Abstract
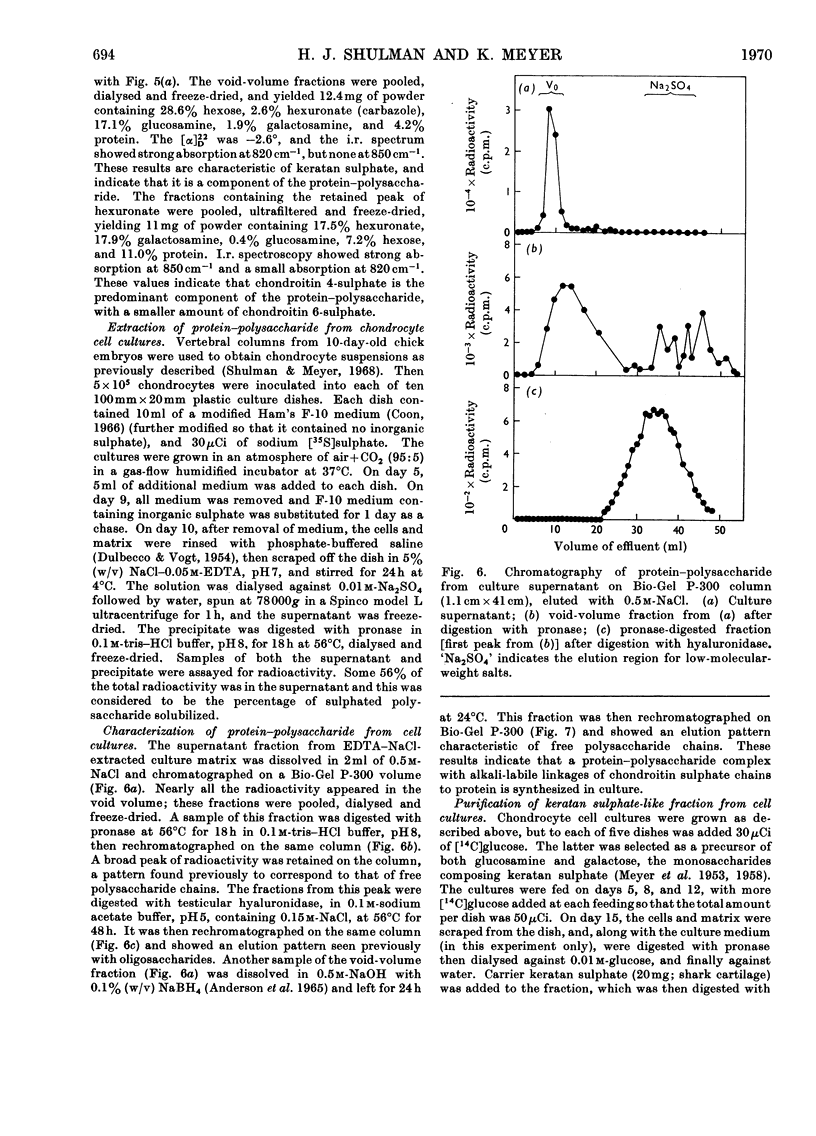
The nature of the matrix produced by embryonic chicken chondrocytes in cell culture was studied, and compared with adult and embryonic chicken cartilage. Adult chicken cartilage contains a protein–polysaccharide easily extracted with EDTA–sodium chloride at 4°C. Purification of this macromolecule on Bio-Gel P-300 and Bio-Gel A-50m yielded a progressively more homogeneous species in the ultracentrifuge. It contained mostly chondroitin 4-sulphate, some chondroitin 6-sulphate, and keratan sulphate. Embryonic chicken cartilage was previously shown to contain mostly chondroitin 4-sulphate, some chondroitin 6-sulphate and essentially no keratan sulphate. The matrix produced in chondrocyte cell cultures was shown to contain a protein–polysaccharide with alkali-labile linkages of chondroitin 4-sulphate to the protein core. A fraction was isolated from the matrix with many properties of keratan sulphate.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDERSON B., HOFFMAN P., MEYER K. THE O-SERINE LINKAGE IN PEPTIDES OF CHONDROITIN 4- OR 6-SULFATE. J Biol Chem. 1965 Jan;240:156–167. [PubMed] [Google Scholar]
- Abbott J., Holtzer H. The loss of phenotypic traits by differentiated cells, V. The effect of 5-bromodeoxyuridine on cloned chondrocytes. Proc Natl Acad Sci U S A. 1968 Apr;59(4):1144–1151. doi: 10.1073/pnas.59.4.1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbott J., Holtzer H. The loss of phenotypic traits by differentiated cells. 3. The reversible behavior of chondrocytes in primary cultures. J Cell Biol. 1966 Mar;28(3):473–487. doi: 10.1083/jcb.28.3.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryan J. Studies on clonal cartilage strains. I. Effect of contaminant non-cartilage cells. Exp Cell Res. 1968 Oct;52(2):319–326. doi: 10.1016/0014-4827(68)90473-4. [DOI] [PubMed] [Google Scholar]
- Bryan J. Studies on clonal cartilage strains. II. Selective effects of different growth conditions. Exp Cell Res. 1968 Oct;52(2):327–337. doi: 10.1016/0014-4827(68)90474-6. [DOI] [PubMed] [Google Scholar]
- Chacko S., Abbott J., Holtzer S., Holtzer H. The loss of phenotypic traits by differentiated cells. VI. Behavior of the progeny of a single chondrocyte. J Exp Med. 1969 Aug 1;130(2):417–442. doi: 10.1084/jem.130.2.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coon H. G., Cahn R. D. Differentiation in vitro: effects of Sephadex fractions of chick embryo extract. Science. 1966 Sep 2;153(3740):1116–1119. doi: 10.1126/science.153.3740.1116. [DOI] [PubMed] [Google Scholar]
- DAVIDSON E., HOFFMAN P., LINKER A., MEYER K. The acid mucopolysaccharides of connective tissue. Biochim Biophys Acta. 1956 Sep;21(3):506–518. doi: 10.1016/0006-3002(56)90188-3. [DOI] [PubMed] [Google Scholar]
- DULBECCO R., VOGT M. Plaque formation and isolation of pure lines with poliomyelitis viruses. J Exp Med. 1954 Feb;99(2):167–182. doi: 10.1084/jem.99.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingman C. W., Peacock A. C. Analytical studies on nuclear ribonucleic acid using polyacrylamide gel electrophoresis. Biochemistry. 1968 Feb;7(2):659–668. doi: 10.1021/bi00842a022. [DOI] [PubMed] [Google Scholar]
- Elson L. A., Morgan W. T. A colorimetric method for the determination of glucosamine and chondrosamine. Biochem J. 1933;27(6):1824–1828. doi: 10.1042/bj0271824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GLICK M. C., STOCKDALE F. E. DIFFERENCES IN GLYCOSAMINOGLYCANS DERIVED FROM CHICK EMBRYO CHONDROCYTES GROWN IN VITRO AND IN VIVO. Biochim Biophys Acta. 1964 Mar 2;83:61–68. doi: 10.1016/0926-6526(64)90051-5. [DOI] [PubMed] [Google Scholar]
- HOFFMAN P., LINKER A., MEYER K. The acid mucopolysaccharides of connective tissue. III. the sulfate linkage. Biochim Biophys Acta. 1958 Oct;30(1):184–185. doi: 10.1016/0006-3002(58)90256-7. [DOI] [PubMed] [Google Scholar]
- Hascall V. C., Sajdera S. W. Proteinpolysaccharide complex from bovine nasal cartilage. The function of glycoprotein in the formation of aggregates. J Biol Chem. 1969 May 10;244(9):2384–2396. [PubMed] [Google Scholar]
- Hoffman P., Mashburn T. A., Jr, Meyer K., Bray B. A. Proteinpolysaccharide of bovine cartilage. I. Extraction and electrophoretic studies. J Biol Chem. 1967 Sep 10;242(17):3799–3804. [PubMed] [Google Scholar]
- Holthausen H. S., Chacko S., Davidson E. A., Holtzer H. Effect of 5-bromodeoxyuridine on expression of cultured chondrocytes grown in vitro. Proc Natl Acad Sci U S A. 1969 Jul;63(3):864–870. doi: 10.1073/pnas.63.3.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzer H., Abbott J., Lash J., Holtzer S. THE LOSS OF PHENOTYPIC TRAITS BY DIFFERENTIATED CELLS IN VITRO, I. DEDIFFERENTIATION OF CARTILAGE CELLS. Proc Natl Acad Sci U S A. 1960 Dec;46(12):1533–1542. doi: 10.1073/pnas.46.12.1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAPLAN D., MEYER K. Ageing of human cartilage. Nature. 1959 May 2;183(4670):1267–1268. doi: 10.1038/1831267a0. [DOI] [PubMed] [Google Scholar]
- Kraemer P. M. Production of heparin related glycosaminoglycans by an extablished mammalian cell line. J Cell Physiol. 1968 Apr;71(2):109–120. doi: 10.1002/jcp.1040710202. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MALAWISTA I., SCHUBERT M. Chondromucoprotein: new extraction method and alkaline degradation. J Biol Chem. 1958 Jan;230(1):535–544. [PubMed] [Google Scholar]
- MATHEWS M. B. Isomeric chondroitin sulphates. Nature. 1958 Feb 8;181(4606):421–422. doi: 10.1038/181421a0. [DOI] [PubMed] [Google Scholar]
- MEYER K., HOFFMAN P., LINKER A. Mucopolysaccharides of costal cartilage. Science. 1958 Oct 17;128(3329):896–896. doi: 10.1126/science.128.3329.896. [DOI] [PubMed] [Google Scholar]
- MEYER K., LINKER A., DAVIDSON E. A., WEISSMANN B. The mucopolysaccharides of bovine cornea. J Biol Chem. 1953 Dec;205(2):611–616. [PubMed] [Google Scholar]
- Mathews M. B., Glagov S. Acid mucopolysaccharide patterns in aging human cartilage. J Clin Invest. 1966 Jul;45(7):1103–1111. doi: 10.1172/JCI105416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nameroff M., Holtzer H. The loss of phenotypic traits by differentiated cells. IV. Changes in polysaccharides produced by dividing chondrocytes. Dev Biol. 1967 Sep;16(3):250–281. doi: 10.1016/0012-1606(67)90026-7. [DOI] [PubMed] [Google Scholar]
- PROCKOP D. J., PETTENGILL O., HOLTZER H. INCORPORATION OF SULFATE AND THE SYNTHESIS OF COLLAGEN BY CULTURES OF EMBRYONIC CHONDROCYTES. Biochim Biophys Acta. 1964 Jul 7;83:189–196. doi: 10.1016/0926-6526(64)90034-5. [DOI] [PubMed] [Google Scholar]
- Pal S., Doganges P. T., Schubert M. The separation of new forms of the proteinpolysaccharides of bovine nasal cartilage. J Biol Chem. 1966 Sep 25;241(18):4261–4266. [PubMed] [Google Scholar]
- Pawelek J. M. Effects of thyroxine and low oxygen tension on chondrogenic expression in cell culture. Dev Biol. 1969 Jan;19(1):52–72. doi: 10.1016/0012-1606(69)90070-0. [DOI] [PubMed] [Google Scholar]
- Robinson H. C., Dorfman A. The sulfation of chondroitin sulfate in embryonic chick cartilage epiphyses. J Biol Chem. 1969 Jan 25;244(2):348–352. [PubMed] [Google Scholar]
- SEIFTER S., DAYTON S. The estimation of glycogen with the anthrone reagent. Arch Biochem. 1950 Jan;25(1):191–200. [PubMed] [Google Scholar]
- SHATTON J., SCHUBERT M. Isolation of a mucoprotein from cartilage. J Biol Chem. 1954 Dec;211(2):565–573. [PubMed] [Google Scholar]
- Sajdera S. W., Hascall V. C. Proteinpolysaccharide complex from bovine nasal cartilage. A comparison of low and high shear extraction procedures. J Biol Chem. 1969 Jan 10;244(1):77–87. [PubMed] [Google Scholar]
- Shulman H. J., Meyer K. Cellular differentiation and the aging process in cartilaginous tissues. Mucopolysaccharide synthesis in cell cultures of chondrocytes. J Exp Med. 1968 Dec 1;128(6):1353–1362. doi: 10.1084/jem.128.6.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steven F. S. The effect of chelating agents on collagen interfibrillar matrix interactions in connective tissue. Biochim Biophys Acta. 1967 Aug 15;140(3):522–528. doi: 10.1016/0005-2795(67)90526-0. [DOI] [PubMed] [Google Scholar]


