Abstract
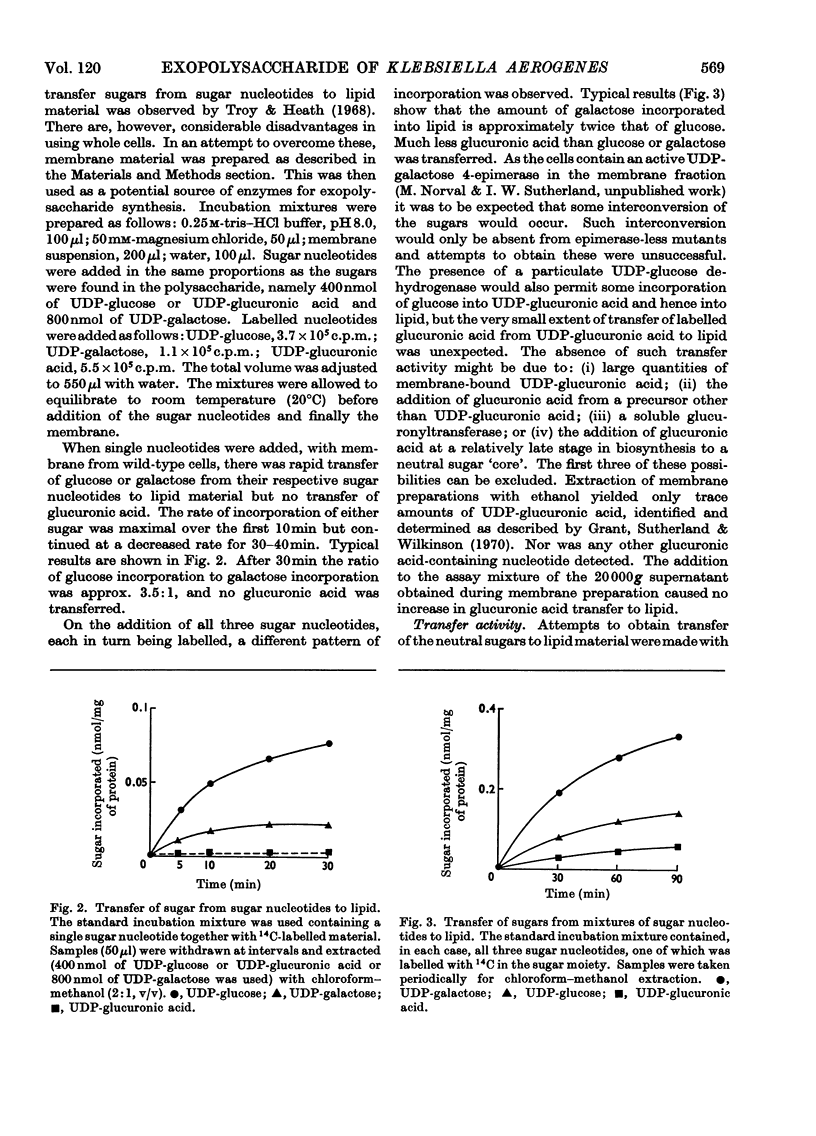
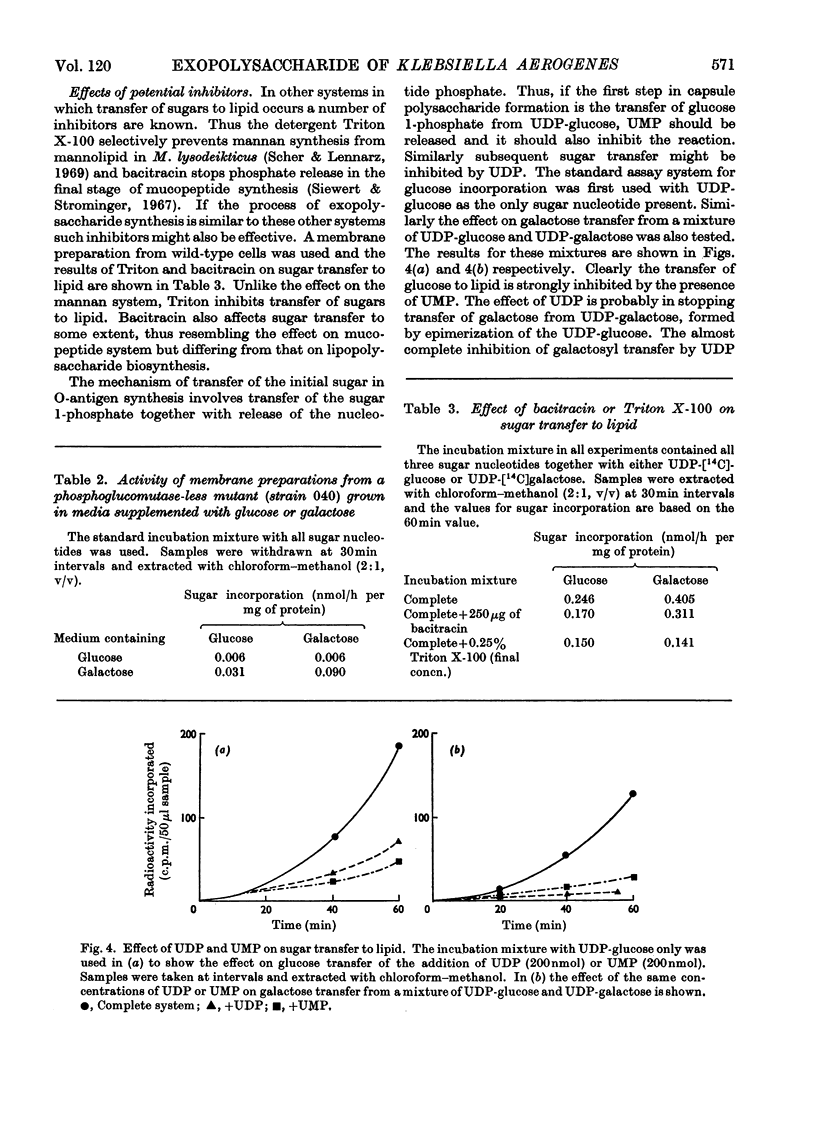
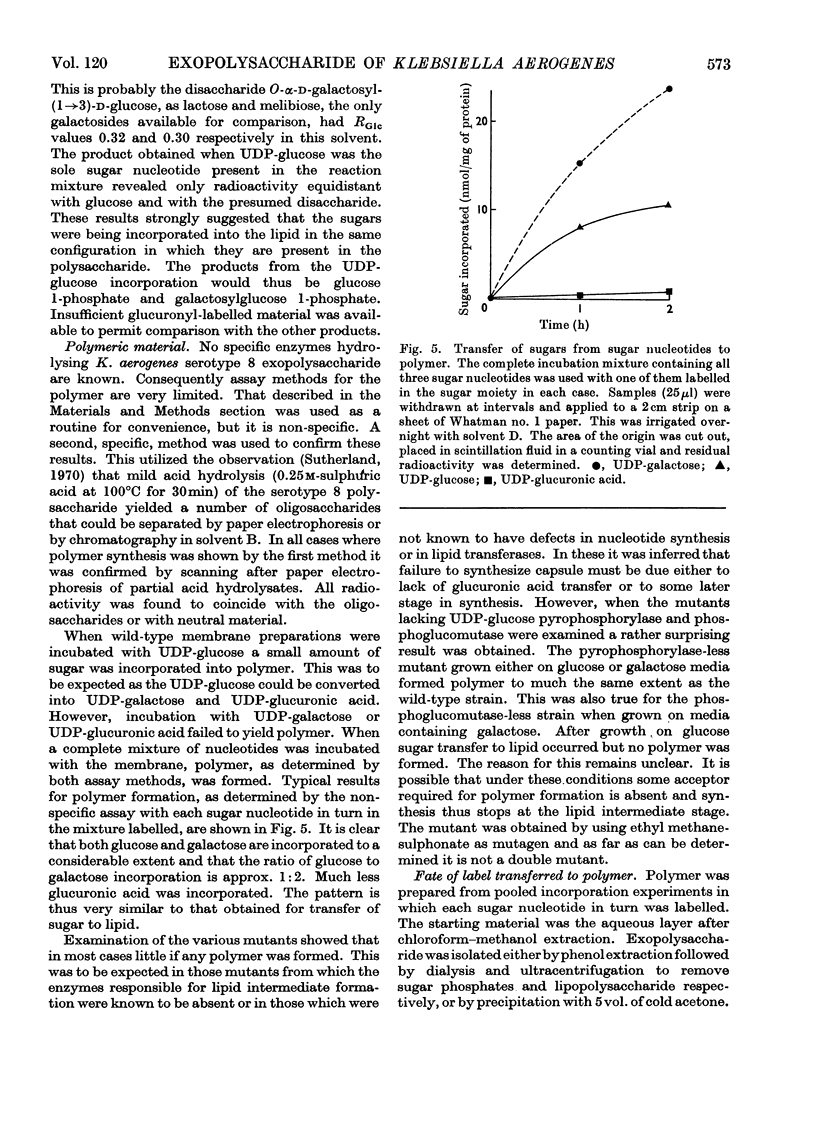
1. Membrane preparations from Klebsiella aerogenes type 8 were shown to transfer glucose and galactose from their uridine diphosphate derivatives to a lipid and to polymer. The ratio of glucose to galactose transfer in both cases was 1:2. This is the same ratio in which these sugars occur in native polysaccharide. Galactose transfer was dependent on prior glucosylation of the lipid. Mutants were obtained lacking (a) glucosyltransferase and (b) galactosyltransferase. The transferase activities in a number of non-mucoid mutants was examined. 2. Glucose transfer was partially inhibited by uridine monophosphate, and incorporation of either glucose or galactose into lipid was decreased in the presence of uridine diphosphate. The sugars are thought to be linked to a lipid through a pyrophosphate bond, and treatment of the lipid intermediates with phenol yielded water-soluble compounds. These could be dephosphorylated with alkaline phosphatase. Transfer of glucuronic acid to lipid or polymer from uridine diphosphate glucuronic acid was much lower than that of the other two sugars. 3. The fate of sugars incorporated into polymer was also followed. Some conversion of glucose into galactose and glucuronic acid occurred. Mutants unable to transfer glucose or galactose to lipid were unable to form polymer. Other mutants capable of lipid glycosylation were in some cases unable to form polymer. A model for capsular polysaccharide synthesis is proposed and its similarity to the formation of other polymers outside the cell membrane is discussed.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Caccam J. F., Jackson J. J., Eylar E. H. The biosynthesis of mannose-containing glycoproteins: a possible lipid intermediate. Biochem Biophys Res Commun. 1969 May 22;35(4):505–511. doi: 10.1016/0006-291x(69)90375-1. [DOI] [PubMed] [Google Scholar]
- Conrad H. E., Bamburg J. R., Epley J. D., Kindt T. J. The structure of the Aerobacter aerogenes A3(S1) polysaccharide. II. Sequence analysis and hydrolysis studies. Biochemistry. 1966 Sep;5(9):2808–2817. doi: 10.1021/bi00873a005. [DOI] [PubMed] [Google Scholar]
- DUDMAN W. F., WILKINSON J. F. The composition of the extracellular polysaccharides of Aerobacter-Klebsiella strains. Biochem J. 1956 Feb;62(2):289–295. doi: 10.1042/bj0620289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FISCHER F. G., DORFEL H. Die Polyuronsäuren der Braunalgen. Hoppe Seylers Z Physiol Chem. 1955 Dec 22;302(4-6):186–203. [PubMed] [Google Scholar]
- Grant W. D., Sutherland I. W., Wilkimson J. F. Control of colanic acid synthesis. J Bacteriol. 1970 Jul;103(1):89–96. doi: 10.1128/jb.103.1.89-96.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HANES C. S., ISHERWOOD F. A. Separation of the phosphoric esters on the filter paper chromatogram. Nature. 1949 Dec 31;164(4183):1107-12, illust. doi: 10.1038/1641107a0. [DOI] [PubMed] [Google Scholar]
- Higashi Y., Strominger J. L., Sweeley C. C. Structure of a lipid intermediate in cell wall peptidoglycan synthesis: a derivative of a C55 isoprenoid alcohol. Proc Natl Acad Sci U S A. 1967 Jun;57(6):1878–1884. doi: 10.1073/pnas.57.6.1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hungerer D., Jann K., Jann B., Orskov F., Orskov I. Immunochemistry of K antigens of Escherichia coli. 4. The K antigen of E. coli O 9:K30:H12. Eur J Biochem. 1967 Jul;2(1):115–126. doi: 10.1111/j.1432-1033.1967.tb00115.x. [DOI] [PubMed] [Google Scholar]
- Kent J. L., Osborn M. J. Properties of the O-specific hapten formed in vivo by mutant strains of Salmonella typhimurium. Biochemistry. 1968 Dec;7(12):4396–4408. doi: 10.1021/bi00852a036. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lahav M., Chiu T. H., Lennarz W. J. Studies on the biosynthesis of mannan in Micrococcus lysodeikticus. II. The enzymatic synthesis of mannosyl-l-phosphoryl-undecaprenol. J Biol Chem. 1969 Nov 10;244(21):5890–5898. [PubMed] [Google Scholar]
- Lennarz W. J., Talamo B. The chemical characterization and enzymatic synthesis of mannolipids in Micrococcus lysodeikticus. J Biol Chem. 1966 Jun 10;241(11):2707–2719. [PubMed] [Google Scholar]
- Nikaido H. Biosynthesis of cell wall lipopolysaccharide in gram-negative enteric bacteria. Adv Enzymol Relat Areas Mol Biol. 1968;31:77–124. doi: 10.1002/9780470122761.ch3. [DOI] [PubMed] [Google Scholar]
- PALADINI A. C., LELOIR L. F. Studies on uridine-diphosphate-glucose. Biochem J. 1952 Jun;51(3):426–430. doi: 10.1042/bj0510426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scher M., Lennarz W. J. Studies on the biosynthesis of mannan in Micrococcus lysodeikticus. I. Characterization of mannan-14C formed enzymatically from mannosyl-1-phosphoryl-undecaprenol. J Biol Chem. 1969 May 25;244(10):2777–2789. [PubMed] [Google Scholar]
- Scher M., Lennarz W. J., Sweeley C. C. The biosynthesis of mannosyl-1-phosphoryl-polyisoprenol in Micrococcus lysodeikticus and its role in mannan synthesis. Proc Natl Acad Sci U S A. 1968 Apr;59(4):1313–1320. doi: 10.1073/pnas.59.4.1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siewert G., Strominger J. L. Bacitracin: an inhibitor of the dephosphorylation of lipid pyrophosphate, an intermediate in the biosynthesis of the peptidoglycan of bacterial cell walls. Proc Natl Acad Sci U S A. 1967 Mar;57(3):767–773. doi: 10.1073/pnas.57.3.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland I. W. Structural studies on colanic acid, the common exopolysaccharide found in the enterobacteriaceae, by partial acid hydrolysis. Oligosaccharides from colanic acid. Biochem J. 1969 Dec;115(5):935–945. doi: 10.1042/bj1150935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland I. W., Wilkinson J. F. The composition of lipopolysaccharides of Klebsiella aerogenes and Aerobacter cloacae. Biochim Biophys Acta. 1966 Mar 28;117(1):261–263. doi: 10.1016/0304-4165(66)90175-9. [DOI] [PubMed] [Google Scholar]
- Tovey K. C., Roberts R. M. An artefact in the chromatography of sugar nucleotides using solvents containing ammonium acetate. J Chromatogr. 1970 Mar 4;47(2):287–290. doi: 10.1016/0021-9673(70)80043-7. [DOI] [PubMed] [Google Scholar]
- WILKINSON J. F., DUGUID J. P., EDMUNDS P. N. The distribution of polysaccharide production in Aerobacter and Escherichia strains and its relation to antigenic character; with a note on the influence of potassium deficiency upon production of polysaccharide by Aerobacter aerogenes. J Gen Microbiol. 1954 Aug;11(1):59–72. doi: 10.1099/00221287-11-1-59. [DOI] [PubMed] [Google Scholar]
- Wright A., Dankert M., Fennessey P., Robbins P. W. Characterization of a polyisoprenoid compound functional in O-antigen biosynthesis. Proc Natl Acad Sci U S A. 1967 Jun;57(6):1798–1803. doi: 10.1073/pnas.57.6.1798. [DOI] [PMC free article] [PubMed] [Google Scholar]


