Abstract
Purpose
Cancer stem cells are associated with unfavorable prognosis in hepatocellular carcinoma (HCC). However, existing stemness-related biomarkers and prognostic models are limited.
Methods
The stemness-related signatures were derived from taking the union of the results obtained by performing WGCNA and CytoTRACE analysis at the bulk RNA-seq and scRNA-seq levels, respectively. Univariate Cox regression and the LASSO were applied for filtering prognosis-related signatures and selecting variables. Finally, ten gene signatures were identified to construct the prognostic model. We evaluated the differences in survival, genomic alternation, biological processes, and degree of immune cell infiltration in the high- and low-risk groups. pRRophetic and Tumor Immune Dysfunction and Exclusion (TIDE) algorithms were utilized to predict chemosensitivity and immunotherapy response. Human Protein Atlas (HPA) database was used to evaluate the protein expressions.
Results
A stemness-related prognostic model was constructed with ten genes including YBX1, CYB5R3, CDC20, RAMP3, LDHA, MTHFS, PTRH2, SRPRB, GNA14, and CLEC3B. Kaplan–Meier and ROC curve analyses showed that the high-risk group had a worse prognosis and the AUC of the model in four datasets was greater than 0.64. Multivariate Cox regression analyses verified that the model was an independent prognostic indicator in predicting overall survival, and a nomogram was then built for clinical utility in predicting the prognosis of HCC. Additionally, chemotherapy drug sensitivity and immunotherapy response analyses revealed that the high-risk group exhibited a higher likelihood of benefiting from these treatments.
Conclusion
The novel stemness-related prognostic model is a promising biomarker for estimating overall survival in HCC.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00432-023-05202-2.
Keywords: Hepatocellular carcinoma, scRNA-seq, Prognostic model, Immunotherapy, Stemness, Drug sensitivity
Introduction
Hepatocellular carcinoma (HCC) is the major type of primary liver cancer, accounting for 80–90% of all cases and ranking as the fourth leading cause of cancer-related deaths worldwide (Li et al. 2021). Due to the fact that HCC is often diagnosed at an advanced stage, surgical treatments such as curative liver resection or transplantation are not suitable options, resulting in poor prognosis and high rates of recurrence (Forner et al. 2018). The intricate molecular mechanisms driving HCC tumor progression are highly complex, and analyzing the multifaceted interplay among genes, proteins, and other molecules for comprehensive elucidation of these mechanisms requires still difficult. The emergence of high-throughput sequencing technologies has facilitated the identification of gene signatures that effectively predict HCC risk and prognosis. Constructing a prognostic model based on gene signatures is a promising approach to predicting the response of HCC patients to different treatments, including chemotherapy, targeted drug therapy, and immune checkpoint inhibitors. This personalized approach can help enhance patient outcomes by tailoring the treatment plan to the individual patient’s molecular profile (Weston and Hood 2004; Thorgeirsson et al. 2006).
Cancer stem cells (CSCs) are a unique subset of cancer cells with stem cell-like properties that contribute to treatment resistance and tumor recurrence (Lee et al. 2022). The regulatory programs controlling stem cell function are highly active in cancer, endowing it with characteristics that promote tumor progression and therapy resistance. Stemness, as a stem cell-like tumor phenotype, is negatively correlated with anti-cancer immunity and associated with poor prognosis in various cancers (Miranda et al. 2019). Previous studies have identified liver cancer tumor stem cell markers including CD24, CD13, CD44, etc., which provide new targets for cancer treatment and enrich the therapeutic options for liver cancer (Lee et al. 2022). Therefore, it is necessary to understand the key features and mechanisms of liver CSCs and to construct a liver cancer prognostic model to predict and improve the prognosis of HCC patients.
In recent years, researchers have developed some algorithms (Sun et al. 2022; Wang et al. 2022) that integrate bulk RNA-seq and scRNA-seq data to construct cancer prognostic models (Gulati et al. 2020; Zhu et al. 2021; Chen et al. 2022; P. Song et al. 2022) to leverage the advantages of bulk RNA-seq and scRNA-seq. Integrating bulk RNA-seq and scRNA-seq data in building prognostic models can address the shortcomings of relying solely on bulk RNA-seq data and these prognostic models have better predictive performance than using one data type. While prognostic modeling based on bulk RNA-seq data and scRNA-seq data has demonstrated promising results in other types of cancer, there are still notable research gaps when it comes to integrating these two types of data to construct prognostic models specifically in HCC. To predict the prognosis of HCC patients, investigate molecular feature differences among different risk groups, and enhance precision medicine and clinical decision-making, this study successfully integrated bulk RNA-seq and scRNA-seq data. Consequently, a prognostic model for HCC was constructed. Additionally, a comprehensive analysis of molecular features and personalized treatment strategies for HCC patients was conducted, contributing to a deeper understanding of the disease and providing valuable insights for personalized care.
This study utilized WGCNA and CytoTRACE to identify gene signatures associated with stemness characteristics from both bulk RNA-seq and scRNA-seq data. Subsequently, 10 prognostic gene signatures were selected through univariate Cox regression and LASSO, and a stemness-related risk score model was constructed. To evaluate the predictive performance of the model, patients from four datasets were divided into high- and low-risk groups based on the median risk score and used for survival analysis and ROC curve analysis to validate the stability and reliability of the model. In addition, this study also conducted independent prognostic factor analysis, immune therapy difference research, drug sensitivity analysis, and protein expression verification using the HPA database. The results confirmed the predictive performance of the model and its ability to guide personalized treatment of HCC patients. The developed prognostic model holds the potential to assist healthcare professionals and patients in predicting disease progression and treatment outcomes.
Materials and methods
Data collection and preprocessing
Gene expression data and the corresponding clinical datasheets for 424 HCC samples were obtained from the Cancer Genome Atlas (TCGA) using R package TCGAbiolinks. And 364 primary HCC samples with survival data were retained for subsequent analysis. The gene expression profile matrix files from GSE14520 based on platform GPL571 (including 225 HCC samples) and GSE149614 based on GPL24676 (including 10 HCC samples) were downloaded from the Gene Expression Omnibus (GEO) database (https://www.ncbi.nlm.nih.gov/geo/). ICGC-LIRI cohort (including 240 HCC samples) was obtained from the International Cancer Genome Consortium(ICGC) database (https://dcc.icgc.org/projects/LIRI-JP). LICH-CN cohort (including 159 Chinese HCC patients) was downloaded from the literature (Gao et al. 2019).Table 1 presents the clinical characteristics of the bulk RNA-seq dataset utilized in the study.
Table 1.
Clinical characteristics of hepatocellular carcinoma from multiple cohorts
| TCGA-LIHC | LIRI-JP | GSE14520 | LIHC-CN | |
|---|---|---|---|---|
| N = 364 | N = 240 | N = 221 | N = 159 | |
| Age | ||||
| < = 59 | 164 (45.1%) | 44 (18.3%) | 178 (80.5%) | 106 (66.7%) |
| > 59 | 200 (54.9%) | 196 (81.7%) | 43 (19.5%) | 53 (33.3%) |
| Gender | ||||
| Male | 246 (67.6%) | 179 (74.6%) | 191 (86.4%) | 128 (80.5%) |
| Female | 118 (32.4%) | 61 (25.4%) | 30 (13.6%) | 31 (19.5%) |
| Stage | ||||
| I | 170 (46.7%) | 36 (15.0%) | 93 (42.1%) | 91 (57.2%) |
| II | 83 (22.8%) | 109 (45.4%) | 77 (34.8%) | 14 (8.81%) |
| III | 83 (22.8%) | 74 (30.8%) | 49 (22.2%) | 52 (32.7%) |
| IV | 4 (1.10%) | 21 (8.75%) | 0 (0.00%) | 2 (1.26%) |
| Unknown | 24 (6.59%) | 0 (0.00%) | 2 (0.90%) | 0 (0.00%) |
| OS | ||||
| Alive | 234 (64.3%) | 197 (82.1%) | 136 (61.5%) | 103 (64.8%) |
| Dead | 130 (35.7%) | 43 (17.9%) | 85 (38.5%) | 56 (35.2%) |
Identification of stemness-related genes utilizing bulk RNA-seq data and scRNA-seq data
To identify the stemness-related signatures in bulk RNA-seq data, we first used the OCLR algorithm (Malta et al. 2018) to calculate the stemness score of each sample for TCGA-LIHC cohort. The calculation process of this algorithm included the following three steps: firstly, downloading and standardizing stem cell training set data from Progenitor Cell Biology Consortium (https://www.synapse.org); Secondly, constructing a single-class logistic regression model to obtain the weight of each gene using the “gelnet” function in the R package gelnet; Finally, calculating the correlation between the weight and gene expression based on Spearman method and standardizing the results to 0–1 to predict the stemness score of new samples. A higher stemness score represents a higher stemness of the sample. Weighted gene co-expression network analysis (WGCNA) (Langfelder and Horvath 2008) could discover gene modules with collaborative expression and explore the relationship between gene networks and phenotypes of interest, as well as hub genes in the network. Therefore, we subsequently used the WGCNA to identify phenotype-related core genes according to the following steps. Firstly, calculating the Pearson correlation coefficient between two genes based on the expression profile data, and constructing a similarity matrix. Secondly, according to the soft threshold β, converting the similarity matrix into a topological matrix using the topological overlap measure (TOM), which represents the degree of connection between two genes. Next, clustering the genes based on the 1-TOM distance, and identifying the gene module using the dynamic tree-cut method. Finally, calculate the correlation between different gene modules and stemness and clinical traits.
The analysis of scRNA-seq data was performed using the R package Seurat and CytoTrace. Firstly, we performed quality control (QC) of the raw counts by calculating the percentage of mitochondrial or ribosomal genes and excluding low-quality cells. Subsequently, the “FindVariableFeatures” function was adopted to filter the top 1500 highly variable genes after QC. And “RunPCA” function was used to reduce the dimension of PCA for the first 1500 highly variable genes screened above. Uniform manifold approximation and projection (UMAP) (Becht et al. 2018) was used for dimensionality reduction and cluster identification. The “FeaturePlot” function was used for visualizing HCC stem cell marker genes. Finally, “FindAllMarkers” function was used to screen the marker genes of each subgroup with logfc = 1 (differential multiples). Finally, we used the corrected p < 0.05 to screen the marker genes. R package CytoTRACE was applied to calculate the CytoTRACE scores for malignant cells. CytoTRACE scores range from 0 to 1, while higher scores indicate higher stemness and vice versa.
Single cell RNA sequence analysis
Cell annotation was performed by the SingleR package (Aran et al. 2019). Single-cell pseudotime trajectories were generated using the Monocle package (Qiu et al. 2017). Transcription factors (TFs) were predicted using the SCENIC package (Aibar et al. 2017). The communicating interactions between cells and identify the mechanism of the communicating molecules were explored using the CellChat package (Jin et al. 2021).
Construction and validation of the stemness-related prognostic model
We utilized the TCGA-LIHC cohort to establish a prediction model, and 364 samples were randomly divided into training and internal testing sets in 7:3 ratio. Firstly, univariate Cox regression analysis was performed to screen for prognostic-related genes. Subsequently, to avoid overfitting and reduce variables, we conducted a survival analysis of the LASSO Cox proportional hazards regression model using the “cv.glmnet” function in R package glmnet (Tibshirani 1997), and selected the optimal model by tenfold cross-validation. We extracted the coefficients of genes with non-zero coefficients from the analysis results of the LASSO Cox proportional hazards regression model and calculated the risk score using the following formula.
where represents the number of genes, represents the -th gene, denotes the coefficient of the -th gene, and represents the expression of the -th gene.
Survival analysis was performed using the Kaplan–Meier method using the R package survminer. In addition, receiver operating characteristic (ROC) curve analysis was conducted using the R package survivalROC (Heagerty and Zheng 2005) and the area under the curve (AUC) was calculated for predicting 1-, 3-, and 5-year OS. the same methods were applied to calculate the risk score and conduct Kaplan–Meier survival analysis and ROC curve analysis in three independent external datasets to validate the predictive performance and robustness of the stemness-related prognostic model.
To validate that the risk score could serve as an independent prognostic factor, multivariate Cox regression analysis was performed using the R package survival for age, gender, tumor grade, and risk score. And the R package regplot was used to construct and visualize a nomogram of 10 prognostic genes by conducting a multivariate Cox regression analysis. To verify the predictive performance of the nomogram, 1-, 3-, and 5-year OS were calculated using 1000 bootstrap resampling and the calibration curve was plotted using the R package rms.
Copy number variation and functional enrichment analysis
We used R package maftools to explore the difference between two risk groups and drew the waterfall of them. In order to investigate the biological difference and understand the different pathways involved in two risk groups, Gene Set Enrichment Analysis (GSEA) was performed with the R package clusterProfiler. Gene set variation analysis (GSVA) was used to further analyze the difference of pathways between the different risk groups using R package GSVA.
Immune infiltration and immune therapy response analysis
The infiltration rate of immune cells was assessed by CIBERSORT algorithm (Newman et al. 2019) with the default reference data set (LM22) to calculate the infiltration abundance of 22 type immune cells in the TCGA-LIHC cohort. Tumor mutation burden (TMB) is defined as the number of somatic mutations per million bases in the genome sequence. TMB and neoantigen load (NAL) are important factors that affect the response to immunotherapy. We calculated the levels of TMB and NAL in the high- and low-risk groups of the TCGA-LIHC cohort and tested the significance of differences between the two groups using the Wilcoxon test. To explore the response difference to immunotherapy in four cohorts, we used the Tumor Immune Dysfunction and Exclusion (TIDE) algorithm (http://tide.dfci.harvard.edu/) to predict the response to immunotherapy in four cohorts.
Drug sensitivity analysis
We included drug sensitivity information (IC50) from the CTRP and PRISM databases and screened drugs through three steps. Firstly, calculating the correlation between each drug and risk scores in the two databases. Secondly, predicting drug sensitivity using the ridge regression method in the R package pRRophetic and selecting drugs with significantly higher sensitivity using a cutoff of log2foldchange > 0.1 and p < 0.05. Finally, intersecting the results of the correlation analysis in the first step with the screened drugs in the second step to obtain candidate drugs.
Statistical analysis
All statistical analyses and visualization were performed using R software v4.1.1 (https://www.r-project.org/). Kaplan–Meier analysis with the log-rank test was used to detect differences in OS between different groups. Categorized variables between different risk groups were compared by the Wilcoxon t-test. P < 0.05 was set as a significant threshold. Benjamini–Hochberg was implemented to adjust the P-value for multiple testing using the R function “p.adjust”.
Results
Using WGCNA to identify stemness-related genes based on bulk RNA-seq data
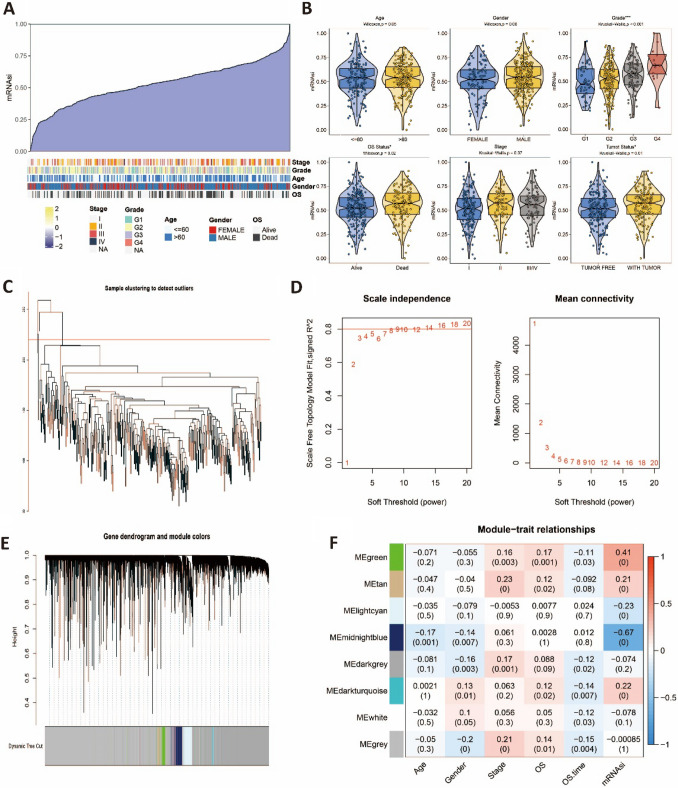
To identify stemness-associated gene signatures in bulk RNA-seq data, we first calculated the stemness score (mRNAsi) of each sample in the TCGA-LIHC dataset using the OCLR algorithm (Fig. 1A). We initially investigated the correlation between stemness scores and clinical variables. Our findings revealed that patients with higher histological grades, deceased patients, and patients with tumors exhibited higher stemness scores (Fig. 1B). However, there were no significant differences in stemness scores among different age groups, genders, and TNM stages (Fig. 1B). Then, we used WGCNA to explore stemness-related modules and core genes through the following steps. Sample clustering and static pruning were performed to remove four outlier samples, resulting in 360 samples being retained (Fig. 1C). For the selection of the soft-thresholding parameter, we chose 14 as the soft-thresholding parameter since the scale-free fit index reached 0.8 when the R2 was greater than or equal to 0.8, and the mean connectivity no longer decreased after the threshold of 14 (Fig. 1D). Through dynamic tree pruning and merging of similar modules, eight gene modules were obtained (Fig. 1E). Correlation analysis revealed that although the overall correlation between each module and the trait was low, they were highly correlated with mRNAsi. Eventually, 1 620 genes from the green and midnight blue modules were selected as the feature genes for subsequent analysis, based on the screening criteria of |r|≥ 0.3 and p < 0.05 (Fig. 1F).
Fig. 1.
Identification of stemness-related signatures based on bulk RNA-seq data A Summary of the relationship between mRNAsi and clinical variables. B mRNAsi in different groups. C Hierarchical cluster plot of TCGA-LIHC cohort. D The scale-free fit index for soft thresholding powers. The left panel illustrates the relationship between and R2. The right panel illustrates the relationship between and mean connectivity. E Dendrogram of genes and module colors. F The heatmap of the relationship between modules and clinical traits. Symbols: ***, 0 < p-value < 0.001; **0.001 ≤ p-value < 0.01; *0.01 ≤ p-value < 0.05; ns, 0.05 ≤ p-value < 1
Using CytoTRACE to identify stemness-related genes based on scRNA-seq data
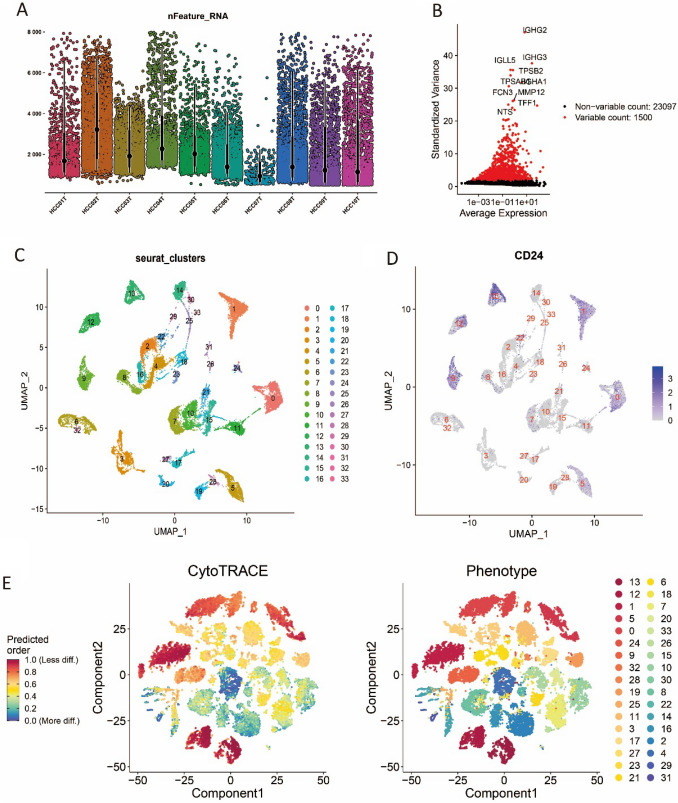
To identify stemness-associated gene signatures at the scRNA-seq level, we obtained the scRNA-seq dataset GSE149614 from the GEO database, which included 10 tumor samples comprising a total of 34 414 cells. A total of 24 363 cells were identified after QC (Fig. 2A). 34 distinct clusters were identified after PCA and UMAP analysis (Fig. 2C). To identify the cluster with the highest stemness, CD24 was selected as the stem cell marker from literature (Lee et al. 2022). The result showed that CD24 was mainly highly expressed in cluster 0, 1, 9, 12, and 13 (Fig. 2D), suggesting that these clusters had higher stem cell properties. CytoTRACE analysis indicated that top ten clusters with the highest stemness were 13, 12, 1, 5, 0, 24, 9, 32, 28, and 19 (Fig. 2E). The results of cytoTRACE showed that the clusters with high stemness features identified by marker-based discovery highly overlapped with those identified by CytoTRACE. Five clusters (0, 1, 9, 12, and 13) with high stemness were identified by taking the intersection of these two methods. Performing differential expression analysis of the above five clusters, 975 DEGs associated with high stemness were identified for further analysis.
Fig. 2.
Identification of stemness-related signatures based on single-cell RNA sequencing data A Violin plots to display a number of RNA features of ten tumor samples. B Variable features plot. C UMAP plot of the clustering result. D Expression of cancer stem cell marker CD24. E Result of CytoTRACE and corresponding phenotype
Single-cell analysis revealed heterogeneity of stemness-related cell populations
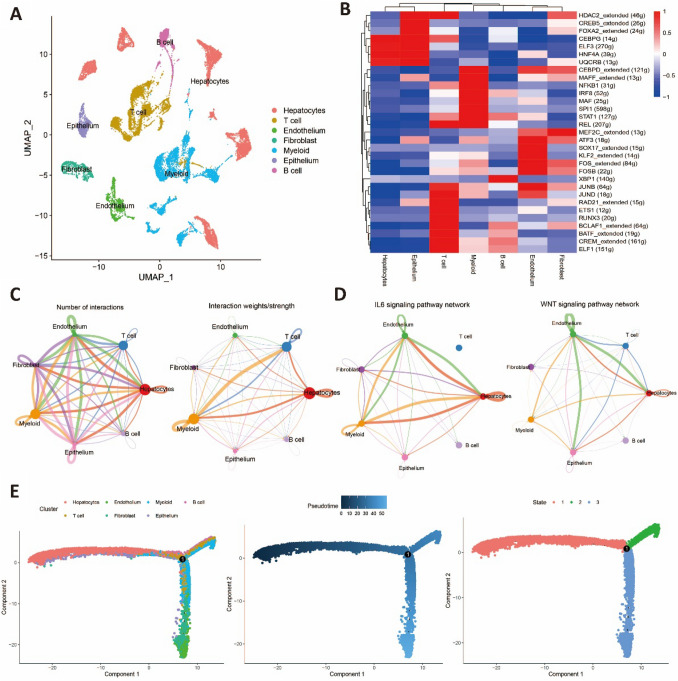
To investigate the heterogeneity between cell populations with high stemness characteristics and other cell populations, we utilized the singleR package to annotate cells into seven cell types (Fig. 3A). Combining these results with previous findings, we identified hepatocytes and epithelial cells as the cell populations exhibiting high stemness characteristics. Transcription factor dysregulation is closely associated with the development of liver cancer. SCENIC was employed to identify the key activated transcription factors in each cell population. We found that in hepatocytes, the transcription factors CEBPG, ELF3, HNF4A, and UQCRB were significantly activated. In epithelial cells, in addition to the aforementioned transcription factors, the transcription factors HDAC2_extended, CREB5_extended, and FOXA2_extended were also observed to be activated (Fig. 3B). The activation of ELF3 and HNF4A has been shown to promote cancer cell proliferation and lead to poor prognosis (Zheng et al. 2018; Takashima et al. 2018). The CellChat algorithm was employed to explore the intercellular interactions. The results revealed a highly complex communication network among the seven cell types, involving numerous ligands and receptors. This finding underscores the intricate changes in the microenvironment during tumor initiation and progression (Fig. 3C). Moreover, a cell–cell communication network was constructed based on the IL6 and WNT pathways, which are associated with stemness. Notably, hepatocytes exhibited strong signaling communication with other cell populations within the network (Fig. 3D). Lastly, we employed the monocle2 package to reconstruct the differentiation trajectory of cell populations within the HCC. The results revealed that hepatocytes and epithelial cells occupied the upstream position of the developmental trajectory (Fig. 3E) and exhibited strong differentiation capacity. The results mentioned above indicate a significant heterogeneity between stemness-related cell populations and other cell clusters. These stemness-related cell populations exhibited a higher degree of differentiation potential and increased activity during the differentiation process.
Fig. 3.
Single-cell analysis reveals heterogeneity of stemness-related cell populations A UMAP plots of annotated cell types. B Heatmap of transcription factors activation in different cell types. C Overview of intercellular communication. D Intercellular communication of IL6 and WNT pathways. E Pseudotime trajectory analysis of different cell types
Constructing and verifying a 10-gene prognostic model based on stemness-related signatures
A total of 2482 genes were obtained by taking a concatenation of the results of the scRNA-seq data and the bulk RNA-seq to construct the prognostic model in the TCGA-LIHC dataset. After a univariate Cox regression analysis, 307 survival-related genes were screened out with a Bonferroni-corrected p-value of 0.05 as the filtering criterion. LASSO was performed and ten gene signatures were ultimately identified to construct the prognostic model (Fig. 4A, B), including six risk genes and four protective genes (Fig. 4C). Finally, we extracted the coefficients of each gene from the LASSO results to construct a prognostic signature, and the calculation formula is shown below:
Fig. 4.
Construction of 10-gene prognostic model A Ten-fold cross-validations for screening of the optimal parameter. B LASSO coefficient profiles determined by the optimal lambda. C Forest of ten prognostic genes. D Kaplan–Meier curves of TCGA training cohort, TCGA test cohort and TCGA whole cohort. E ROC curves of TCGA training cohort, TCGA test cohort and TCGA whole cohort. The Kaplan–Meier curves (F) and ROC curves (G) of three external cohort
Patients in the TCGA training set, TCGA internal test set, and TCGA dataset were classified into high-risk and low-risk groups based on median risk scores. Kaplan–Meier survival analysis showed that patients in the high-risk group had poorer OS in the TCGA training set (P-value < 0.001), TCGA internal test set (P-value = 0.003), and TCGA dataset (P-value < 0.001) (Fig. 4D). To assess the predictive accuracy of the prognostic model, we calculated the area under the curve (AUC) values of the receiver Operating Characteristic (ROC) curves for OS at 1, 3, and 5 years for the TCGA training set, internal test set, and TCGA dataset (Fig. 4E). And the results show that the AUC of three datasets were all greater than 0.7.
To verify the robustness of the model, we used three external datasets, LIHC-CN, ICGC-LIRI, and GSE14520 for validation. All patients were classified into high-risk and low-risk groups using the same method as in the TCGA-LIHC dataset. Consistent results obtained in TCGA-LIHC dataset were observed, the high-risk group had poorer OS in all three external datasets, with p-values less than 0.001 (Fig. 4F). Additionally, our analysis yielded AUC values ranging from 0.73 to 0.79 for the ICGC-LIRI dataset (Fig. 4G), 0.69 to 0.76 for the LIHC-CN dataset (Fig. 4F), and 0.64 to 0.69 for the GSE14520 dataset (Fig. 4G). The AUC values in the validation datasets were all greater than 0.6, with the ICGC-LIRI dataset achieving the best predictive performance with an AUC of 0.79 (Fig. 4G). The results evince that the constructed prognostic model exhibits a robust predictive capacity.
Among the 10 genes, Y-box binding protein 1 (YB-1/YBX1) was associated with prognosis and drug resistance in HCC, and is strongly associated with prognosis and drug resistance in HCC (Xu et al. 2020; Tao et al. 2019). CDC20 functions as a regulatory protein in several parts of the cell cycle and is highly expressed in patients with HCC (Jeong et al. 2022). The expression of receptor activity modifying protein 3 (RAMP3) is significantly downregulated in HCC tissues, and increased RAMP3 expression is beneficial for OS (Fang et al. 2018). LDHA may mediate tumor growth and metastasis in HCC and lead to poor prognosis (Serra et al. 2022). PTRH2 is a potential oncogene that promotes malignancy and metastasis (Corpuz et al. 2020). The expression of GNA14 in HCC expression is downregulated and it is negatively associated with hepatitis B virus (HBV) infection, vascular invasion, and prognosis of HCC (Song et al. 2021). Downregulation of CLEC3B in HCC promotes migration, invasion, and epithelial-mesenchymal transition of tumor cells and endothelial cells, ultimately leading to poor prognosis (Dai et al. 2019). Although no studies have shown that CYB5R3 and SRPRB are associated with HCC, these genes may serve as potential prognostic factors for HCC.
Relationship between the stemness-related prognostic model and clinical parameters or patient outcome
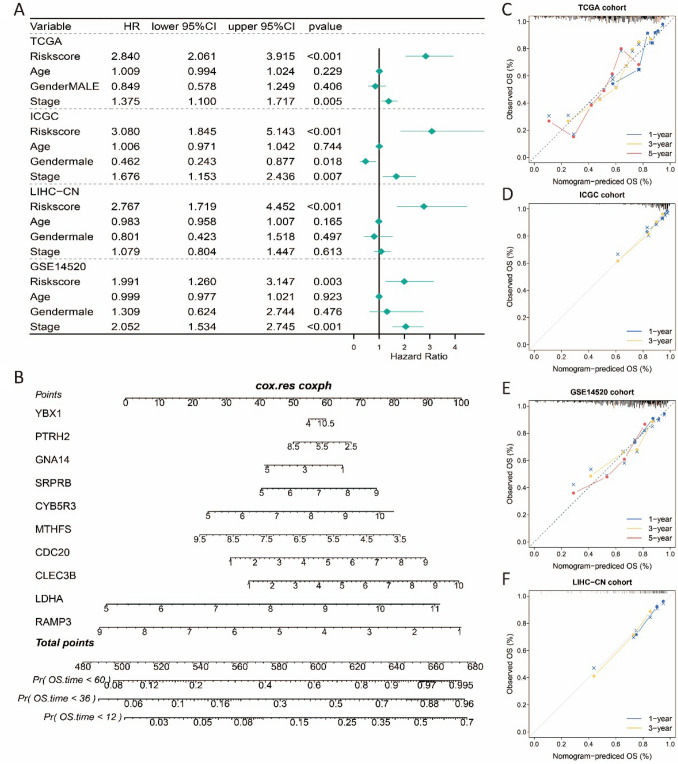
To explore whether the stemness-related risk score constructed in this study can serve as an independent prognostic factor, we conducted multivariate Cox regression analysis on age, sex, tumor grade, and risk score in the training and validation datasets. The results showed that the risk score could serve as an independent prognostic factor in all four datasets, including TCGA-LIHC (HR = 2.840, 95% CI 2.061–3.915, P-value < 0.001), ICGC-LIRI (HR = 3.080, 95% CI 3.845–5.143, P-value < 0.001), LIHC-CN (HR = 2.767, 95% CI 1.719–4.252, P-value < 0.001), and GSE14520 (HR = 1.991, 95% CI 1.620–3.147, P-value = 0.003) (Fig. 5A). To facilitate the clinical prediction of 1-, 3-, and 5-year OS of HCC patients, we constructed a nomogram based on the ten identified stemness-related prognostic gene signatures (Fig. 5B). The calibration curve results showed that the prognostic model’s predictions of 1-, 3-, and 5-year OS probabilities were consistent with the ideal predictions (gray line) across all datasets (Fig. 5C–F). The obtained results demonstrate that the nomogram developed using stemness-related gene signatures holds significant utility as a predictive tool for assessing the prognosis of patients with HCC.
Fig. 5.
Independence analysis, Nomogram construction and calibration plot validations A Forest plot of risk score, age, gender and stage in four cohorts. B Nomogram based on ten prognostic genes for 1-, 3- and 5-year OS predictions. C–F Calibration curves for testing the agreement between 1-, 3- and 5-year predicted overall survival and actual observations in TCGA-LIHC cohort, ICGC-LIRI cohort, GSE14520 cohort and LIHC-CN cohort
To investigate the relationship between the risk score and clinical variables, we generated heatmaps of the expression of stemness-related gene signatures (Fig. S2 A-D). The heatmaps from the four datasets showed that protective genes were highly expressed in the low-risk group, while risk genes were highly expressed in the high-risk group. In addition, patients with higher tumor grades were mainly in the high-risk group, and those who experienced survival events were mainly in the high-risk group. The distribution of risk scores and survival status showed that the high-risk group had a shorter survival time and more OS events (Fig S2 E–H).
Somatic mutation landscape and GSEA analysis between high and low stemness-risk groups
The high-risk group had a higher proportion of alterations (80.11%, Fig. S1 A) than low-risk group (68.16%, Fig. S1 B). And the high-risk group had a higher percentage of TP53 mutations (37% vs 21%, Fig S1 A, B). The tumor suppressor gene TP53 is the most commonly mutated gene. Wild-type p53 can suppress tumor progression through multiple pathways. However, mutations in TP53 and the resulting p53 inactivation allow evasion of tumor cell death and rapid tumor progression (Bykov et al. 2018). We therefore hypothesize that TP53 mutations are the main cause affecting high- and low- risk groups.
To characterize the molecular features and biological functions of differentially expressed genes in high- and low-risk groups, differential expression analysis was performed using the R package limma (Ritchie et al. 2015), and gene set enrichment analysis (GSEA) was performed using the hallmark gene set as the background set. A total of 24 pathways were significantly upregulated in the high-risk group and 4 pathways were significantly upregulated in the low-risk group (Fig. 6A, B). Among the upregulated pathways in high-risk group, 11 pathways were associated with the promotion of cancer development (Fig. 6A). And we found that the pathway HALLMARK_INTERFERON_GAMMA_RESPONSE showed upregulation, indicating that the high-risk group was more likely to respond to immunotherapy (Grasso et al. 2020). Meanwhile, the pathways that were significantly enriched in the low-risk group were mainly associated with bile acid metabolism, coagulation, fatty acid metabolism, and xenobiotic metabolism (Fig. 6B). And GSVA was used to further explore the differences of upregulated pathways in different risk groups, and the results are shown in Fig. 6C.
Fig. 6.

Differentially functional pathways between different risk groups in TCGA A Upregulated pathways in high-risk group. B Upregulated pathways in low-risk group. C Heatmap of enrichment level calculated by GSVA for upregulated pathways
Immunotherapy response and chemotherapy sensitivity among different risk groups
Tumor immunotherapy has changed the treatment paradigm for HCC and improved survival rates. In this study, we investigated the benefits of immunotherapy in high- and low-risk groups. First, we used the CIBERSORT algorithm to calculate the abundance of 22 immune cell types. The low-risk group had a higher proportion of activated CD4 memory T cells, CD8 T cells, resting mast cells, and M1 macrophages, while the high-risk group had a higher proportion of activated dendritic cells (Fig. 7A). CD8 T cells, resting mast cells, and M1 macrophages have a tumor-inhibiting effect, and their higher infiltration may be a potential factor for better prognosis in the low-risk group (Fig. 7A). Dendritic cells are responsible for antigen presentation and can enhance the host's innate and adaptive immunity. The higher proportion of activated dendritic cells in the high-risk group suggests that this group may be more likely to respond to immunotherapy. Considering that tumor mutation burden (TMB) and tumor neoantigen load (TNL) are indicative of immunotherapy response, we calculated and compared the TMB and TNL levels in the high- and low-risk groups. The results showed that the high-risk group had a higher TMB level (P-value = 0.0177, Fig. 7B), while TNL did not show a significant difference (P-value = 0.9528, Fig. 7C). In addition, we examined the expression of immune checkpoints in the high- and low-risk groups. We observed that in the TCGA-LIHC, ICGC-LIRI, and LIHC-CN cohorts, the expression levels of many immune checkpoint genes were higher in the high-risk group. This suggests that patients in the high-risk group from these three datasets may benefit more from immunotherapy (Fig. 7D).
Fig. 7.
Relation between risk score and immune A Immune cell infiltration proportion in different risk groups. B-C Boxplot of TMB and NAL in TCGA cohort. D Expression of immune checkpoint gene in four cohorts. E Treatment response rates of immunotherapy in high- and low-risk groups in TCGA-LIHC cohort, ICGC-LIRI cohort, LIHC-CN cohort and GSE14520 cohort. Symbols: ***0 < p-value < 0.001; **0.001 ≤ p-value < 0.01; *, 0.01 ≤ p-value < 0.05; ns, 0.05 ≤ p-value < 1
To further compare the differences in immunotherapy response between the high- and low-risk groups, we used the TIDE algorithm to predict the response to immunotherapy in four datasets. In the TCGA-LIHC and ICGC-LIRI datasets, high-risk group was more likely to respond to immunotherapy (68.13% and 68.33%, respectively, with P-value < 0.001 and P-value = 0.008). Although the same trend was observed in the GSE14520 dataset, it was not significant (P-value = 0.059). In the LIHC-CN dataset, no significant difference in immunotherapy response was observed between the high-risk and low-risk groups. (P-value = 0.87, Fig. 7E).
Subsequently, we explored the sensitivity of the high-risk and low-risk groups to chemotherapy drugs by analyzing the drug sensitivity data obtained from the CTRP and PRISM databases. We selected eight candidate drugs from the CTRP database (Fig. S3A) and four candidate drugs from the PRISM database (Fig. S3B). Among these 12 drugs, high-risk group had higher sensitivity and were more likely to benefit from these drugs. All of these results suggest that patients in the high-risk group are more likely to respond to immunotherapy and have a higher sensitivity to chemotherapy drugs. Therefore, the stemness-related risk score can potentially serve as an indicator to predict the potential benefit of both immunotherapy and chemotherapy for patients, thereby aiding in personalized treatment decision-making.
The expression patterns of stemness-related gene signatures
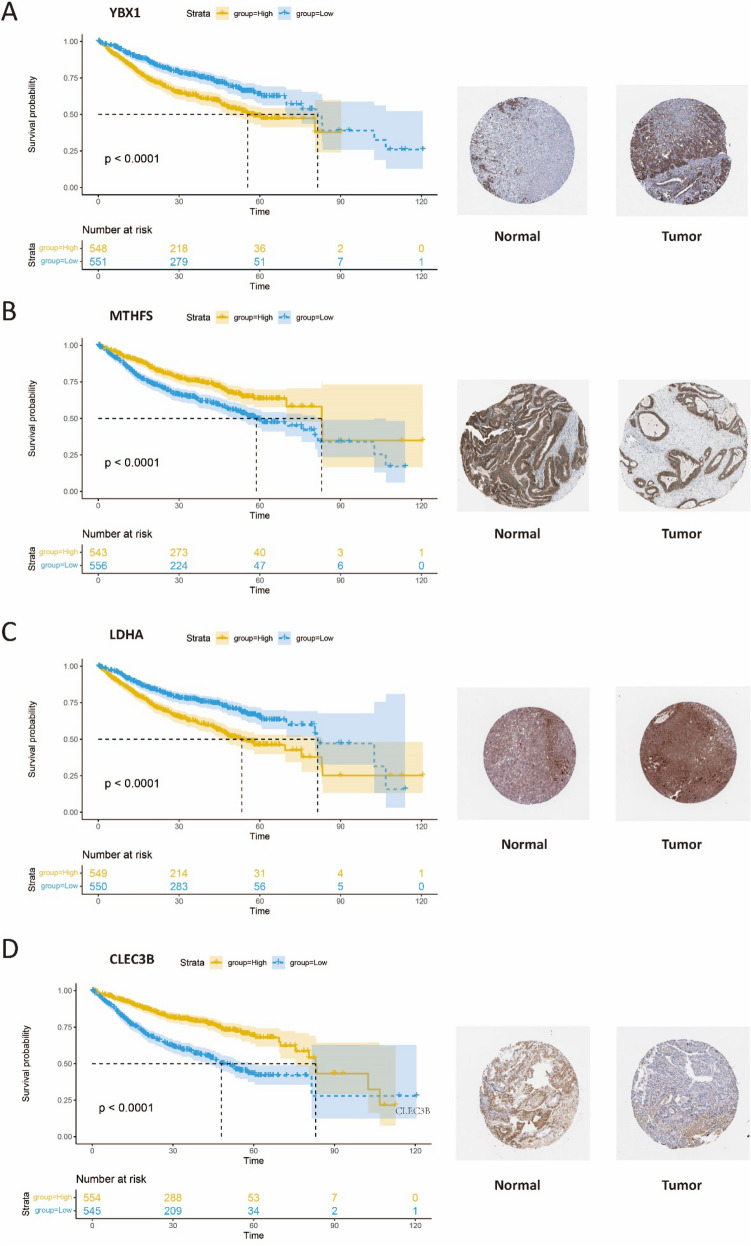
To validate the prognostic significance of the identified gene signatures at the proteomic level, we downloaded the immunohistochemistry results of 10 prognostic genes from the HPA database (https://www.proteinatlas.org/). Patients were divided into high- and low-expression groups based on the median expression levels, and Kaplan–Meier survival analysis was performed. The immunohistochemistry results of four genes, namely YBX1, LDHA, MTHFS, and CLEC3B, were consistent with our study results. Univariate analysis revealed that YBX1 and LDHA were risk genes, and high expression levels of these genes were associated with poorer prognosis (P-value < 0.0001, Figs. 8A, B), while MTHFS and CLEC3B were protective genes, and high expression levels of these genes were associated with better prognosis (Figs. 8C, D). Additionally, the translated protein of YBX1 and LDHA were found to have higher levels in tumor tissues (Figs. 8A, B), while MTHFS and CLEC3B were more highly expressed in normal tissues (Figs. 8C, D). These findings suggest that the risk genes identified in our study may exert their effect on tumor prognosis through the modulation of specific protein expression levels. Our study provides robust support for further investigation into the mechanistic roles of these genes in tumorigenesis and progression.
Fig. 8.
HPA database validation A The Kaplan–Meier curve and the protein expression of YBX1. B The Kaplan–Meier curve and the protein expression of MTHFS. C The Kaplan–Meier curve and the protein expression of LDHA. D The Kaplan–Meier curve and the protein expression of CLEC3B
Discussion
Tumor stem cells (CSCs) can regenerate all the assets of a tumor due to their unique stem cell-like self-renewal and differentiation capacity. It has been observed that CSCs contribute to tumor growth and are responsible for tumor recurrence and treatment resistance (Yamashita and Wang 2013). Tumor stemness has been associated with a poorer prognosis in various solid tumors (Ben-Porath et al. 2008). Consequently, several studies have focused on constructing prognostic models based on tumor stemness in different cancer types (Li et al. 2020; Yue et al. 2022; Weng et al. 2022). However, there are no studies that integrate scRNA-seq data and bulk RNA-seq data to construct prognostic models for HCC.
In this study, stemness-related prognostic signatures were extracted from bulk RNA-seq data and scRNA-seq data based on WGCNA and CytoTRACE, respectively. To make full use of both scRNA-seq data features and bulk RNA-seq data features, we merged the genes obtained in both, and a total of 10 genes were obtained for the construction of the prognostic model by performing univariate Cox and LASSO. Among the 10 genes examined, only CYB5R3 and SRPRB lack evidence suggesting their association with hepatocellular carcinoma (HCC). Nonetheless, these genes show promise as potential predictive factors and drug targets, awaiting validation in future investigation. A notable aspect of this study is the balanced utilization of both bulk RNA-seq data and scRNA-seq data. Among the 10 genes included in the prognostic model, five were derived from bulk RNA-seq data while the other five were obtained from scRNA-seq data. This comprehensive integration of both data types allowed for a more comprehensive and robust characterization of the stemness-related gene signatures associated with HCC. Receiver operating characteristic analysis suggested that the prognostic model has high statistical power. K–M survival analysis showed that the high-risk group with higher stemness had a worse prognosis. Additionally, multivariate Cox regression analyses revealed that the prognostic value of the prognostic model was independent of clinical characteristics. These findings suggest that the stemness-related risk score may serve as a valuable indicator of patient outcomes in HCC. To improve the predictive efficacy of the signature and facilitate clinical application, we subsequently constructed and validated a nomogram based stemness-related signatures for clinical practicality to predict OS.
CSCs have been associated with aggressive tumor behavior, including vascular invasion, metastasis, and poor prognosis (Yamashita and Wang 2013). Furthermore, CSCs possess immunosuppressive properties and can create an immunosuppressive tumor microenvironment, which hampers the efficacy of immunotherapy. CSCs can evade immune recognition and destruction through various mechanisms, including altered antigen presentation, upregulation of immune checkpoint molecules, and secretion of immunosuppressive factors (Chen et al. 2013). To investigate the relationship between stemness risk scores and immunotherapy, we first investigated the abundance of immune cell infiltration in the high- and low-risk groups in TCGA-LIHC. The high-risk group had a higher proportion of CD8 T cells and macrophages and may be more likely to respond to immunotherapy. TMB and NAL have emerged as informative indicators of immunotherapy response, and significantly higher TMB levels in the high-risk group, suggesting a higher likelihood of response to immunotherapy. Using the TIDE algorithm, we conducted predictions on the immunotherapy response of patients in four different datasets. Consistent with the findings from tumor immune infiltration, TMB analysis, and immune checkpoint gene expression, the high-risk group demonstrated a higher likelihood of responding to immunotherapy. Additionally, the analysis of drug sensitivity revealed that the high-risk group had a greater potential for benefiting from chemotherapy drugs. These results collectively highlight the utility of the stemness-related prognostic model in guiding personalized treatment approaches for patients with HCC. All of these results suggest that the stemness-related prognostic model could guide the personalized treatment of HCC patients.
However, there are some limitations in this study. First, it was a retrospective study based on public sequencing data, and the sample capacity of the tissue microarray verification cohort was insufficient. Second, although the HPA database was used for validation at the proteomic level, not all genes were validated and reliability may be lacking. Finally, although the risk score could predict patient response to chemotherapy and immunotherapy, statistically significant differences were not observed in all datasets for immunotherapy response.
In general, the main contribution of this study is the comprehensive utilization of both bulk RNA-seq and scRNA-seq data and the use of various methods to construct a predictive model based on stemness characteristics. This model can provide guidance and reference for the treatment of HCC patients and provide new ideas and inspiration for the prognosis research of HCC.
Conclusion
In summary, this study successfully integrated scRNA-seq data and bulk RNA-seq data to identify stemness-related gene signatures and construct a prognostic model consisting of ten genes. Furthermore, the robustness of this prognostic model was validated in three independent datasets, enhancing its reliability and generalizability. By stratifying patients into high- and low-risk groups based on the prognostic model, we investigated the differences between these groups in terms of clinical variables, immunotherapy response, and chemotherapy drug sensitivity. These findings provide valuable insights and guidance for personalized treatment strategies in HCC patients, facilitating improved patient care and outcomes.
Supplementary Information
Below is the link to the electronic supplementary material.
Author contributions
XW, XC: methodology, data curation, formal analysis, writing—original draft preparation. MZ, GL, and DC: validation and formal analysis; JF and FY: conceptualization, writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
National Natural Science Foundation of China [No.81973145, No. 82273735]. Key R&D Program of Jiangsu Province (Social Development) (BE2020694).
Data availability
The data used to support the findings of this study are available from the corresponding author upon request.
Declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Xin Wang and Xinyi Chen have contributed equally to this work.
Contributor Information
Fangrong Yan, Email: f.r.yan@163.com.
Jingya Fang, Email: jyfang1904@163.com.
References
- Aibar S, Gonzalez-Blas CB, Moerman T, Huynh-Thu VA, Imrichova H, Hulselmans G et al (2017) SCENIC: single-cell regulatory network inference and clustering. Nat Methods 14(11):1083–1086. 10.1038/nmeth.4463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aran D, Looney AP, Liu L, Wu E, Fong V, Hsu A et al (2019) Reference-based analysis of lung single-cell sequencing reveals a transitional profibrotic macrophage. Nat Immunol 20(2):163–172. 10.1038/s41590-018-0276-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becht E, McInnes L, Healy J, Dutertre CA, Kwok IWH, Ng LG et al (2018) Dimensionality reduction for visualizing single-cell data using UMAP. Nat Biotechnol. 10.1038/nbt.4314 [DOI] [PubMed] [Google Scholar]
- Ben-Porath I, Thomson MW, Carey VJ, Ge R, Bell GW, Regev A et al (2008) An embryonic stem cell-like gene expression signature in poorly differentiated aggressive human tumors. Nat Genet 40(5):499–507. 10.1038/ng.127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bykov VJN, Eriksson SE, Bianchi J, Wiman KG (2018) Targeting mutant p53 for efficient cancer therapy. Nat Rev Cancer 18(2):89–102. 10.1038/nrc.2017.109 [DOI] [PubMed] [Google Scholar]
- Chen K, Huang YH, Chen JL (2013) Understanding and targeting cancer stem cells: therapeutic implications and challenges. Acta Pharmacol Sin 34(6):732–740. 10.1038/aps.2013.27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K, Liu X, Liu W, Wang F, Tian X, Yang Y (2022) Development and validation of prognostic and diagnostic model for pancreatic ductal adenocarcinoma based on scRNA-seq and bulk-seq datasets. Hum Mol Genet 31(10):1705–1719. 10.1093/hmg/ddab343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corpuz AD, Ramos JW, Matter ML (2020) PTRH2: an adhesion regulated molecular switch at the nexus of life, death, and differentiation. Cell Death Discov 6(1):124. 10.1038/s41420-020-00357-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai W, Wang Y, Yang T, Wang J, Wu W, Gu J (2019) Downregulation of exosomal CLEC3B in hepatocellular carcinoma promotes metastasis and angiogenesis via AMPK and VEGF signals. Cell Commun Signal 17(1):113. 10.1186/s12964-019-0423-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang A, Zhou S, Su X, Liu C, Chen X, Wan Y et al (2018) RAMP3 is a prognostic indicator of liver cancer and might reduce the adverse effect of TP53 mutation on survival. Future Oncol 14(25):2615–2625. 10.2217/fon-2018-0296 [DOI] [PubMed] [Google Scholar]
- Forner A, Reig M, Bruix J (2018) Hepatocellular carcinoma. Lancet 391(10127):1301–1314. 10.1016/s0140-6736(18)30010-2 [DOI] [PubMed] [Google Scholar]
- Gao Q, Zhu H, Dong L, Shi W, Chen R, Song Z et al (2019) Integrated proteogenomic characterization of HBV-related hepatocellular carcinoma. Cell 179(2):561-577.e22. 10.1016/j.cell.2019.08.052 [DOI] [PubMed] [Google Scholar]
- Grasso CS, Tsoi J, Onyshchenko M, Abril-Rodriguez G, Ross-Macdonald P, Wind-Rotolo M et al (2020) Conserved interferon-γ signaling drives clinical response to immune checkpoint blockade therapy in melanoma. Cancer Cell 38(4):500-515.e3. 10.1016/j.ccell.2020.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulati GS, Sikandar SS, Wesche DJ, Manjunath A, Bharadwaj A, Berger MJ et al (2020) Single-cell transcriptional diversity is a hallmark of developmental potential. Science 367(6476):405–411. 10.1126/science.aax0249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heagerty PJ, Zheng Y (2005) Survival model predictive accuracy and ROC curves. Biometrics 61(1):92–105. 10.1111/j.0006-341X.2005.030814.x [DOI] [PubMed] [Google Scholar]
- Jeong SM, Bui QT, Kwak M, Lee JY, Lee PC (2022) Targeting Cdc20 for cancer therapy. Biochim Biophys Acta Rev Cancer 1877(6):188824. 10.1016/j.bbcan.2022.188824 [DOI] [PubMed] [Google Scholar]
- Jin S, Guerrero-Juarez CF, Zhang L, Chang I, Ramos R, Kuan CH et al (2021) Inference and analysis of cell-cell communication using Cell Chat. Nat Commun 12(1):1088. 10.1038/s41467-021-21246-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langfelder P, Horvath S (2008) WGCNA: an R package for weighted correlation network analysis. BMC Bioinformatics 9:559. 10.1186/1471-2105-9-559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee TK, Guan XY, Ma S (2022) Cancer stem cells in hepatocellular carcinoma-from origin to clinical implications. Nat Rev Gastroenterol Hepatol 19(1):26–44. 10.1038/s41575-021-00508-3 [DOI] [PubMed] [Google Scholar]
- Li X, Li Y, Yu X, Jin F (2020) Identification and validation of stemness-related lncRNA prognostic signature for breast cancer. J Transl Med 18(1):331. 10.1186/s12967-020-02497-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Ramadori P, Pfister D, Seehawer M, Zender L, Heikenwalder M (2021) The immunological and metabolic landscape in primary and metastatic liver cancer. Nat Rev Cancer 21(9):541–557. 10.1038/s41568-021-00383-9 [DOI] [PubMed] [Google Scholar]
- Malta TM, Sokolov A, Gentles AJ, Burzykowski T, Poisson L, Weinstein JN et al (2018) Machine learning identifies stemness features associated with oncogenic dedifferentiation. Cell 173(2):338-354e15. 10.1016/j.cell.2018.03.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda A, Hamilton PT, Zhang AW, Pattnaik S, Becht E, Mezheyeuski A et al (2019) Cancer stemness, intratumoral heterogeneity, and immune response across cancers. Proc Natl Acad Sci USA 116(18):9020–9029. 10.1073/pnas.1818210116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman AM, Steen CB, Liu CL, Gentles AJ, Chaudhuri AA, Scherer F et al (2019) Determining cell type abundance and expression from bulk tissues with digital cytometry. Nat Biotechnol 37(7):773–782. 10.1038/s41587-019-0114-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu X, Mao Q, Tang Y, Wang L, Chawla R, Pliner HA et al (2017) Reversed graph embedding resolves complex single-cell trajectories. Nat Methods 14(10):979–982. 10.1038/nmeth.4402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie ME, Phipson B, Wu D, Hu Y, Law CW, Shi W et al (2015) Limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res 43(7):e47. 10.1093/nar/gkv007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serra M, Di Matteo M, Serneels J, Pal R, Cafarello ST, Lanza M et al (2022) Deletion of lactate dehydrogenase-A impairs oncogene-induced mouse hepatocellular carcinoma development. Cell Mol Gastroenterol Hepatol 14(3):609–624. 10.1016/j.jcmgh.2022.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song G, Zhu X, Xuan Z, Zhao L, Dong H, Chen J et al (2021) Hypermethylation of GNA14 and its tumor-suppressive role in hepatitis B virus-related hepatocellular carcinoma. Theranostics 11(5):2318–2333. 10.7150/thno.48739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song P, Li W, Wu X, Qian Z, Ying J, Gao S et al (2022) Integrated analysis of single-cell and bulk RNA-sequencing identifies a signature based on B cell marker genes to predict prognosis and immunotherapy response in lung adenocarcinoma. Cancer Immunol Immunother 71(10):2341–2354. 10.1007/s00262-022-03143-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun D, Guan X, Moran AE, Wu LY, Qian DZ, Schedin P et al (2022) Identifying phenotype-associated subpopulations by integrating bulk and single-cell sequencing data. Nat Biotechnol 40(4):527–538. 10.1038/s41587-021-01091-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takashima Y, Horisawa K, Udono M, Ohkawa Y, Suzuki A (2018) Prolonged inhibition of hepatocellular carcinoma cell proliferation by combinatorial expression of defined transcription factors. Cancer Sci 109(11):3543–3553. 10.1111/cas.13798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao Z, Ruan H, Sun L, Kuang D, Song Y, Wang Q et al (2019) Targeting the YB-1/PD-L1 axis to enhance chemotherapy and antitumor immunity. Cancer Immunol Res 7(7):1135–1147. 10.1158/2326-6066.CIR-18-0648 [DOI] [PubMed] [Google Scholar]
- Thorgeirsson SS, Lee JS, Grisham JW (2006) Functional genomics of hepatocellular carcinoma. Hepatology 43(2 Suppl 1):S145–S150. 10.1002/hep.21063 [DOI] [PubMed] [Google Scholar]
- Tibshirani R (1997) The lasso method for variable selection in the Cox model. Stat Med 16(4):385–395 [DOI] [PubMed] [Google Scholar]
- Wang R, Zheng X, Wang J, Wan S, Song F, Wong MH et al (2022) Improving bulk RNA-seq classification by transferring gene signature from single cells in acute myeloid leukemia. Brief Bioinform. 10.1093/bib/bbac002 [DOI] [PubMed] [Google Scholar]
- Weng M, Li T, Zhao J, Guo M, Zhao W, Gu W et al (2022) mRNAsi-related metabolic risk score model identifies poor prognosis, immunoevasive contexture, and low chemotherapy response in colorectal cancer patients through machine learning. Front Immunol 13:950782. 10.3389/fimmu.2022.950782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weston AD, Hood L (2004) Systems biology, proteomics, and the future of health care: toward predictive, preventative, and personalized medicine. J Proteome Res 3(2):179–196. 10.1021/pr0499693 [DOI] [PubMed] [Google Scholar]
- Xu J, Ji L, Liang Y, Wan Z, Zheng W, Song X et al (2020) CircRNA-SORE mediates sorafenib resistance in hepatocellular carcinoma by stabilizing YBX1. Signal Transduct Target Ther 5(1):298. 10.1038/s41392-020-00375-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita T, Wang XW (2013) Cancer stem cells in the development of liver cancer. J Clin Invest 123(5):1911–1918. 10.1172/JCI66024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue Y, Tao J, An D, Shi L (2022) Exploring the role of tumor stemness and the potential of stemness-related risk model in the prognosis of intrahepatic cholangiocarcinoma. Front Genet 13:1089405. 10.3389/fgene.2022.1089405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng L, Xu M, Xu J, Wu K, Fang Q, Liang Y et al (2018) ELF3 promotes epithelial-mesenchymal transition by protecting ZEB1 from miR-141–3p-mediated silencing in hepatocellular carcinoma. Cell Death Dis 9(3):387. 10.1038/s41419-018-0399-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu L, Liu J, Chen J, Zhou Q (2021) The developing landscape of combinatorial therapies of immune checkpoint blockade with DNA damage repair inhibitors for the treatment of breast and ovarian cancers. J Hematol Oncol 14(1):206. 10.1186/s13045-021-01218-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.