Abstract
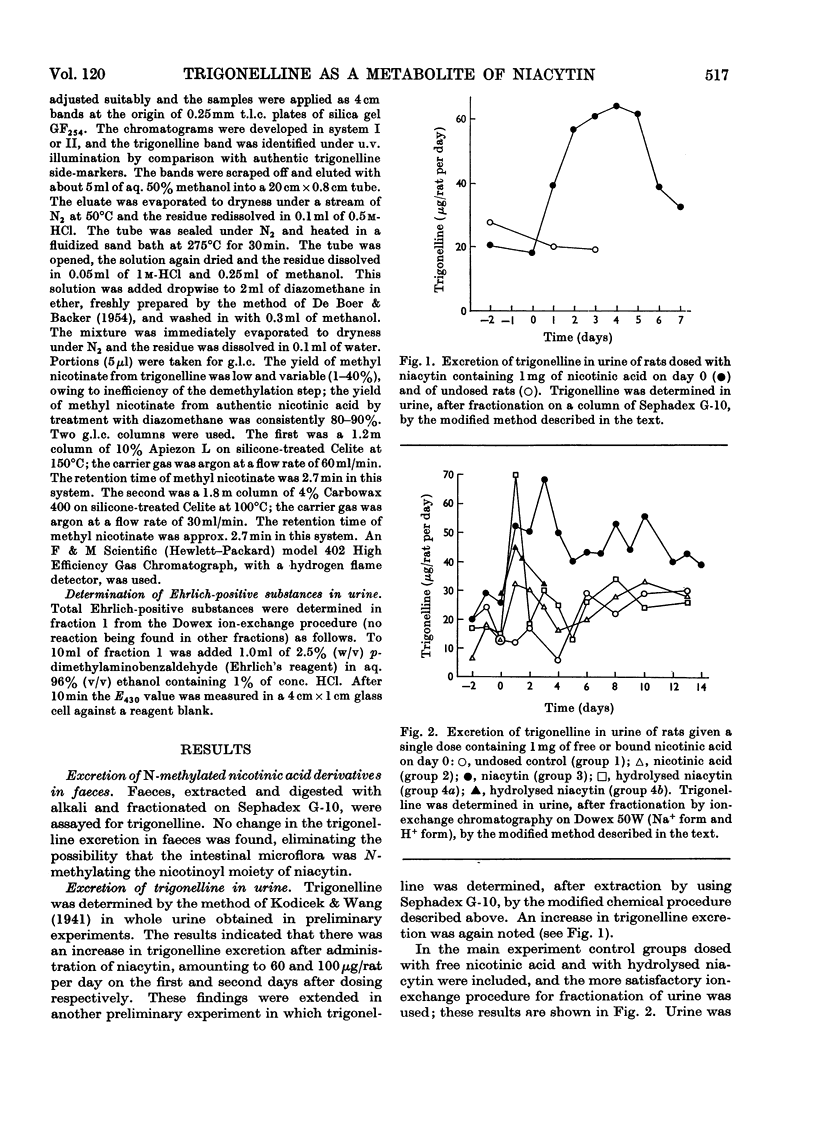
1. To investigate the fate of orally administered niacytin, urine and faeces of rats given single niacytin doses were examined for nicotinic acid derivatives methylated on the pyridine nitrogen atom, determined as trigonelline. 2. Methods were devised for the extraction of trigonelline from urine and faeces and for its differentiation from N′-methylnicotinamide. 3. A prolonged elevation of the excretion of trigonelline in the urine of rats dosed with niacytin was detected colorimetrically, in contrast with the urinary excretion in control groups given free nicotinic acid or hydrolysed niacytin. The total conversion of the nicotinoyl moiety of niacytin into trigonelline was 30–40%. 4. The identity of this metabolite as trigonelline was established by t.l.c., by its u.v. spectrum and by g.l.c. after conversion into methyl nicotinate. 5. The excretion of Ehrlich-positive substances was also increased in urine after administration of niacytin, the increase being approximately parallel to the trigonelline excretion. 6. No increase in the excretion of trigonelline in faeces was found after administration of niacytin. 7. These results suggest a metabolic path-way for niacytin in the rat involving methylation of the pyridine nitrogen without prior release of free nicotinic acid. This hypothesis explains the absence of biological activity of niacytin. An endogenous source of urinary trigonelline was also demonstrated.
Full text
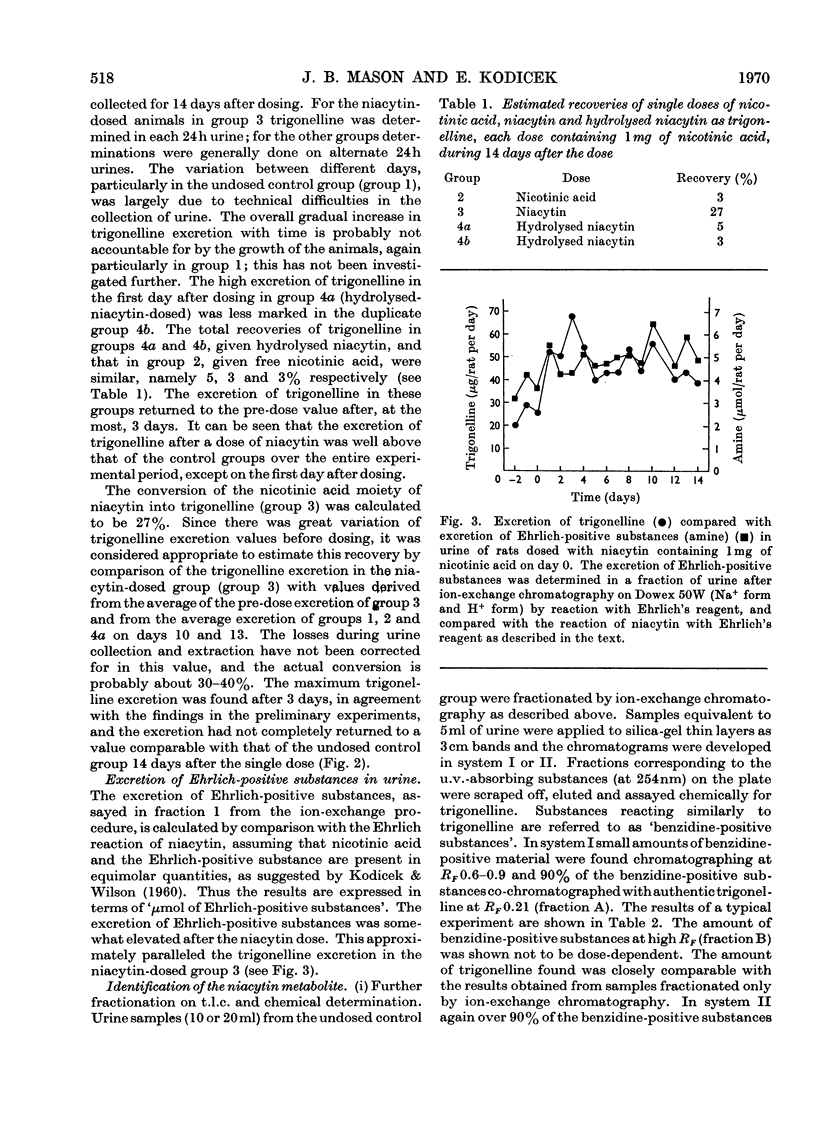
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CANTONI G. L. Methylation of nicotinamide with soluble enzyme system from rat liver. J Biol Chem. 1951 Mar;189(1):203–216. [PubMed] [Google Scholar]
- Ellinger P., Fraenkel G., Kader M. M. The utilization of nicotinamide derivatives and related compounds by mammals, insects and bacteria. Biochem J. 1947;41(4):559–568. [PMC free article] [PubMed] [Google Scholar]
- JOSHI J. G., HANDLER P. Metabolism of trigonelline. J Biol Chem. 1962 Oct;237:3185–3188. [PubMed] [Google Scholar]
- MCKENNIS H., Jr, BOWMAN E. R., HORVATH A., BEDERKA J. P., Jr METABOLIC RELEASE OF METHYL GROUPS FROM A SERIES OF N-METHYLPYRIDINIUM COMPOUNDS. Nature. 1964 May 16;202:699–700. doi: 10.1038/202699a0. [DOI] [PubMed] [Google Scholar]
- Mason J. B., Kodicek E. The metabolism of niacytin in the rat. Studies of the excretion of nicotinic acid metabolites. Biochem J. 1970 Dec;120(3):509–513. doi: 10.1042/bj1200509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer H., Preiss B., Bauer S. The oxidation of fatty acids by a particulate fraction from desert-locust (Schistocerca gregaria) thorax tissues. Biochem J. 1960 Jul;76(1):27–35. doi: 10.1042/bj0760027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrack B., Greengard P., Kalinsky H. On the relative efficacy of nicotinamide and nicotinic acid as precursors of nicotinamide adenine dinucleotide. J Biol Chem. 1966 May 25;241(10):2367–2372. [PubMed] [Google Scholar]


