Abstract
Background
The role of mitophagy in various cancer-associated biological processes is well recognized. Nonetheless, the comprehensive implications of mitophagy in clear cell renal cell carcinoma (ccRCC) necessitate further exploration.
Methods
Based on the transcriptomic data encompassing 25 mitophagy-related genes (MRGs), we identified the distinct mitophage patterns in 763 ccRCC samples. Subsequently, a mitophage-related predictive signature with machine learning algorithms was constructed, designated as RiskScore, to quantify the individual mitophagy status in ccRCC patients. Employing multispectral immunofluorescence (mIF) and immunohistochemistry (IHC) staining, we detected the effect of PTEN-induced putative kinase 1 (PINK1) in the prognosis and immune microenvironment of ccRCC.
Results
Our analysis initially encompassed a comprehensive assessment of the expression profiling, genomic variations, and interactions among the 25 MRGs in ccRCC. Subsequently, the consensus clustering algorithm was applied to stratify ccRCC patients into three clusters with distinct prognostic outcomes, tumor microenvironment (TME) characteristics, and underlying biological pathways. We screened eight pivotal genes (CLIC4, PTPRB, SLC16A12, ENPP5, FLRT3, HRH2, PDK4, and SCD5) to construct a mitophagy-related predictive signature, which showed excellent prognostic value for ccRCC patients. Moreover, patient subgroups divided by the RiskScore showed contrasting expression levels of immune checkpoints (ICPs), abundance of immune cells, and immunotherapy response. Additionally, a nomogram was established with robust predictive power integrating the RiskScore and clinical features. Notably, we observed that PINK1 expression markedly correlated with favorable treatment response and advanced maturation stages of tertiary lymphoid structures, which potentially shed light on enhancing anti-tumor immunity of ccRCC.
Conclusion
Collectively, this study initially developed a signature associated with mitophagy, which demonstrated an excellent ability to predict the clinical prognosis, TME characterization, and responsiveness to targeted therapy and immunotherapy for ccRCC patients. Of particular note is the pivotal role of PINK1 in mediating the treatment response and immune microenvironment for ccRCC patients.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00432-023-05349-y.
Keywords: Clear cell renal cell carcinoma, Mitophagy, Prognosis, Tumor microenvironment, Immunotherapy response, PINK1
Introduction
Renal cell carcinoma (RCC) is the most common type of kidney cancer, accounting for approximately 90% of cases worldwide. Its incidence varies globally, with higher rates reported in developed countries (Zheng et al. 2016; Siegel et al. 2023). It is estimated that there are around 403,000 new cases of RCC diagnosed worldwide each year. In the US, RCC represents approximately 2–3% of all adult malignancies (Siegel et al. 2023). The disease bears significant responsibility for worldwide cancer-related mortality, with roughly 175,000 annual deaths attributed to RCC (Braun et al. 2021).
Renal cell carcinoma has several histological subtypes, including clear cell renal cell carcinoma (ccRCC), papillary renal cell carcinoma, chromophobe renal cell carcinoma, and other rare subtypes (Li 2022a). Predominant among these is clear cell carcinoma, encompassing 70–80% of RCC occurrences. Treatment of ccRCC depends on various factors, including the clinical staging, pathological grading, and histological subtype of the tumor. Despite therapeutic advancements, resistance to therapy and disease relapse remain challenges in managing advanced ccRCC (Chevrier et al. 2017). Molecular biomarkers, such as von Hippel–Lindau (VHL) gene mutation status, loss of heterozygosity (LOH) in specific chromosomal regions, and gene expression levels (e.g., PBRM1, BAP1, and SETD2), have exhibited associations with patient outcomes and responsiveness to targeted treatments (Xu et al. 2022; Xu, et al. 2022a; Zaffuto et al. 2016). The integration of these molecular biomarkers into prognostic models holds potential for precise risk stratification and personalized therapeutic strategies (Peter et al. 2021; Senbabaoglu et al. 2016). Therefore, the identification of dependable biomarkers or models for early diagnosis, prognostic assessment, and treatment response evaluation constitutes a persistent challenge in RCC research. Individualized precision medicine could help improve diagnostic accuracy, guide treatment decisions, and monitor disease progression of ccRCC (Vesely et al. 2022; Xu et al. 2019a).
Mitophagy, which is the selective degradation of damaged or dysfunctional mitochondria, plays a pivotal role in maintaining cellular homeostasis and preventing the accumulation of defective mitochondria (Doblado et al. 2021). In the context of carcinoma, arising from epithelial cells, mitophagy's impact on tumor genesis and progression embodies dual facets of benefit and detriment (Poole and Macleod 2021). In its normative role, mitophagy serves as a tumor suppressive mechanism, eliminating compromised mitochondria that amass reactive oxygen species (ROS) and incite genomic instability, precipitating carcinogenesis (Panigrahi et al. 2020; Korolchuk et al. 2017). Through the removal of dysfunctional mitochondria, mitophagy preserves mitochondrial integrity, counteracting mutational buildup that might foment cancer emergence.
Nonetheless, excess or dysregulated mitophagy can promote tumor advancement, counteracting its initial tumor suppressive role. In some cases, enhanced mitophagy can contribute to the survival and growth of cancer cells by eliminating damaged mitochondria that trigger programmed or immunogenic cell death (Poole and Macleod 2021; Xu 2023). This allows cancer cells to evade cell death signals and proliferate uncontrollably. In cancer cells, mitophagy can also support metabolic adaptation by selectively removing damaged mitochondria that produce less ATP and replacing them with functional mitochondria that enhance energy production. This metabolic rewiring empowers cancer cells to meet augmented energy demands and support tumor growth. Some studies suggest mitophagy's involvement in conferring resistance to therapies in carcinoma (Luo 2023). Cancer cells with elevated mitophagy level may be better equipped to survive harsh conditions, such as nutrient deprivation or exposure to chemotherapy and radiation (Poole and Macleod 2021). By eliminating damaged mitochondria and maintaining mitochondrial function, cancer cells augment survival and withstand the impacts of anti-cancer interventions.
RCC is characterized by hypoxic conditions resulting from aberrant tumor vascularization (Xu et al. 2021a; Qu et al. 2022). Within a hypoxic milieu, low oxygen concentrations stimulate mitophagy as an adaptive response to maintain mitochondrial quality and function, and mitophagy can help to preserve cellular energy and promote cell survival (Gilkes et al. 2014). However, excessive or prolonged mitophagy can induce mitochondrial dysfunction and promote tumor aggressiveness. In this study, we aim to explore the effect of mitophagy on ccRCC malignancy in a large multi-ethnic population and establish a novel approach for facilitating individualized ccRCC diagnosis and treatment through the integration of multi-omics data and machine learning algorithms.
Materials and methods
Patients collection and data normalization
We utilized the publicly available transcriptome data and clinical information of 763 ccRCC patients from TCGA database (https://portal.gdc.cancer.gov/repository), CPTAC database (https://cptac-data-portal.georgetown.edu/studysummary/S050), ICGC database (https://icgc.org/), and EMBL database (https://www.ebi.ac.uk/arrayexpress/), including TCGA-KIRC, CPTAC-3, ICGC RECA-EU, and E-MTAB-3267 cohorts (Clark et al. 2019; Marquardt et al. 2021). The expression levels of the RNA-seq samples were transformed from fragments per kilobase of transcript per million mapped reads (FPKM) to transcripts per million (TPM), and log2(TPM + 1) was taken. Batch effects were corrected using the combat algorithm of the “sva” package in R software (Leek et al. 2012). In addition, copy number variations (CNVs) files, somatic mutation data, and tumor mutation burden (TMB) data were obtained from the TCGA database. The detailed baseline clinical data of ccRCC patients are summarized in Table S1.
This study included 94 Chinese patients with advanced ccRCC, who underwent first-line sunitinib at the Department of Urology of Fudan University Shanghai Cancer Center, during the period from January 2008 to December 2019. Pathological examinations identified tumor and tumor-adjacent tissue regions, with clinicopathological characteristics documented in Table S2.
Collection of mitophagy-related genes (MRGs)
MRGs set was required from the Molecular Signatures Database (MSigDB; https://www.gsea-msigdb.org/gsea/msigdb/human/search.jsp). We selected “REACTOME_MITOPHAGY” gene set and intersected the same genes between the gene set and the transcriptome data of above integrated cohort for further analyses (Table S2).
Unsupervised clustering analysis
Unsupervised clustering was executed to stratify patients into distinct molecular subtypes with the “ConsensusClusterPlus” package in R software (Hu et al. 2022). The consensus matrix, cumulative distribution function (CDF), and relative change in area under the CDF curve determined the optimal cluster number.
Exploration of the immune landscape in distinct subgroups
The CIBERSORT algorithm quantified immune cell proportions within the tumor microenvironment (TME) of each ccRCC sample (Hu et al. 2022). Expression levels of immune checkpoints (ICPs) were evaluated and contrasted among distinct clusters. Additionally, stromal and immune compositions in ccRCC samples were determined using the ESTIMATE algorithm (Xu et al. 2019b).
Identificationof differential expression genes (DEGs) and functional enrichment analysis
DEGs amid diverse mitophagy-related clusters were identified through the “limma” package in R software, applying criteria of adjusted P value < 0.05 and |log2-fold change (FC)|> 0.8. Gene Ontology (GO) annotation and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analyses were carried out via the “clusterProfiler” package in R software, with thresholds of p value and q value < 0.05. Gene set variation analysis (GSVA) using the KEGG gene set (c2.cp.kegg.v7.5.1) was executed across clusters, employing criteria of |log2-fold change (FC)|> 0.15 and adjusted P value < 0.001 (Xu et al. 2021b; Hanzelmann et al. 2013).
Construction and validation of a mitophagy-related predictive signature
Univariate Cox regression analysis with the DEGs identified the prognosis-related genes. Statistically significant DEGs (P < 0.001) were then used for the subsequent least absolute shrinkage and selector operation (LASSO) analysis and multivariate Cox analysis to construct the predictive signature (RiskScore) (Xu et al. 2019b). The RiskScore was computed with the RiskGenes as described: RiskScore = h0(t)* exp (∑expression of RiskGene* corresponding coefficient). Patients were stratified into low or high RiskGroup based on the median RiskScore.
Prognostic significance of the signature was assessed with Kaplan–Meier analysis, and stratified survival analyses for the patients in different clinical subgroups were conducted. Tumor immune dysfunction and exclusion (TIDE) analysis (http://tide.dfci.harvard.edu/) predicted patients' immunotherapy response, with lower TIDE scores indicating better response (Xu et al. 2021c; Jiang et al. 2018). Validation of immunotherapy response using the mitophagy-related model was performed on the immune checkpoint inhibitors (ICIs) received David Liu cohort, comprising 121 metastatic melanoma patients treated with nivolumab or pembrolizumab.
Development and validation of a nomogram
A nomogram was constructed combining the RiskScore and clinical traits to predict the 1-, 3-, and 5-year overall survival (OS) for ccRCC patients using the “rms,” “regplot,” and “survival” packages in R software. The reliability of the nomogram was assessed through ROC and calibration curves.
Immunohistochemistry (IHC) assays
Histopathological assessment of PINK1 expression on ccRCC slides followed established protocols, with independent evaluation by experienced pathologists. The slides of ccRCC tissue or adjacent normal kidney tissues were selected for staining, and the slides were placed on a baking machine at 56 ℃ for about 1 h. Immunohistochemical staining goes through a series of steps, including dewaxing, removal of endogenous peroxidase, antigen repair, washing, sealing, primary antibody incubation, secondary antibody incubation, stained with diaminobenzidine solution (DAB), dehydration, neutral gum sealing, and photography. The primary PINK1 polyclonal antibody (PA5-13402, Invitrogen, USA) applied to the tissue section and incubated overnight or for a designated period at an appropriate temperature. The specific implementation methods are as previously described (Zheng et al. 2021).
Multispectral immunofluorescence (mIF) staining assays
Tissue slides were prepared with primary and secondary antibodies, while excluding fluorophores, to establish negative controls for autofluorescence evaluation. Multiplex stained slides were scanned using a Vectra Polaris Quantitative Pathology Imaging System (Akoya Biosciences) at 20-nm wavelength intervals from 440 to 780 nm with a fixed exposure time and an absolute magnification of ✕200. All scans for each slide were then superimposed to obtain a single image. Multilayer images were imported to inForm v.2.4.8 (Akoya Biosciences) for quantitative image analysis. For the analysis of ccRCC immune microenvironment, specifically the context of tertiary lymphoid structures (TLS) maturation, pathological slides were costained for CD20, CD23, CD3, CD68, CDC163, pan-CK, and DAPI.
Statistical analysis
All statistical analyses and graph visualizations were implemented in R v4.1.3. The cutoff value was defined via “survminer” R package or median threshold. Statistical significance was set at p < 0.05 (two sides) unless otherwise indicated.
Results
Differential expression and genomic mutations of mitophagy-related genes (MRGs) in ccRCC
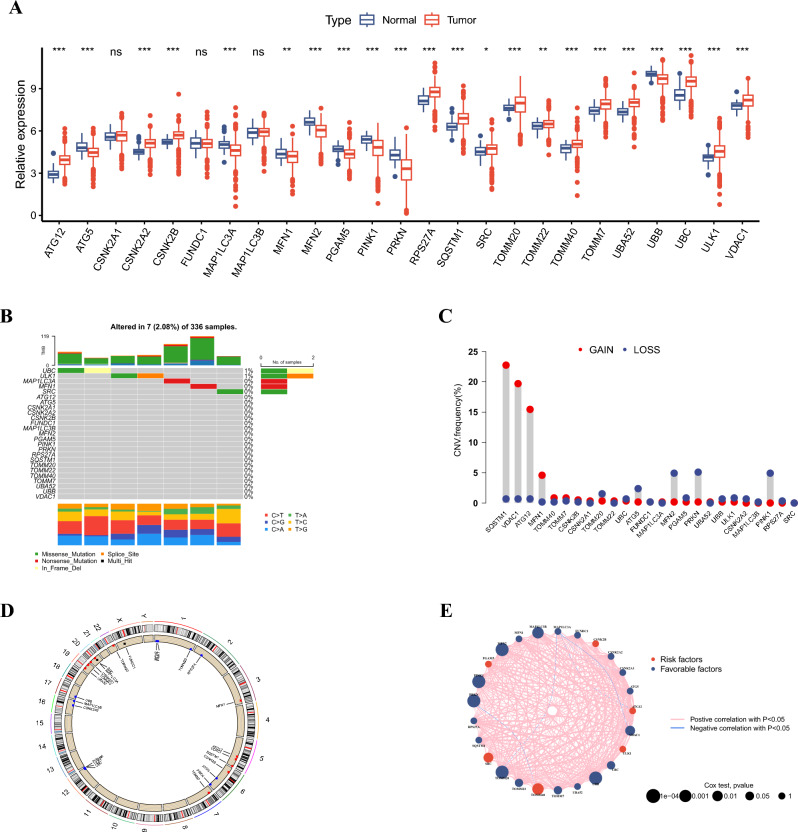
In our study, we analyzed the mRNA expression levels of 25 MRGs across ccRCC and normal tissues sourced from TCGA database (Fig. 1A). A total of 22 MRGs were differentially expressed at mRNA level, among which 14 MRGs were upregulated and eight MGRs were downregulated in ccRCC. Only seven in 336 ccRCC samples had mutations of the 25 MGRs, and UBC and ULK1 had the highest mutations frequencies (Fig. 1B). Additionally, we ascertained a profile of CNVs associated with the 25 MRGs, revealing noteworthy amplification tendencies in genes such as SQSTM1, VDAC1, ATG12, and MFN1. Conversely, genes such as TOMM20, ATG5, MFN2, PRKN, and PINK1 exhibited a greater frequency of deletion within CNVs (Fig. 1C). The chromosomal distribution of these 25 MRGs is displayed in Fig. 1D. Besides, an encompassing visualization of the interactions, correlations, and prognostic implications of the 25 MRGs is depicted in Fig. 1E.
Fig. 1.
Landscape of expression and genetic variations of MRGs in ccRCC. A Expression levels of MRGs in tumor and adjacent tissues of ccRCC patients from the TCGA-KIRC cohort. B Mutation frequency and types of MRGs in ccRCC from the TCGA-KIRC cohort. C CNV frequency of MRGs in ccRCC from the TCGA-KIRC cohort. D Location of CNV alteration of MRGs on chromosomes in ccRCC from the TCGA-KIRC cohort. E The interactions of MRGs in ccRCC from the TCGA-KIRC cohort
Identification of mitophagy-related clusters and characterization of distinct clusters
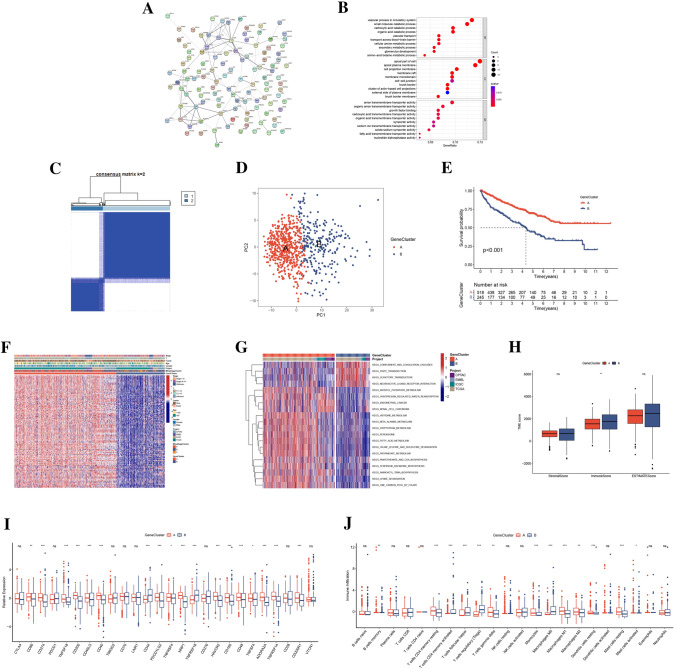
To further explore the role of mitophagy in ccRCC, we applied the unsupervised clustering algorithm to stratify ccRCC patients based on the mRNA expressions of the 25 MRGs. The optimal number of the identified clusters was 3 (370 patients in MitophagyCluster A, 305 patients in MitophagyCluster B, and 88 patients in MitophagyCluster C) (Fig. 2A and Table S3). The result was defined by the least crossover of the consensus matrix, the smooth trend of the CDF, and no significant shift of the area under the curve (Figure S2). Principal component analysis (PCA) further corroborated the discernible divergence among the three MitophagyClusters (Fig. 2B). Survival analysis revealed that patients in MitophagyCluster A had best prognosis (Fig. 2C). The varying clinicopathological features and expression profiles of the 25 MRGs among MitophagyClusters were captured within a heatmap, with patients in MitophagyCluster A displaying heightened MRG expression levels (Fig. 2D). Functional enrichment analysis of GSVA results unveiled distinct pathway enrichments in MitophagyClusters A, B, and C, elucidating diverse biological contexts (Fig. 2E–G). These pathways encompassed mTOR signaling, ubiquitin-mediated proteolysis, renal cell carcinoma, aminoacyl tRNA biosynthesis within MitophagyCluster A, and underscoring the intricate interplay of molecular processes. Moreover, an in-depth exploration of the TME features, particularly the immune landscape, was undertaken across the different MitophagyClusters. It emerged that MitophagyCluster A exhibited elevated stromal scores alongside reduced immune scores (Fig. 2H and Table S4). Patients in MitophagyCluster A had lowest expression of CTLA4, PDCD1, TMIGD2, CD70, and TNFRSF18 and highest expression of CD274, TNFSF18, CD200, CD40, PDCD1LG2, NRP1, HAVCR2, CD160, TNFSF4, ADORA2A, TNFRSF14, and VTCN1 (Fig. 2I). The CIBERSORT analysis showed that the abundance of immune cells was varied among distinct MitophagyClusters (Fig. 2J and Table S5). Collectively, the MitophagyClusters unveiled distinct immunophenotypes with pronounced implications for the immune microenvironment.
Fig. 2.
Clinical and TME characteristics of mitophagy-related clusters. A Consensus matrix heatmap of unsupervised clustering analysis. B PCA analysis of MRGs expression difference among three MitophagyClusters. C Survival analysis of three MitophagyClusters. D Heatmap of clinicopathological features and expressions of MRGs among MitophagyClusters. E–G GSVA of enriching biological pathways among three MitophagyClusters. H The differences in estimate, stromal, and immune scores of MitophagyClusters. I The differences in expressions of ICPs among three MitophagyClusters. J The difference in infiltrating abundance of immune cells among three MitophagyClusters
Functional enrichment and identification of gene subgroups based on the DEGs
To further explore the biological characteristics of the MitophagyClusters, we identified 112 MitophagyClusters-related DEGs through the “limma” package (Table S6). Elucidating the interconnectivity among these DEGs, a protein–protein interaction (PPI) network was constructed utilizing the STRING database (Fig. 3A). Subsequent GO enrichment analysis highlighted enrichment within biological processes such as vascular process in circulatory system, small-molecule catabolic process, apical part of cell, apical plasma membrane, anion transmembrane transporter activity, and growth factor binding, etc. (Fig. 3B). Following this, uniCox analysis unveiled a pool of 110 prognosis-related DEGs, offering a comprehensive perspective on the clinical relevance of these genes.
Fig. 3.
Functional enrichment analysis and identification of GeneClusters. A PPI network of the DEGs from STRING database. B GO enrichment analysis of DEGs between two GeneClusters. C Consensus matrix heatmap of unsupervised clustering analysis. D PCA analysis of expression difference in prognosis-related genes between two GeneClusters. E Survival analysis of two GeneClusters. F Heatmap of clinicopathological features and expression of prognosis-related genes between two GeneClusters. E–G GSVA of enriching biological pathways between two GeneClusters. H The differences in estimate, stromal, and immune scores of GeneClusters. I The differences in expression of ICPs between two GeneClusters. J The difference in infiltrating abundance of immune cells between two GeneClusters
Based on the 110 prognosis-related DEGs, we conducted the unsupervised clustering algorithm again to stratify the patients into different subgroups, termed as GeneCluster (Fig. 3C and Table S3). We displayed the CDF plot, delta plot, and tracking plot in Figure S3. PCA analysis underscored substantial transcriptome distinctions between GeneCluster A and GeneCluster B (Fig. 3D). Furthermore, superior clinical prognosis was demonstrated among patients situated within GeneCluster A (Fig. 3E). We found that patients in GeneCluster A were tended to be stagesI–II, T1–T2, and in MitophagyCluster A (Fig. 3F). Pathway enrichment analysis through GSVA illuminated an array of enriched pathways, serving to delineate the functional contexts of GeneCluster A and GeneCluster B (Fig. 3G). Furthermore, we analyzed the immune TME features between the GeneClusters A and B. We found that patients in GeneCluster A had lower immune score, implying lower abundance of immune fraction (Fig. 3H). Besides, we compared the expressions of ICPs and abundance of infiltrating immune cells between the two GeneClusters. Patients in GeneCluster A had low expressed of CD44 and TNFRSF18 and high expressed of CD86, CD274, TNFSF18, CD200, CD40LG, CD40, PDCD1LG2, TNFRSF4, NRP1, HAVCR2, CD160, CD48, TNFSF4, etc. (Fig. 3I). Patients in GeneCluster B showed higher infiltrating level of T cells CD4 memory activated, Tregs, macrophages M0, and neutrophils and lower abundance of T cells gamma delta, monocytes, macrophages M1, and macrophages M2 (Fig. 3J).
Development of the mitophagy-related prognostic signature
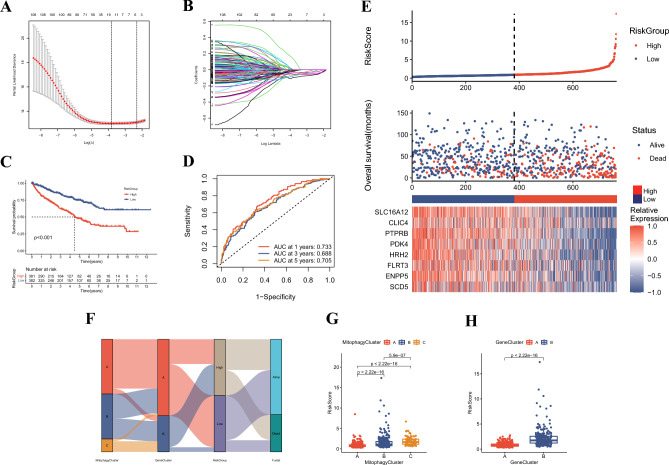
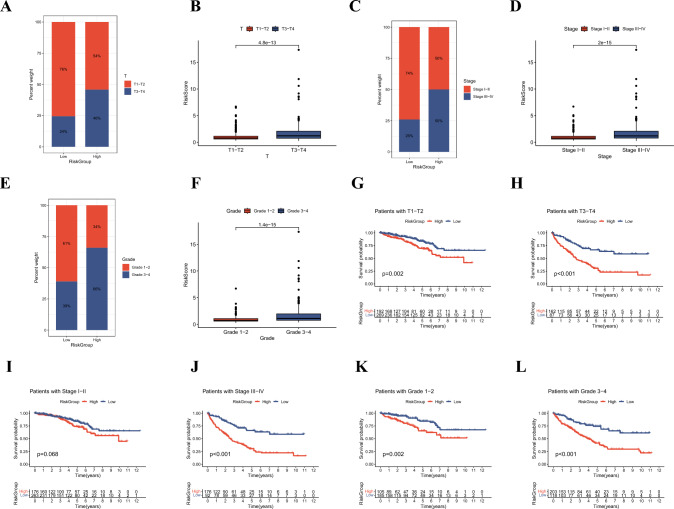
To comprehensively quantify the mitophagy-related pattern in ccRCC, we applied multivariate Cox and LASSO analyses to construct a prognostic predictive signature, termed as RiskScore (Fig. 4A, B and Table S7). We identified eight risk genes with most prognostic significance, including CLIC4, PTPRB, SLC16A12, ENPP5, FLRT3, HRH2, PDK4, and SCD5. The patients were divided into high RiskGroup and low RiskGroup by the median RiskScore (Table S3). Survival analysis revealed that patients in high RiskGroup suffered worse prognosis (Fig. 4C). The AUCs of 1, 3, and 5 years of ROC curves were 0.733, 0.688, and 0.705, implying excellent predictive power of the model (Fig. 4D). The distribution plot showed that as the RiskScore increased, the mortality rate of ccRCC patients went up (Fig. 4E). Figure 4F displays the distribution of patients across MitophagyCluster, GeneCluster, and RiskGroup. We found that patients in MitophagyCluster A and GeneCluster A had lower RiskScore, consistent with the improved clinical prognosis (Fig. 4 G-H). To further evaluate the predictive ability of the prognostic signature, we stratified patients into two subgroups according to different clinical characteristics and analyzed the difference in prognosis between the two subgroups. We found that male patients with advanced T stage, TNM stage, and grade had higher RiskScore, and no significant difference was found for age (Figs. 5A–F and S4A-D). Besides, across all subgroups except stagesI–II, patients in the high RiskGroup consistently exhibited diminished prognoses (Figs. 5G–L and S4E-H).
Fig. 4.
Construction of the mitophagy-related predictive signature. A-B Coefficient profiles of the prognosis-related genes and identification of the best parameter in LASSO analysis. C Kaplan–Meier analysis of the OS between two RiskGroups. D ROCs analysis of RiskScore in predicting the 1-, 3-, and 5-year OS. E Distribution plot of RiskScore, OS status, and expression of risk genes. F Alluvial diagram of patients distributions in MitophagyClusters, GeneClusters, and RiskGroups. G Differences in RiskScore among three MitophagyClusters. H Differences in RiskScore between two GeneClusters
Fig. 5.
Correlation between RiskGroup and clinical characteristics. A-F Proportion of clinical features (T stage, TNM stage, and grade) in low and high RiskGroup. G-L Survival analysis of ccRCC patients between two RiskGroups in different subgroups stratified by clinical features (T stage, TNM stage, and grade)
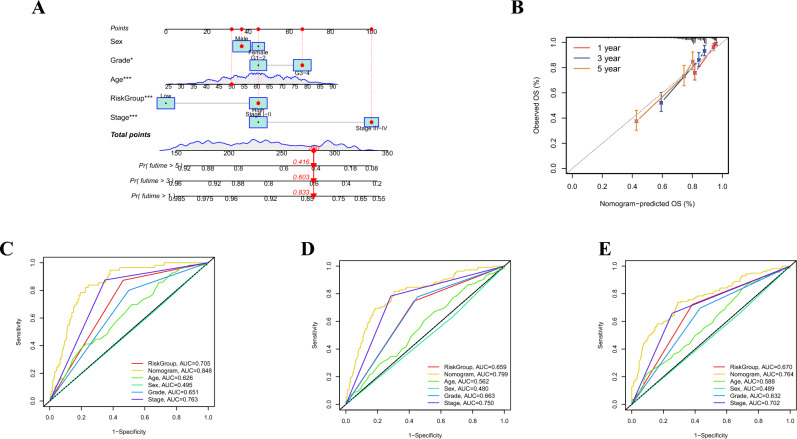
Construction and validation of a prognostic nomogram
Subsequent to integrating the RiskScore alongside salient clinical attributes, an insightful nomogram was developed to predict the 1-, 3-, and 5-year overall survival (OS) for ccRCC patients. We found that age, grade, TNM stage, and RiskGroup were significant predictive features of the nomogram (Fig. 6A). The calibration plot effectively demonstrated an excellent alignment between actual OS and the nomogram-predicted OS for ccRCC patients (Fig. 6B). Moreover, the predictive potency of the nomogram was further assessed through the utilization of ROC curves. Impressively, the respective AUCs for 1-, 3-, and 5-year OS were 0.848, 0.799, and 0.764, thereby underscoring the nomogram's robust predictive efficacy (Fig. 6C–E).
Fig. 6.
Establishment and validation of a nomogram. A The nomogram for predicting the 1-, 3-, and 5-year OS based on the RiskGroup and clinicopathological features. B Calibration curves for validation of the nomogram. C-E ROCs of the nomogram for predicting the 1-, 3-, and 5-year OS
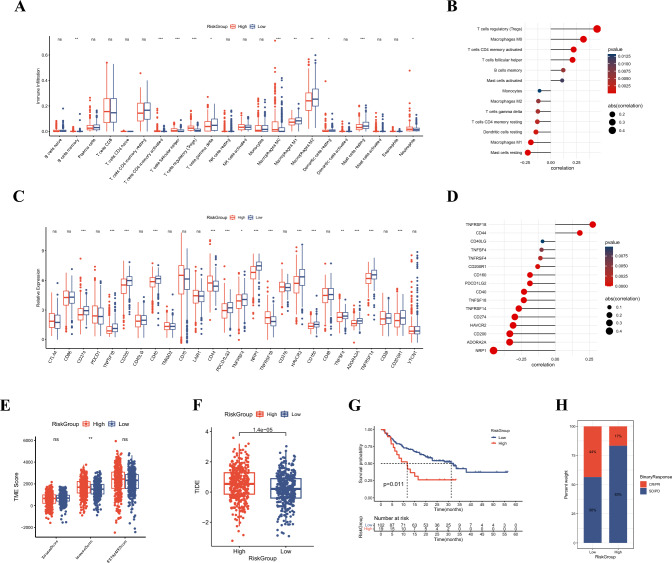
Exploration the immune microenvironment and immunotherapy response of the riskgroups
We first analyzed the abundance of infiltrating immune cells between the high and low RiskGroups. This analysis illuminated the heightened infiltration of Tregs, macrophages M0, and T cells CD4 memory activated within the high RiskGroup, demonstrating a positive correlation with RiskScore. Conversely, mast cells resting, macrophages M1, T cells gamma delta, and macrophages M2 exhibited reduced infiltration within the high RiskGroup, correlating negatively with the RiskScore (Fig. 7A, B). Considering the significance of ICIs in the clinical treatment for ccRCC, we compared the difference in expression levels of ICPs between the two RiskGroups. TNFRSF18 and CD44 were high expressed in high RiskGroup and positively correlated with the RiskScore (Fig. 7C, D). In contrast, NRP1, ADORA2A, CD200, CD274, and PDCD1LG2 exhibited reduced expression within the high RiskGroup, coupled with a negative correlation to RiskScore (Fig. 7C, D). Employing the ESTIMATE algorithm, we computed stromal, immune, and estimate scores, which indicated higher immune scores within the high RiskGroup, indicative of elevated immune fractions (Fig. 7E). Subsequently, we conducted TIDE algorithm to assess the immunotherapy response of the RiskScore. Patients in low RiskGroup had lower TIDE score, indicative of heightened sensitivity to immunotherapy (Fig. 7F). To rigorously evaluate the predictive utility of the RiskScore within the context of immunotherapy response, external validation was performed within the David Liu cohort. This validation corroborated that the high RiskGroup was associated with poorer prognosis and a diminished rate of complete or partial response (CR/PR), thereby affirming the predictive power of the RiskScore in immunotherapy response (Fig. 7G, H).
Fig. 7.
Evaluation of immune TME and immunotherapy response of RiskScore. A The difference in infiltrating abundance of immune cells between two RiskGroups. B The correlation between RiskScore and infiltrating abundance of immune cells. C The differences in expression of ICPs between two RiskGroups. D The correlation between RiskScore and expression of ICPs. E The differences in estimate, stromal, and immune scores of RiskGroups. F The differences in TIDE score between two RiskGroups. G Survival analysis of patients stratified by RiskScore in David Liu cohort. H The differences in immunotherapy response between two RiskGroups in David Liu cohort
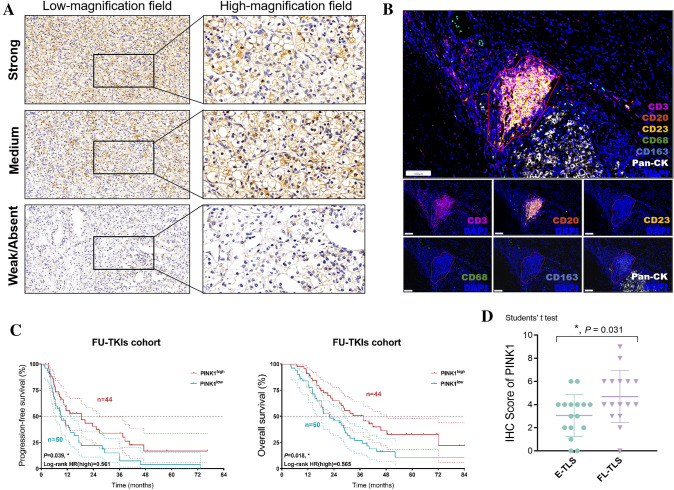
Role of PTEN-induced putative kinase 1 (PINK1) in clinical prognosis and immune microenvironment of ccRCC
PTEN-induced putative kinase 1 (PINK1)-mediated mitophagy is a specific pathway involved in the selective removal of damaged or dysfunctional mitochondria. While PINK1-mediated mitophagy has been extensively studied in the context of neurodegenerative diseases, such as Parkinson's disease. However, its role within the realm of cancer, including ccRCC, remains an area of ongoing elucidation. In light of this, we endeavored to elucidate the clinical ramifications of PINK1-mediated mitophagy within ccRCC, offering potential insights into therapeutic strategies. Thus, we assessed expression of PINK1 in ccRCC slides and displayed the images in both high- and low-magnification fields (Fig. 8A). To evaluate the maturation dynamics of tertiary lymphoid structures (TLS), co-staining of slides for CD3, CD20, CD21, and CD23 was performed (Helmink et al. 2020; Horeweg et al. 2022; Xu et al. 2022b). CD68 and CD163 signals were also detected on the relationship between PINK1 expression and M2 macrophages infiltration. All dense lymphocytic aggregates irrespective of CD45/CD20/CD21/CD23 signals were imaged as multispectral high-power field (HPF) on the Vectra 3.0 imaging system (PerkinElmer). Each HPF image was assessed for the TLS maturation stage as follows: early TLS (E-TLS), dense lymphocytic aggregates with mixed B and T cells, lacking CD23 signals; follicle-like TLS (FL-TLS), dense lymphocytic aggregates with CD23 signals, indicating the presence of a germinal center (both FDC and GC reaction) (Fig. 8B) (Silina et al. 2018). Subsequently, we validated our observations within an external cohort enrolling a total of 94 Chinese patients with ccRCC receiving tyrosine kinase inhibitors (TKIs), particularly sunitinib, post-partial, or radical nephrectomy in the FUSCC. The results suggested that ccRCC patients with higher expression of PINK1 exhibited significantly enhanced PFS (P = 0.039, HR = 0.561) and OS (P = 0.018, HR = 0.565) (Fig. 8C). In more mature TLS, immune cells become better equipped to recognize and attack tumor cells. B cells can produce antibodies targeting tumor antigens, T cells can be activated to directly attack cancer cells, and dendritic cells can present tumor antigens to other immune cells, initiating a coordinated immune response. Interestingly, we found a trend that elevated PINK1 expression markedly correlated with advanced maturation stages of TLS, which may shed light on augmented anti-tumor immune milieu of the ccRCC sample (Fig. 8D).
Fig. 8.
Effects of PINK1 in the clinical prognosis and immune microenvironment of ccRCC. A The images of PINK1 expression in both high- and low-magnification fields using IHC staining analysis. B Evaluation of the maturation stage of tertiary lymphoid structures (TLS) by multispectral immunofluorescence analysis. C Survival analysis of patients stratified by PINK1 in FUSCC cohort receiving TKIs. D Differential PINK1 expression with advanced maturation stages of TLS
Discussion
With rapid advancement in precision medicine and high-throughput approaches, several prognostic models incorporating various clinical and pathological factors, such as tumor stage, grade, size, and lymph node involvement, have been developed to assess the risk and estimate prognosis in ccRCC patients (Qu et al. 2022; Chen et al. 2016). Well-established prognostic models include the Memorial Sloan Kettering Cancer Center (MSKCC), the International Metastatic Renal Cell Carcinoma Database Consortium (IMDC) scores, and other multi-omics models (Li 2022a; Yang et al. 2019). In our study, we estimated the predictive potency and biological ramifications of mitophagy in ccRCC with multi-omics data from large-scale populations, which facilitates patient prognosis estimation and guides therapeutic decision-making.
Targeted therapies have revolutionized the landscape of ccRCC therapeutics. Inhibitors targeting the vascular endothelial growth factor (VEGF) pathway, such as sunitinib, pazopanib, and axitinib, have demonstrated efficacy in advanced and metastatic ccRCC. Additionally, TKIs, such as everolimus and temsirolimus, have shown remarkable efficacy in certain patient populations (Capitanio et al. 2021). These targeted therapies improve progression-free survival and overall survival rates compared to traditional chemotherapy. In this study, we incorporated a validation cohort enrolling 94 Chinese ccRCC patients, all recipients of TKIs, specifically sunitinib, following partial or radical nephrectomy in FUSCC. ccRCC patients exhibiting elevated PINK1 expression experience notably favorable clinical outcomes in contrast with their counterparts demonstrating subdued PINK1 expression. This discernment underscores the potential of PINK1-mediated mitophagy as a predictive marker for positive responses and extended survival within the ambit of adjuvant targeted therapies.
Mitophagy in ccRCC can play a role in metabolic adaptation within the TME. Dysfunctional mitochondria generate reactive oxygen species (ROS) and contribute to metabolic perturbations (Xu et al. 2022c; Li et al. 2023). Mitophagy orchestrates the targeted elimination of damaged mitochondria, thereby sustaining mitochondrial integrity and cellular energy homeostasis. This process allows cancer cells to selectively eliminate mitochondria with impaired function and replace them with functional mitochondria, thereby supporting the increased energy demands of tumor cells in the TME (Geissler et al. 2015). Dysregulated mitophagy, which results in the accumulation of damaged mitochondria, increased levels of reactive oxygen species (ROS), and cellular stress, has been implicated in promoting tumor progression and aggressiveness in cancers (Siska et al. 2017). While dysregulated mitophagy has been linked to promoting tumor progression and aggressiveness in certain cancers like ccRCC, it is not a universal predictor for all cancer patients. The interplay between mitophagy and tumor behavior is highly complex and influenced by multiple factors. Therefore, while understanding mitophagy's role can provide valuable insights into cancer biology, it cannot serve as a definitive predictor of tumor behavior for all individuals with cancer. Further research is needed to explore the specifics of this relationship in various cancer types and to develop a deeper understanding of how mitophagy contributes to cancer progression in different contexts.
In ccRCC, dysregulated mitophagy can contribute to the altered metabolic state of tumor cells and provide a growth advantage. Mitophagy may influence the redox balance and oxidative stress within the TME of ccRCC. Dysfunctional mitochondria contribute to the accumulation of ROS, which, in turn, can promote tumorigenesis and tumor progression (Luo 2023). By selectively eliminating damaged mitochondria through mitophagy, ccRCC cells can reduce ROS levels and maintain redox homeostasis. This can impact the oxidative stress levels in the TME and potentially influence tumor cell proliferation and survival.
Mitochondria are critical organelles involved in various cellular processes, including energy production and programmed cell death. If the mitochondria are dysfunctional or not functioning optimally due to abnormal mitophagy, cancer cells might become less susceptible to treatment-induced apoptosis. This cellular heterogeneity could contribute to variable responses to treatment and the emergence of drug-resistant populations within the tumor (Siska et al. 2017). Understanding these mechanisms is crucial for developing effective treatment strategies that can overcome or exploit this potential resistance mechanism. The impact of PINK1-mediated mitophagy within ccRCC extends to the realm of immune responses within the TME (Xu et al. 2022c; Wang et al. 2018). Mitochondria interface with immune signaling pathways, capable of releasing danger-associated molecular patterns (DAMPs) that activate immune responses. Mitophagy may modulate the release of DAMPs alongside metabolic dysregulation, thereby influencing immune responses and interactions between tumor and stromal components within the TME (Zheng et al. 2021; Tang et al. 2023; Anwaier et al. 2023). The regulation of mitophagy in ccRCC cells can mediate immune surveillance, immune cell infiltration, and potentially impact the response to immunotherapies (Hunter et al. 2021; Tian et al. 2023).
Within the realm of ccRCC treatment, ICIs, particularly those targeting the programmed cell death protein 1 (PD-1) pathway, have demonstrated remarkable efficacy (Braun et al. 2021). Agents such as nivolumab and pembrolizumab have demonstrated durable responses and improved survival outcomes, especially in patients with advanced and metastatic disease. Combination therapies involving ICIs and targeted agents are being explored to enhance therapeutic efficacy (Cai et al. 2019). Our utilization of the TIDE algorithm revealed that patients in the low RiskGroup exhibited lower TIDE scores, implying heightened sensitivity to immunotherapy (Jiang et al. 2018). Furthermore, external validation within the David Liu cohort reaffirmed the correlation between high RiskGroup categorization and adverse prognostic outcomes. This underscores the significance of the mitophagy-related signature in predicting responses to standard adjuvant therapies, such as the combination of nivolumab and ipilimumab. Besides, the treatment of ccRCC is increasingly moving toward combination therapies, leveraging the benefits of different treatment modalities, and the PINK1-mediated mitophagy contributed to elevated frequency and advanced maturation of TLS (Meylan et al. 2022; Munoz-Erazo et al. 2020). It is mutually confirmed that, by eliminating damaged mitochondria, ccRCC cells can remove sources of cellular stress and enhance their survival in the face of anticancer treatments (Kim et al. 2021). This resilience to therapeutic interventions holds potential implications for the efficacy of conventional treatments, inclusive of targeted therapies and immunotherapies (Vesely et al. 2022; Li 2022b).
Conclusion
Collectively, this study first comprehensively demonstrated the effects of mitophagy on the TME characteristics, clinical prognosis, and treatments response in ccRCC patients, which may contribute to more precise treatment strategies for ccRCC patients. Besides, we found that PINK1 markedly correlated with treatment response and reshaped immune TME in congruence with advanced maturation stages of tumor-associated tertiary lymphoid structures in ccRCC.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file1 SUPPLEMENTARY FIGURE 1. Survival analysis of ccRCC patients stratified by the expression of MRGs. (A-H) Kaplan–Meier analysis of the OS of ccRCC patients stratified by the expression of MRGs. SUPPLEMENTARY FIGURE 2. Generation of mitophagy-related clusters by unsupervised clustering analysis. (A-H) Consensus matrix heatmaps of unsupervised clustering from k = 2 to k = 9. (I-K) The cumulative distribution function plot, delta plot, and tracking plot from k = 2 to k = 9. SUPPLEMENTARY FIGURE 3. Development of GeneClusters by unsupervised clustering analysis. (A-H) Consensus matrix heatmaps of unsupervised clustering from k = 2 to k = 9. (I-K) The cumulative distribution function plot, delta plot, and tracking plot from k = 2 to k = 9. SUPPLEMENTARY FIGURE 4. Correlation between RiskGroup and clinical features. (A-F) Proportion of clinical features (age and gender) in low and high RiskGroup. (G-L) Survival analysis of ccRCC patients between two RiskGroups in different subgroups stratified by clinical features (age and gender). (PDF 1834 KB)
Acknowledgements
We are grateful to all patients for their dedicated participation in the study.
Abbreviations
- ccRCC
Clear cell renal cell carcinoma
- CDF
Cumulative distribution function
- CNVs
Copy number variations
- CPTAC
Clinical Proteomic Tumor Analysis Consortium
- DAMPs
Danger-associated molecular patterns
- DCA
Decision curve analysis
- DEGs
Differential expression genes
- EMBL
European Molecular Biology Laboratory
- FPKM
Fragments per kilobase of transcript per million
- FUSCC
Fudan University Shanghai Cancer Center
- GO
Gene Ontology
- GSVA
Gene set variation analysis
- ICGC
International Cancer Genome Consortium
- ICIs
Immune checkpoint inhibitors
- ICPs
Immune checkpoints
- IHC
Immunohistochemistry
- IMDC
International Metastatic Renal Cell Carcinoma Database Consortium
- KEGG
Kyoto Encyclopedia of Genes and Genomes
- LASSO
Least absolute shrinkage and selector operation
- LOH
Loss of heterozygosity
- mIF
Multispectral immunofluorescence
- MRGs
Mitophagy-related genes
- MSKCC
Memorial Sloan Kettering Cancer Center
- OS
Overall survival
- PCA
Principal component analysis
- PD-1
Programmed cell death protein 1
- PINK1
PTEN-induced putative kinase 1
- PPI
Protein–protein interaction
- RCC
Renal cell carcinoma
- ROC
Receiver operating characteristic
- ROS
Reactive oxygen species
- ssGSEA
Single-sample gene set enrichment analysis
- TCGA
The cancer genome atlas
- TIDE
Tumor immune dysfunction and exclusion
- TKI
Tyrosine kinase inhibitors
- TMB
Tumor mutation burden
- TME
Tumor microenvironment
- TPM
Transcripts per million
- VEGF
Vascular endothelial growth factor
- VHL
Von Hippel–Lindau
Author contributions
JY, JX, and WL: helped in conceptualization. JX, WL, and SL: contributed to data curation and formal analysis. JX, WL, and HT: worked in investigation and methodology. HT and TW: worked in resources and software. JY, HT, and TW: worked in supervision. JX, JY, and WL: contributed to original draft. JY, HT, and TW: helped in editing.
Funding
This study was partially supported by the grant of Longhua Hospital of Shanghai University of Traditional Chinese Medicine (YW002.017).
Data availability
The datasets used and/or analyzed in the study are available from the corresponding author upon reasonable request.
Declarations
Conflict of interest
The authors report no potential conflicts of interest.
Ethical approval and consent to participate
The study design and test procedures followed the Helsinki Declaration II. The ethics approval and consent to participate of the Urology and Pathology departments in this study were approved by the ethics committee of Renji Hospital, Shanghai Jiao Tong University School of Medicine.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jianfeng Xiang and Wangrui Liu Contribute equally to this work.
Contributor Information
Tao Wang, Email: 13061931996@163.com.
Haidan Tang, Email: m13481699318@163.con.
Jianfeng Yang, Email: yangjianfeng@shutcm.edu.cn.
References
- Anwaier A et al (2023) Tumor microenvironment-based signatures distinguish intratumoral heterogeneity, prognosis, and immunogenomic features of clear cell renal cell carcinoma. J Natl Cancer Center. 10.1016/j.jncc.2023.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun DA et al (2021) Progressive immune dysfunction with advancing disease stage in renal cell carcinoma. Cancer Cell 39(5):632-648 e8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai J et al (2019) The role of PD-1/PD-L1 axis and macrophage in the progression and treatment of cancer. J Cancer Res Clin Oncol 145(6):1377–1385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capitanio U et al (2021) Parenchymal biopsy in the management of patients with renal cancer. World J Urol 39(8):2961–2968 [DOI] [PubMed] [Google Scholar]
- Chen W et al (2016) Cancer statistics in China, 2015. CA Cancer J Clin 66(2):115–132 [DOI] [PubMed] [Google Scholar]
- Chevrier S et al (2017) An immune atlas of clear cell renal cell carcinoma. Cell 169(4):736-749 e18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark DJ et al (2019) Integrated proteogenomic characterization of clear cell renal cell carcinoma. Cell 179(4):964-983 e31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doblado L et al (2021) Mitophagy in human diseases. Int J Mol Sci 22(8):3903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geissler K et al (2015) Immune signature of tumor infiltrating immune cells in renal cancer. Oncoimmunology 4(1):e985082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilkes DM, Semenza GL, Wirtz D (2014) Hypoxia and the extracellular matrix: drivers of tumour metastasis. Nat Rev Cancer 14(6):430–439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanzelmann S, Castelo R, Guinney J (2013) GSVA: gene set variation analysis for microarray and RNA-seq data. BMC Bioinformatics 14:7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmink BA et al (2020) B cells and tertiary lymphoid structures promote immunotherapy response. Nature 577(7791):549–555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horeweg N et al (2022) Tertiary lymphoid structures critical for prognosis in endometrial cancer patients. Nat Commun 13(1):1373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X et al (2022) Bioinformatics-led discovery of osteoarthritis biomarkers and inflammatory infiltrates. Front Immunol 13:871008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter MV et al (2021) Spatially resolved transcriptomics reveals the architecture of the tumor-microenvironment interface. Nat Commun 12(1):6278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang P et al (2018) Signatures of T cell dysfunction and exclusion predict cancer immunotherapy response. Nat Med 24(10):1550–1558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MC et al (2021) Updates on immunotherapy and immune landscape in renal clear cell carcinoma. Cancers (Basel) 13(22):5856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korolchuk VI et al (2017) Mitochondria in cell senescence: is mitophagy the weakest link? EBioMedicine 21:7–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leek JT et al (2012) The sva package for removing batch effects and other unwanted variation in high-throughput experiments. Bioinformatics 28(6):882–883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y et al (2022a) Histopathologic and proteogenomic heterogeneity reveals features of clear cell renal cell carcinoma aggressiveness. Cancer Cell 41(1):139–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X et al (2022b) Immune checkpoint blockade in pancreatic cancer: trudging through the immune desert. Semin Cancer Biol. 10.1016/j.semcancer.2022.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y et al (2023) PINK1-mediated mitophagy promotes oxidative phosphorylation and redox homeostasis to induce drug-tolerant persister cancer cells. Cancer Res 83(3):398–413 [DOI] [PubMed] [Google Scholar]
- Luo J et al (2023) Enhanced mitophagy driven by ADAR1-GLI1 editing supports the self-renewal of cancer stem cells in hepatocellular carcinoma. Hepatology. 10.1097/HEP.0000000000000299 [DOI] [PubMed] [Google Scholar]
- Marquardt A et al (2021) Subgroup-independent mapping of renal cell carcinoma-machine learning reveals prognostic mitochondrial gene signature beyond histopathologic boundaries. Front Oncol 11:621278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meylan M et al (2022) Tertiary lymphoid structures generate and propagate anti-tumor antibody-producing plasma cells in renal cell cancer. Immunity 55(3):527-541 e5 [DOI] [PubMed] [Google Scholar]
- Munoz-Erazo L et al (2020) Tertiary lymphoid structures in cancer—considerations for patient prognosis. Cell Mol Immunol 17(6):570–575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panigrahi DP et al (2020) The emerging, multifaceted role of mitophagy in cancer and cancer therapeutics. Semin Cancer Biol 66:45–58 [DOI] [PubMed] [Google Scholar]
- Peter MR et al (2021) Investigating urinary circular RNA biomarkers for improved detection of renal cell carcinoma. Front Oncol 11:814228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole LP, Macleod KF (2021) Mitophagy in tumorigenesis and metastasis. Cell Mol Life Sci 78(8):3817–3851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu Y et al (2022) A proteogenomic analysis of clear cell renal cell carcinoma in a Chinese population. Nat Commun 13(1):2052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senbabaoglu Y et al (2016) Tumor immune microenvironment characterization in clear cell renal cell carcinoma identifies prognostic and immunotherapeutically relevant messenger RNA signatures. Genome Biol 17(1):231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel RL et al (2023) Cancer statistics, 2023. CA Cancer J Clin 73(1):17–48 [DOI] [PubMed] [Google Scholar]
- Silina K et al (2018) Germinal centers determine the prognostic relevance of tertiary lymphoid structures and are impaired by corticosteroids in lung squamous cell carcinoma. Cancer Res 78(5):1308–1320 [DOI] [PubMed] [Google Scholar]
- Siska PJ et al (2017) Mitochondrial dysregulation and glycolytic insufficiency functionally impair CD8 T cells infiltrating human renal cell carcinoma. JCI Insight. 10.1172/jci.insight.93411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang H et al (2023) Heterogeneity and function of cancer-associated fibroblasts in renal cell carcinoma. J Natl Cancer Center 3(2):100–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian X et al (2023) Special issue “The advance of solid tumor research in China”: Multi-omics analysis based on 1311 clear cell renal cell carcinoma samples identifies a glycolysis signature associated with prognosis and treatment response. Int J Cancer 152(1):66–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vesely MD, Zhang T, Chen L (2022) Resistance Mechanisms to Anti-PD Cancer Immunotherapy. Annu Rev Immunol 40:45–74 [DOI] [PubMed] [Google Scholar]
- Wang L et al (2018) PTEN-L is a novel protein phosphatase for ubiquitin dephosphorylation to inhibit PINK1-Parkin-mediated mitophagy. Cell Res 28(8):787–802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu WH et al (2019a) Prognostic implications of aquaporin 9 expression in clear cell renal cell carcinoma. J Transl Med 17(1):363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu WH et al (2019b) Prognostic value and immune infiltration of novel signatures in clear cell renal cell carcinoma microenvironment. Aging (Albany NY) 11(17):6999–7020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W et al (2021a) Prognostic immunophenotyping clusters of clear cell renal cell carcinoma defined by the unique tumor immune microenvironment. Front Cell Dev Biol 9:785410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W et al (2021b) Systematic genome-wide profiles reveal alternative splicing landscape and implications of splicing regulator DExD-box helicase 21 in aggressive progression of adrenocortical carcinoma. Phenomics 1(6):243–256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W et al (2021c) Hexokinase 3 dysfunction promotes tumorigenesis and immune escape by upregulating monocyte/macrophage infiltration into the clear cell renal cell carcinoma microenvironment. Int J Biol Sci 17(9):2205–2222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W et al (2022) The unique genomic landscape and prognostic mutational signature of Chinese clear cell renal cell carcinoma. J Natl Cancer Center. 10.1016/j.jncc.2022.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W et al (2022a) Genomic alteration of MTAP/CDKN2A predicts sarcomatoid differentiation and poor prognosis and modulates response to immune checkpoint blockade in renal cell carcinoma. Front Immunol 13:953721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W et al (2022b) Tumor-associated macrophage-derived chemokine CCL5 facilitates the progression and immunosuppressive tumor microenvironment of clear cell renal cell carcinoma. Int J Biol Sci 18(13):4884–4900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y et al (2022c) PINK1 deficiency in gastric cancer compromises mitophagy, promotes the Warburg effect, and facilitates M2 polarization of macrophages. Cancer Lett 529:19–36 [DOI] [PubMed] [Google Scholar]
- Xu W et al (2023) Stimuli-responsive nanodelivery systems for amplifying immunogenic cell death in cancer immunotherapy. Immunol Rev. 10.1111/imr.13237 [DOI] [PubMed] [Google Scholar]
- Yang JF et al (2019) Screening, identification and validation of CCND1 and PECAM1/CD31 for predicting prognosis in renal cell carcinoma patients. Aging (Albany NY) 11(24):12057–12079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaffuto E, Karakiewicz PI, Capitanio U (2016) Complete response after treatment with first-line targeted anti-vascular endothelial growth factor therapy in metastatic renal cancer: what next? Ann Transl Med 4(15):291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng J et al (2021) Traditional Chinese medicine Bu-Shen-Jian-Pi-Fang attenuates glycolysis and immune escape in clear cell renal cell carcinoma: results based on network pharmacology. Biosci Rep. 10.1042/BSR20204421 [DOI] [PMC free article] [PubMed]
- Zheng Y et al (2021) STOML2 potentiates metastasis of hepatocellular carcinoma by promoting PINK1-mediated mitophagy and regulates sensitivity to lenvatinib. J Hematol Oncol 14(1):16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng R et al (2022) Cancer incidence and mortality in China, 2016. J Natl Cancer Center. 10.1016/j.jncc.2022.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Zheng J et al (2021) Traditional Chinese medicine Bu-Shen-Jian-Pi-Fang attenuates glycolysis and immune escape in clear cell renal cell carcinoma: results based on network pharmacology. Biosci Rep. 10.1042/BSR20204421 [DOI] [PMC free article] [PubMed]
Supplementary Materials
Supplementary file1 SUPPLEMENTARY FIGURE 1. Survival analysis of ccRCC patients stratified by the expression of MRGs. (A-H) Kaplan–Meier analysis of the OS of ccRCC patients stratified by the expression of MRGs. SUPPLEMENTARY FIGURE 2. Generation of mitophagy-related clusters by unsupervised clustering analysis. (A-H) Consensus matrix heatmaps of unsupervised clustering from k = 2 to k = 9. (I-K) The cumulative distribution function plot, delta plot, and tracking plot from k = 2 to k = 9. SUPPLEMENTARY FIGURE 3. Development of GeneClusters by unsupervised clustering analysis. (A-H) Consensus matrix heatmaps of unsupervised clustering from k = 2 to k = 9. (I-K) The cumulative distribution function plot, delta plot, and tracking plot from k = 2 to k = 9. SUPPLEMENTARY FIGURE 4. Correlation between RiskGroup and clinical features. (A-F) Proportion of clinical features (age and gender) in low and high RiskGroup. (G-L) Survival analysis of ccRCC patients between two RiskGroups in different subgroups stratified by clinical features (age and gender). (PDF 1834 KB)
Data Availability Statement
The datasets used and/or analyzed in the study are available from the corresponding author upon reasonable request.