Abstract
1. The purification of creatine kinase (ATP–creatine phosphotransferase, EC 2.7.3.2) from ox brain by a method that is quicker, simpler and gives much higher yields than other published procedures is described. 2. Stoicheiometric inhibition studies with iodoacetate showed that the enzyme, like that from muscle, has two reactive thiol groups that are essential for enzyme activity. 3. The amino acid sequence around the essential thiol groups was determined and found to be virtually identical with that in creatine kinases from rabbit and ox muscle, and very similar to that found in arginine kinase; the evolutionary significance of this is discussed. 4. The identification of DNS-amino acids on thin layers of silica gel was found to have, in many cases, distinct advantages over that on polyamide layers.
Full text
PDF

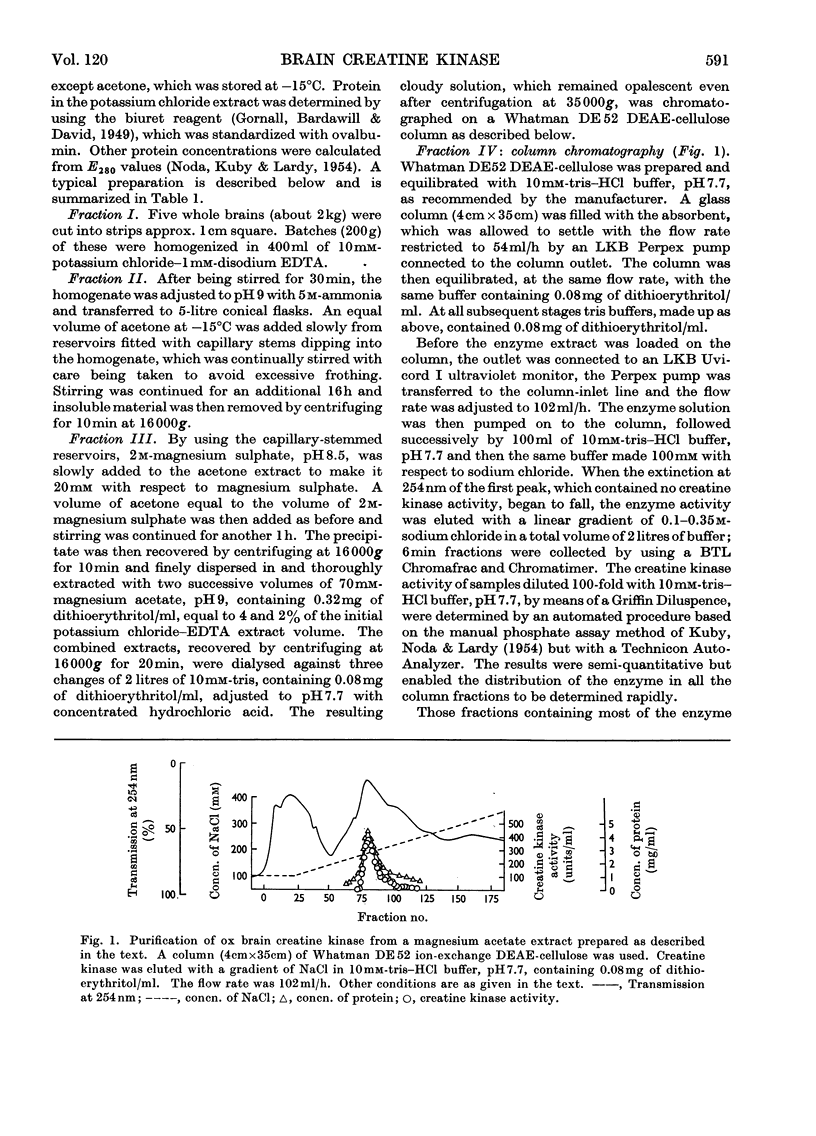
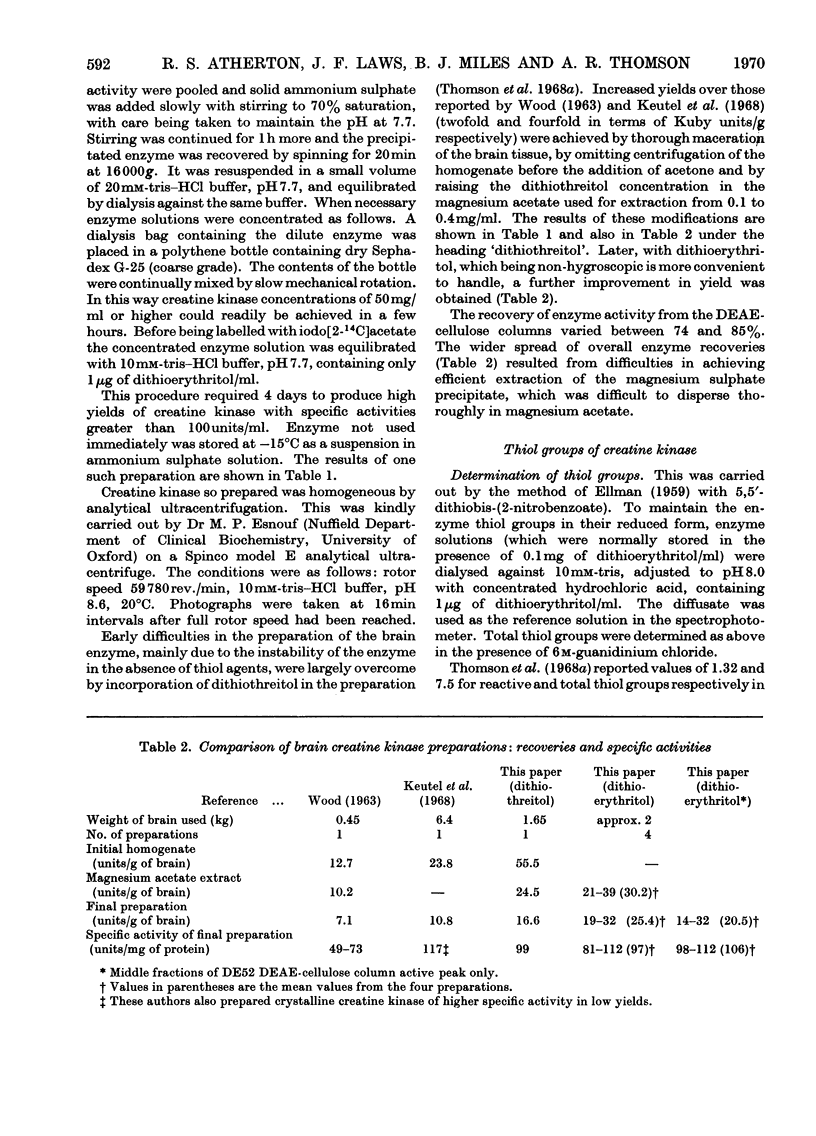
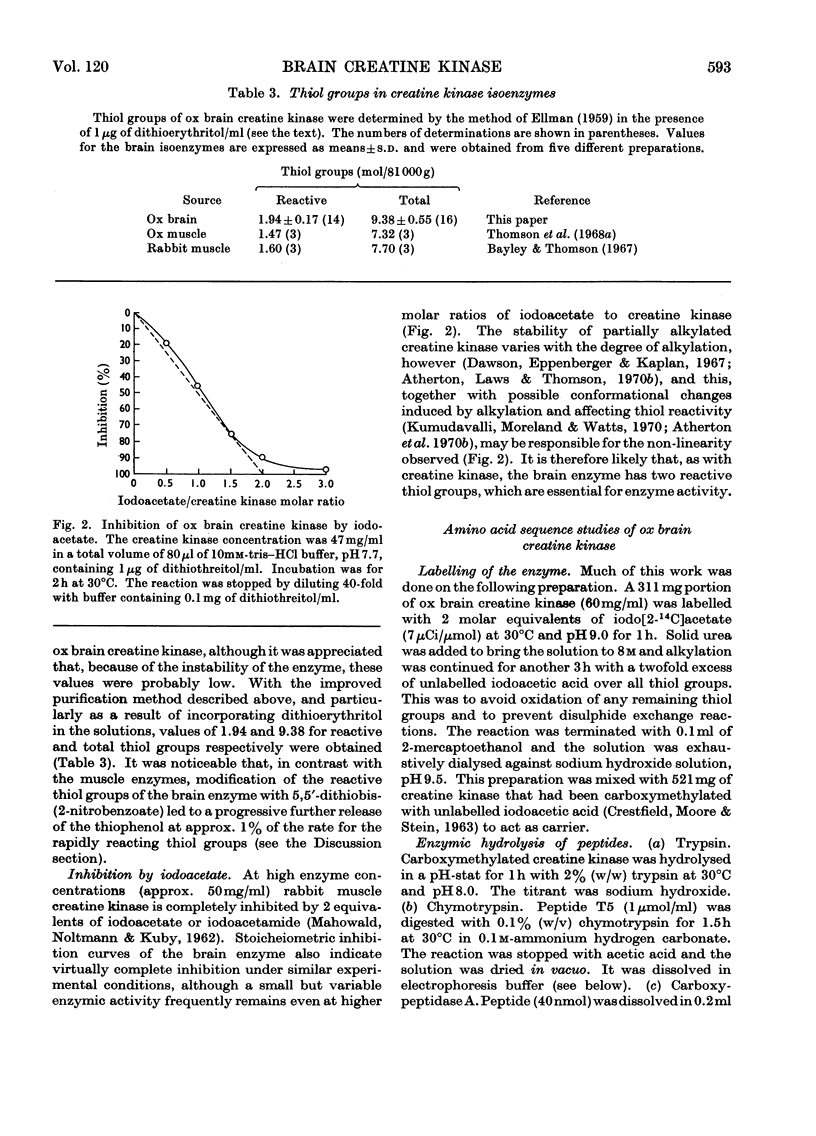
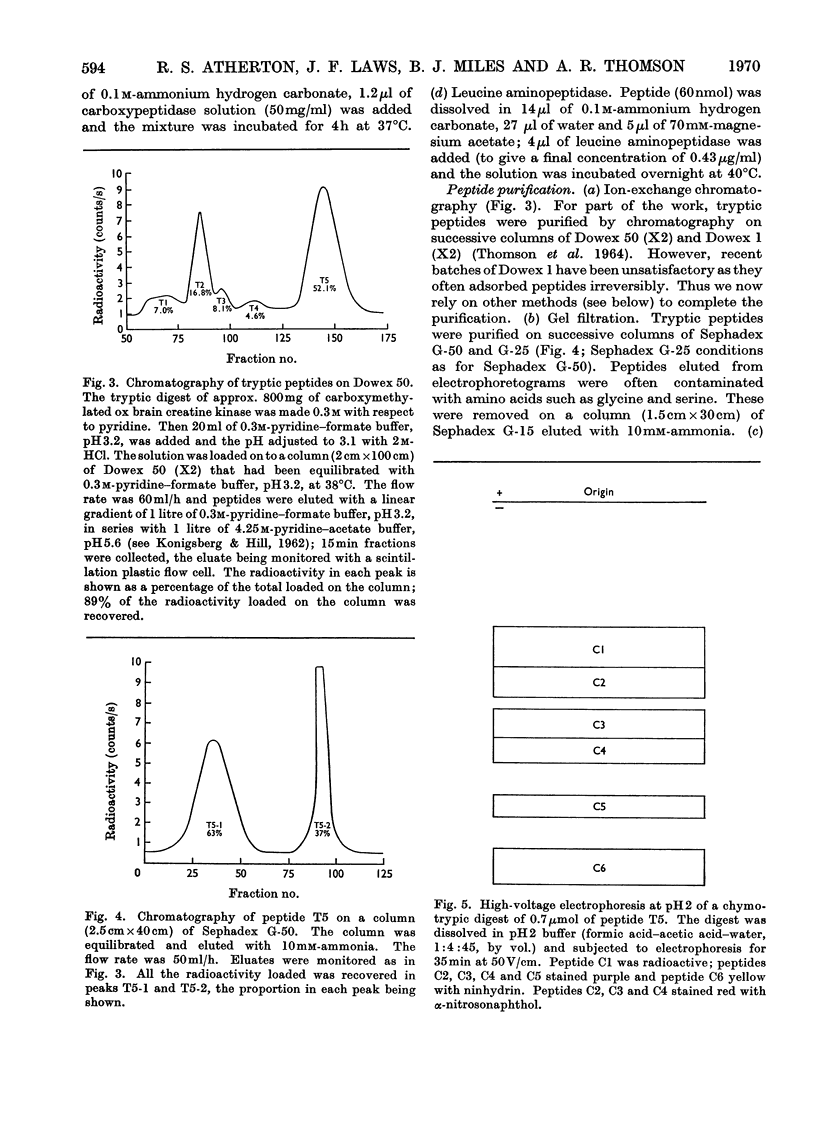




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams M. J., Ford G. C., Koekoek R., Lentz P. J., McPherson A., Jr, Rossmann M. G., Smiley I. E., Schevitz R. W., Wonacott A. J. Structure of lactate dehydrogenase at 2-8 A resolution. Nature. 1970 Sep 12;227(5263):1098–1103. doi: 10.1038/2271098a0. [DOI] [PubMed] [Google Scholar]
- Atherton R. S., Laws J. F., Thomson A. R. Alkylation of bovine brain creatine kinase. Biochem J. 1970 Aug;118(5):903–904. doi: 10.1042/bj1180903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atherton R. S., Thomson A. R. 'Fingerprinting' of 1-dimethylaminonaphthalene-5-sulphonyl-labelled protein digests. Biochem J. 1969 Mar;111(5):797–798. doi: 10.1042/bj1110797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CRESTFIELD A. M., MOORE S., STEIN W. H. The preparation and enzymatic hydrolysis of reduced and S-carboxymethylated proteins. J Biol Chem. 1963 Feb;238:622–627. [PubMed] [Google Scholar]
- Dawson D. M., Eppenberger H. M., Kaplan N. O. The comparative enzymology of creatine kinases. II. Physical and chemical properties. J Biol Chem. 1967 Jan 25;242(2):210–217. [PubMed] [Google Scholar]
- Der Terrossian E., Pradel L. A., Kassab R., Thoai N. V. Comparative structural studies of the active site of ATP-guanidine phosphotransferases. The essential cysteine tryptic peptide of arginine kinase from Homarus vulgaris muscle. Eur J Biochem. 1969 Dec;11(3):482–490. doi: 10.1111/j.1432-1033.1969.tb00798.x. [DOI] [PubMed] [Google Scholar]
- Deyl Z., Rosmus J. Thin layer chromatography of Dansyl amino acid derivatives. J Chromatogr. 1965 Dec;20(3):514–520. doi: 10.1016/s0021-9673(01)97453-9. [DOI] [PubMed] [Google Scholar]
- ELLMAN G. L. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959 May;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- ELODI P., SZORENYI E. Properties of crystalline arginine-phosphoferase isolated from Crustacean muscle. Acta Physiol Acad Sci Hung. 1956;9(4):367–379. [PubMed] [Google Scholar]
- EPPENBERGER H. M., EPPENBERGER M., RICHTERICH R., AEBI H. THE ONTOGENY OF CREATINE KINASE ISOZYMES. Dev Biol. 1964 Aug;10:1–16. doi: 10.1016/0012-1606(64)90002-8. [DOI] [PubMed] [Google Scholar]
- Edstrom R. D. Rapid elution of materials from paper chromatograms. Anal Biochem. 1968 Oct 10;26(1):204–205. doi: 10.1016/0003-2697(68)90050-x. [DOI] [PubMed] [Google Scholar]
- Eppenberger H. M., Dawson D. M., Kaplan N. O. The comparative enzymology of creatine kinases. I. Isolation and characterization from chicken and rabbit tissues. J Biol Chem. 1967 Jan 25;242(2):204–209. [PubMed] [Google Scholar]
- KONIGSBERG W., HILL R. J. The structure of human hemoglobin. III. The sequence of amino acids in the tryptic peptides of the alpha chain. J Biol Chem. 1962 Aug;237:2547–2561. [PubMed] [Google Scholar]
- KUBY S. A., NODA L., LARDY H. A. Adenosinetriphosphate-creatine transphosphorylase. I. Isolation of the crystalline enzyme from rabbit muscle. J Biol Chem. 1954 Jul;209(1):191–201. [PubMed] [Google Scholar]
- Kassab R., Pradel L. A., Terrossian E der, Thoai N. V. Comparaison des groupes SH reactifs des ATP: guanidines phosphotransferases. Biochim Biophys Acta. 1967 Mar 15;132(2):347–360. doi: 10.1016/0005-2744(67)90154-4. [DOI] [PubMed] [Google Scholar]
- Keutel H. J., Jacobs H. K., Okabe K., Yue R. H., Kuby S. A. Studies on adenosine triphosphate transphosphorylases. VII. Isolation of the crystalline adenosine triphosphate--creatine transphosphorylase from calf brain. Biochemistry. 1968 Dec;7(12):4283–4290. doi: 10.1021/bi00852a020. [DOI] [PubMed] [Google Scholar]
- Kress L. F., Bono V. H., Jr, Noda L. The sulfhydryl groups of rabbit muscle adenosine triphosphate-adenosine monophosphate phosphotransferase. Activity of enzyme treated with mercurials. J Biol Chem. 1966 May 25;241(10):2293–2300. [PubMed] [Google Scholar]
- Kress L. F., Noda L. Inactivation of rabbit muscle adenosine triphosphate-adenosine 5'-phosphate phosphotransferase by alkylation of methionine residues. J Biol Chem. 1967 Feb 25;242(4):558–564. [PubMed] [Google Scholar]
- Kumudavalli I., Moreland B. H., Watts D. C. Properties and reaction with iodoacetamide of adenosine 5'-triphosphate-creatine phosphotransferase from human skeletal muscle. Further evidence about the role of the essential thiol group in relation to the mechanism of action. Biochem J. 1970 Apr;117(3):513–523. doi: 10.1042/bj1170513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAHOWALD T. A., NOLTMANN E. A., KUBY S. A. Studies on adenosine triphosphate transphosphorylases. III. Inhibition reactions. J Biol Chem. 1962 May;237:1535–1548. [PubMed] [Google Scholar]
- MAHOWALD T. A. THE AMINO ACID SEQUENCE AROUND THE "REACTIVE" SULFHYDRYL GROUPS IN ADENOSINE TRIPHOSPHOCREATINE PHOSPHOTRANSFERASE. Biochemistry. 1965 Apr;4:732–740. doi: 10.1021/bi00880a019. [DOI] [PubMed] [Google Scholar]
- Morse D., Horecker B. L. Thin-layer chromatographic separation of DNS-amino acids. Anal Biochem. 1966 Mar;14(3):429–433. doi: 10.1016/0003-2697(66)90285-5. [DOI] [PubMed] [Google Scholar]
- NODA L., KUBY S. A., LARDY H. A. Adenosinetriphosphate-creatine transphosphorylase. II. Homogeneity and physicochemical properties. J Biol Chem. 1954 Jul;209(1):203–210. [PubMed] [Google Scholar]
- Pradel L. A., Kassab R., Conlay C., Nguyen Van Thoai Propriétés et composition en acides aminés de l'ATP: guanidinoacétate phosphotransférase purifiée. Biochim Biophys Acta. 1968 Feb 19;154(2):305–314. [PubMed] [Google Scholar]
- Seiler N., Wiechmann J. Zum Nachweis von Aminosäuren im 10-10-Mol-Massetab. Trennung von 1-Dimethylamino-naphthalin-5-sulfonyl-aminosäuren auf Dünnschichtchromatogrammen. Experientia. 1964 Oct 15;20(10):559–560. doi: 10.1007/BF02150289. [DOI] [PubMed] [Google Scholar]
- Smith E., Morrison J. F. Kinetic studies on the arginine kinase reaction. J Biol Chem. 1969 Aug 10;244(15):4224–4234. [PubMed] [Google Scholar]
- THOMSON A. R., EVELEIGH J. W., MILES B. J. AMINO-ACID SEQUENCE AROUND THE REACTIVE THIOL GROUPS OF ADENOSINE TRIPHOSPHATE--CREATINE PHOSPHOTRANSFERASE. Nature. 1964 Jul 18;203:267–269. doi: 10.1038/203267a0. [DOI] [PubMed] [Google Scholar]
- Virden R., Watts D. C. The role of thiol groups in the structure and mechanism of action of arginine kinase. Biochem J. 1966 Apr;99(1):162–172. doi: 10.1042/bj0990162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WATTS D. C., RABIN B. R. A study of the 'reactive' sulphydryl groups of adenosine 5'-triphosphate-creatine phosphotransferase. Biochem J. 1962 Dec;85:507–516. doi: 10.1042/bj0850507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOOD T. Adenosine triphosphate-creatine phosphotransferase from ox brain: purification and isolation. Biochem J. 1963 Jun;87:453–462. doi: 10.1042/bj0870453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts D. C., Rabin B. R., Crook E. M. The number of catalytic sites in creatine phosphokinase as determined by a study of its reactive sulphydryl groups. Biochem J. 1962 Mar;82(3):412–417. doi: 10.1042/bj0820412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts R. L., Watts D. C. Gene duplication and the evolution of enzymes. Nature. 1968 Mar 23;217(5134):1125–1130. doi: 10.1038/2171125a0. [DOI] [PubMed] [Google Scholar]
- Weeds A. G., Noda L. Amino acid sequences aroung the thiol groups of myokinase. Biochem J. 1968 Mar;107(2):311–312. doi: 10.1042/bj1070311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods K. R., Wang K. T. Separation of dansyl-amino acids by polyamide layer chromatography. Biochim Biophys Acta. 1967 Feb 21;133(2):369–370. doi: 10.1016/0005-2795(67)90078-5. [DOI] [PubMed] [Google Scholar]



