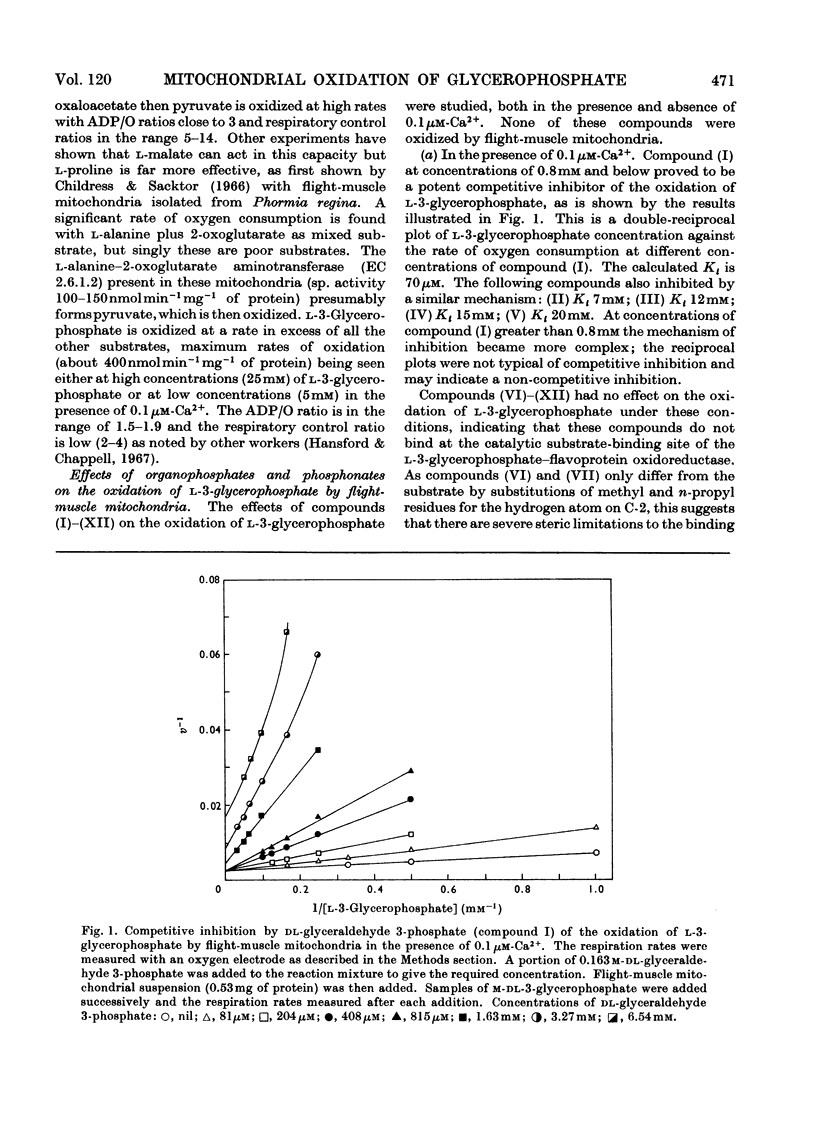
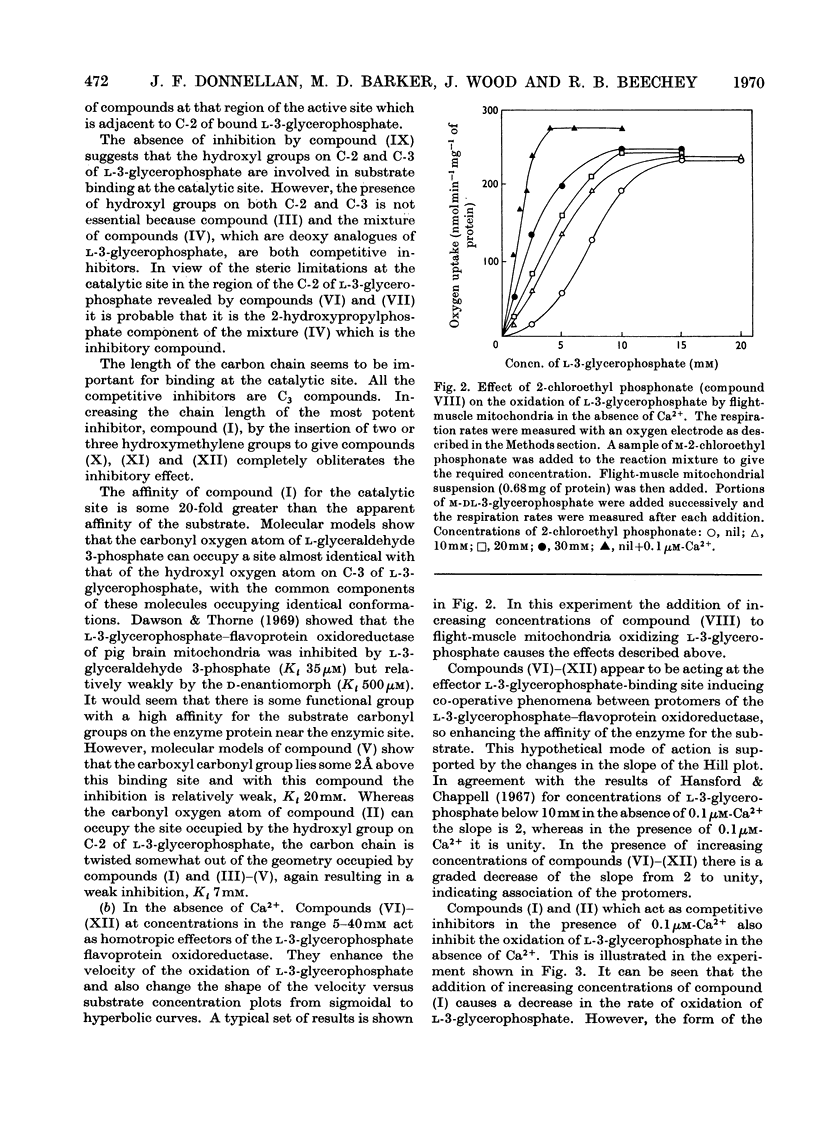
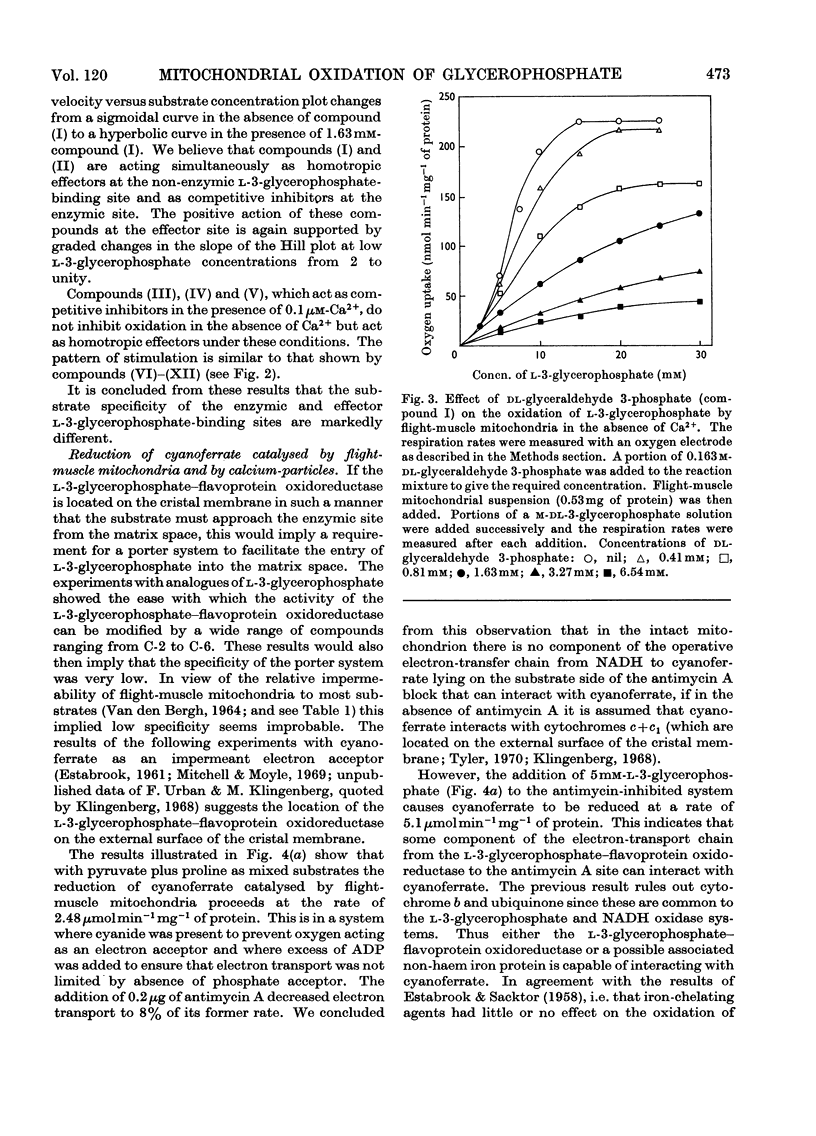
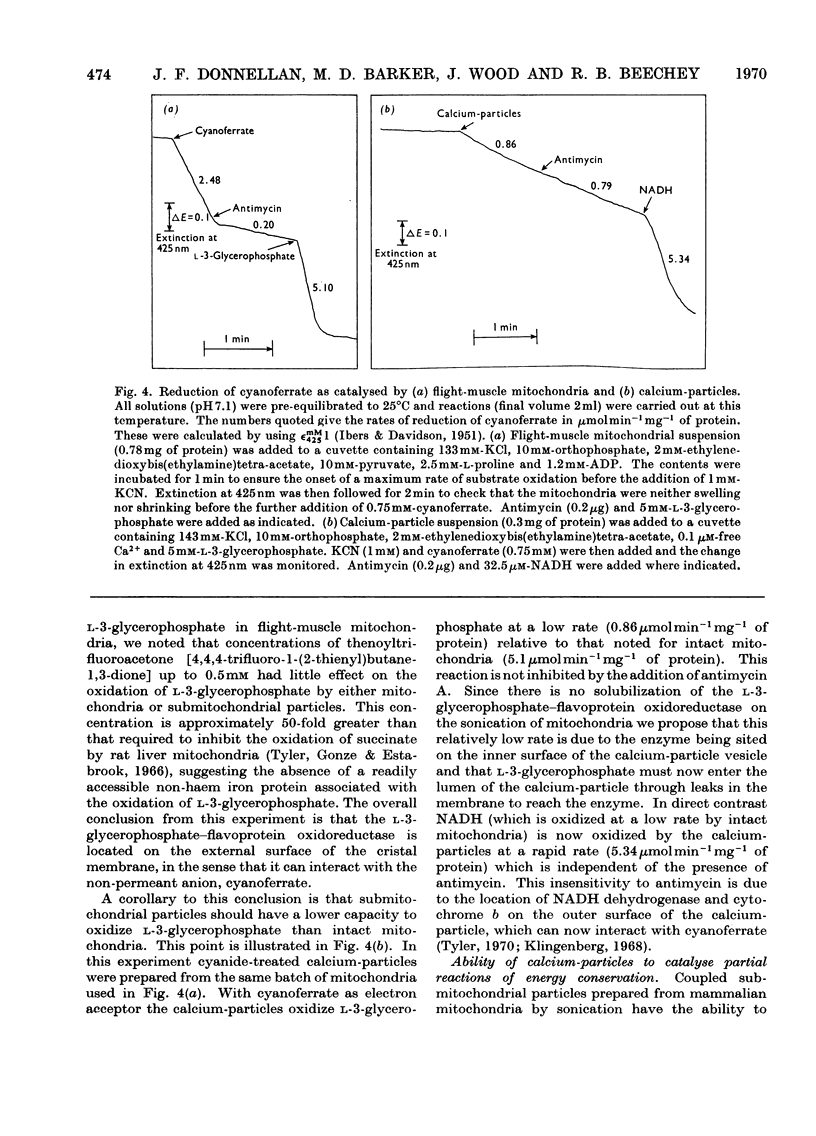
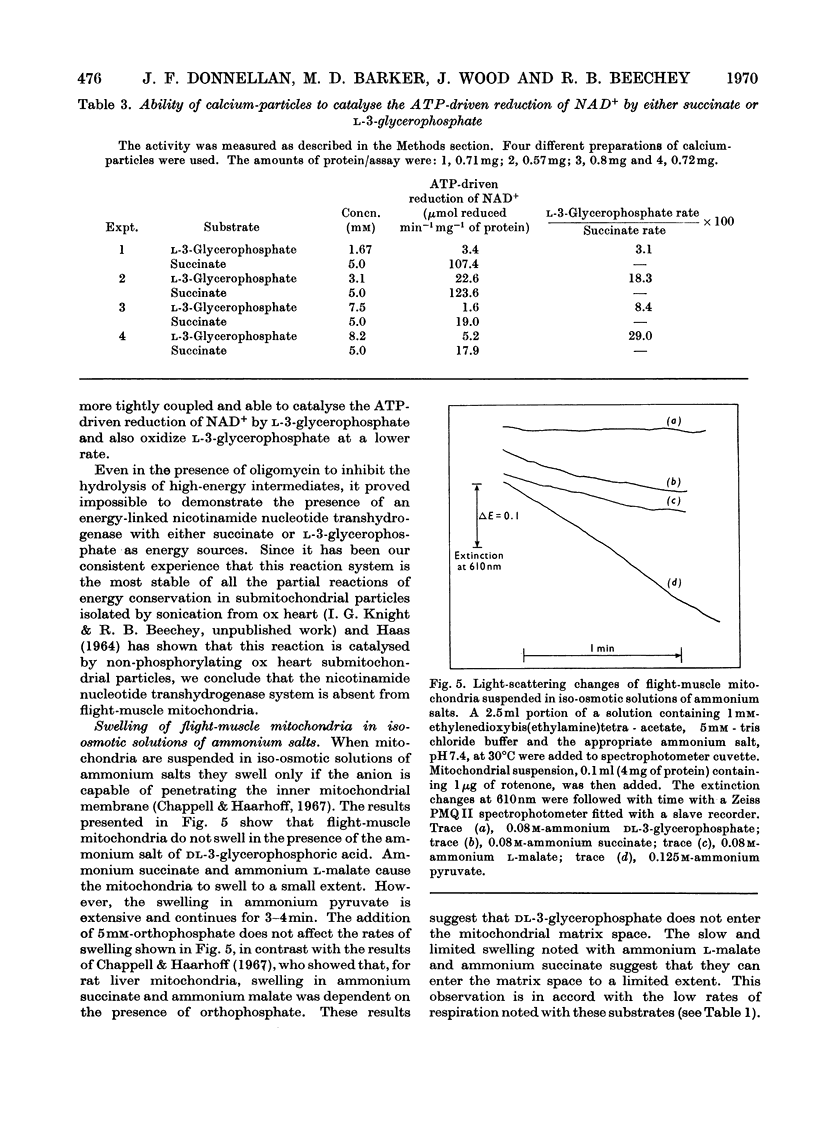
1. The oxidation of l-3-glycerophosphate by flight-muscle mitochondria isolated from the flesh fly Sarcophaga barbata has been studied. Use of substrate analogues indicates that the catalytic and effector l-3-glycerophosphate binding sites on the allosteric l-3-glycerophosphate–flavoprotein oxidoreductase differ markedly in specificity. 2. The l-3-glycerophosphate–cyanoferrate oxidoreductase system in these mitochondria is antimycin-insensitive whereas the corresponding NADH–cyanoferrate oxidoreductase is extremely sensitive to this respiratory-chain inhibitor. Also no swelling is observed when these mitochondria are suspended in iso-osmotic solutions of ammonium glycerophosphate in contrast with the extensive swelling seen in similar solutions of ammonium pyruvate. These observations indicate that l-3-glycerophosphate does not penetrate the mitochondrial matrix whereas pyruvate does. 3. Submitochondrial particles catalyse the ATP-driven reduction of NAD+ by l-3-glycerophosphate but at a far lower rate than that seen when succinate is the electron donor. These particles do not have an energy-linked pyridine nucleotide transhydrogenase activity. 4. We conclude that the l-3-glycerophosphate–flavoprotein oxidoreductase is located on the outer surface of the inner membrane of the flight-muscle mitochondria.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRUNI A., LUCIANI S., CONTESSA A. R. INHIBITION BY ATRACTYLOSIDE OF THE BINDING OF ADENINE-NUCLEOTIDES TO RAT-LIVER MITOCHONDRIA. Nature. 1964 Mar 21;201:1219–1220. doi: 10.1038/2011219a0. [DOI] [PubMed] [Google Scholar]
- Beechey R. B., Roberton A. M., Holloway C. T., Knight I. G. The properties of dicyclohexylcarbodiimide as an inhibitor of oxidative phosphorylation. Biochemistry. 1967 Dec;6(12):3867–3879. doi: 10.1021/bi00864a033. [DOI] [PubMed] [Google Scholar]
- Dawson A. P., Thorne C. J. Preparation and some properties of L-3-glycerophosphate dehydrogenase from pig brain mitochondria. Biochem J. 1969 Jan;111(1):27–34. doi: 10.1042/bj1110027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnellan J. F., Barker M. D., Beechey R. B. Localization of the L-glycerol 1-phosphate-flavoprotein oxidoreductase on the outer surface of the inner membrane of insect flight-muscle mitochondria. Biochem J. 1970 Feb;116(4):31P–31P. doi: 10.1042/bj1160031pa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnellan J. F., Beechey R. B. Factors affecting the oxidation of glycerol-1-phosphate by insect flight-muscle mitochondria. J Insect Physiol. 1969 Mar;15(3):367–372. doi: 10.1016/0022-1910(69)90283-2. [DOI] [PubMed] [Google Scholar]
- ESTABROOK R. W., SACKTOR B. alpha-Glycerophosphate oxidase of flight muscle mitochondria. J Biol Chem. 1958 Oct;233(4):1014–1019. [PubMed] [Google Scholar]
- ESTABROOK R. W. Studies of oxidative phosphorylation with potassium ferricyanide as electron acceptor. J Biol Chem. 1961 Nov;236:3051–3057. [PubMed] [Google Scholar]
- HAAS D. W. ENERGY-LINKED REACTIONS IN THE KEILIN AND HARTREE HEART-MUSCLE PREPARATION. Biochim Biophys Acta. 1964 Sep 18;89:543–544. doi: 10.1016/0926-6569(64)90081-1. [DOI] [PubMed] [Google Scholar]
- HAYASHI S., KOCH J. P., LIN E. C. ACTIVE TRANSPORT OF L-ALPHA-GLYCEROPHOSPHATE IN ESCHERICHIA COLI. J Biol Chem. 1964 Sep;239:3098–3105. [PubMed] [Google Scholar]
- Hansford R. G., Chappell J. B. The effect of Ca2+ on the oxidation of glycerol phosphate by blowfly flight-muscle mitochondria. Biochem Biophys Res Commun. 1967 Jun 23;27(6):686–692. doi: 10.1016/s0006-291x(67)80090-1. [DOI] [PubMed] [Google Scholar]
- Klingenberg M. Localization of the glycerol-phosphate dehydrogenase in the outer phase of the mitochondrial inner membrane. Eur J Biochem. 1970 Apr;13(2):247–252. doi: 10.1111/j.1432-1033.1970.tb00924.x. [DOI] [PubMed] [Google Scholar]
- Mitchell P., Moyle J. Translocation of some anions cations and acids in rat liver mitochondria. Eur J Biochem. 1969 Jun;9(2):149–155. doi: 10.1111/j.1432-1033.1969.tb00588.x. [DOI] [PubMed] [Google Scholar]
- PORTZEHL H., CALDWELL P. C., RUEEGG J. C. THE DEPENDENCE OF CONTRACTION AND RELAXATION OF MUSCLE FIBRES FROM THE CRAB MAIA SQUINADO ON THE INTERNAL CONCENTRATION OF FREE CALCIUM IONS. Biochim Biophys Acta. 1964 May 25;79:581–591. doi: 10.1016/0926-6577(64)90224-4. [DOI] [PubMed] [Google Scholar]
- RINGLER R. L., SINGER T. P. Studies on the mitochondrial alpha-glycerophosphate dehydrogenase. I. Reaction of the dehydrogenase with electron acceptors and the respiratory chain. J Biol Chem. 1959 Aug;234(8):2211–2217. [PubMed] [Google Scholar]
- Tyler D. D. Evidence of a phosphate-transporter system in the inner membrane of isolated mitochondria. Biochem J. 1969 Mar;111(5):665–678. doi: 10.1042/bj1110665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler D. D., Gonze J., Estabrook R. W. Observations on the inhibitor sensitivity of the choline oxidation system. Arch Biochem Biophys. 1966 Aug;115(2):373–384. doi: 10.1016/0003-9861(66)90287-6. [DOI] [PubMed] [Google Scholar]
- Tyler D. D. Spatial arrangement of respiratory-chain components in mitochondrial membranes. Biochem J. 1970 Feb;116(4):30P–31P. doi: 10.1042/bj1160030p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZEBE E. C., MCSHAN W. H. Lactic and alpha-glycerophosphate dehydrogenases in insects. J Gen Physiol. 1957 May 20;40(5):779–790. doi: 10.1085/jgp.40.5.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den BERGH S., SLATER E. C. The respiratory activity and permeability of housefly sarcosomes. Biochem J. 1962 Feb;82:362–371. doi: 10.1042/bj0820362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Bergh S. G. Pyruvate oxidation and the permeability of housefly sarcosomes. Biochem J. 1964 Oct;93(1):128–136. doi: 10.1042/bj0930128. [DOI] [PMC free article] [PubMed] [Google Scholar]


