Abstract
Purpose
We aimed to assess the role of adjuvant FOLFIRINOX, in comparison with other adjuvant therapy, in patients who received neoadjuvant FOLFIRINOX and surgery for borderline resectable or locally advanced pancreatic cancer (BRPC or LAPC).
Methods
Our target population was patients with BRPC or LAPC, who received adjuvant therapy following neoadjuvant FOLFIRINOX and surgery between June 2013 and October 2020. Multivariable Cox proportional-hazard model was used to identify factors associated with overall survival (OS) and recurrence free survival (RFS).
Results
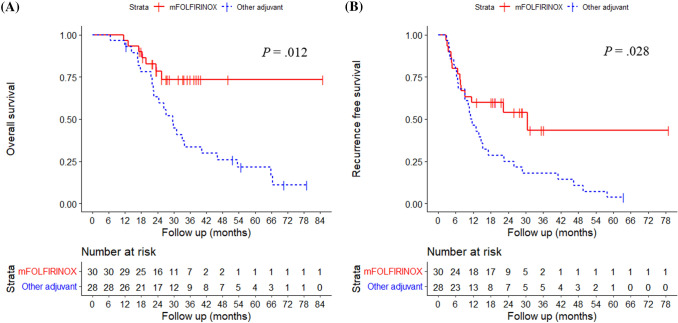
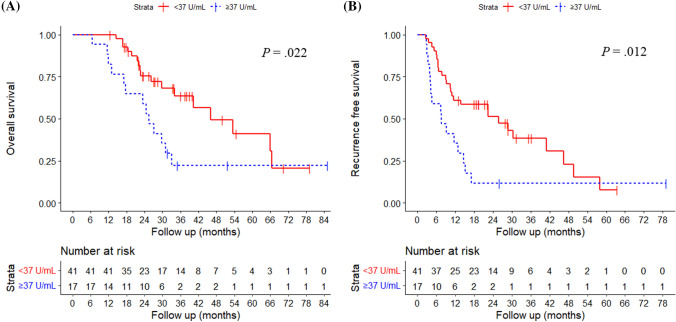
Among 244 patients with BRPC or LAPC who received neoadjuvant FOLFIRINOX, 79 patients underwent subsequent surgery. Among them, 58 who received adjuvant therapy [median age, 63 years; 33 females (56.9%)] were included. Thirty patients received adjuvant modified FOLFIRINOX (mFOLFIRINOX), while 28 received adjuvant therapy other than FOLFIRINOX. In multivariable analysis, mFOLFIRINOX and post-treatment carbohydrate antigen 19-9 (CA 19-9) were significantly associated with OS and RFS. According to mFOLFIRINOX vs. other adjuvant therapy, median OS was not reached at 37.5 months of follow-up vs. 29.7 months (P = .012); and median RFS was 30.5 vs. 11.0 months (P = .028). According to post-treatment CA 19-9 (< 37 vs. ≥ 37 U/mL), median OS was 46.0 vs. 25.5 months (P = .022); and median RFS was 25.9 vs. 7.6 months (P = .012).
Conclusion
Continued adjuvant mFOLFIRINOX and post-treatment CA 19-9 level were associated with survival in patients with BRPC or LAPC who received neoadjuvant FOLFIRINOX and surgery. Continued adjuvant mFOLFIRINOX after neoadjuvant FOLFIRINOX could be considered for patients with good performance.
Keywords: Pancreatic cancer, Neoadjuvant chemotherapy, Pancreatectomy, Adjuvant chemotherapy, Survival
Introduction
Pancreatic cancer was the seventh leading cause of global cancer-related death in 2020, with gradually increasing incidence and mortality rates in many countries (Sung et al. 2021). While surgical resection is the only curative therapy for pancreatic cancer, less than 20% of patients are eligible for upfront surgery at their initial presentation (Ryan et al. 2014; Sohal et al 2014). Even after surgery with curative intent, the prognosis remains dismal, with a reported recurrence rate of about 75% within 2 years (Groot et al. 2018).
There is now a widely shared consensus that neoadjuvant therapy improves oncologic outcomes of borderline resectable or locally advanced pancreatic cancer (BRPC or LAPC) by controlling micrometastasis and increasing R0 resection achievement (Ferrone et al. 2015). Evidence for neoadjuvant treatment is accumulating, as shown by two recent randomized controlled studies that included patients with BRPC (Jang et al. 2018; Versteijne et al. 2020). National Comprehensive Cancer Network (NCCN) guideline also endorses neoadjuvant therapy and recommends subsequent surgery in patients without disease progression during neoadjuvant therapy (Tempero et al. 2021) despite the lack of a firmly established chemotherapy regimen.
Fluorouracil, leucovorin, irinotecan, and oxaliplatin (FOLFIRINOX) combination therapy, based on its proven efficacy in patients with both resected and metastatic pancreatic cancer (Conroy et al. 2011; Conroy et al. 2018), is now widely used as a neoadjuvant regimen (Hackert et al. 2016). However, adjuvant chemotherapy regimen following neoadjuvant FOLFIRINOX and surgery is less well-established.
To this end, we aimed to identify the predictors of survival in patients who received neoadjuvant FOLFIRINOX and subsequent surgery for BRPC and LAPC, with an emphasis on the role of continued adjuvant modified FOLFIRINOX in comparison with adjuvant therapy other than mFOLFIRINOX.
Methods
Study design and patients
This retrospective cohort study was approved by the Institutional Review Board (IRB No. B-2106/688-110), which waived the requirement for patient informed consent due to the retrospective study design. The study took place in a single tertiary medical center. Eligible population was patients initially diagnosed as having BRPC or LAPC, and received FOLFIRINOX chemotherapy between June 2013 and October 2020.
The exclusion criteria were as follows: (1) no subsequent surgery; (2) less than six months follow-up after surgery at the time of investigation; (3) no adjuvant treatment; (4) periampullary cancer other than pancreatic head cancer; (5) use of FOLFIRINOX as an adjuvant regimen only, as a second-line or later regimen before surgery, or as a palliative regimen. We had weekly multidisciplinary meetings where decisions regarding surgery were made. Decisions regarding the number of cycles and dose reduction for neoadjuvant FOLFIRINOX were left to discretion of the attending physicians.
Treatment regimen
Neoadjuvant FOLFIRINOX regimen in this study was identical to the palliative FOLFIRINOX regimen (oxaliplatin 85 mg per square meter, leucovorin 400 mg per square meter, irinotecan 180 mg per square meter, intravenous bolus fluorouracil 400 mg per square meter, and continuous intravenous infusion of 2400 mg per square meter every 2 weeks) (Conroy et al. 2011).
The attending physicians determined the need for adjuvant therapy based on postoperative pathological outcomes and patient performance. For adjuvant chemotherapy, patients received either mFOLFIRINOX, or other agents including but not limited to gemcitabine-based chemotherapy. Adjuvant mFOLFIRINOX regimen were oxaliplatin 85 mg per square meter, leucovorin 400 mg per square meter, irinotecan 150 mg per square meter, and continuous intravenous infusion of fluorouracil 2400 mg per square meter every 2 weeks (Conroy et al. 2018). Dose adjustment for the regimen could be made by attending physicians. The reduced dose of FOLFIRINOX was calculated according to a previous study performed by our group (Lee et al. 2017).
Resectability adjudication and pathologic review
A radiologist (HYK) reviewed all available imaging studies and classified the initial and re-staged resectability in each patient according to the NCCN guidelines version 2.2021 (Tempero et al. 2021). Imaging studies for initial staging were performed at the time of diagnosis before any definitive treatment of pancreatic cancer, and those for re-staging were performed typically within 2 weeks before the surgery.
A dedicated hepatobiliary pathologist (HYN) categorized the TNM staging in accordance with the American Joint Committee on Cancer Staging 8th edition (Chun et al. 2018) and assessed the surgical margin status of pancreatic cancer. Tumor regression grade after neoadjuvant chemotherapy was also assessed according to the College of American Pathologists (CAP) 4-tier grading system (Chatterjee et al. 2012), where a higher grade indicates a poorer response.
Statistical analysis
We used Mann–Whitney U test for comparison of continuous variables, and Fisher’s exact test for categorical variables. We used the Kaplan–Meier method to calculate the overall survival (OS) and recurrent free survival (RFS), and log-rank tests for the comparisons. Variables with P < 0.05 in univariable analysis were further analyzed using multivariable Cox proportional-hazards regression. A statistical significance was defined as P < 0.05. We used SPSS®, version 25.0 (IBM SPSS Statistics Software for Windows, Version 25.0; IBM, Armonk, New York, USA) and R statistical software, version 4.0.2 (R Project for Statistical Computing, Version 4.0.2; R Foundation, Vienna, Austria).
Results
Enrolled patients
Among 244 patients with BRPC or LAPC who received FOLFIRINOX, 165 patients did not receive surgery due to reasons as follows: 69 with disease progression, 42 with poor performance, 42 who did not receive surgery (22 who refused surgery, and 20 who were still on neoadjuvant FOLFIRINOX), eight who were lost to follow-up, and four who were transferred to another hospital. Among 79 patients who underwent subsequent surgery, 21 did not receive any adjuvant therapy. Finally, 58 patients were enrolled in this study. Of them, 30 patients received mFOLFIRINOX. Remaining 28 received adjuvant therapy other than mFOLFIRINOX as follows: 17 with gemcitabine only, 2 with gemcitabine and capecitabine, 2 with gemcitabine and nab-paclitaxel, 1 with gemcitabine and erlotinib, 1 with fluorouracil and leucovorin, and 5 with concurrent chemoradiotherapy with fluorouracil, gemcitabine, or capecitabine (Fig. 1).
Fig. 1.
Flowchart showing the selection of study population. BRPC borderline resectable pancreatic cancer; LAPC locally advanced pancreatic cancer; FOLFIRINOX fluorouracil, leucovorin, irinotecan, oxaliplatin; mFOLFIRINOX, modified FOLFIRINOX
Baseline characteristics
At the time of initial assessment, the median age (range) was 63 (40–81) years (Table 1). There were more females than males (33, 56.9%). All patients had an Eastern Cooperative Oncology Group (ECOG) performance score of 0 or 1. Most patients had borderline resectable pancreatic cancer at initial assessment (50, 86.2%). Tumor was most frequently noted at the pancreatic head (40, 69.0%) and the median size was 3.0 cm (cT). The median carbohydrate antigen 19-9 (CA19-9) was 124 (range 5–7900) U/ml, and two-thirds of patients (41, 70.7%) had an elevated 19–9 level (≥ 37 U/mL). The median duration of follow-up after diagnosis was 37.5 months. There was no significant difference in the baseline characteristics between the two groups, except for in sex distribution.
Table 1.
Baseline characteristics
| mFOLFIRINOX (n = 30) | Other adjuvants (n = 28) | All (n = 58) | P | |
|---|---|---|---|---|
| Agea | 63 (40–81) | 64 (47–76) | 63 (40–81) | 0.400 |
| Sex | 0.037 | |||
| Male | 9 (30.0) | 16 (57.1) | 25 (43.1) | |
| Female | 21 (70.0) | 12 (42.9) | 33 (56.9) | |
| Baseline ECOG score | 0.630 | |||
| 0 | 19 (63.3) | 16 (57.1) | 35 (60.3) | |
| 1 | 11 (36.7) | 12 (42.9) | 23 (39.7) | |
| Primary tumor site | 0.060 | |||
| Head | 24 (80.0) | 16 (57.1) | 40 (69.0) | |
| Body or tail | 6 (20.0) | 12 (42.9) | 18 (31.0) | |
| Tumor sizea (cT) | 2.8 (1.3–7.5) | 3.3 (1.6–7.5) | 3.0 (1.3–7.5) | 0.186 |
| Initial resectability | 0.999 | |||
| BRPC | 26 (86.7) | 24 (85.7) | 50 (86.2) | |
| LAPC | 4 (13.3) | 4 (14.3) | 8 (13.8) | |
| Baseline CA 19-9a | 117 (5–7900) | 139 (5–3400) | 124 (5–7900) | 0.493 |
| ≥ 37 U/mL | 19 (63.3) | 22 (78.6) | 41 (70.7) | 0.203 |
| Follow-up, monthsa | 31.7 (11.6–85.1) | 70.5 (6.6–79.1) | 37.5 (6.6–85.1) | 0.586 |
Values in parentheses are percentages unless indicated otherwise
ECOG Eastern cooperative oncology group, BRPC borderline resectable pancreatic cancer, LAPC locally advanced pancreatic cancer, CA 19-9 carbohydrate antigen 19-9
aValues are median (range)
Post-treatment characteristics
The median number of cycles and duration of neoadjuvant FOLFIRINOX were eight cycles and 4.3 months, respectively (Table 2). Eight patients (13.7%) underwent preoperative radiation therapy; one (1.7%) with conventional radiation therapy, one (1.7%) with intensity modulated radiation therapy, and the other six (10.3%) with stereotactic body radiation therapy. After neoadjuvant FOLFIRINOX, there were 13 patients with RPC, 42 with BRPC, and three with LAPC according to re-staged resectability. Pancreaticoduodenectomy was performed in 39 patients (67.3%), more frequently in patients who received mFOLFIRINOX.
Table 2.
Post-treatment characteristics
| mFOLFIRINOX (n = 30) | Other adjuvants (n = 28) | All (n = 58) | P | |
|---|---|---|---|---|
| Preoperative therapy | ||||
| Chemotherapy | ||||
| Cyclesa | 8 (5–15) | 9 (2–16) | 8 (2–16) | 0.591 |
| Duration, monthsa | 4.1 (1.9–8.1) | 4.7 (0.4–11.7) | 4.3 (0.4–11.7) | 0.234 |
| Radiation | ||||
| Conventional | 0 | 1 (3.6) | 1 (1.7) | |
| IMRT | 0 | 1 (3.6) | 1 (1.7) | |
| SBRT | 3 (10.0) | 3 (10.7) | 6 (10.3) | |
| Re-staged resectability | 0.337 | |||
| RPC | 8 (26.7) | 5 (17.9) | 13 (22.4) | |
| BRPC | 21 (70.0) | 21 (75.0) | 42 (72.4) | |
| LAPC | 1 (3.3) | 2 (7.1) | 3 (5.2) | |
| Surgery | 0.021 | |||
| Pancreaticoduodenectomy | 24 (80.0) | 15 (53.6) | 39 (67.3) | |
| Distal pancreatectomy | 6 (20.0) | 11 (39.3) | 17 (29.3) | |
| Subtotal or total pancreatectomy | 0 (0.0) | 2 (7.1) | 2 (3.4) | |
| Postoperative ECOG performance score | 0.354 | |||
| 0–1 | 26 (86.7) | 27 (96.4) | 53 (91.4) | |
| ≥ 2 | 4 (13.3) | 1 (3.6) | 5 (8.6) | |
| Cellular differentiation | 0.607 | |||
| WD to MD | 23 (76.7) | 23 (82.1) | 46 (79.3) | |
| PD | 7 (23.3) | 5 (17.9) | 12 (20.7) | |
| Resection margin | 0.648 | |||
| Negative (R0) | 24 (80.0) | 21 (75.0) | 45 (77.6) | |
| Positive (R1 or R2) | 6 (20.0) | 7 (25.0) | 13 (22.4) | |
| Tumor sizea (ypT) | 2.0 (0.1–5.0) | 3.0 (1.0–7.7) | 2.5 (0.1–7.7) | 0.001 |
| ≥ 2 cm | 18 (60.0) | 26 (92.9) | 44 (75.9) | 0.003 |
| LN metastasis (ypN) | 0.096 | |||
| Negative (N0) | 15 (50.0) | 8 (28.6) | 23 (39.7) | |
| Positive (N1 or N2) | 15 (50.0) | 20 (71.4) | 35 (60.3) | |
| TNM stageb | 0.064 | |||
| I | 15 (50.0) | 8 (28.6) | 23 (39.7) | |
| II | 13 (43.3) | 15 (53.6) | 28 (48.3) | |
| III | 2 (6.7) | 5 (17.8) | 7 (12.0) | |
| CAP score | 0.051 | |||
| 1 | 3 (10.0) | 1 (3.5) | 4 (6.9) | |
| 2 | 21 (70.0) | 15 (53.6) | 36 (62.1) | |
| 3 | 6 (20.0) | 12 (42.9) | 18 (31.0) | |
| Adjuvant therapy | ||||
| Duration, monthsa | 2.3 (0.1–7.7) | 3.4 (0.3–5.4) | 3.2 (0.1–7.7) | 0.840 |
| Dose reduction | 25 (83.3) | 10 (35.7) | 35 (60.3) | 0.001 |
| Percentage of reductiona | 20 (0–50) | 0 (0–45) | 15 (0–50) | 0.002 |
| Post-treatment CA 19-9a | 12 (5–3100) | 16 (5–11,100) | 14 (5–11,100) | 0.174 |
| ≥ 37 U/mL | 8 (26.7) | 9 (32.1) | 17 (29.3) | 0.647 |
| Recurrence | 0.005 | |||
| No | 16 (53.3) | 5 (17.9) | 21 (36.2) | |
| Yes | 14 (46.7) | 23 (82.1) | 37 (63.8) | |
Values in parentheses are percentages unless indicated otherwise
aValues are median (range)
bDefined according to the AJCC Cancer Staging Manual, 8th edition
IMRT intensity modulated radiation therapy, SBRT stereotactic body radiation therapy, RPC resectable pancreatic cancer, BRPC borderline resectable pancreatic cancer, LAPC locally advanced pancreatic cancer, ECOG Eastern cooperative oncology group, WD well differentiated, MD moderately differentiated, PD, poorly differentiated, CAP score College of American pathologists score, CA 19–9 carbohydrate antigen 19–9
After surgery, 53 patients (91.4%) had an ECOG score of 0 or 1. There were 46 patients (79.3%) with well or moderately differentiated adenocarcinoma. A negative resection margin (R0) was achieved in 45 patients (77.6%); and R1, R2 resection margin in 12 patients (20.7%) and 1 patient (1.7%), respectively. The median size of the primary tumor was 2.5 (0.1–7.7) cm, and 14 patients (24.1%) had tumors less than 2 cm in size (ypT1). The median size of the primary tumor was larger in patients with adjuvant therapy other than mFOLFIRINOX (2.0 cm vs. 3.0 cm, P = 0.001), with a higher proportion of patients with tumor size ≥ 2 cm (60.0% vs. 92.9%, P = 0.003). Twenty-three patients (39.7%) had negative lymph node metastasis (ypN0). Twenty-three patients had stage I (39.7%), 28 had stage II (48.3%), and seven had stage III (12.0%) cancer. The CAP grade was 1 in four patients, 2 in 36 patients, and 3 in 18 patients. The median duration of adjuvant therapy (range) was 3.2 months (0.1–7.7), without a significant difference between the two groups. Chemotherapy dose was reduced in 35 patients (60.3%), and the reduction was more common in the mFOLFILINOX group (83.3% vs. 35.7%, P = 0.001). The median percentage of dose reduction was also higher in the mFOLFIRINOX group (20% vs. 0%, P = 0.002). The median (IQR) level of CA 19–9 was 14 (5–11,100) U/mL, and 17 patients (29.3%) had an elevated CA 19-9 (≥ 37 U/mL) after the adjuvant therapy. Recurrence occurred in 37 patients (63.8%) and was significantly more common in patients with adjuvant therapy other than mFOLFIRINOX.
Survival outcomes
The median OS and RFS were 33.9 and 13.9 months. The OS rates at 1- and 2-year were 94.8% and 70.6%, respectively. Of various clinical factors associated with survival, adjuvant therapy other than mFOLFIRINOX [hazard ratio (HR), 2.91; 95% confidence interval (CI), 1.24–6.81; P = 0.014] and elevated post-treatment CA 19–9 ≥ 37 U/mL (HR, 2.39; 1.14–5.03; P = 0.021) were independent risk factors for overall survival (Table 3). The RFS rates at 1- and 2-year were 53.4% and 39.6%, respectively. Adjuvant therapy other than mFOLFIRINOX (HR 1.95; 1.02–3.74; P = 0.043) and elevated post-treatment CA 19–9 ≥ 37 U/mL (HR 1.96; 1.02–3.80; P = 0.045) were also independent risk factors for RFS (Table 4). Nodal metastasis was an independent risk factor for OS and RFS in univariable analysis, but not in multivariable analysis.
Table 3.
Univariable and multivariable Cox regression analyses for overall survival
| Univariable analysis | Multivariable analysis | |||
|---|---|---|---|---|
| Hazard ratio | P value | Hazard ratio | P value | |
| Age | 1.05 (1.00–1.10) | 0.053 | ||
| Sex | ||||
| Male | 1.00 (Reference) | |||
| Female | 1.01 (0.49–2.08) | 0.979 | ||
| Adjuvant therapy | ||||
| mFOLFIRINOX | 1.00 (Reference) | 1.00 (Reference) | ||
| Other adjuvants | 2.87 (1.22–6.77) | 0.016 | 2.91 (1.24–6.81) | 0.014 |
| Re-staged resectability | ||||
| RPC | 1.00 (Reference) | |||
| BRPC | 2.37 (0.82–6.89) | 0.112 | ||
| LAPC | 4.43 (0.78–25.26) | 0.093 | ||
| Post-treatment CA 19–9 | ||||
| < 37 U/mL | 1.00 (Reference) | 1.00 (Reference) | ||
| ≥ 37 U/mL | 2.29 (1.11–4.73) | 0.026 | 2.39 (1.14–5.03) | 0.021 |
| Tumor size (ypT) | ||||
| < 2 cm | 1.00 (Reference) | |||
| ≥ 2 cm | 2.34 (0.80–6.84) | 0.119 | ||
| LN metastasis (ypN) | ||||
| Negative (N0) | 1.00 (Reference) | 1.00 (Reference) | ||
| Positive (N1 or N2) | 2.42 (1.07–5.49) | 0.034 | 1.93 (0.84–4.41) | 0.121 |
| Resection margin | ||||
| Negative | 1.00 (Reference) | |||
| Positive | 1.17 (0.50–2.75) | 0.713 | ||
| CAP score | ||||
| 1 | 1.00 (Reference) | |||
| 2 | 1.91 (0.25–14.48) | 0.534 | ||
| 3 | 5.78 (0.74–45.35) | 0.095 | ||
Values in parentheses are 95 per cent confidence intervals. Variables with P < 0.05 in univariable analysis were included in the multivariable model
mFOLFIRINOX modified FOLFIRINOX, BRPC borderline resectable pancreatic cancer, LAPC locally advanced pancreatic cancer, RPC resectable pancreatic cancer, CA 19–9 carbohydrate antigen 19–9, CAP score College of American Pathologists score
Table 4.
Univariable and multivariable Cox regression analyses for recurrence free survival
| Univariable analysis | Multivariable analysis | |||
|---|---|---|---|---|
| Hazard ratio | P value | Hazard ratio | P value | |
| Age | 1.04 (1.00–1.08) | 0.028 | ||
| Sex | ||||
| Male | 1.00 (Reference) | |||
| Female | 1.01 (0.54–1.89) | 0.983 | ||
| Adjuvant therapy | ||||
| mFOLFIRINOX | 1.00 (Reference) | 1.00 (Reference) | ||
| Other adjuvants | 2.04 (1.06–3.93) | 0.032 | 1.95 (1.02–3.74) | 0.043 |
| Re-staged resectability | ||||
| RPC | 1.00 (Reference) | |||
| BRPC | 1.93 (0.85–4.39) | 0.117 | ||
| LAPC | 2.85 (0.58–13.95) | 0.196 | ||
| Post-treatment CA 19-9 | ||||
| < 37 U/mL | 1.00 (Reference) | 1.00 (Reference) | ||
| ≥ 37 U/mL | 2.24 (1.18–4.27) | 0.014 | 1.96 (1.02–3.80) | 0.045 |
| Tumor size (ypT) | ||||
| < 2 cm | 1.00 (Reference) | |||
| ≥ 2 cm | 2.33 (0.97–5.61) | 0.058 | ||
| LN metastasis (ypN) | ||||
| Negative (N0) | 1.00 (Reference) | 1.00 (Reference) | ||
| Positive (N1 or N2) | 2.21 (1.14–4.30) | 0.020 | 1.91 (0.97–3.78) | 0.063 |
| Resection margin | ||||
| Negative | 1.00 (Reference) | |||
| Positive | 1.17 (0.56–2.45) | 0.684 | ||
| CAP score | ||||
| 1 | 1.00 (Reference) | |||
| 2 | 3.88 (0.52–29.01) | 0.186 | ||
| 3 | 11.71 (1.51–91.05) | 0.019 | ||
Values in parentheses are 95 per cent confidence intervals. Variables with P < 0.05 in univariable analysis were included in the multivariable model; mFOLFIRINOX modified FOLFIRINOX, BRPC borderline resectable pancreatic cancer, LAPC locally advanced pancreatic cancer, RPC resectable pancreatic cancer, CA 19-9 carbohydrate antigen 19-9, CAP score College of American pathologists score
According to mFOLFIRINOX vs. other adjuvant therapy, the median OS was not reached at 37.5 months of follow-up vs. 29.7 months (P = 0.012, Fig. 2a); and the median RFS was 30.5 vs. 11.0 months (P = 0.028, Fig. 2b). According to post-treatment CA 19–9 (< 37 vs. ≥ 37 U/mL), the median OS was 46.0 vs. 25.5 months (P = 0.022, Fig. 3a); and the median RFS was 25.9 vs. 7.6 months (P = 0.012., Fig. 3b), respectively.
Fig. 2.
Overall survival and recurrent free survival according to adjuvant therapy. In patients with mFOLFIRINOX vs. other adjuvant therapy, a the median overall survival was not reached vs. 29.7 months (P = .012), and b the median recurrence free survival was 30.5 vs. 11.0 months (P = .028), respectively. mFOLFIRINOX, modified fluorouracil, leucovorin, irinotecan, oxaliplatin
Fig. 3.
Overall survival and recurrent free survival according to post-adjuvant CA 19-9 level. In patients with CA 19-9 < 37 U/mL vs. ≥ 37 U/mL, a the median overall survival was 46.0 vs. 25.5 months (P = .022), and b the median recurrence free survival was 25.9 vs. 7.6 months (P = .012), respectively. CA 19-9, carbohydrate antigen 19-9
Discussion
In this study, continued use of mFOLFIRINOX as adjuvant therapy and post-treatment CA19-9 were two significant predictors for survival in patients who received neoadjuvant FOLFIRINOX and surgery for borderline resectable or locally advanced pancreatic cancer. We compared mFOLFIRINOX with other adjuvant therapy, the majority of which was gemcitabine-based regimen, and showed that continued use of mFOLFIRINOX was associated with better survival outcomes in patients who received neoadjuvant FOLFIRINOX and surgery for pancreatic cancer. The results were consistent in both OS and RFS by multivariable regression analysis. Our results have important clinical implications, since there is currently no standardized regimen of adjuvant therapy in patients who receive neoadjuvant therapy for pancreatic cancer. To our knowledge, no study has formally compared FOLFIRINOX with other regimen as adjuvant therapy following neoadjuvant FOLFIRINOX.
Lack of standardized regimen for adjuvant therapy in patients who receive neoadjuvant therapy may be attributable to inadequate evidence supporting the use of adjuvant therapy under such circumstance. Whereas the evidence supporting the use of neoadjuvant therapy for BRPC is accumulating (Gemenetzis et al. 2019; Hackert et al. 2016; Michelakos et al. 2019; Rangelova et al. 2021), the results of previous studies on additional use of adjuvant therapy following neoadjuvant therapy have been more discordant. In comparison with studies that supported beneficial role of adjuvant therapy (Groot et al. 2019; Perri et al. 2020), a few other studies suggested that the benefit was observed only in selected patients who had suboptimal response to neoadjuvant therapy in terms of pathological findings or CA 19-9 (Liu et al. 2020; Roland et al. 2015; van Roessel et al. 2020). A latest multinational retrospective cohort study including 520 patients showed that benefits of adjuvant therapy were present only in pathology-proven node-positive disease. In that study, the most frequently used regimen was gemcitabine-based chemotherapy (59%), followed by FOLFIRINOX (20%) (van Roessel et al. 2020).
Our results showing superior survival in patients who received mFOLFIRINOX as adjuvant therapy should be approached cautiously. Even though there was no statistically significant difference in most patient characteristics, there was a tendency for postoperative pathological outcomes such as CAP score or nodal status to be better in patients who received mFOLFIRINOX than in those who received other agents. Dose reduction of chemotherapy was also more frequent in patients who received mFOLFIRINOX. Our results, as important as they are, should be validated via more rigorously conducted studies including a larger patient sample, preferably of prospective design.
CA 19-9 is the only widely accepted biomarker in pancreatic cancer (BallehaninnaandChamberlain 2012; Scara et al. 2015). Post-treatment CA 19-9, but not baseline CA 19-9, was an independent prognostic predictor in our study. Previous studies have also shown that perioperative (preoperative or postoperative) CA 19-9 was associated with the survival of patients with neoadjuvant therapy (Aldakkak et al. 2015; Aoki et al. 2019; Tsai et al. 2020). Our results are also consistent with the previous studies where normalization of CA 19-9, rather than CA 19-9 level at initial presentation or the magnitude of its change, was associated with a better prognosis. However, post-treatment CA 19-9 was more informative than postoperative CA 19-9 in this study.
Previous studies have reported the nodal status as one of the most important prognostic factors (Roland et al. 2015; van Roessel et al. 2020). Lymph node metastasis was not significantly associated with survival in this study, although there was a trend of association. Insignificant hazard ratio of the lymph node metastasis in this study may be attributable to low statistical power caused by the small sample size.
Our study had several limitations. First, it was a retrospective single-center study. Consequently, there was a possibility of selection bias involving the choice of the adjuvant regimen. Although there was no significant between-group difference in most patient characteristics including the ECOG performance score, there was a tendency for postoperative pathological outcomes to be better in the mFOLFIRINOX group as described earlier. Second, the study sample was relatively small and thus statistically underpowered to prove association of pathologic responses such as nodal metastasis or surgical margin status with survival (Klaiber et al. 2021; Michelakos et al. 2019). Third, we included both BRPC and LAPC, thus creating heterogeneity of the study population. Such pooling of both BRPC and LAPC has also been performed in other previous studies (Michelakos et al. 2019; Rangelova et al. 2021; Versteijne et al. 2020; Wijetunga et al. 2021). Finally, the study did not include patients without adjuvant therapy. The number of patients who were excluded because they did not receive adjuvant therapy was very small in this study. The concept of determining whether to proceed with adjuvant therapy according to pathological outcomes has only recently been adapted in our center (van Roessel et al. 2020).
In conclusion, mFOLFIRINOX in comparison with other adjuvant therapy, and post-treatment CA 19–9 level were significantly associated with longer OS and RFS in patients who received neoadjuvant FOLFIRINOX and surgery for pancreatic cancer. Continued adjuvant mFOLFIRINOX treatment following neoadjuvant FOLFIRINOX could be considered for patients with good performance score. Further studies are necessary for this unsolved yet important issue.
Author contributions
JP: study conception and design; acquisition, analysis, or interpretation of data; drafting of the manuscript; and critical revision of the manuscript for intellectual content. HYK: acquisition, analysis, or interpretation of data; drafting of the manuscript; and critical revision of the manuscript for intellectual content. HYN: drafting of the manuscript; and critical revision of the manuscript for intellectual content. JSL: drafting of the manuscript; and critical revision of the manuscript for intellectual content. JCL: drafting of the manuscript; and critical revision of the manuscript for intellectual content. JWK: drafting of the manuscript; and critical revision of the manuscript for intellectual content. YSY: drafting of the manuscript; and critical revision of the manuscript for intellectual content. JHH: drafting of the manuscript; and critical revision of the manuscript for intellectual content. HSH: drafting of the manuscript; and critical revision of the manuscript for intellectual content. JK: full access to all data in the study; responsibility for the integrity of the data and the accuracy of the data; study conception and design; acquisition, analysis, or interpretation of data; study supervision; drafting of the manuscript; and critical revision of the manuscript for intellectual content.
Funding
This work did not receive any funding.
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Conflict of interest
The authors have no conflicts of interest to declare.
Ethical approval
This study was approved by the Institutional Review Board of Seoul National University Bundang Hospital (IRB No. B-2106/688-110).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jaewoo Park and Hae Young Kim contributed equally to this work as first authors.
References
- Aldakkak M, Christians KK, Krepline AN, George B, Ritch PS, Erickson BA, Johnston FM, Evans DB, Tsai S (2015) Pre-treatment carbohydrate antigen 19–9 does not predict the response to neoadjuvant therapy in patients with localized pancreatic cancer. HPB 17:942–952. 10.1111/hpb.12448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki S, Motoi F, Murakami Y, Sho M, Satoi S, Honda G, Uemura K, Okada KI, Matsumoto I, Nagai M, Yanagimoto H, Kurata M, Fukumoto T, Mizuma M, Yamaue H, Unno M, Multicenter Study Group of Pancreatobiliary S (2019) Decreased serum carbohydrate antigen 19–9 levels after neoadjuvant therapy predict a better prognosis for patients with pancreatic adenocarcinoma: a multicenter case-control study of 240 patients. BMC Cancer 19:252. 10.1186/s12885-019-5460-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballehaninna UK, Chamberlain RS (2012) The clinical utility of serum CA 19–9 in the diagnosis, prognosis and management of pancreatic adenocarcinoma: an evidence based appraisal. J Gastrointest Oncol 3:105–119. 10.3978/j.issn.2078-6891.2011.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee D, Katz MH, Rashid A, Varadhachary GR, Wolff RA, Wang H, Lee JE, Pisters PW, Vauthey JN, Crane C, Gomez HF, Abbruzzese JL, Fleming JB, Wang H (2012) Histologic grading of the extent of residual carcinoma following neoadjuvant chemoradiation in pancreatic ductal adenocarcinoma: a predictor for patient outcome. Cancer 118:3182–3190. 10.1002/cncr.26651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun YS, Pawlik TM, Vauthey JN (2018) 8th edition of the AJCC cancer staging manual: pancreas and hepatobiliary cancers. Ann Surg Oncol 25:845–847. 10.1245/s10434-017-6025-x [DOI] [PubMed] [Google Scholar]
- Conroy T, Desseigne F, Ychou M, Bouche O, Guimbaud R, Becouarn Y, Adenis A, Raoul JL, Gourgou-Bourgade S, de la Fouchardiere C, Bennouna J, Bachet JB, Khemissa-Akouz F, Pere-Verge D, Delbaldo C, Assenat E, Chauffert B, Michel P, Montoto-Grillot C, Ducreux M, Groupe Tumeurs Digestives of U, & Intergroup P (2011) FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 364:1817–1825. 10.1056/NEJMoa1011923 [DOI] [PubMed] [Google Scholar]
- Conroy T, Hammel P, Hebbar M, Ben Abdelghani M, Wei AC, Raoul JL, Chone L, Francois E, Artru P, Biagi JJ, Lecomte T, Assenat E, Faroux R, Ychou M, Volet J, Sauvanet A, Breysacher G, Di Fiore F, Cripps C, Kavan P, Texereau P, Bouhier-Leporrier K, Khemissa-Akouz F, Legoux JL, Juzyna B, Gourgou S, O’Callaghan CJ, Jouffroy-Zeller C, Rat P, Malka D, Castan F, Bachet JB (2018) FOLFIRINOX or gemcitabine as adjuvant therapy for pancreatic cancer. N Engl J Med 379:2395–2406. 10.1056/NEJMoa1809775 [DOI] [PubMed] [Google Scholar]
- Ferrone CR, Marchegiani G, Hong TS, Ryan DP, Deshpande V, McDonnell EI, Sabbatino F, Santos DD, Allen JN, Blaszkowsky LS, Clark JW, Faris JE, Goyal L, Kwak EL, Murphy JE, Ting DT, Wo JY, Zhu AX, Warshaw AL, Lillemoe KD, Fernandez-del Castillo C (2015) Radiological and surgical implications of neoadjuvant treatment with FOLFIRINOX for locally advanced and borderline resectable pancreatic cancer. Ann Surg 261:12–17. 10.1097/SLA.0000000000000867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gemenetzis G, Groot VP, Blair AB, Laheru DA, Zheng L, Narang AK, Fishman EK, Hruban RH, Yu J, Burkhart RA, Cameron JL, Weiss MJ, Wolfgang CL, He J (2019) Survival in locally advanced pancreatic cancer after neoadjuvant therapy and surgical resection. Ann Surg 270:340–347. 10.1097/SLA.0000000000002753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groot VP, Blair AB, Gemenetzis G, Ding D, Burkhart RA, Yu J, BorelRinkes IHM, Molenaar IQ, Cameron JL, Weiss MJ, Wolfgang CL, He J (2019) Recurrence after neoadjuvant therapy and resection of borderline resectable and locally advanced pancreatic cancer. Eur J Surg Oncol 45:1674–1683. 10.1016/j.ejso.2019.04.007 [DOI] [PubMed] [Google Scholar]
- Groot VP, Rezaee N, Wu W, Cameron JL, Fishman EK, Hruban RH, Weiss MJ, Zheng L, Wolfgang CL, He J (2018) Patterns, timing, and predictors of recurrence following pancreatectomy for pancreatic ductal adenocarcinoma. Ann Surg 267:936–945. 10.1097/SLA.0000000000002234 [DOI] [PubMed] [Google Scholar]
- Hackert T, Sachsenmaier M, Hinz U, Schneider L, Michalski CW, Springfeld C, Strobel O, Jager D, Ulrich A, Buchler MW (2016) Locally advanced pancreatic cancer: neoadjuvant therapy with FOLFIRINOX results in resectability in 60% of the patients. Ann Surg 264:457–463. 10.1097/SLA.0000000000001850 [DOI] [PubMed] [Google Scholar]
- Jang JY, Han Y, Lee H, Kim SW, Kwon W, Lee KH, Oh DY, Chie EK, Lee JM, Heo JS, Park JO, Lim DH, Kim SH, Park SJ, Lee WJ, Koh YH, Park JS, Yoon DS, Lee IJ, Choi SH (2018) Oncological benefits of neoadjuvant chemoradiation with gemcitabine versus upfront surgery in patients with borderline resectable pancreatic cancer: a prospective, randomized, open-label, multicenter phase 2/3 trial. Ann Surg 268:215–222. 10.1097/SLA.0000000000002705 [DOI] [PubMed] [Google Scholar]
- Klaiber U, Schnaidt ES, Hinz U, Gaida MM, Heger U, Hank T, Strobel O, Neoptolemos JP, Mihaljevic AL, Buchler MW, Hackert T (2021) Prognostic factors of survival after neoadjuvant treatment and resection for initially unresectable pancreatic cancer. Ann Surg 273:154–162. 10.1097/SLA.0000000000003270 [DOI] [PubMed] [Google Scholar]
- Lee JC, Kim JW, Ahn S, Kim HW, Lee J, Kim YH, Paik KH, Kim J, Hwang JH (2017) Optimal dose reduction of FOLFIRINOX for preserving tumour response in advanced pancreatic cancer: using cumulative relative dose intensity. Eur J Cancer 76:125–133. 10.1016/j.ejca.2017.02.010 [DOI] [PubMed] [Google Scholar]
- Liu H, Zenati MS, Rieser CJ, Al-Abbas A, Lee KK, Singhi AD, Bahary N, Hogg ME, Zeh HJ 3rd, Zureikat AH (2020) CA19-9 Change during neoadjuvant therapy may guide the need for additional adjuvant therapy following resected pancreatic cancer. Ann Surg Oncol 27:3950–3960. 10.1245/s10434-020-08468-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michelakos T, Pergolini I, Castillo CF, Honselmann KC, Cai L, Deshpande V, Wo JY, Ryan DP, Allen JN, Blaszkowsky LS, Clark JW, Murphy JE, Nipp RD, Parikh A, Qadan M, Warshaw AL, Hong TS, Lillemoe KD, Ferrone CR (2019) Predictors of resectability and survival in patients with borderline and locally advanced pancreatic cancer who underwent neoadjuvant treatment with FOLFIRINOX. Ann Surg 269:733–740. 10.1097/SLA.0000000000002600 [DOI] [PubMed] [Google Scholar]
- Perri G, Prakash L, Qiao W, Varadhachary GR, Wolff R, Fogelman D, Overman M, Pant S, Javle M, Koay EJ, Herman J, Kim M, Ikoma N, Tzeng CW, Lee JE, Katz MHG (2020) Postoperative chemotherapy benefits patients who received preoperative therapy and pancreatectomy for pancreatic adenocarcinoma. Ann Surg 271:996–1002. 10.1097/SLA.0000000000003763 [DOI] [PubMed] [Google Scholar]
- Rangelova E, Wefer A, Persson S, Valente R, Tanaka K, Orsini N, Segersvard R, Arnelo U, Del Chiaro M (2021) Surgery improves survival after neoadjuvant therapy for borderline and locally advanced pancreatic cancer: a single institution experience. Ann Surg 273:579–586. 10.1097/SLA.0000000000003301 [DOI] [PubMed] [Google Scholar]
- Roland CL, Katz MH, Tzeng CW, Lin H, Varadhachary GR, Shroff R, Javle M, Fogelman D, Wolff RA, Vauthey JN, Crane CH, Lee JE, Fleming JB (2015) The addition of postoperative chemotherapy is associated with improved survival in patients with pancreatic cancer treated with preoperative therapy. Ann Surg Oncol 22(Suppl 3):S1221-1228. 10.1245/s10434-015-4854-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan DP, Hong TS, Bardeesy N (2014) Pancreatic adenocarcinoma. N Engl J Med 371:1039–1049. 10.1056/NEJMra1404198 [DOI] [PubMed] [Google Scholar]
- Scara S, Bottoni P, Scatena R (2015) CA 19–9: biochemical and clinical aspects. Adv Exp Med Biol 867:247–260. 10.1007/978-94-017-7215-0_15 [DOI] [PubMed] [Google Scholar]
- Sohal DP, Walsh RM, Ramanathan RK, Khorana AA (2014) Pancreatic adenocarcinoma: treating a systemic disease with systemic therapy. J Natl Cancer Inst. 10.1093/jnci/dju011 [DOI] [PubMed] [Google Scholar]
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F (2021) Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71:209–249. 10.3322/caac.21660 [DOI] [PubMed] [Google Scholar]
- Tempero MA, Malafa MP, Al-Hawary M, Behrman SW, Benson AB, Cardin DB, Chiorean EG, Chung V, Czito B, Del Chiaro M, Dillhoff M, Donahue TR, Dotan E, Ferrone CR, Fountzilas C, Hardacre J, Hawkins WG, Klute K, Ko AH, Kunstman JW, LoConte N, Lowy AM, Moravek C, Nakakura EK, Narang AK, Obando J, Polanco PM, Reddy S, Reyngold M, Scaife C, Shen J, Vollmer C, Wolff RA, Wolpin BM, Lynn B, George GV (2021) Pancreatic adenocarcinoma, version 2.2021, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 19:439–457. 10.6004/jnccn.2021.0017 [DOI] [PubMed] [Google Scholar]
- Tsai S, George B, Wittmann D, Ritch PS, Krepline AN, Aldakkak M, Barnes CA, Christians KK, Dua K, Griffin M, Hagen C, Hall WA, Erickson BA, Evans DB (2020) Importance of normalization of CA19-9 levels following neoadjuvant therapy in patients with localized pancreatic cancer. Ann Surg 271:740–747. 10.1097/SLA.0000000000003049 [DOI] [PubMed] [Google Scholar]
- van Roessel S, van Veldhuisen E, Klompmaker S, Janssen QP, Abu Hilal M, Alseidi A, Balduzzi A, Balzano G, Bassi C, Berrevoet F, Bonds M, Busch OR, Butturini G, Del Chiaro M, Conlon KC, Falconi M, Frigerio I, Fusai GK, Gagniere J, Griffin O, Hackert T, Halimi A, Klaiber U, Labori KJ, Malleo G, Marino MV, Mortensen MB, Nikov A, Lesurtel M, Keck T, Kleeff J, Pande R, Pfeiffer P, Pietrasz D, Roberts KJ, Sa Cunha A, Salvia R, Strobel O, Tarvainen T, Bossuyt PM, van Laarhoven HWM, Wilmink JW, Groot Koerkamp B, Besselink MG, European-African Hepato-Pancreato-Biliary A (2020) Evaluation of adjuvant chemotherapy in patients with resected pancreatic cancer after neoadjuvant FOLFIRINOX treatment. JAMA Oncol 6:1733–1740. 10.1001/jamaoncol.2020.3537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Versteijne E, Suker M, Groothuis K, Akkermans-Vogelaar JM, Besselink MG, Bonsing BA, Buijsen J, Busch OR, Creemers GM, van Dam RM, Eskens F, Festen S, de Groot JWB, Groot Koerkamp B, de Hingh IH, Homs MYV, van Hooft JE, Kerver ED, Luelmo SAC, Neelis KJ, Nuyttens J, Paardekooper G, Patijn GA, van der Sangen MJC, de Vos-Geelen J, Wilmink JW, Zwinderman AH, Punt CJ, van Eijck CH, van Tienhoven G, Dutch Pancreatic Cancer G (2020) Preoperative chemoradiotherapy versus immediate surgery for resectable and borderline resectable pancreatic cancer: results of the Dutch Randomized Phase III PREOPANC trial. J Clin Oncol 38:1763–1773. 10.1200/JCO.19.02274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijetunga AR, Chua TC, Nahm CB, Pavlakis N, Clarke S, Chan DL, Diakos C, Maloney S, Ashrafi-Zadeh A, Kneebone A, Hruby G, Jamieson NB, Gill A, Mittal A, Samra JS (2021) Survival in borderline resectable and locally advanced pancreatic cancer is determined by the duration and response of neoadjuvant therapy. Eur J Surg Oncol 47:2543–2550. 10.1016/j.ejso.2021.04.005 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.