Abstract
The immutans (im) variegation mutant of Arabidopsis has green and white leaf sectors due to the action of a nuclear recessive gene, IMMUTANS (IM). This gene encodes the IM protein, which is a chloroplast homolog of the mitochondrial alternative oxidase. Because the white sectors of im accumulate the noncolored carotenoid, phytoene, IM likely serves as a redox component in phytoene desaturation. In this paper, we show that IM has a global impact on plant growth and development and is required for the differentiation of multiple plastid types, including chloroplasts, amyloplasts, and etioplasts. IM promoter activity and IM mRNAs are also expressed ubiquitously in Arabidopsis. IM transcript levels correlate with carotenoid accumulation in some, but not all, tissues. This suggests that IM function is not limited to carotenogenesis. Leaf anatomy is radically altered in the green and white sectors of im: Mesophyll cell sizes are dramatically enlarged in the green sectors and palisade cells fail to expand in the white sectors. The green im sectors also have significantly higher than normal rates of O2 evolution and elevated chlorophyll a/b ratios, typical of those found in “sun” leaves. We conclude that the changes in structure and photosynthetic function of the green leaf sectors are part of an adaptive mechanism that attempts to compensate for a lack of photosynthesis in the white leaf sectors, while maximizing the ability of the plant to avoid photodamage.
Variegation mutants provide an excellent system to explore the nature of communication between the nucleus-cytoplasm, chloroplast, and mitochondrial genetic compartments (for review, see León et al., 1998; Rodermel, 2001). The leaves of these mutants have green and white (or yellow) sectors that arise as a consequence of mutations in nuclear or organellar genes (Tilney-Bassett, 1975). Whereas the green sectors contain cells with morphologically normal chloroplasts, cells in the white sectors contain plastids that lack pigments and normal lamellar structures. One common mechanism of variegation involves the induction of defective mitochondria or chloroplasts by mutations in nuclear genes for organelle proteins. This is sometimes due to transposable element activity, in which case the green and white cells have different genotypes. In other cases, the two types of cells have the same (mutant) genotype, indicating that the gene defined by the mutation codes for a product that is required for organelle biogenesis in some, but not all, cells of the mutant.
Despite the large number of mutant screens that have been conducted in Arabidopsis, surprisingly few nuclear “variegation” loci have been reported. These include cab underexpressed (cue1), chloroplast mutator (chm), differential development of vascular-associated cells (dov), immutans (im), pale cress (pac), var1, and var2 (e.g. Rédei, 1963, 1967, 1973; Röbbelen, 1968; Martínez-Zapater et al., 1992; Reiter et al., 1994; Li et al., 1995; Grevelding et al., 1996; Sakamoto et al., 1996; Kinsman and Pyke, 1998; López-Juez et al., 1998; Meurer et al., 1998; Streatfield et al., 1999; Tirlapur et al., 1999). Of these, we have focused on im (Wetzel et al., 1994; Meehan et al., 1996; Wetzel and Rodermel, 1998; Wu et al., 1999) and var2 (Chen et al., 1999, 2000); im is the topic of the present investigation. im was first isolated and partially characterized nearly 40 years ago by Rédei (1963, 1967) and Röbbelen (1968). Sectoring in im is due to the action of a nuclear recessive gene, and white sector formation is promoted by growth in elevated light or temperature (Rédei, 1963; Röbbelen, 1968; Wetzel et al., 1994). Visually white reproductive structures of im give rise to variegated progeny that are predominantly green or white, again depending on growth illumination and temperature. Because of this apparent phenotypic reversibility and an inability of the mutant to convert permanently from an all-green (“wild-type-like”) to an albino phenotype, Rédei (1975) called the mutant immutans (for “immutable”). Consistent with this reversibility, abnormal plastids are not maternally inherited in im, suggesting that the plastid defect can be cured (Wetzel et al., 1994).
Biochemical analyses revealed that im white sectors accumulate phytoene, a colorless C40 carotenoid intermediate (Wetzel et al., 1994). This suggests that the mutant is impaired in the activity of phytoene desaturase (PDS), the plastid enzyme that converts phytoene to β-carotene (Bartley et al., 1991). We cloned the IMMUTANS (IM) gene by map-based methods and found that it codes for a plastid homolog of the mitochondrial alternative oxidase (AOX; Wu et al., 1999); a transposon-tagged im allele has also been reported (Carol et al., 1999). AOX is an inner mitochondrial membrane protein that functions as a terminal oxidase in the alternative (cyanide-resistant) pathway of mitochondrial respiration where it generates water from ubiquinol (for review, see Siedow and Umbach, 1995; Vanlerberghe and McIntosh, 1997). This similarity to AOX suggested that the IM protein may be a component of a redox pathway that functions in the desaturation of phytoene (Beyer et al., 1989; Mayer et al., 1990, 1992; Schulz et al., 1993; Nievelstein et al., 1995; Norris et al., 1995). Consistent with this interpretation, IM has quinol:oxygen oxidoreductase activity when expressed in Escherichia coli (Josse et al., 2000).
We were interested in determining the physiological function of IM and the mechanism of im variegation. A powerful way to gain insight into IM function is to examine the phenotype of im plants. Because previous studies of im have focused on leaf variegation, we were interested in determining whether im has other phenotypes. In this report, we show that the mutant is impaired in its growth and development, and that this impairment is due, in part, to a blockage of plastid differentiation in diverse cell types. IM expression appears to be ubiquitous, but expression levels are not always correlated with carotenoid accumulation, opening the possibility that IM serves as a general electron sink in plastid membranes. Mesophyll cell morphogenesis is affected in both the green and white sectors of im. The disruptions in leaf morphogenesis in the white sectors are consistent with the idea that the expression of IM is required for the transmission of a plastid signal(s) to regulate leaf developmental programming. The green im sectors, on the other hand, have higher than normal photosynthetic rates, and the anatomical alterations in these sectors may be part of an adaptive strategy to compensate for a lack of photosynthesis in the white sectors.
RESULTS
Phenotype of immutans
We have sequenced three IM alleles and all are predicted to be null (Wu et al., 1999). For the present studies, we used the spotty allele. We have reported previously that im seeds germinate normally under all light conditions (Wetzel et al., 1994), and that depending on the illumination conditions, germinated seedlings have green, variegated, or white cotyledons and true leaves (Rédei, 1967; Röbbelen, 1968; Wetzel et al., 1994). Other normally green organs, including stems and sepals, are also variegated. Whereas im flowers are morphologically normal, siliques are smaller than wild type and are either variegated or all white. White siliques lack seeds and variegated siliques have significantly fewer seeds than normal.
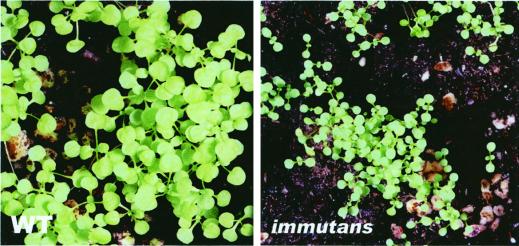
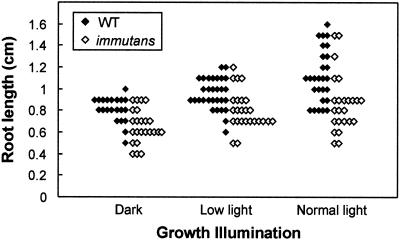
Under low-light conditions that promote the formation of nearly all-green plants, im grew more slowly than the wild type (Fig. 1). Yet, im ultimately attained the stature of wild-type plants. Shoot growth was similarly retarded in mutant plants maintained under normal light conditions. However, in normal light it is difficult to ascribe the growth impairment to a lack of IM per se because it can be argued that these plants have white sectors and, consequently, that there is less green tissue than normal to support growth. Figure 2 shows that wild-type and im roots increased in length as a function of growth illumination. Whereas both types of roots have a similar size distribution in darkness and under low-light conditions, there was a tendency for the wild-type to have longer roots than im under normal light conditions. Considered together, Figures 1 and 2 indicate that a lack of IM impacts root and shoot development.
Figure 1.
Growth of wild type and im. Plants were maintained under low-light conditions (15 μmol m−2 s−1) and photographed 8 weeks after germination. The wild type has an average of four true leaves and im an average of two true leaves. The seeds germinated at the same time. A magnification of 5× applies for both left and right panels.
Figure 2.
Root growth in wild type and im. Root lengths were measured after 4 d of growth on Murashige and Skoog medium supplemented with 1% (w/v) Suc. The plants were maintained under normal light (100 μmol m−2 s−1), low light (15 μmol m−2 s−1), and in darkness. Each data point represents an individual plant.
Expression of IM
The phenotype of im suggests that IM is expressed not only in leaves, but also in other Arabidopsis tissues and organs. To determine the developmental and tissue specificity of IM expression, we investigated the patterns of IM promoter activity in transgenic plants that bear an IM promoter: β-glucuronidase (GUS) reporter gene fusion (Fig. 3). Seeds from each line were germinated on Murashige and Skoog medium or in soil and GUS activity assays were carried out at different stages of development. The expression patterns were identical for each of five independently transformed lines; the results in Figure 4 are from one of the lines.
Figure 3.
pPZP/IMGUS, the IM promoter: GUS fusion construct. pPZP/IMGUS contains an approximately 3-kb upstream region of IM fused to the GUS (β-glucuronidase) gene and nos terminator. The selectable marker is an NPTII gene fused to 35S promoter/nos terminator elements. Twenty-five amino acids in the fusion protein are from the IM protein (Wu et al., 1999). RB, Right border; LB, left border.
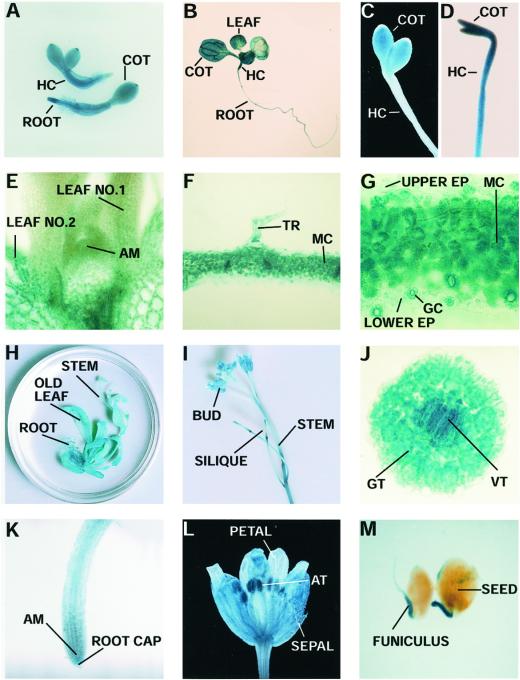
Figure 4.
Expression patterns of the IM promoter: GUS transgene during development. A, One-day-old light-grown seedlings. B, Seven-day-old light-grown seedling. C, Dark-field microscopy of a 4-d-old etiolated seedling (5× objective). D, Four-day-old etiolated seedling (35S promoter: GUS fusion; 5× objective). E, Cross-section of a shoot meristem of a 10-d-old light-grown seedling (25× objective). F and G, Cross-sections of first true leaves of 10-d-old light-grown seedlings (10× and 25× objectives, respectively). H, Six-week-old rosette. I, Bolt from a flowering plant. J, Cross-section of a hypocotyl of a 10-d-old light-grown seedling (25× objective). K, Root tip of a 10-d-old light-grown seedling. L, Dark-field microscopy of a flower (5× objective). M, Young seeds. AM, Apical meristem; AT, anther; COT, cotyledon; EP, epidermis; GC, guard cell; GT, ground tissues; HC, hypocotyl; MC, mesophyll cell; VT, vascular tissues; TR, trichome.
GUS activity was first observed in 1-d-old light-grown seedlings immediately after seed coat breakage (Fig. 4A). All of the tissues (roots, hypocotyls, and cotyledons) were heavily stained. This pattern was maintained throughout vegetative development, as illustrated by the presence of GUS staining in roots, cotyledons, hypocotyls, and developing first leaves of 7-d-old light-grown seedlings (Fig. 4B). High levels of GUS activity were also found in the cotyledons of dark-grown seedlings; however, the hypocotyls were barely stained (Fig. 4C). This is in contrast to control experiments performed with transgenic 35S promoter: GUS seedlings, in which the hypocoyls and cotyledons were uniformly stained (Fig. 4D).
GUS activity appeared to increase during early leaf development. It was low in the shoot apical meristem (Fig. 4E) and in very young expanding leaves (leaf no. 1 in Fig. 4E). As the leaves continued to expand, GUS activity increased (leaf no. 2 in Fig. 4E). Mesophyll cells, guard cells, and trichomes were stained in young leaves, whereas epidermal cells lacked significant staining (Fig. 4, F and G). GUS activity was present in old leaves of 6-week-old mature rosettes (Fig. 4H). Stems also had appreciable GUS activity (Fig. 4I). A cross-section of a hypocotyl reveals that staining was very high in the vascular tissues, but lower in the ground tissues (Fig. 4J). GUS activity was also present throughout the root (Fig. 4K). Staining was observed in all flower parts, including the sepals, petals, and anthers (Fig. 4L), and also in green silique coats (Fig. 4I). In young seeds, GUS was expressed specifically in the funiculus (Fig. 4M). All tissues except seed coats were stained with GUS in the control 35S promoter: GUS fusion plants (as in Fig. 4D).
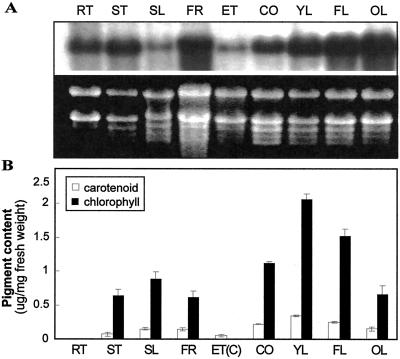
To obtain a quantitative estimate of IM mRNA levels, we performed northern-blot analyses on total cell RNAs isolated from various Arabidopsis tissues and organs. Figure 5A shows that IM mRNAs are present in all of the RNA samples analyzed. IM transcripts were most abundant in leaves, cotyledons, flowers, and stems, and least abundant in etiolated seedlings and siliques. Based on the distribution of GUS staining, it is likely that most of the IM mRNAs in etiolated seedlings were present in the cotyledons. IM mRNAs increase in amount during leaf development. These experiments validate the results of the IM promoter:GUS assays and indicate that IM is expressed ubiquitously in Arabidopsis tissues and organs throughout development.
Figure 5.
Expression analysis of IM mRNA and pigment levels in Arabidopsis. A, RNA gel-blot analyses were performed as described in “Materials and Methods.” The RNA gel is stained with ethidium bromide to show rRNA (loading control). The blot was probed with a radiolabeled IM cDNA (Wu et al., 1999). B, Total carotenoids and chlorophylls were extracted from Arabidopsis as described in “Materials and Methods.” Values are an average of three separate experiments ±sd. The samples in A and B are from 4- to 5-week-old plants grown under normal light conditions (100 μmol m−2 s−1), with the exception of the samples from dark-grown seedlings (ET). RT, Root; ST, stem; SL, green silique; FR, flowers (petals + green sepals); ET, 7-d-old etiolated seedling (cotyledon + hypocotyl); ET(C), cotyledons from 7-d-old etiolated seedlings; CO, 7-d-old cotyledon; YL, young leaf (5-mm length); FL, just fully expanded leaf (40-mm length); OL, senescing, late fully expanded leaf.
Pigment Analyses
Carotenoid and chlorophyll levels were examined in the same organs and tissues as the RNA gel-blot analyses to determine whether there was a correlation between IM mRNA accumulation and pigment content (Fig. 5B). In general, all the green organs of the plant had relatively high levels of IM mRNAs and carotenoids. The cotyledons of etiolated seedlings, which were stained with GUS (Fig. 4C), also accumulated carotenoids, in contrast to etiolated hypocotyls, which lacked GUS staining (Fig. 4C) and did not accumulate carotenoids (data not shown). Despite the general correspondence between IM expression and carotenoid content, this correlation does not hold for all Arabidopsis tissues. For instance, IM mRNAs are nearly as abundant in roots as in cotyledons and stems, but roots contain only trace pigment amounts. IM mRNAs also increase progressively during leaf development, whereas carotenoid and chlorophyll levels decline. We conclude that the patterns of IM mRNA expression and pigment accumulation do not necessarily correspond in Arabidopsis tissues and organs and during development.
Plastid Ultrastructure
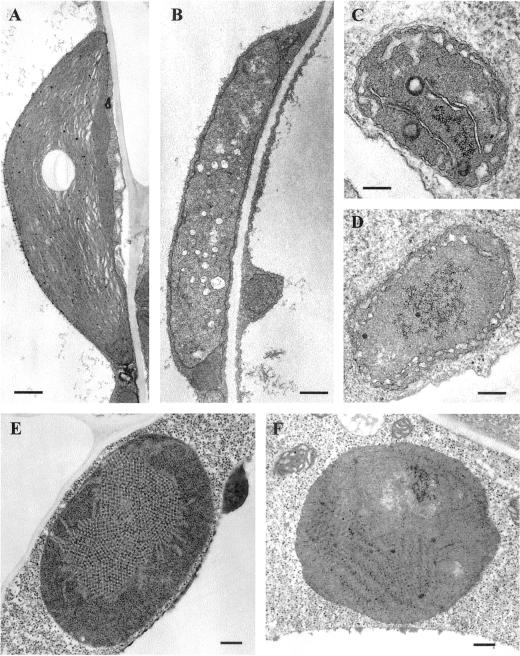
We previously have examined the ultrastructure of plastids in the green and white leaf sectors of im (Wetzel et al., 1994). Because of the ubiquity of IM expression, we wanted to determine whether IM is required for the biogenesis of plastids in organs other than leaves. As shown in Figure 6, normal chloroplasts were present in wild-type cotyledons and in the green sectors of im cotyledons (Fig. 6A), whereas the white sectors of im cotyledons contained vacuolated plastids that lacked organized lamellar structures (Fig. 6B). The latter plastids were the size of normal chloroplasts, i.e. much larger (approximately 6 μm) than undifferentiated proplastids in meristem cells (0.5–1 μm; Bowman, 1994). These findings are similar to transmission electron microscopy analyses of plastids in wild-type and im leaves (Wetzel et al., 1994).
Figure 6.
Plastid ultrastructure. Wild-type and im seedlings were grown on Murashige and Skoog plates for 7 d under normal light conditions (A, B, C, and D) or in darkness (E and F). A, Chloroplast from a wild-type cotyledon (bar = 500 nm). B, Chloroplast from an im cotyledon (bar = 500 nm). C, Amyloplast from a wild-type root (bar = 200 nm). D, Amyloplast from an im root (bar = 200 nm). E, Etioplast from a wild-type cotyledon (bar = 200 nm). F, Etioplast from an im cotyledon (bar = 200 nm).
Amyloplasts are small (approximately the size of proplastids), irregularly shaped plastids in roots (Bowman, 1994). They usually contain starch granules and a few extended lamellar structures. Figure 6C shows that roots from wild-type Arabidopsis contain typical amyloplasts. Examination of a large number of plastids in sections of im roots revealed that some resembled wild-type amyloplasts, but that most were devoid of extended lamellae and starch granules (Fig. 6C). This heterogeneity in structure suggests that cells in im root tissues have a heteroplastidic amyloplast population. Cells in the white leaf sectors of im are also heteroplastidic (Wetzel et al., 1994).
Etioplasts are achlorophyllous plastids found in dark-grown seedlings (for review, see von Wettstein et al., 1995). They contain a distinctive paracrystalline lattice of interconnected membrane tubules (the prolamellar body [PLB]). Figure 6E shows a representative etioplast from a dark-grown wild-type cotyledon; it has a single large PLB. In contrast, etioplasts from dark-grown im seedlings did not contain PLBs, but rather have a large, organized molecular array (Fig. 6F). A large number of sections of im etioplasts have been examined, and PLB-like structures have not been observed (i.e. the molecular array structure is not an artifact of sectioning). One possibility is that this structure represents an unassembled intermediate of the PLB. Taken together, the data in Figure 6 indicate that IM is required for the normal development of mutliple plastid types in Arabidopsis.
Anatomy of im Leaves
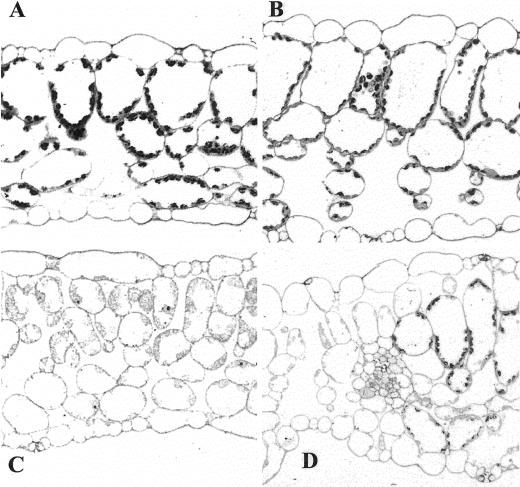
Although a lack of the IM protein results in variegated green organs and retards plant growth, light microscopy of tissue sections revealed that the morphology of nongreen im organs (e.g. roots, hypocotyls, and cotyledons of etiolated seedlings) is not detectably perturbed (data not shown). This is in contrast to green organs such as leaves. We have shown previously that chloroplast development is impaired in the white sectors of im leaves, but that the green leaf sectors contain morphologically normal chloroplasts (Wetzel et al., 1994). Figure 7 shows representative tissue sections of wild-type leaves, green im sectors, white im sectors, and a transition zone between green and white sectors. The wild-type leaves had typical epidermal, columnar palisade mesophyll and spongy mesophyll cell layers; the latter two layers had densely staining chloroplasts (Fig. 7A). In contrast, the tissue organization of the green and white sectors of im leaves was perturbed. In particular, the green sectors were thicker than normal due to a marked enlargement in the sizes of the mesophyll cells, epidermal cells, and air spaces (Fig. 7B). The white leaf sectors had a normal thickness, but the palisade cells failed to expand normally (Fig. 7C). The distinctive characteristics of the white and green sectors were apparent in regions where the two tissue types abut and overlay one another (Fig. 7D).
Figure 7.
Light microscopy of fully expanded leaves from wild-type and im plants grown under normal light conditions. A magnification of 25× applies to A through D. The white sectors stain less intensely than green sectors because their plastids are deficient in internal structures. A, Wild type. B, Green leaf sector of im. C, White leaf sector of im. D, Adjacent green and white sectors of im.
The significant anatomical differences between the wild-type and im green sectors raise the question of whether the two types of green tissue have similar photosynthetic rates. As a first approach to address this question, we measured the amount of oxygen evolved from im versus wild-type plants on a per- chlorophyll basis. We analyzed the response of plants germinated and maintained under both low light and normal light conditions. We found that the im green sectors evolved approximately twice as much oxygen as the wild type under both illumination conditions (Fig. 8A). In normal light conditions, the enhancement in oxygen evolution was accompanied by a significantly enhanced chlorophyll a/b ratio (Fig. 8B). Similar chlorophyll a/b ratios were observed under low-light conditions.
Figure 8.
Photosynthetic oxygen evolution and chlorophyll a/b ratios in wild-type leaves and im green leaf sectors. Plants were grown under normal light (100 μmol m−2 s−1) or low light (15 μmol m−2 s−1). Oxygen evolution (A) and chlorophyll a/b ratios (B) were determined as described in “Materials and Methods.” Each graph represents an average ± sd of three different leaf samples for each illumination condition.
DISCUSSION
IM Plays an Important Role in Plant Development
Previous morphological, biochemical, and molecular analyses of im have focused on leaves (Rédei, 1963, 1967; Röbbelen, 1968; Wetzel et al., 1994). These studies showed that IM is required for normal chloroplast biogenesis in some, but not all, plastids and cells of the expanding leaf. In the present study, we observed phenotypic alterations in other major organ systems of the mutant. This was true for both green organs (e.g. cotyledons, stems, and siliques) and nongreen organs (e.g. roots, etiolated hypocotyls and cotyledons). These data suggest that IM has a global impact on plant physiology and development. Support for this conclusion is provided by our IM promoter: GUS fusion and northern-blot analyses showing that the IM promoter is active and that IM mRNAs are expressed ubiquitously in Arabidopsis tissues and organs throughout development. Chloroplast, amyloplast, and etioplast development were also impaired in im. In etioplasts this impairment results in less than 50% the normal accumulation of carotenoids (data not shown), indicating that IM is necessary for carotenoid deposition in plastid types other than chloroplasts (Wetzel et al., 1994). The impact of IM could be direct or indirect. For instance, because carotenoids are precursors of ABA, an inhibition of carotenoid biosynthesis in tissues lacking IM could result in a disruption of ABA synthesis with consequent downstream effects on development.
Role of IM in Plastid Metabolism
The accumulation of phytoene in im white sectors suggests that PDS activity is impaired in im and that IM plays an important role in carotenoid biosynthesis (Wetzel et al., 1994). Cloning and sequencing of IM revealed that the gene product is a chloroplast membrane protein with homology to the AOX class of inner mitochondrial membrane terminal oxidases (Carol et al., 1999; Wu et al., 1999). IM also has quinol oxidase activity when expressed in E. coli (Josse et al., 2000). Considered together, these data suggest that IM is a redox component of a phytoene desaturation pathway involving PDS, plastoquinol, and oxygen as a terminal acceptor (Beyer et al., 1989; Mayer et al., 1990, 1992; Schulz et al., 1993; Nievelstein et al., 1995; Norris et al., 1995).
In support of the central role of IM in carotenogenesis, our data show that the IM promoter is active and that IM transcripts are abundant in Arabidopsis tissues that accumulate high levels of carotenoids, including cotyledons of light- and dark-grown plants, leaves, stems, siliques, and flowers. In the same manner, some tissues that do not accumulate carotenoids, e.g. hypocotyls of dark-grown seedlings, have low levels of IM expression. In further support of the idea that IM expression and carotenoid accumulation are frequently coordinated is the finding that transcripts from an IM ortholog in tomato (Lycopersicon esculentum) are abundantly expressed during tomato fruit ripening (Josse et al., 2000; R. Bae and S. Rodermel, unpublished data). During ripening, chloroplasts are converted into carotenoid-accumulating chromoplasts; Arabidopsis, versus tomato, does not have an abundant chromoplast population.
Despite the apparent coordination of IM mRNA expression and carotenogenesis in many Arabidopsis tissues, one of the central findings of this paper was that this is not always the case. For example, carotenoids do not accumulate in roots, despite high levels of IM mRNA. IM is also up-regulated during the progression of leaf development, as carotenoid levels fall. In Arabidopsis and other dicots, photosynthetic rates reach a maximum early in leaf development in the expanding leaf, then progressively decline during a prolonged senescent phase in the fully expanded leaf (Gan and Amasino, 1997; Miller et al., 1997, 2000; A. Miller, D. Stessman, M. Spalding, and S. Rodermel, unpublished data). During senescence, chloroplasts are converted into gerontoplasts and resources are mobilized to growing parts of the plant. Both anabolic and catabolic processes are responsible for reductions that occur in many plastid components during the senescence process (Matile, 1992). The up-regulation of IM expression in the face of declining carotenoid production is consistent with the hypothesis that IM participates in oxidative activities that occur during this phase of leaf ontogeny.
Because all plastid types synthesize carotenoids, the possibility cannot be ruled out that IM is an electron transfer component involved solely in carotenogenesis. Nevertheless, our data point the way toward a more global role of this protein in plastid metabolism. In agreement with this hypothesis, recent evidence in Chlamydomonas reinhardtii suggests that IM serves as a terminal oxidase in chlororespiration (Cournac et al., 2000). In the context of its importance in plastid metabolism and its ubiquitous expression in all plastid types, it is interesting that IM does not seem to be required in cyanobacteria, because BLAST searches show that IM (and AOX) are not present in this evolutionary precursor of chloroplasts. AOX is present in plant, but not animal, mitochondria and is encoded by a small multigene family in the nucleus (Vanlerberghe and McIntosh, 1997). IM, on the other hand, is only distantly related to the AOX class of proteins and is present as a single nuclear gene, at least in Arabidopsis and tomato (Wu et al., 1999; R. Bae and S. Rodermel, unpublished data). Thus, our working hypothesis is that a plant nuclear gene for an enzyme with terminal oxidase activity arose that contained an organelle targeting signal, and that this gene is the progenitor of an extended gene family whose products became functional in mitochondria and plastids as redox components in multiple metabolic pathways. It will be interesting to examine the evolution of this protein class as more IM and AOX genes are isolated and characterized.
Plastid-to-Nucleus Communication Regulates Leaf Development
A considerable body of evidence supports the notion that the transcription of nuclear genes for many photosynthetic proteins is controlled by the developmental state of the plastid (the “plastid signal” hypothesis; for review, see Taylor, 1989; Susek and Chory, 1992; León et al., 1998; Rodermel, 2001). Consistent with this hypothesis, we have reported that plastids in the white sectors of im have reduced rates of Lhcb transcription and decreased Lhcb mRNA levels (Meehan et al., 1996). In addition to plastid signals that regulate the transcription of nuclear photosynthetic genes, it has been proposed that the metabolic state of the plastid controls tissue and organ developmental programming. Identification of this type of communication has come from an examination of a handful of nuclear gene-induced pigment mutants whose white leaf tissues have abnormal plastids and cells, and altered palisade and/or spongy mesophyll cell layer organizations. These include dag of Antirrhinum majus (Chatterjee et al., 1996), dcl of tomato (Keddie et al., 1996), and several Arabidopsis mutants, including cla1 (Mandel et al., 1996; Estévez et al., 2000), cue1 (Li et al., 1995; Streatfield et al., 1999), and pac (Reiter et al., 1994; Meurer et al., 1998). Because the products of the genes defined by these mutations reside in the plastid, it has been argued that these proteins are not independently required for plastid development and cell differentiation (and consequently for proper leaf morphogenesis), but that the effects on mesophyll cell differentiation are a consequence of incomplete chloroplast differentiation. How the status of the plastid is sensed is not known. Regardless, we conclude that im should be added to the list of mutants in which this type of plastid-to-nucleus communication is impaired.
Adaptations in the Green im Sectors
Our anatomical studies showed that the green leaf sectors of im are thicker than normal due to an enhancement in mesophyll cell size and intercellular air space volume (Fig. 7). Analyses of fluorescence-activated cell sorter-purified cells previously demonstrated that cells from green im leaf sectors have more chlorophyll than similarly sized cells from wild-type plants (Meehan et al., 1996). As illustrated in Figure 8, the im green sectors also have significantly elevated rates of oxygen evolution on a chlorophyll basis. Oxygen evolution is frequently taken as a measure of photosynthesis (e.g. Van and Spalding, 1999), and thus one interpretation of our data is that the green sectors have enhanced rates of photosynthesis, perhaps as part of a complex mechanism whereby the photosynthetic potential of the im green sectors is enhanced to compensate for a lack of photosynthesis in the white sectors. One way that higher rates of photosynthesis could be attained is by enhancing the activities of regulatory enzymes of photosynthetic carbon assimilation such as Rubisco (e.g. Huner et al., 1998).
We also found that the green im cells have significantly higher chlorophyll a/b ratios than wild-type cells under normal light conditions. High chlorophyll a/b ratios are typically found in “sun” versus “shade” plants and are indicative of smaller light-harvesting complexes and/or an altered stoichiometry of photosystem I and photosystem II (for review, see Stitt, 1991). These are typically adaptations to avoid light stress. Our working hypothesis is that a lack of IM gives rise to morphological and biochemical adaptations in the green sectors that make the leaf more “sun”-like, perhaps as a way to avoid photooxidative damage.
MATERIALS AND METHODS
Plant Material and Growth Conditions
Seeds from wild-type Arabidopsis (Columbia ecotype) and the spotty allele of im (Wetzel et al., 1994) were germinated and grown at 25°C under continuous illumination, either at 100 μmol m−2 s−1 (normal light) or at 15 μmol m−2 s−1 (low light). Samples were collected from various tissues and organs from 4- to 5-week-old plants. To measure root lengths, wild-type and im seeds were plated on Murashige and Skoog medium (pH 5.7) supplemented with 1% (w/v) Suc. Before plating, the seeds were surface sterilized for 1 min in 70% (v/v) ethanol, 10 min in 5% (w/v) NaCl, and washed five times for 1 min each with sterile distilled water. The plates were incubated in a vertical position under normal or low-light conditions or in continuous darkness.
RNA and Pigment Analyses
Total RNA isolation and RNA gel-blot analyses were performed according to procedures described previously (Wetzel et al., 1994). The formaldehyde gels contained equal amounts of RNA per gel lane. The blots were probed with an IM cDNA (Wu et al., 1999). The analyses were repeated twice to confirm the reproducibility of the results. Pigment extractions and calculations of pigment concentrations were performed essentially as described by Lichtenthaler (1987). Leaf tissues were extracted with several changes of 95% (v/v) ethanol in the dark at 4°C, and absorbance measurements were made at 664, 649, and 470 nm.
IM Promoter: GUS Fusion Constructs
Transgenic Arabidopsis were generated that contained either an IM promoter: GUS fusion or a cauliflower mosaic virus 35S promoter: GUS fusion. The IM promoter: GUS fusion was derived from the binary plasmid, pPZP/IMGUS. To generate pPZP/IMGUS, an approximately 2.1-kb SmaI/EcoRI fragment of pBI121 (CLONTECH, Palo Alto, CA), which contains the GUS gene (Jefferson et al., 1987) fused to the nos terminator, was subcloned into pPZP211 (Hajdukiewicz et al., 1994), a binary vector that contains the NPTII gene driven by the 35S promoter. This gave rise to pPZP/GUS. The IM promoter is a BclI/XbaI fragment that includes a portion of the N-terminal transit sequence of IM (25 amino acids) and approximately 3 kb of upstream sequence (Fig. 3). It was subcloned from a Ler lambda genomic library (Voytas et al., 1990) and inserted as a PstI/XbaI fragment into pPZPGUS. The resulting construct (pPZP/IMGUS) is a translational fusion between the approximately 25 amino acids of the transit sequence and the GUS protein. The 35S promoter-GUS fusion sequence was derived from the binary plasmid, pPZP/35SGUS. In this construct, the XbaI/SacI GUS-containing sub-fragment of pPZPGUS was replaced by the 2.7-kb PstI/SacI sub-fragment of pBI121, which contains the GUS gene fused to the 35S promoter.
pPZP/IMGUS and pPZP/35SGUS were introduced into the Agrobacterium tumefaciens strain C58CI by electroporation, and flowering Arabidopsis plants (Columbia ecotype) were transformed by the floral dip method (Clough and Bent, 1998). After flowering, the T1 seeds were collected and germinated on selective Murashige and Skoog medium (50 μg mL−1 kanamycin). Forty-two T1 lines of the IM promoter: GUS fusion were screened for the presence of the foreign DNA by Southern hybridization (procedures described by Wetzel et al., 1994), and five lines were identified with single-copy T-DNA insertions at different sites in the genome. Each of these contained an intact reporter gene fusion. Similar procedures were carried out with the 35S promoter: GUS lines. Two T1 lines were identified that had single intact inserts.
GUS activity assays were conducted on transgenic plants with a single IM promoter: GUS insertion. For these assays, T2 seeds were sown on Murashige and Skoog plates in the dark for 2 d at 4°C. To obtain etiolated seedlings, the plates were maintained in darkness for another 4 d, but at 22°C. To obtain light-grown seedlings, the plates were transferred from the cold to a growth chamber (100 μmol m−2 s−1 continuous illumination, 22°C). To obtain mature plants, the seedlings were transplanted to soil, then maintained in a growth chamber. Plants of different developmental stages were collected and analyzed for GUS activity as described by Horvath et al. (1993). In some experiments, the stained plant tissues were embedded in 4% (w/v) agarose, and sections (approximately 50 μm) were examined by light microscopy.
Light and Electron Microscopy
For transmission electron microscopy, cotyledon and root samples were obtained from 7-d-old seedlings grown on Murashige and Skoog medium under either normal light conditions or darkness. The samples were fixed, stained, and examined as in Horner and Wagner (1980). Samples for light microscopy were obtained from fully expanded leaves or roots from wild-type and im plants grown under normal light conditions in the growth chamber. They were cut into 1-mm pieces, vacuum infiltrated with fixative (1% [w/v] paraformaldehyde and 2% [w/v] glutaraldehyde), and then incubated overnight at 4°C. After washing in 0.1 m cacodylate buffer, the samples were dehydrated through an ethanol series and embedded in Spurr's resin. Sections (1.5 μm) were attached to glass slides, stained with 1% (w/v) toluidine blue, and observed in bright field with a light microscope (Leitz Orthoplan, Iowa City).
Measurements of Oxygen Evolution
Leaves from 4- to 5-week-old wild-type and im plants (prebolting) grown under normal and low-light conditions were used for oxygen evolution experiments as described by Van and Spalding (1999). Single plants were cut in half from the bottom to the top of the plant. One-half of the plant was used for chlorophyll determinations, as described above, while the other half was cut into 1- to 2-mm-sized pieces and immersed into 1 mL of 10 mm NaHCO3. After vacuum infiltration for 15 min, the sample was placed in a Clark O2 electrode chamber and O2 evolution was measured under 500 μmol m−2 s−1 of incident light at 25°C. The amount of oxygen evolved was calculated as described by Allen and Holmes (1986).
Footnotes
This work was supported by the U.S. Department of Energy (Energy Biosciences; grant no. DE–FG02–94ER20147 to S.R.R.). This is journal paper no. J–19348 of the Iowa Agricultural Experiment Station (Ames), project no. 2987.
LITERATURE CITED
- Allen JF, Holmes NG. Electron transport and redox titration. In: Hipkins MF, Baker NR, editors. Photosynthesis Energy Transduction: A Practical Approach. Oxford: IRL Press; 1986. pp. 103–140. [Google Scholar]
- Bartley GE, Viitanen PV, Pecker I, Chamovitz D, Hirschberg J, Scolnik PA. Molecular cloning and expression in photosynthetic bacteria of a soybean cDNA coding for phytoene desaturase, an enzyme of the carotenoid biosynthesis pathway. Proc Natl Acad Sci USA. 1991;88:6532–6536. doi: 10.1073/pnas.88.15.6532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyer P, Mayer M, Kleinig H. Molecular oxygen and the state of geometric isomerism of intermediates are essential in the carotene desaturation and cyclization reactions in daffodil chromoplasts. Eur J Biochem. 1989;184:141–150. doi: 10.1111/j.1432-1033.1989.tb15000.x. [DOI] [PubMed] [Google Scholar]
- Bowman J. Arabidopsis: An Atlas of Morphology and Development. New York: Springer-Verlag; 1994. [Google Scholar]
- Carol P, Stevenson D, Bisanz C, Breitenbach J, Sandmann G, Mache R, Coupland G, Kuntz M. Mutations in the Arabidopsis gene IMMUTANS cause a variegated phenotype by inactivating a chloroplast terminal oxidase associated with phytoene desaturation. Plant Cell. 1999;11:57–68. doi: 10.1105/tpc.11.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee M, Sparvoli S, Edmunds C, Garosi P, Findlay K, Martin C. DAG, a gene required for chloroplast differentiation and palisade development in Antirrhinum majus. EMBO J. 1996;15:4194–4207. [PMC free article] [PubMed] [Google Scholar]
- Chen M, Choi YD, Voytas D, Rodermel S. Mutations in the Arabidopsis VAR2 locus cause leaf variegation due to the loss of a chloroplast FtsH protease. Plant J. 2000;22:303–313. doi: 10.1046/j.1365-313x.2000.00738.x. [DOI] [PubMed] [Google Scholar]
- Chen M, Jensen M, Rodermel S. The yellow variegated mutant of Arabidopsis is plastid autonomous and delayed in chloroplast biogenesis. J Heredity. 1999;90:207–214. doi: 10.1093/jhered/90.1.207. [DOI] [PubMed] [Google Scholar]
- Clough SJ, Bent AF. Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998;16:735–743. doi: 10.1046/j.1365-313x.1998.00343.x. [DOI] [PubMed] [Google Scholar]
- Cournac L, Redding K, Ravenel J, Rumeau D, Josse E-M, Kuntz M, Peltier G. Electron flow between photosystem II and oxygen in chloroplasts of photosystem-deficient algae is mediated by a quinol oxidase involved in chlororespiration. J Biol Chem. 2000;275:17256–17262. doi: 10.1074/jbc.M908732199. [DOI] [PubMed] [Google Scholar]
- Estévez JM, Cantero A, Romero C, Kawaide H, Jiménez LF, Kuzuyama T, Seto H, Kamiya Y, León P. Analysis of the expression of CLA1, a gene that encodes the 1-deoxyxylulose 5-phosphate synthase of the 2-C-methyl-D-erythritol-4-phosphate pathway in Arabidopsis. Plant Physiol. 2000;124:95–103. doi: 10.1104/pp.124.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan S, Amasino RM. Making sense of senescence. Plant Physiol. 1997;113:313–319. doi: 10.1104/pp.113.2.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grevelding C, Suter-Crazzolara C, von Menges A, Kemper E, Masterson R, Schell J, Reiss B. Characterization of a new allele of pale cress and its role in greening in Arabidopsis thaliana. Mol Gen Genet. 1996;251:532–541. doi: 10.1007/BF02173642. [DOI] [PubMed] [Google Scholar]
- Hajdukiewicz P, Svab Z, Maliga P. The small, versatile pPZP family of Agrobacterium binary vectors for plant transformation. Plant Mol Biol. 1994;25:989–994. doi: 10.1007/BF00014672. [DOI] [PubMed] [Google Scholar]
- Horner HT, Wagner BL. The association of druse crystals with the developing stomium of Capsicum annum (Solanaceae) anthers. Am J Bot. 1980;67:1347–1360. [Google Scholar]
- Horvath DP, McLarney BK, Thomashow MF. Regulation of Arabidopsis thaliana L. (Heyn) cor78 in response to low temperature. Plant Physiol. 1993;103:1047–1053. doi: 10.1104/pp.103.4.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huner NPA, Oquist G, Sarhan F. Energy balance and acclimation to light and cold. Trends Plant Sci. 1998;3:224–230. [Google Scholar]
- Jefferson RA, Kavanagh TA, Bevan MW. GUS fusions: β-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J. 1987;6:3901–3907. doi: 10.1002/j.1460-2075.1987.tb02730.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josse E-M, Simkin AJ, Gaffé J, Laboré A-M, Kuntz M, Carol P. A plastid terminal oxidase associated with carotenoid desaturation during chromoplast differentiation. Plant Physiol. 2000;123:1427–1436. doi: 10.1104/pp.123.4.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keddie JS, Carroll B, Jones JDG, Gruissem W. The DCL gene of tomato is required for chloroplast development and palisade cell morphogenesis in leaves. EMBO J. 1996;15:4208–4217. [PMC free article] [PubMed] [Google Scholar]
- Kinsman EA, Pyke KA. Bundle sheath cells and cell-specific plastid development in Arabidopsis leaves. Development. 1998;125:1815–1822. doi: 10.1242/dev.125.10.1815. [DOI] [PubMed] [Google Scholar]
- León P, Arroyo A, Mackenzie S. Nuclear control of plastid and mitochondrial development in higher plants. Annu Rev Plant Physiol Plant Mol Biol. 1998;49:453–480. doi: 10.1146/annurev.arplant.49.1.453. [DOI] [PubMed] [Google Scholar]
- Li H, Culligan K, Dixon RA, Chory J. CUE1: a mesophyll cell-specific positive regulator of light-controlled gene expression in Arabidopsis. Plant Cell. 1995;7:1599–1610. doi: 10.1105/tpc.7.10.1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtenthaler HK. Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. In: Packer L, Douce R, editors. Methods in Enzymology. San Diego: Academic Press; 1987. pp. 350–382. [Google Scholar]
- López-Juez E, Jarvis RP, Takeuchi A, Page AM, Chory J. New Arabidopsis cue mutants suggest a close connection between plastid and phytochrome regulation of nuclear gene expression. Plant Physiol. 1998;118:803–815. doi: 10.1104/pp.118.3.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandel MA, Feldmann KA, Herrera-Estrella L, Rocha-Sosa M, León P. CLA1, a novel gene required for chloroplast development, is highly conserved in evolution. Plant J. 1996;9:649–658. doi: 10.1046/j.1365-313x.1996.9050649.x. [DOI] [PubMed] [Google Scholar]
- Martínez-Zapater JM, Gil P, Capel J, Somerville CR. Mutations at the Arabidopsis CHM locus promote rearrangements of the mitochondrial genome. Plant Cell. 1992;4:889–899. doi: 10.1105/tpc.4.8.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matile P. Chloroplast senescence. In: Baker NR, Thomas H, editors. Crop Photosynthesis: Spatial and Temporal Determinants. New York: Elsevier Science Publishers B.V.; 1992. pp. 413–441. [Google Scholar]
- Mayer MP, Beyer P, Kleinig H. Quinone compounds are able to replace molecular oxygen as terminal electron acceptor in phytoene desaturation in chromoplasts of Narcissus pseudonarcissus L. Eur J Biochem. 1990;191:359–363. doi: 10.1111/j.1432-1033.1990.tb19130.x. [DOI] [PubMed] [Google Scholar]
- Mayer MP, Nievelstein V, Beyer P. Purification and characterization of a NADPH dependent oxidoreductase from chromoplasts of Narcissus pseudonarcissus: a redox-mediator possibly involved in carotene desaturation. Plant Physiol Biochem. 1992;30:389–398. [Google Scholar]
- Meehan L, Harkins K, Chory J, Rodermel S. Lhcb transcription is coordinated with cell size and chlorophyll accumulation. Plant Physiol. 1996;112:953–963. doi: 10.1104/pp.112.3.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meurer J, Grevelding C, Westhoff P, Reiss B. The PAC protein affects the maturation of specific chloroplast mRNAs in Arabidopsis thaliana. Mol Gen Genet. 1998;258:342–351. doi: 10.1007/s004380050740. [DOI] [PubMed] [Google Scholar]
- Miller A, Schlagnhaufer C, Spalding M, Rodermel S. Carbohydrate regulation of leaf development: prolongation of leaf senescence in Rubisco antisense mutants of tobacco. Photosynth Res. 2000;63:1–8. doi: 10.1023/A:1006367719639. [DOI] [PubMed] [Google Scholar]
- Miller A, Tsai C-H, Hemphill D, Endres M, Rodermel S, Spalding M. Elevated CO2 effects during leaf ontogeny: a new perspective on acclimation. Plant Physiol. 1997;115:1195–1200. doi: 10.1104/pp.115.3.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nievelstein V, Vandekerkchove J, Tadros MH, Lintig JV, Nitschke W, Beyer P. Carotene desaturation is linked to a respiratory redox pathway in Narcissus pseudonarcissus chromoplast membranes: involvement of a 23-kDa oxygen-evolving-complex-like protein. Eur J Biochem. 1995;233:864–872. doi: 10.1111/j.1432-1033.1995.864_3.x. [DOI] [PubMed] [Google Scholar]
- Norris SR, Barrette TR, DellaPenna D. Genetic dissection of carotenoid synthesis in Arabidopsis defines plastoquinone as an essential component of phytoene desaturation. Plant Cell. 1995;7:2139–2149. doi: 10.1105/tpc.7.12.2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rédei GP. Somatic instability caused by a cysteine-sensitive gene in Arabidopsis. Science. 1963;139:767–769. doi: 10.1126/science.139.3556.767. [DOI] [PubMed] [Google Scholar]
- Rédei GP. Biochemical aspects of a genetically determined variegation in Arabidopsis. Genetics. 1967;56:431–443. doi: 10.1093/genetics/56.3.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rédei GP. Extra-chromosomal mutability determined by a nuclear gene locus in Arabidopsis. Mutation Res. 1973;18:149–162. [Google Scholar]
- Rédei GP. Arabidopsis as a genetic tool. Annu Rev Genet. 1975;9:111–127. doi: 10.1146/annurev.ge.09.120175.000551. [DOI] [PubMed] [Google Scholar]
- Reiter RS, Coomber SA, Bourett TM, Bartley GE, Scolnik PA. Control of leaf and chloroplast development by the Arabidopsis gene pale cress. Plant Cell. 1994;6:1253–1264. doi: 10.1105/tpc.6.9.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Röbbelen G. Genbedingte rotlicht-empfindlichkeit der chloroplastendifferenzierung bei Arabidopsis. Planta. 1968;80:237–254. [Google Scholar]
- Rodermel SR (2001) Regulatory interactions between the nucleus and the plastid: plastid-to-nucleus signaling. Trends Plant Sci (in press) [DOI] [PubMed]
- Sakamoto W, Kondo H, Murata M, Motoyoshi F. Altered mitochondrial gene expression in a maternal distorted leaf mutant of Arabidopsis induced by chloroplast mutator. Plant Cell. 1996;8:1377–1390. doi: 10.1105/tpc.8.8.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz A, Ort O, Beyer P, Kleinig H. SC-0051, a 2-benzoyl-cyclohexane-1,3-dione bleaching herbicide, is a potent inhibitor of the enzyme p-hydroxyphenylpyruvate dioxygenase. FEBS Lett. 1993;318:162–166. doi: 10.1016/0014-5793(93)80013-k. [DOI] [PubMed] [Google Scholar]
- Siedow JN, Umbach AL. Plant mitochondrial electron transfer and molecular biology. Plant Cell. 1995;7:821–831. doi: 10.1105/tpc.7.7.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stitt M. Rising CO2 levels and their potential significance for carbon flow in photosynthetic cells. Plant Cell Environ. 1991;14:741–762. [Google Scholar]
- Streatfield SJ, Weber A, Kinsman EA, Häusler RE, Li J, Post-Beittenmiller D, Kaiser WM, Pyke KA, Flügge U-I, Chory J. The phosphoenolpyruvate/phosphate translocator is required for phenolic metabolism, palisade cell development, and plastid-dependent nuclear gene expression. Plant Cell. 1999;11:1609–1621. doi: 10.1105/tpc.11.9.1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susek RE, Chory J. A tale of two genomes: role of a chloroplast signal in coordinating nuclear and plastid genome expression. Aust J Plant Physiol. 1992;19:387–399. [Google Scholar]
- Taylor WE. Regulatory interactions between nuclear and plastid genomes. Annu Rev Plant Physiol Plant Mol Biol. 1989;40:211–233. [Google Scholar]
- Tilney-Bassett RAE. Genetics of variegated plants. In: Birky CW, Perlman PS, Byers TJ, editors. Genetics and Biogenesis of Mitochondria and Chloroplasts. Ohio State University Press, Columbus. 1975. pp. 268–308. [Google Scholar]
- Tirlapur UK, Dahse I, Reiss B, Meurer J, Oelmuller R. Characterization of the activity of a plastid-targeted green fluorescent protein in Arabidopsis. Eur J Cell Biol. 1999;78:233–240. doi: 10.1016/S0171-9335(99)80056-9. [DOI] [PubMed] [Google Scholar]
- Van K, Spalding M. Periplasmic carbonic anhydrase structural gene (cah1) mutant in Chlamydomonas reinhardtii. Plant Physiol. 1999;120:767–764. doi: 10.1104/pp.120.3.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanlerberghe GC, McIntosh L. Alternative oxidase: from gene to function. Annu Rev Plant Physiol Plant Mol Biol. 1997;48:703–734. doi: 10.1146/annurev.arplant.48.1.703. [DOI] [PubMed] [Google Scholar]
- von Wettstein D, Gough S, Kannangara CG. Chlorophyll biosynthesis. Plant Cell. 1995;7:1039–1057. doi: 10.1105/tpc.7.7.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voytas DF, Konieczny A, Cummings MP, Ausubel FM. The structure, distribution and evolution of the Ta1 retrotransposable element family of Arabidopsis thaliana. Genetics. 1990;126:713–721. doi: 10.1093/genetics/126.3.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetzel CM, Jiang CZ, Meehan LJ, Voytas DF, Rodermel SR. Nuclear-organelle interactions: the immutans variegation mutant of Arabidopsis is plastid autonomous and impaired in carotenoid biosynthesis. Plant J. 1994;6:161–175. doi: 10.1046/j.1365-313x.1994.6020161.x. [DOI] [PubMed] [Google Scholar]
- Wetzel CM, Rodermel SR. Regulation of phytoene desaturase expression is independent of leaf pigment content in Arabidopsis thaliana. Plant Mol Biol. 1998;37:1045–1053. doi: 10.1023/a:1006021522259. [DOI] [PubMed] [Google Scholar]
- Wu D, Wright DA, Wetzel C, Voytas DF, Rodermel S. The IMMUTANS variegation locus of Arabidopsis defines a mitochondrial alternative oxidase homolog that functions during early chloroplast biogenesis. Plant Cell. 1999;11:43–55. doi: 10.1105/tpc.11.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]