Abstract
Purpose
Soft tissue sarcomas (STS) are diagnosed in 4–6 cases per 100 000 people a year and are associated with an unfavorable prognosis. Around one-third of patients will develop metastatic disease that requires palliative systemic therapy. Current therapeutic options have limited activity, and new treatments are tested, mainly in phase II trials. There is high variability and no standardization of phase II designs. We aimed to analyze the current landscape of phase II studies in STS and evaluate how its statistical design can affect the results.
Methods
Full-text phase II studies published in STS patients between 2005 and 2020 were identified and analyzed.
Results
We have identified 102 trials, of which 77.4% were single-arm trials, 16.7% were randomized comparative trials (RCT), and 5.9% were randomized noncomparative trials. Including multiple cohorts, 22 randomized and 128 single-arm cohorts were analyzed. Nearly 80% of trials reported full statistical bases of the design. Over 20 different primary endpoints were used, with PFS as the most common in RCT trials (81.8%) and ORR (36.7%) and 3-months progression-free survival (PFS) rate (21.9%) in single-arm trials. Overall, 27.3% of RCT and 37.5% of single-arm trials were positive. Among single-arm trials, studies using 3- or 6-month rates were more often positive than those based on ORR.
Conclusions
There is high heterogeneity in sarcoma trial designs, mainly in primary-endpoint and hypotheses used for size calculation. There is an unmet need for standardization that will incorporate factors associated with the rarity of the disease, outcomes detected in previous trials and real-life studies, and specific characteristics of new therapeutic agents.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00432-022-04149-0.
Keywords: Sarcoma, STS, Clinical trial, Phase II, Primary endpoint
Introduction
Soft tissue sarcomas (STS) are malignant tumors with a mesenchymal origin with an annual incidence of 4–6 cases per 100,000 people. According to the recent classification by World Health Organization, it is a highly heterogeneous group of malignancies including around 100 different subtypes (The WHO Classification of Tumours Editorial Board 2020). General treatment strategies are similar for all subtypes and, in the case of the localized disease, include surgical resection with or without neoadjuvant or adjuvant radiotherapy and/or chemotherapy (Network et al. 2021; Gronchi et al. 2021). Despite optimal surgery, around one-third of patients will recur, either in the form of local relapse or distant spread. The prognosis is advanced/metastatic disease is inferior, with estimated median overall survival (OS) of around 18–24 months, depending on the histological subtype and response to doxorubicin-based chemotherapy, which is the standard of care in the first line (Gronchi et al. 2021; Sobczuk et al. 2020).
There is a high and unmet need for new therapeutic options for patients with STS. With more investment in preclinical and clinical research, a portfolio of potential therapeutic agents is constantly growing, resulting in an increasing number of clinical trials (Gresham et al. 2020). New drugs are introduced, including immunotherapy, monoclonal antibodies or tyrosine kinase inhibitors that have become a standard of care in many cancer types. A positive trend in a growing number of trials is also visible in the field of sarcoma; however, they did not result in significant improvement in treatment outcomes. In the past ten years, only four new therapies have been approved by the Food and Drug Administration (FDA) to treat patients with soft tissue sarcoma—pazopanib, eribulin, tazemetostat, and nab-sirolimus.
Multiple reasons can explain the unsatisfactory results of most trials in patients with sarcoma. First, the pathogenesis of this class of tumors is poorly understood, with little information about driving molecular events causing the development of sarcoma. Second, the high heterogeneity of sarcoma subtypes affects the natural course of the disease and response to different agents, which can impact clinical trial results, especially when they are not histo-type specific. Third, the rarity of the disease and a low number of patients eligible for clinical trials may cause the enrolment of some studies to be prolonged or not completed. At the same time, some potential drugs might not even be tested due to difficulties in recruiting the necessary number of patients (Augustine et al. 2013). And last but not least, the design of clinical trials has a significant impact on the progress in treating patients with STS.
Optimal design, which considers the number of available patients, limited resources, and poor outcomes with conventional therapy, is crucial in rare diseases, especially in phase II trials that aim to select potentially effective agents for further testing in phase III studies. Inappropriately designed phase 2 trials carry risks that effective therapy will be discarded if a trial lacks statistical power or ineffective treatment will be considered effective due to incorrect selection of endpoints or not rigorous statistical design (Mick et al. 2000; Rubinstein et al. 2005). It is worth underlining that many drugs, mainly in rare and ultrarare diseases, have been approved based on phase II trials without confirmatory studies; thus appropriate methodological approach is essential at this stage (Gaddipati et al. 2012). Most commonly, phase II trials are single-arm, nonrandomized open-label trials, but new strategies like two-stage, adaptive or Bayesian design are becoming more common to foster progress and reduce the pitfalls of traditional studies. The selection of primary outcomes is another major issue with high variability between all registered trials. From patients' perspectives, overall survival and quality of life are the most optimal endpoints since these factors directly impact their life and well-being. However, most phase II clinical trials use surrogate endpoints that should correlate with overall survival but are easier to observe during the study. The most common surrogate endpoints include objective response rate (ORR), progression-free survival (PFS), and PFS rates at specified time points. There is no uniform use of primary endpoints in clinical trials in sarcoma, even though Van Glabbeke (Glabbeke et al. 2002) and Soft Tissue and Bone Sarcoma Group of the European Organization for Research and Treatment of Cancer (EORTC STBSG) have suggested a 3- or 6-month PFS rates as optimal endpoints for such studies. For first-line therapy, a 6-month PFR of ≥ 30–56% (depending on histology) and ≥ 2-line treatment, a 3-month PFR of ≥ 40% were suggested as reference values to suggest drug activity and further studies.
This study aimed to analyze the current landscape of published phase II trials conducted in patients with soft tissue sarcoma. The particular interest was put on statistical consideration, selecting primary endpoints, and reporting the results. Moreover, we aimed to analyze if different designs and selection of endpoints based on EORTC STBSG recommendations would affect the overall outcome of the studies.
Methods
Studies selection
The primary investigator (PS) performed a literature search on 21 July 2021 using the PubMed and Scopus databases. Terms that were used for the search included the following: “sarcoma,” “sarcomas,” “clinical trial,” and “phase II.” All primarily selected studies were screened independently for inclusion and exclusion criteria by at least two investigators (HB, PW, KI). In terms of discordance, the primary investigator made the final decision. Moreover, references or selected articles and conference materials were screened to identify eligible studies.
Inclusion criteria included: articles in English published between 2005 and 2020, trials with systemic therapy (chemotherapy, targeted therapy, immunotherapy, cell therapy) performed in patients with soft tissue sarcoma, phase II or phase I/II trials with separate results for phase II portion. Exclusion criteria included studies enrolling patients with bone sarcoma, chondrosarcoma, Ewing sarcoma, rhabdomyosarcoma, Kaposi sarcoma, gastrointestinal stromal tumours (GIST), combinations with radiotherapy or surgery, studies in neoadjuvant or adjuvant settings, noninferiority trials, hyperthermia trials, the clinical trial protocol only publications, conference abstract only, exploratory or translational analyses. For multicohort studies with a separate design for each cohort, inclusion and exclusion criteria were applied to each cohort separately. For randomized noncomparative trials, each arm that used experimental regimens was considered a single-arm trial for easy reporting. Control arms, which used standard of care regimens and were introduced to the trial only to verify historical data or statistical assumptions, were excluded.
Data analysis and statistical methods
The full-text publications were used to extract all data. Information about trial design and reported outcomes were extracted from each publication. This included details of the study population (sarcoma subtypes eligible, line of therapy, number of enrolled patients), information about the design of the trial (primary and secondary endpoints, null and alternative hypotheses, statistical power—1-β, significance—α, calculated number of required patients and details of two-stage design, if applicable). Specific data regarding outcomes were collected, if available in the publication, including results for primary endpoint, median overall survival (OS), median progression-free survival (PFS), objective response rate (ORR), and disease control rate (DCR) according to RECIST 1.1 or other criteria (e.g., Choi), 3- and 6-month PFS rates, 12- and 24- month OS rates. Only literally reported rates and survivals were included in the database.
Descriptive statistics were used to summarize data about trial designs and reported outcomes. Due to the high variability of used endpoints, the following rules were adopted for transparent reporting and analyses. PFS rates and DCR rates at specific time points were considered equivalent; PFS and TTP were considered equivalent. Different time point reporting was used in analyzed trials (measures in weeks or months); thus, trials were combined into the same groups based on the following assumptions: 8 weeks was considered equivalent to 2 months, 12 weeks to 3 months, 16–18 weeks to 4 months and 24–26 weeks equivalent to 6 months.
To evaluate how different statistical considerations would affect the trial’s overall conclusion (positive or negative), we used 3- or 6-month PFS rates as a recommended endpoint for phase II trials by EORTC STBSG and applied them as hypothetical primary endpoints (Glabbeke et al. 2002). For first-line treatment, the threshold for a 6-month PFS rate of 40% (the original benchmark of 30–56% depending on histology was simplified to 40%) was considered as a reference value to suggest drug activity, < 20% would indicate no activity, while for ≥ 2-line 3-month PFS rate of 40% and 20% were used for suggesting activity and lack of activity, respectively. Since the EORTC suggestions are mainly applicable to single-arm trials, we used only experimental arm results for modeling for analyses of randomized comparative trials. Calculations were performed only for trials that directly reported necessary outcomes and for which the current group size would warrant 1-β and α of at least 80% and 10%, respectively; this is a minimum of 29 patients. For trials including patients in all lines of therapy (≥ 1), we applied two models: (1) with the same rules as for 1st line treatment only or (2) as for ≥ 2 lines. Moreover, trials that reported PFS rates > 40%, but were conducted in a population smaller than 29 patients, were included if a post-hoc statistical power was ≥ 80%, with p ≤ 10%.
Results
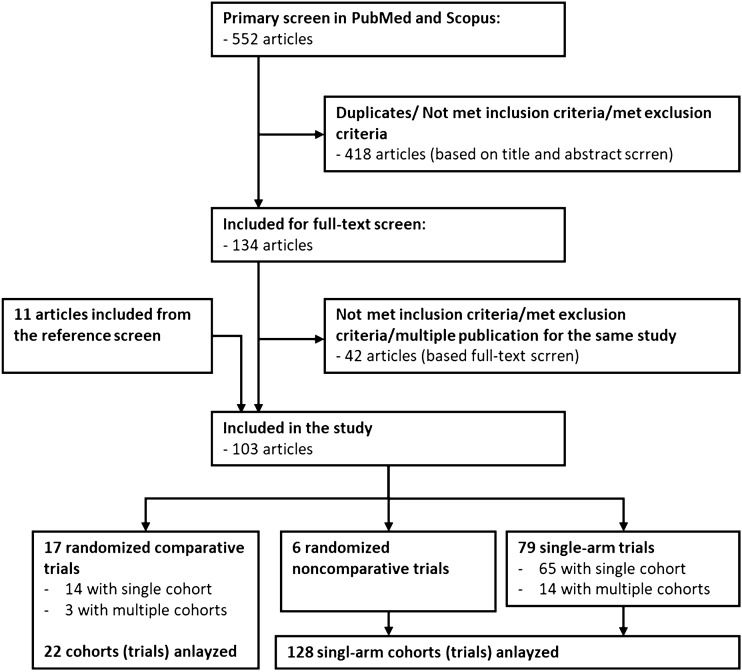
The primary screen has identified 552 records (Fig. 1). After screening in titles and abstracts, 133 papers met eligibility criteria, but 42 were excluded due to exclusion criteria detected in full-text publication or duplication (multiple publications for the same study). Additional 11 studies have been identified through the reference screen. Finally, we have included 102 phase II trials in STS published between January 2015 and December 2020 (Appendix 1). Majority, 77.4% (79) were single-arm trials, 16.7% (17) consisted randomized comparative trials and 5.9% (6) were randomized noncomparative trials.
Fig. 1.
A work flow of studies selection
Randomized comparative trials
We have 17 randomized comparative trials, three of them have included separate cohorts for different sarcoma subtypes with randomization within the cohort and statistical assumptions made for each. Therefore, each cohort was analyzed as separate trials, resulting in 22 randomized comparative trials in total.
Trials design
The majority of trials were conducted as open-label—59.1% (13), while the remaining rials were double-blinded. Only 27.3% (6) trials were performed in a specific single subtype of sarcoma, 18.2% (4) trials included selected subtypes, and 54.5% (12) trials included a wide population of different advanced STS. 27.3% (6) of trials were focused only on 1st line therapy and used doxorubicin in the control arm. Next, 18.2% (4) included patients treated in ≥ 1 line—3 trials used drugs or combinations that showed previous activity in sarcoma (doxorubicin, gemcitabine, gemcitabine + docetaxel), while 1 used investigational drug with no proof of activity in STS. 54.5% (12) were conducted in ≥ 2 line, with placebo or best supportive care in the comparative arm in 8 or approved agents (gemcitabine, trabectedin, dacarbazine) in 4.
PFS was the most common primary endpoint in 81.8% (18), followed by ORR (9.2%, 2, trials designed based on single-arm trial bases), 3-month PFS rate (4.5%, 1, one-sample binominal design), and 6-month PFS rate (4.5%, 1, Bayesian design). Details of statistical bases and sample size calculations were published for all trials except one that set PFS difference as a primary endpoint. For PFS-based trials, planned HR for the primary endpoint varied between 0.33 and 0.67, with a median of 0.5. The statistical power (1-β) was between 80 and 95%, and α 2.5% and 20% (median 5%).
The median trial size was 86 (range 27–270). The most common was 1:1 randomization in 18 trials and 2:1 in 4. Among studies with available sample size calculations (22), 77.3% (17) enrolled a prespecified number of patients; and 13.6% (3) closed prematurely at futility analyses. At the same time, 9.1% (2) recruited < 90% of planned patients and did not explain this protocol deviation.
Trials outcomes
Overall, 27.3% (6) of trials met the primary endpoint, including one trial in 1st line setting and 4 in ≥ 2 line. Five positive studies were based on PFS/TTP and one on 3-month PFS. All studies reported data on median PFS; 77.3% (17) reported HR calculation for PFS—5 significant improvements with tested compounds. Seventeen studies reported OS and HR for OS, with only three studies showing significant improvement in OS. All studies reported ORR ranging from 0 to 25% (aldoxorubicin).
Modeling of trial outcomes with an alternative primary endpoint
Model 1: 12 trials provided sufficient information about 3- or 6-month PFS rates, and 8 of them had a sample size adequate to apply our model. Six trials would meet the primary endpoint, including one in 1st line and five in ≥ 2 line. 4 of 6 trials (66.6%) that met their primary endpoint would also meet our hypothetical calculations, while two were not assessable due to sample size difference. Additionally, two primarily negative trials would be positive in this setting (one with PFS and one with ORR as the primary endpoint). Model 2 yield the same results.
Single-arm and randomized noncomparative trials
We have identified 79 single-arm trials and five randomized noncomparative trials. Nine single-arm studies were created from noncomparative randomized trials. Since multiple single-arm trials were conducted with various cohorts with specific statistical assumptions, each cohort was considered a single study. Overall, 128 single-arm studies/cohorts were analyzed.
Trials design
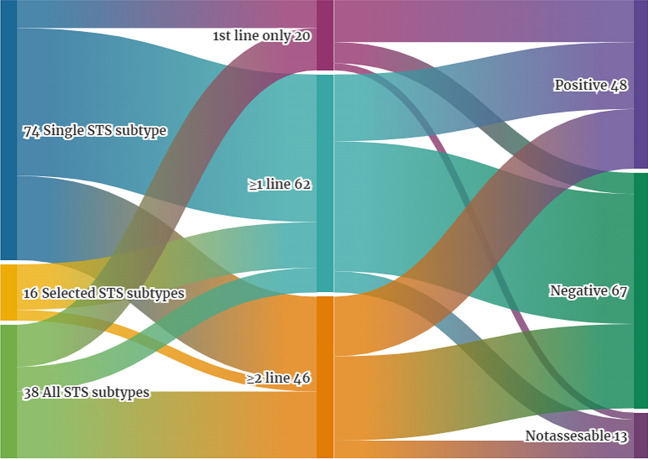
All of the trials were open-label. The majority of studies were performed in a specified single subtype of sarcoma—57.8% (74), 12.5% (16) trials included selected subtypes, and 29.7% (38) were conducted in a wide population of different advanced STS (Fig. 2). Studies in single subtype were mainly dedicated to patients with leiomyosarcoma or liposarcoma, 37.8% (28, including 11 trials in uterine LMS) and 14.8% of studies, respectively (Table 1).
Fig. 2.
Characteristic of single-arm and nonrandomized clinical trials including eligible subtypes of sarcoma, line of treatment and overall result of the trial. (Created in https://flourish.studio/)
Table 1.
Number of single-arm and nonrandomized clinical trials conducted in patients with selected sarcoma subtypes depending on histotype
| Sarcoma type (n = 74) | n | % |
|---|---|---|
| Leiomyosarcoma | 28 | 37.8% |
| Uterine leiomyosarcoma | 11 | 14.9% |
| Liposarcoma | 11 | 14.8% |
| Angiosarcoma | 6 | 8.1% |
| Alveolar soft part sarcoma | 6 | 8.1% |
| Synovial sarcoma | 6 | 8.1% |
| Undifferentiated pleomorphic sarcoma | 6 | 8.1% |
| Malignant peripheral nerve sheath tumor | 4 | 5.4% |
| Solitary fibrous tumor | 3 | 4.1% |
| Clear-cell sarcoma | 2 | 2.7% |
| Epithelioid sarcoma | 1 | 1.4% |
| Fibrosarcoma | 1 | 1.4% |
Only 15.6% (20) of trials included treatment-naïve patients solely in 1st line therapy, while the majority allowed participation of patients in any line – 48.4% (62). 36% (46) trials included patients in ≥ 2 line (Fig. 2). Out of 20 trials conducted in 1st line, three were evaluating new anthracycline-based compounds (13‐Deoxy, 5‐iminodoxorubicin; non-pegylated liposomal doxorubicin; amrubicin), two evaluated drugs that were not previously tested or did not show activity in pretreated patients with sarcoma (brostallicin, temsirolimus), while most (15) were conducted with drugs active in 2nd or other lines, or included backbone of active agents that can be used in treatment-naïve patients. List of all tested drugs is provided in Table 2.
Table 2.
List of compounds used in Phase II trials (randomized and single-arm trials), divided by main classes of drugs
| Chemotherapy | Targeted agents | Immunotherapy | Cellular therapy | |
|---|---|---|---|---|
| Tyrosine kinase inhibitors (TKIs) | Other | |||
|
13‐Deoxy, 5‐iminodoxorubicin (GPX‐150) Aldoxorubicin Amrubicin Bendamustin Brostallicin Dacarbazine Docetaxel Doxorubicin Eribulin Evofosfamide Exatecan Gemcatabine Ifosfamide Ixabepilone Liposomal doxorubicin Lurbinectedin Non-pegylated liposomal doxorubicin Paclitaxel Pemetrexed Plitidepsin Soblidotin Thalidomide Trabectedin Trofosfamide |
Alisertib Anlotynib Axitinib Brivanib Cediranib Crizotinib Dasatinib ENMD-2076 Gefitinib Pazopanib Regorafenib Sorafenib Sunitinib Tivozanib |
Aflibercept Bevacizumab Conatumumab Ganetespib Letrozole Olaratumab Ontuxizumab Palbociclib Panobinbostat Perifosine Ridaforolimus Selumetinib Sirolimus Tazemetostant Temsirolimus Thrombospondin-1-Mimetic Angiogenesis Inhibitor ABT-510 Trebananib Vorinostat |
Nivolumab Pembrolizumab Talimogene laherparepvec (T-VEC) |
NY-ESO-1c259T Cells |
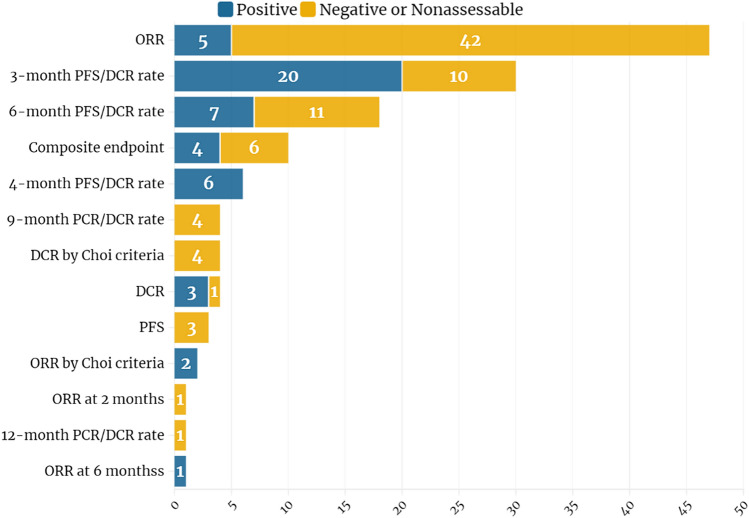
Over 20 different primary endpoints were identified among the analyzed trials. After grouping trials according to the assumptions described in the methods, ORR was the most commonly used primary endpoint in 36.7% (47), followed by 3-month and 6-month PFS rates, 21.9% (28) and 13.3% (17) of trials, respectively (Fig. 3). Composite endpoints consisting of 2 or 3 single endpoints were used in 7.8% (10) studies.
Fig. 3.
Endpoints used in single-arm and nonrandomized clinical trials and their correlation with trial results. (Created in https://flourish.studio/)
Full details of statistical bases and sample size calculation (including statistical power, level of significance, null and alternative hypotheses, size calculation, and rules for 2-stage design) were published for 78.1% (100) trials, 14.8% (19) had single information missing, while 7.0% (9) had no information about statistical design. The majority, 64.1% (82), of trials used a double-stage design, 3.1% (4) used a Bayesian method, while the remaining were classic single-stage phase II trials. Further details of the statistical design are presented for non-Bayesian trials (124), separately for 2-stage (82) and single-stage design (42) in Table 3. 87.2% (102/1117) of trials recruited a calculated number of patients, 6.8% (8/121) recruited < 90% of planned patients.
Table 3.
Details of statistical design for single-arm and nonrandomized clinical trials stratified by study type (single- or two-stage design) primary endpoint
| Single-stage design (n = 31*) | Two-stage design (n = 65)** | |||
|---|---|---|---|---|
| Median | Range | Median | Range | |
| ORR as primary endpoint | n = 6 | n = 25 (4 in 1st line, 1 in 2nd line, 19 in ≥ 1 line, 1 in ≥ 2 line) | ||
| H0 | 5% | 5–10% | 5% | 5–20% |
| H1 | 17.5% | 15%-25% | 25% | 17–40% |
| 1-β | 85% | 81–91% | 90% | 85–91% |
| α | 9.5% | 4–10% | 10% | 3–10% |
| Calculated trial size | 12 | 12–50 |
Stage 1: 12 Stage2: 36 |
Stage 1: 9–25 Stage2: 24–51 |
| Progress to stage 2 | 9/25 | |||
| Actual trial size | 17 | 14–42 |
Stage 1 only: 12 Stage 1 + 2: 37 |
Stage 1 only: 8–23 Stage 1 + 2: 29–43 |
| 3-month PFS/DCR rate as primary endpoint | n = 8 (3 ≥ 1 line, 5 in ≥ 2 line) | n = 18 (9 in ≥ 1 line, 9 in ≥ 2 line) | ||
| H0 | 20% | 19–40% | 20% | 20% |
| H1 | 40% | 40%-60% | 40% | 40% |
| 1-β | 85% | 80–94% | 90% | 80–90% |
| α | 6% | 5–15% | 10% | 5–10% |
| Calculated trial size | 35 | 25–50 |
Stage 1: 17 Stage2: 37 |
Stage 1: 13–17 Stage2: 23–43 |
| Progress to stage 2 | 7/18 | |||
| Actual trial size | 38.5 | 27–60 |
Stage 1 only: 19 Stage 1 + 2: 40 |
Stage 1 only: 7–27 Stage 1 + 2: 32–47 |
| 6-month PFS/DCR rate as primary endpoint | n = 8 (3 in 1st line, 4 in ≥ 1 line, 1 in ≥ 2 line) | n = 6 (3 in 1st line, 3 in ≥ 2 line) | ||
| H0 | 25% | 14–40% | 20.5% | 10–25% |
| H1 | 40% | 25%-60% | 40% | 30–50% |
| 1-β | 90% | 80–90% | 88% | 80–91% |
| α | 10% | 5–10% | 10% | 5–12% |
| Calculated trial size | 30 | 21–109 |
Stage 1: 12 Stage2: 22.5 |
Stage 1: 10–43 Stage2: 20–71 |
| Progress to stage 2 | 3/6 | |||
| Actual trial size | 34.5 | 19–126 |
Stage 1 only: 12 Stage 1 + 2: 25 |
Stage 1 only: 10–29 Stage 1 + 2: 20–78 |
*11 trials not provided complete statistical assumptions; **17 trials not provided full statistical assumptions
Trials outcomes
Overall, 37.5% (48) trials met the primary endpoint, while in 10.2% (13) available data did not allow appropriate assessment due to missing hypotheses and statistical assumptions. Among positive trials, 41.7% (20) were based on 3-month PFS as a primary endpoint, 10.4% (5) on ORR, and on 6-month PFS/DCR or 4-month PFS/DCR rate endpoints 14.6% (7), and 12.5% (6), respectively. From another perspective, only 10.6% of trials designed with ORR as the primary endpoint met the prespecified hypothesis. For trials based on 3-, 4- or 6-month PFS/DCR rates, the positive outcomes were observed in 66.7%, 100%, and 38.9%, respectively. Moreover, 40% of trials with composite endpoints were positive; all of them included PFS/DCR rates as one of the endpoints and did not include ORR.
Analyzing two-stage design trials, 46.3% (38/82) have progressed to the second stage, of which 55.3% (21) had positive results showing activity of tested drugs. Overall, 25.6% (21/38) of two-stage trials met the primary endpoint compared to 40.5% (17/42) of single-stage trials.
76.7% (99) of trials reported median OS data, ranging from 4.9 to 49.8, with a median of 14.1 months. 89.1% (114) trials published median PFS data, ranging from 1.3 to 21.0 with a median of 3.4 months. Median PFS in trials conducted in the 1st line was 5.8 months, in the 2nd line 4.8 months, in patients in any line 3.2 months, and in ≥ 2 line 3.2 months. 95.3% (122) of trials reported ORR, ranging from 0 to 59.6%, with a median of 4.9%. Median reported ORR for 1st and 2nd line trials were 17.1% and 23.8%, while for ≥ 1 and ≥ 2 line trials 2.5 and 3.2%, respectively. 70.3% (90) of studies reported DCR, ranging from 0 to 100%, with a median of 53.1%. Median reported DCR for 1st and 2nd line trials were 64.5% and 65%, while for ≥ 1 and ≥ 2 line trials, 50.7% and 48%, respectively. 3-month PFS rates were reported in 53.9% (69) of trials, 61.7% (79) reported 6-month PFS rates. Only 19.5% (25) of trials reported 1-year OS data and 15.6% (20) 2-year OS rates.
Modeling of trial outcomes with the alternative primary endpoint
We applied our hypothetical models as described above to evaluate how different statistical considerations would affect the results.
Model 1: 64.1% (82) of trials provided sufficient information about 3- or 6-month PFS rates, and 46.9% (60) had a sample size adequate to apply our model. After applying our model, only 36.7% (22/60) of studies used endpoints recommended by EORTC. Thirty-five trials have met the original primary endpoint (58.3%), and 28 of them would also meet our hypothetical endpoint. Notably, 7 of primarily 18 negative trials would meet the endpoint if set as a 3- or 6-month PFS rate. 6/7 were designed with ORR as a primary endpoint. Seven primarily positive trials were negative under this model.
Model 2: 56.3% (72) of trials provided sufficient information about 3- or 6-month PFS rates, and 48.4% (62) had a sample size adequate to apply our model. After applying our model, only 48.4% (30/62) of studies used appropriate endpoints according to EORTC. Thirty-four trials (54.8%) have met the original primary endpoint, and all of them would also meet our hypothetical endpoint (Table 4). Moreover, 14 of 22 primarily negative trials would meet the endpoint if set as a 3- or 6-month PFS rate. 13/14 were designed with ORR as a primary endpoint, one with 9-month PFS/DCR rate.
Table 4.
Impact of application of alternative primary endpoint for the outcome of single-arm and nonrandomized clinical trials in relation to original endpoint
| Results (n) | Model endpoint 1* | Model endpoint 2** | |||
|---|---|---|---|---|---|
| Negative | Positive | Negative | Positive | ||
| Original endpoint | Negative | 11 | 7 | 8 | 14 |
| positive | 7 | 28 | 0 | 34 | |
| nonassesable | 2 | 5 | 0 | 6 | |
*6-month PFS rate for 1st line only and ≥ 1 line trials; 3-month PFS rate for ≥ 2 line trials; H0 20%, H1 40%
**6-month PFS rate for 1st line only trials; 3-month PFS rate for ≥ 1 and ≥ 2 line trials; H0 20%, H1 40%
Discussion
Despite the rarity of the disease, the landscape of clinical trials in sarcoma patients is broad and diverse. It includes a few dedicated phase I trials, dozens of various Phase II trials, and some randomized phase III trials. We have analyzed published phase II trials in patients with STS conducted after introducing RECIST 1.1 criteria that unified assessment of the tumor response. To our knowledge, this is the most extensive analysis performed on phase II trials, which includes a detailed evaluation of statistical design and primary endpoint selection. Most of the previous investigations have focused on randomized trials and, to a lesser extent, on single-arm, consisting of most phase II trials, as shown in our study.
Penel et al. (Que et al. 2018) performed previous analyses and reported data on 53 phase II trials published from 1999 to 2011. The proportion of randomized trials has increased from 5% in their study to 17.5% in current analyses. This is also confirmed by analyses of all registered trials in sarcoma, which showed that the number of randomized and double-blind trials has significantly increased when comparing periods before and after 2008 (Que et al. 2018).
Correct reporting of the study design remains a problem. Around 20% of single arm trials failed to fully report all necessary data to evaluate the statistical hypotheses and trial size calculation fully. A previous French study reported a similar rate of missing information. Also, the quality of reporting the results has increased with time. Considering randomized trials in sarcoma published between 1988 and 2008, 54% of them improperly reported outcome measures (Toulmonde et al. 2011), while in a study by Penel et al. (2013) analyzing phase II trials, this rate was 40%. In our analyses only 10% of trials were not interpretable.
Appropriate design is crucial in conducting phase II trials aimed at identifying drugs with sufficient signals of activity in a specified population to warrant further testing in larger, preferably randomized, phase III trials. The selection of an appropriate primary endpoint can have a crucial role. If the study is designed on a nonmeaningful endpoint may lead to false-positive results suggesting drug activity and endanger patients with treatment with the inferior drug. On the other side, too rigorous endpoint and statistical assumptions may lead to falsely negative results, stopping further assessment of potentially active therapy. Patients with rare tumors are especially vulnerable in terms of trial design due to the lower interest of the pharmaceutical industry and inadequate funding for research and clinical trials. Most of the drugs are tested only in one phase II trial, which result can have bidirectional output on further attempts with tested drug– stop or go. Thus, phase to II trials should be based on a verified primary endpoint with an adequate null hypothesis (threshold suggesting lack of activity) and alternative hypothesis (prespecified activity level). The success or failure of the trial depends on those decisions.
As for phase III trials, the consensus is relatively straightforward and favors definitive endpoints, such as overall survival, over surrogate endpoints, which in many cases are not validated. The situation is less clear for phase II trials, especially single-arm, which rely on external historical data used to generate the null hypothesis. Overall survival is rarely used in these settings due to the longer time of follow-up required to achieve the required number of events that stay against the desire to obtain signals about activity, or lack of it, and move the drug to phase III trial. Moreover, OS can be affected by other factors like previous treatment, cross-over, subsequent therapies, and the impact of comorbidities. It is highly dependent on the distribution of prognostic factors in the enrolled cohort and may be considered with caution outside the setting of randomized trials (Korn et al. 2008). Due to their low size, phase II trials are hard to perform with full control of prognostic factors, especially in heterogeneous diseases like sarcoma. Among analyzed studies, only surrogate endpoints were used. In randomized comparative trials, PFS was the most common endpoint. The expected difference between the experimental and control arm (hazard ratio) should be at least 0.65, according to the European Society for Medical Oncology Magnitude of Clinical Benefit Scale (Cherny et al. 2017), and almost all analyzed trials have met this criterium. It is worth mentioning that the correlation between PFS and OS in patients with sarcoma is not established. A recent meta-analysis of individual patients data from 14 trials in sarcoma shows a moderate correlation of PFS rates as surrogate endpoints for OS (Savina et al. 2018). However, the PFS seems to be the most suitable endpoint for randomized phase II trials in STS.
Higher variability was observed for primary endpoints in single-arm studies, where over 20 different endpoints were identified, with ORR as the most common. A similar proportion of over one-third of trials being based on ORR was reported previously by Penel et al. (2013), but compared to his analyses, the number of trials using 3- or 6-month PFS/DCR rates, as recommended by EORT STBSG, has increased.
ORR was a common endpoint in times of cytotoxic therapies, allowing rapid assessment of drug activity, objectified as tumor shrinkage. However, even with most active doxorubicin-based regimens, ORR rates are very low in patients with sarcoma. Moreover, ORR assessment highly depends on the measurement of lesions on radiographic images, which can be challenging in non–well-defined lesions, such as peritoneal metastases or lesions found in the gastrointestinal tract. In addition, tumor tissue may be replaced by necrotic or fibrotic tissue, and a substantial reduction in viable tumor cell volume may not result in a marked decrease in overall tumor volume (Verweij 2008). With new classes of agents (targeted therapies or immunotherapy) acting through a different mechanism than chemotherapy, objective responses might be less frequently observed. However, clinical benefit, disease stabilization, or improved survival can still be evident. Moreover, ORR thus does not necessarily correlate with overall survival, as shown in sarcoma trials (Penel et al. 2013). Additionally, analysis of positive phase II and proceeding phase II trials showed a trend for phase II trials with PFS or TTP as endpoints to predict positive phase III trials compared with phase II trials based on ORR (Chan et al. 2008).
Considering limitations of traditional RECIST 1.1 criteria for response assessment, alternative methods have been proposed, such as Choi criteria (Choi et al. 2007) for the evaluation of the activity of targeted therapies or immune RECIST (irRECIST) (Seymour et al. 2017) for clinical trials with immunotherapy. These alternatives should be considered in designing clinical trials; however, we have identified only 3 studies (6 cohorts) that used Choi criteria in our cohort.
As mentioned before, 3- and 6-month PFS rates are more commonly used surrogate endpoints. Some studies have shown their correlation with OS (Penel et al. 2013), while other did not. Analyses of randomized trials have failed to show significant surrogacy for 3-month and 6-month PFS rates (Zer et al. 2016; Tanaka et al. 2019) and do not suggest using this endpoint for phase III trials. However, it seems appropriate for phase II single-arm trials that do not have control arms allowing comparison of PFS as continuous variables. This is partially confirmed by our analyses showing that most positive trials used PFS rates as primary endpoints. Due to the high heterogeneity of trials, we have not performed a formal correlation of PFS rates with OS, one of the study limitations.
Surrogate endpoints remain helpful in testing new treatments in earlier drug development stages, such as phase II trials (Redman et al. 2013; LeBlanc and Tangen 2016); however, there is a high need for more unified and consistent use of the most valuable surrogates that have the highest correlation with overall survival in patients with sarcoma, considering different subtypes as well as a line of therapy and previous treatment. EORTC continues its efforts to set benchmarks for PFS rates as suggested endpoints, updating those published previously in 2002 (Glabbeke et al. 2002). New thresholds for LMS patients have been established based on available trials published from 2003 to 2018 (Kantidakis et al. 2021). Under the alternative that the true benefit amounts to a hazard ratio of 0.65, a 6-month PFS rate ≥ 70% should be considered to suggest drug activity in 1st line, while a 6-month PFS rate ≥ 62% or 3-month PFS rate ≥ 44% would suggest drug activity for second and subsequent lines. Recommendations for other subtypes are awaited.
Primary endpoints and statistical hypotheses are used to draw conclusions about drug activity and warrant further testing efforts. We have found 37.5% of nonrandomized and 27.3% of randomized trials to meet the primary endpoint, suggesting drug activity. The numbers can be biased since positive trials are more frequently published in full-text than negative trials (Suñé et al. 2013). A different selection of endpoint or benchmarks can give contrary results, as shown in one of our models, where the rate of positive trials increased from 54 to 87% upon applying EORTC recommendations. Notably, most of those trials used ORR as a primary endpoint, underlining its limitation for trials in patients with sarcoma. Our models can only show the variability of conclusions that can be drawn from the same study, depending on the design but cannot indicate if the studied drug is effective or not in patients with sarcoma. With the lack of other trials with those drugs, the question remained unanswered until new studies with appropriate designs were conducted. Moreover, our models are limited only to studies that reported necessary data; this is approximately 50% of included trials; thus, selection bias can play an important role.
Sample size calculations consist of another step that impacts the feasibility of the study. It is based on null and alternative hypotheses and the possibility of false-positive (α) or false-negative (β) results that researchers can accept (Rubinstein 2014). In the analyzed set, most studies applied the thresholds of 10% for both α and β, but many studies used more strict bases with α of 5%. Some suggestions are recommending lowering the thresholds to 10% for α and 20% for β in trials in rare diseases that can foster recruitment with a smaller number of patients required. e.g., for 5% α and 10% β, a required number of patients is 50 to show a change of endpoint from 20 to 40%, while for 10% α and 20% β, the required number of patients is 29 (Rubinstein 2014). This comes with a cost of a higher risk of false-negative results and termination of the development of a useful agent.
Another typical example of how to tackle the recruitment of patients is a two-stage or adaptive design (Simon 1989; Gagne et al. 2014), where signals of drug activity must be detected in a smaller number of patients to continue recruitment to the estimated number of participants. Nearly two-thirds of studies in our set used such a design, and half of them progressed to second-stage. This reduced the number of required patients by around 20–25% compared to when all terminated studies would recruit total numbers and protect patients from receiving inactive therapy. Newer possibilities include Bayesian design (Dutton et al. 2018), which allows adaptive design, early discontinuation of ineffective treatments, or a factorial method that can reduce sample size by tackling multiple treatment options in a factorial study, in which two (or more) treatment comparisons are carried out simultaneously (Griggs et al. 2009).
Positive result of phase II trial, suggesting the drug activity, should be always verified in pivotal randomized phase III trials. For some drugs, like trabectedin or eribulin (Tap et al. 2016), phase III trials has confirmed the previous finding, while for other, such as aldoxorubicin (Chawla et al. 2017) or olaratumab (Tap et al. 2016), no improvement in survival was noted. As shown in Table 5, some drugs like regorfenib or palbociclib, that has shown activity in phase II, have not been tested in phase III yet. They have also not received marcething authorization based on phase II results. On the other side, tazemetostat has been approved based on the phase II trial, which has not met the primary endpoint (based on the statistical plan ORR of 20% would suggest activity, while ORR of 15% was reported) butndespote that, showed improvement comparing to historical data with other agents (Gounder et al. 2020). Confirmatory phase II trial is currently ongoing.
Table 5.
Results of selected phase III trials with copunds that showed activity in phase II trials
| Compounds active in Phase II trial | Pivotal phase III trial in STS population | Results of Phase III trial | Marketing authorization in STS |
|---|---|---|---|
| Regorafenib | No | NA | No |
| Trabectedin | NCT01343277 | Improvement in PFS, no improvement in OS | Yes |
| Aldoxorubicin | NCT02049905 | No improvement of OS | No |
| Eribulin | NCT01327885 | Improvement in OS | Yes |
| Gemcitabine + sirolimus | No | NA | No |
| Palbociclib | No | NA | No |
| Pazopanib | NCT00753688 | Improvement in PFS, no improvement in OS | Yes |
| Olaratumab | NCT02451943 | No improvement in OS | No |
NA not applicable, OS overall survival, PFS progression-free survival
Our analysis has several limitations. It is based only on published data, and many results not published in the full-text publication could be missed. Similarly, many studies included in the analyses did not provide all the necessary information; thus, some analyses, including our models, are performed on a much smaller number of trials. High variability between studies (different types of therapies, lines of treatment, sarcoma subtypes) limited the possibility to analyze data grouped in more homogenous groups.
Concluding, the landscape of phase II clinical trials is vast and diverse. There is high variability between studies in terms of trial type (randomized comparative, randomized noncomparative, single-arm), design (single-stage, double-stage, Bayesian design), statistical hypotheses, or selection of primary endpoints. The type of primary endpoint seems to be an essential factor affecting the trial results. There is an unmet need for standardization of the design of modern clinical trials in sarcoma that will incorporate factors associated with the rarity of the disease, outcomes detected in previous trials and real-life studies, and specific characteristics of new therapeutic agents.
Supplementary Information
Below is the link to the electronic supplementary material.
Author contribution
Main study conception was created by Pawel Sobczuk. All authors contributed to the study conception and design. Material preparation and data collection and analysis were performed by Hubert Batruk, Paulina Wojcik, Krzystzof Iwaniak and Pawel Sobczuk. Data analyses was performed by Pawel Sobczuk, Katarzyna Kozak and Piotr Rutkowski. The first draft of the manuscript was written by Pawel Sobczuk and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript. Work was supersized by Piotr Rutkowski.
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Conflict of interest
P.S. has received travel grants from MSD, Roche, Novartis, and Pierre Fabre and honoraria for lectures from Swixx BioPharma and BMS. Non-financial interests: European Society of Medical Oncology—Officer; Polish Society of Clinical Oncology—Member of Board of Directors. K.K. has received honoraria for lectures from BMS, MSD, Novartis, Pfizer, and Pierre Fabre. S.K. has received travel grants from Pierre Fabre. P.R. (Piotr Rutkowski): Personal financial interests: Blueprint Medicines, Advisory Board; BMS, invited speaker, honoraria for lectures, Advisory Board; Merck, Advisory Board; Merck, invited speaker, honoraria for lectures; MSD, Advisory Board; Novartis, invited speaker; Pierre Fabre, invited speaker, honoraria for lectures; Pierre Fabre, Advisory Board; Sanofi, Advisory Board, invited speaker. Institutional interests: BMS, funding, financial interest, research grant for institution; Pfizer, research grant, financial interest, research grant for ISS. Non-financial interests: ASCO, Officer; Polish Society of Surgical Oncology, Member of Board of Directors. H.B., P.W. and K.I. declare no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Augustine EF, Adams HR, Mink JW (2013) Clinical trials in rare disease: challenges and opportunities. J Child Neurol 28:1142–1150. 10.1177/0883073813495959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan JK, Ueda SM, Sugiyama VE, Stave CD, Shin JY, Monk BJ, Sikic BI, Osann K, Kapp DS (2008) Analysis of phase II studies on targeted agents and subsequent phase III trials: what are the predictors for success? J Clin Oncol 26:1511–1518. 10.1200/jco.2007.14.8874 [DOI] [PubMed] [Google Scholar]
- Chawla SP, Ganjoo KN, Schuetze S, Papai Z, Van Tine BA, Choy E, Liebner DA, Agulnik M, Chawla S, Wieland S et al (2017) Phase III study of aldoxorubicin vs investigators’ choice as treatment for relapsed/refractory soft tissue sarcomas. J Clin Oncol 35:11000–11000. 10.1200/JCO.2017.35.15_suppl.11000 [Google Scholar]
- Cherny NI, Dafni U, Bogaerts J, Latino NJ, Pentheroudakis G, Douillard JY, Tabernero J, Zielinski C, Piccart MJ, de Vries EGE (2017) ESMO-magnitude of clinical benefit scale version 1.1. Ann Oncol 28:2340–2366. 10.1093/annonc/mdx310 [DOI] [PubMed] [Google Scholar]
- Choi H, Charnsangavej C, Faria SC, Macapinlac HA, Burgess MA, Patel SR, Chen LL, Podoloff DA, Benjamin RS (2007) Correlation of computed tomography and positron emission tomography in patients with metastatic gastrointestinal stromal tumor treated at a single institution with imatinib mesylate: proposal of new computed tomography response criteria. J Clin Oncol 25:1753–1759. 10.1200/jco.2006.07.3049 [DOI] [PubMed] [Google Scholar]
- Dutton P, Love SB, Billingham L, Hassan AB (2018) Analysis of phase II methodologies for single-arm clinical trials with multiple endpoints in rare cancers: an example in Ewing’s sarcoma. Stat Methods Med Res 27:1451–1463. 10.1177/0962280216662070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaddipati H, Liu K, Pariser A, Pazdur R (2012) Rare cancer trial design: lessons from FDA approvals. Clin Cancer Res 18:5172–5178. 10.1158/1078-0432.Ccr-12-1135 [DOI] [PubMed] [Google Scholar]
- Gagne JJ, Thompson L, O’Keefe K, Kesselheim AS (2014) Innovative research methods for studying treatments for rare diseases: methodological review. BMJ 349:g6802. 10.1136/bmj.g6802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gounder M, Schöffski P, Jones RL, Agulnik M, Cote GM, Villalobos VM, Attia S, Chugh R, Chen TW-W, Jahan T et al (2020) Tazemetostat in advanced epithelioid sarcoma with loss of INI1/SMARCB1: an international, open-label, phase 2 basket study. Lancet Oncol 21:1423–1432. 10.1016/s1470-2045(20)30451-4 [DOI] [PubMed] [Google Scholar]
- Gresham G, Meinert JL, Gresham AG, Meinert CL (2020) Assessment of trends in the design, accrual, and completion of trials registered in ClinicalTrials.gov by Sponsor Type, 2000–2019. JAMA Netw Open 3:e2014682. 10.1001/jamanetworkopen.2020.14682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griggs RC, Batshaw M, Dunkle M, Gopal-Srivastava R, Kaye E, Krischer J, Nguyen T, Paulus K, Merkel PA (2009) Clinical research for rare disease: opportunities, challenges, and solutions. Mol Genet Metab 96:20–26. 10.1016/j.ymgme.2008.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronchi A, Miah AB, Dei Tos AP, Abecassis N, Bajpai J, Bauer S, Biagini R, Bielack S, Blay JY, Bolle S et al (2021) Soft tissue and visceral sarcomas: ESMO-EURACAN-GENTURIS Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 10.1016/j.annonc.2021.07.006 [DOI] [PubMed] [Google Scholar]
- Kantidakis G, Litière S, Neven A, Vinches M, Judson I, Schöffski P, Wardelmann E, Stacchiotti S, D’Ambrosio L, Marréaud S et al (2021) Efficacy thresholds for clinical trials with advanced or metastatic leiomyosarcoma patients: a European Organisation for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group meta-analysis based on a literature review for soft-tissue sarcomas. Eur J Cancer 154:253–268. 10.1016/j.ejca.2021.06.025 [DOI] [PubMed] [Google Scholar]
- Korn EL, Liu PY, Lee SJ, Chapman JA, Niedzwiecki D, Suman VJ, Moon J, Sondak VK, Atkins MB, Eisenhauer EA et al (2008) Meta-analysis of phase II cooperative group trials in metastatic stage IV melanoma to determine progression-free and overall survival benchmarks for future phase II trials. J Clin Oncol 26:527–534. 10.1200/jco.2007.12.7837 [DOI] [PubMed] [Google Scholar]
- LeBlanc M, Tangen C (2016) Surrogates for survival or other end points in oncology. JAMA Oncol 2:263–264. 10.1001/jamaoncol.2015.4711 [DOI] [PubMed] [Google Scholar]
- Mick R, Crowley JJ, Carroll RJ (2000) Phase II clinical trial design for noncytotoxic anticancer agents for which time to disease progression is the primary endpoint. Control Clin Trials 21:343–359. 10.1016/S0197-2456(00)00058-1 [DOI] [PubMed] [Google Scholar]
- National Comprehensive Cancer Network. Soft Tissue Sarcoma (Version 2.2021). Availabe online: https://www.nccn.org/professionals/physician_gls/pdf/sarcoma.pdf (accessed on August 30, 2021).
- Penel N, Cousin S, Duhamel A, Kramar A (2013) Activity endpoints reported in soft tissue sarcoma phase II trials: quality of reported endpoints and correlation with overall survival. Crit Rev Oncol Hematol 88:309–317. 10.1016/j.critrevonc.2013.05.004 [DOI] [PubMed] [Google Scholar]
- Que Y, Xiao W, Xu BS, Wen XZ, Weng DS, Zhang X (2018) The changing landscape of phase II/III metastatic sarcoma clinical trials-analysis of ClinicalTrials.gov. BMC Cancer 18:1251. 10.1186/s12885-018-5163-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redman MW, Goldman BH, LeBlanc M, Schott A, Baker LH (2013) Modeling the relationship between progression-free survival and overall survival: the phase II/III trial. Clin Cancer Res 19:2646–2656. 10.1158/1078-0432.Ccr-12-2939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinstein L (2014) Phase II design: history and evolution. Chin Clin Oncol 3:48. 10.3978/j.issn.2304-3865.2014.02.02 [DOI] [PubMed] [Google Scholar]
- Rubinstein LV, Korn EL, Freidlin B, Hunsberger S, Ivy SP, Smith MA (2005) Design issues of randomized phase II trials and a proposal for phase II screening trials. J Clin Oncol 23:7199–7206. 10.1200/jco.2005.01.149 [DOI] [PubMed] [Google Scholar]
- Savina M, Litiere S, Italiano A, Burzykowski T, Bonnetain F, Gourgou S, Rondeau V, Blay JY, Cousin S, Duffaud F et al (2018) Surrogate endpoints in advanced sarcoma trials: a meta-analysis. Oncotarget 9:34617–34627. 10.18632/oncotarget.26166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seymour L, Bogaerts J, Perrone A, Ford R, Schwartz LH, Mandrekar S, Lin NU, Litière S, Dancey J, Chen A et al (2017) iRECIST: guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol 18:e143–e152. 10.1016/s1470-2045(17)30074-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon R (1989) Optimal two-stage designs for phase II clinical trials. Control Clin Trials 10:1–10. 10.1016/0197-2456(89)90015-9 [DOI] [PubMed] [Google Scholar]
- Sobczuk P, Teterycz P, Czarnecka AM, Switaj T, Kosela-Paterczyk H, Kozak K, Falkowski S, Rutkowski P (2020) Systemic treatment for advanced and metastatic malignant peripheral nerve sheath tumors-a sarcoma reference center experience. J Clin Med. 10.3390/jcm9103157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suñé P, Suñé JM, Montoro JB (2013) Positive outcomes influence the rate and time to publication, but not the impact factor of publications of clinical trial results. PLoS ONE 8:e54583. 10.1371/journal.pone.0054583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K, Kawano M, Iwasaki T, Itonaga I, Tsumura H (2019) Surrogacy of intermediate endpoints for overall survival in randomized controlled trials of first-line treatment for advanced soft tissue sarcoma in the pre- and post-pazopanib era: a meta-analytic evaluation. BMC Cancer 19:56. 10.1186/s12885-019-5268-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tap WD, Jones RL, Van Tine BA, Chmielowski B, Elias AD, Adkins D, Agulnik M, Cooney MM, Livingston MB, Pennock G et al (2016) Olaratumab and doxorubicin versus doxorubicin alone for treatment of soft-tissue sarcoma: an open-label phase 1b and randomised phase 2 trial. The Lancet 388:488–497. 10.1016/s0140-6736(16)30587-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- The WHO Classification of Tumours Editorial Board (2020) WHO Classification of Tumours Soft Tissue and Bone Tumours, 5th edn. IARC Press, Lyon [Google Scholar]
- Toulmonde M, Bellera C, Mathoulin-Pelissier S, Debled M, Bui B, Italiano A (2011) Quality of randomized controlled trials reporting in the treatment of sarcomas. J Clin Oncol 29:1204–1209. 10.1200/JCO.2010.30.9369 [DOI] [PubMed] [Google Scholar]
- Van Glabbeke M, Verweij J, Judson I, Nielsen OS (2002) Progression-free rate as the principal end-point for phase II trials in soft-tissue sarcomas. Eur J Cancer 38:543–549. 10.1016/s0959-8049(01)00398-7 [DOI] [PubMed] [Google Scholar]
- Verweij J (2008) Other endpoints in screening studies for soft tissue sarcomas. Oncologist 13(Suppl 2):27–31. 10.1634/theoncologist.13-S2-27 [DOI] [PubMed] [Google Scholar]
- Zer A, Prince RM, Amir E, Razak AA (2016) Evolution of randomized trials in advanced/metastatic soft tissue sarcoma: end point selection, surrogacy, and quality of reporting. J Clin Oncol 34:1469–1475. 10.1200/jco.2015.64.3437 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.