Abstract
Purpose
Although undergoing conventional chemotherapy significantly improves the prognosis of Osteosarcoma, chemoresistance and failure of therapy is still a significant challenge. Furthermore, conventional chemotherapy, like doxorubicin, would upregulate the expression of programmed death-ligand 1 (PD-L1) which caused an immunosuppressive microenvironment and unsatisfied treatment result in Osteosarcoma. Thus, it is urgent to explore a strategy to overcome this disadvantage.
Methods
Human Osteosarcoma cell line MG63 and mouse Osteosarcoma cell line K7 were included in this study. Subcutaneous tumor model was used by injection of K7 cells in BALB/C mice to test the effect of doxorubicin and sorafenib on tumor growth. PD-L1 expression was tested in vitro (flow cytometry, western blot and PCR) and in vivo (flow cytometry and immunohistochemistry). Proportion of immune cells (CD4, CD8, Tregs, and cytotoxic T lymphocytes) in vivo was analyzed with flow cytometry.
Results
Combination of sorafenib and doxorubicin inhibited tumor growth significantly in vivo. Doxorubicin increased PD-L1 expression in vitro and in vivo, while sorafenib inhibited doxorubicin-induced PD-L1 upregulation in vitro and in vivo. Proportion of interferon-γ-secreting CD8 + T lymphocytes in tumor tissue was increased significantly when sorafenib was combined with doxorubicin, while proportion of CD4, CD8, and Tregs was not significantly changed. Extracellular signal-regulated kinases (ERK) pathway could be one of the key mechanisms by which doxorubicin induced upregulation of PD-L1 in Osteosarcoma cells.
Conclusion
Combination of sorafenib and conventional chemotherapeutic reagents is a potent strategy to improve treatment effectiveness by modulating tumor microenvironment in Osteosarcoma through increasing proportion of cytotoxic T lymphocytes.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00432-022-04458-4.
Keywords: Doxorubicin, Sorafenib, Programmed death-ligand 1 (PD-L1), Osteosarcoma, Lymphocyte
Introduction
Osteosarcoma is the most common primary malignant bone tumor in children and adolescents. Following the implementation of chemotherapy, the survival rate of high-grade Osteosarcoma has increased dramatically to 60–70% during the past century (Jeon and Song 2010; Jaffe 2014; Luetke et al. 2014). However, innovations in routine chemotherapy have reached a plateau and no major advances in Osteosarcoma therapy have been made since the 1980s (Takemoto et al. 2022). Currently, traditional chemotherapeutic agents, such as doxorubicin, are still considered the most effective agents for Osteosarcoma treatment (Bielack et al. 2009; Liu et al. 2022), but chemoresistance and failure of therapy has become the primary challenge to the successful treatment of Osteosarcoma (Meltzer and Helman 2021; Liu et al. 2022).
The causes of chemotherapeutic failure are complicated. The proposed mechanisms imply the existence of intrinsic or acquired chemoresistance, most likely due to perturbations in gene expression and signal transduction pathways (Ma et al. 2017) such as increased DNA damage repair activity (Chou and Gorlick 2006) and impaired drug transport (Jeon and Song 2010). Furthermore, our previous study revealed that conventional chemotherapy can upregulate the expression of programmed death-ligand 1 (PD-L1), thus causing an immunosuppressive microenvironment in Osteosarcoma (Wang et al. 2019). However, the potential mechanism of chemotherapy-induced upregulation of PD-L1 in Osteosarcoma is still unknown.
In the past decade, rapid clinical progress in targeted therapies (Chen et al. 2015; Martin-Broto et al. 2017) has led to breakthroughs in the treatment of malignant tumors; these breakthroughs have brought expectations to Osteosarcoma patients. Sorafenib, which is recommended for relapsed/refractory or metastatic Osteosarcoma by the National Comprehensive Cancer Network (NCCN) guidelines (Biermann et al. 2013), is a therapeutic agent that inhibits multiple targets such as the MAPK/ERK pathway (Wilhelm et al. 2004). However, sorafenib alone does not significantly improve prognosis because of transient progression-free survival (PFS) (Grignani et al. 2012). Although sorafenib alone was shown to provide only a brief clinical benefit, it proved to be effective in reducing disease progression in a variety of advanced cases when combined with other drugs that addressed its weaknesses or other aspects of the pathogenesis of Osteosarcoma (Jayson et al. 2016; Coventon 2017). This finding reveals that sorafenib can enhance the efficiency of other therapeutic agents. However, little is known about the mechanism for the increased effectiveness of sorafenib-combination therapies.
In this study, we ascertained that sorafenib significantly decreases doxorubicin-induced upregulation of PD-L1 in Osteosarcoma. This would partially explain the increased effectiveness of sorafenib-combination therapies. Besides this, our data revealed that the ERK pathway could be one of the key mechanisms by which doxorubicin induces upregulation of PD-L1, while sorafenib reduces immunosuppressive status in Osteosarcoma.
Materials and methods
Main materials
Doxorubicin was purchased from Sigma (MO, USA). Sorafenib was purchased from Selleck (Shanghai, China). The anti-mouse PD-L1 antibody used for the immunohistochemistry (IHC) was purchased from CST (Sino Biological, Beijing, China). The ERK1/2 inhibitor was purchased from CST (MA, USA), and the anti-PD-L1 antibody used for Western blotting was purchased from Sigma (MO, USA). Fluorescent antibodies for flow cytometry were purchased from BD Bioscience (NJ, USA).
Cell culture
The human Osteosarcoma cell line MG63, 143B, and U2OS was purchased from the Cell Bank of Type Culture Collection of Chinese Academy of Science (CBTCC, Shanghai, China), and the mouse Osteosarcoma cell line K7 was kindly gifted from Dr. Zhengdong Cai of Shanghai General Hospital. All cells were grown in DMEM supplemented with 10% FBS and maintained in an incubator at 37 °C containing 5% CO2 and 95% air. Doxorubicin (0.4 μM), sorafenib (4 μM), and the ERK1/2 inhibitor PD98059 (10 μM) were used in the indicated groups and incubated with the cells for 48 h.
Western blot analysis
Western blot analyses were performed as previously described (Hu et al. 2017). Cells were lysed in RIPA buffer to extract proteins, which were then separated by SDS-PAGE on a 10% gel and transferred to a nitrocellulose membrane (Millipore, Boston, MA, USA). The membranes were first incubated with blocking buffer followed by overnight incubation with the appropriate primary antibodies at 4 °C. Next, membranes were washed three times with Tris-buffered saline with Tween 20 (TBST) and incubated with secondary antibodies, and the protein bands were visualized using an enhanced chemiluminescence substrate combined with a LI-COR infrared imaging system (LI-COR, NE, USA) following the manufacturer's instructions.
Flow cytometry
Cells were harvested and incubated with the indicated fluorescent antibodies or isotype control antibodies for flow cytometry. The cells were then washed with PBS buffer and analyzed with a CytoFlEX flow cytometer and CytExpert software (Beckman Coulter, CA, USA). Analysis of the subcutaneous tissues is described below.
RNA isolation and real-time PCR
Total RNA was isolated from cells using TRIzol reagent (Invitrogen). RNA samples (500 ng) were subjected to real-time PCR (RT-PCR) using the TaKaRa RT-PCR kit (Takara, Shiga, Japan). Subsequently, RT-PCR was performed on an ABI 7500 Sequence Detection System (Thermo Scientific, Waltham, MA, USA). All procedures were performed according to the manufacturer’s protocols. The primer sequences are listed in Table 1.
Table 1.
Sequences of primers used in real-time PCR
| Gene | Primer sequences (5′-3′) | |
|---|---|---|
| PD-L1 (Mouse) |
Forward Reverse |
AAGCCTCAGCACAGCAACTTCAG TGTAGTCCGCACCACCGTAGC |
| PD-L1 (Human) |
Forward Reverse |
CATCTTATTATGCCTTGGTGTAGCA GGATTACGTCTCCTCCAAATGTG |
| β-actin (Mouse) |
Forward Reverse |
CTACCTCATGAAGATCCTGACC CACAGCTTCTCTTTGATGTCAC |
| β-actin (Human) |
Forward Reverse |
AGCCTCGCCTTTGCCGATCCG CATGCCGGAGCCGTTGTCGAC |
Immunohistochemistry
Paraffin-embedded tissue sections from the subcutaneous tumor models were heated at 60 ℃ overnight, dewaxed in xylene, rehydrated in a graded series of ethanol solutions and treated with 0.01 mol/L citrate buffer (pH 6.0) for antigen retrieval. H2O2 (0.3%) was used to inhibit endogenous peroxidase activity; the slides were then incubated with the appropriate primary antibody overnight at 4 ℃. The detection procedures were performed using a supersensitive IHC detection kit (Bioworld, MN, USA) according to the manufacturer’s instructions.
Subcutaneous Osteosarcoma models
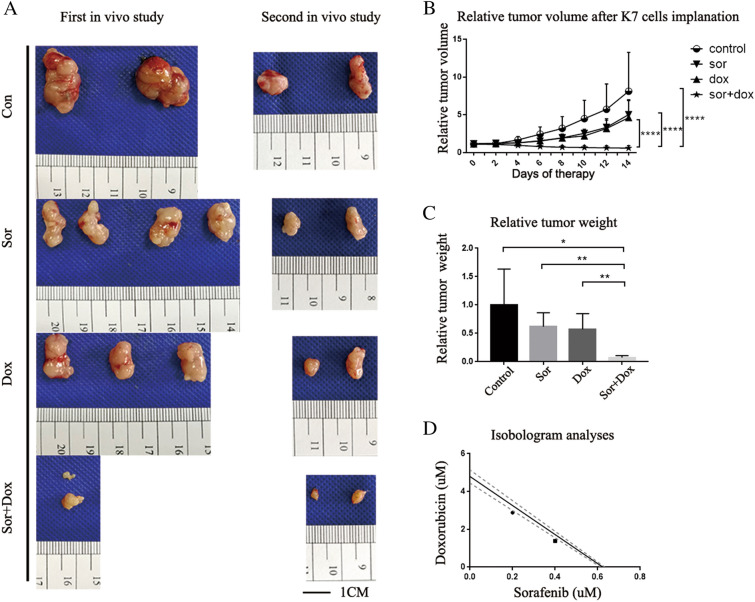
All experiments were carried out in accordance with the recommendations of the Institutes of Laboratory Animal Resources, National Research Council, USA. The protocol was approved by the Committee on the Ethics of Animal Experiments of Shanghai Ruijin Hospital. Six-week-old female BALB/C mice were purchased from Shanghai Laboratory Animal Co. (SLAC, Shanghai, China). Mice received subcutaneous injection of 2 × 106 K7 Osteosarcoma cells (200 μL) into the dorsal gluteal region. Tumor volumes were calculated using the formula (0.5 × length × width2). Treatment began when the tumor size reached 100 mm3. Intraperitoneal injections of control solution (Cremophor EL/ethanol/water (12.5:12.5:75; 95% ethyl alcohol) (Wilhelm et al. 2004)), doxorubicin (3 mg/kg, every 2 days), sorafenib (15 mg/kg, every day), and sorafenib plus doxorubicin were administered for 2 weeks. In our design, there were 4 mice in each group. However, in our first in vivo study, 2 mice dead in the control group, 1 mouse in Dox group and 2 mice in Sor + Dox group could not find tumor growth. So we did second in vivo study, and there were 2 mice in each group. Eventually, there were 4 mice in control group, 6 mice in sorafenib group, 5 mice in doxorubicin group, and 4 mice in Sor + Dox group.
The mice were euthanized following completion of treatment, and tumors were dissected and weighed. The tumor tissues were digested with 1 mg/ml collagenase IV (Sigma-Aldrich) for 60 min at 37 °C to obtain single-cell suspensions. The cells were subsequently stained with antibodies against CD31 and CD45 (to label and remove the pan-lymphocyte and endothelial cells); CD3, CD4, and CD8 (to examine the proportion of T lymphocytes); and PD-L1. A Foxp3 staining kit (Ebioscience, CA, USA) was used to analyze the regulatory T (Treg) cell population according to the manufacturer’s instructions. Antibodies against CD3, CD8, and intracellular IFN-γ were used to examine the activated cytotoxic T lymphocytes (CTLs).
Statistical analysis
Statistical analyses were conducted using GraphPad Prism 7.0 (Aspire Software International). Data were analyzed using T test, one-way, or two-way ANOVA. A statistically significant difference was defined as p < 0.05.
Results
Sorafenib could inhibit the upregulation of PD-L1 induced by doxorubicin in Osteosarcoma cells in vitro
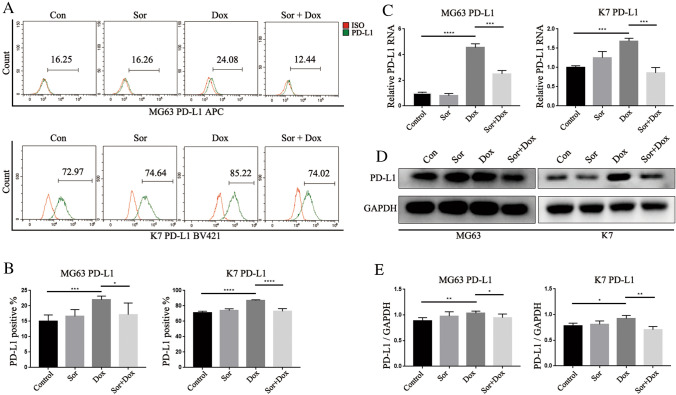
For the first time, we found that sorafenib could inhibit the doxorubicin-induced upregulation of PD-L1 in Osteosarcoma cells. We used flow cytometry, PCR, and Western blotting analysis to analyze the total and surface PD-L1 expression in the human Osteosarcoma cell line MG63 and the mouse Osteosarcoma cell line K7 in vitro.
As shown in Fig. 1A–E and supplemental Fig. 1, compared with the control group, doxorubicin significantly induced PD-L1 expression in Osteosarcoma cells in vitro. PD-L1 expression was decreased in the sorafenib plus doxorubicin group compared with the doxorubicin group. In addition, sorafenib therapy alone did not alter PD-L1 expression compared with the control group.
Fig. 1.
Sorafenib could inhibit the upregulation of PD-L1 induced by doxorubicin in OS cells in vitro. To confirm that PD-L1 upregulation could be induced by doxorubicin and inhibited by sorafenib, PD-L1 expression in OS cell lines was analyzed in vitro: A Flow cytometry analysis and B Quantification of flow cytometry results, C Real-time PCR analysis, D Western blot analysis and E Quantification of western blot results. All data were obtained from at least three independent experiments. All data are presented as the means ± SD. *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001
The effect of combined therapy on Osteosarcoma is better than therapy with sorafenib or doxorubicin alone in vivo
Previous studies revealed that sorafenib can enhance the efficiency of other therapeutic agents (Jayson et al. 2016; Coventon 2017). Our previous study has shown that doxorubicin-induced PD-L1 upregulation on Osteosarcoma cells could suppress the proliferation of CD8 + T Lymphocytes and induce apoptosis in CD8 + T Lymphocytes, thus causing immunosuppressive status in Osteosarcoma (Wang et al. 2019). On the basis of these findings, the combination of sorafenib and doxorubicin may theoretically enhance the antitumor response of the immune system by inhibiting the doxorubicin-induced overexpression of PD-L1.
Therefore, we studied whether the combination of sorafenib and doxorubicin is better than treatment with each agent alone. As shown in Fig. 2A, doxorubicin alone and sorafenib alone inhibited tumor progression compared with the control group; however, the tumor volume increased during the two-week treatment period. In contrast, sorafenib plus doxorubicin dramatically inhibited tumor progression and reduced the tumor volume during the two-week treatment period. As shown in Fig. 2B and Fig. 2C, after two weeks of treatment, the tumor volume and tumor weight in the doxorubicin plus sorafenib group were significantly smaller than those in the sorafenib, doxorubicin, and control groups.
Fig. 2.
The effect of combined therapy on OS is better than therapy with either sorafenib or doxorubicin alone in vivo. A Macroscopic appearance of subcutaneous tumors in BALB/c mice after 14 days of treatment. The in vivo study was performed twice and each group contained at least 4 animals. B Relative tumor volumes were calculated by dividing the tumor volume measurement for each animal by the corresponding day 0 measurement for that animal. P values were calculated by two-way ANOVA. The mean relative tumor volumes (± SD) are shown. C Relative tumor weights were calculated by dividing the tumor weight measurement for each tumor by the average weight for the corresponding control group. P values were calculated by T test. The mean relative tumor weights (± SD) are shown. D Synergistic interaction between sorafenib and doxorubicin was evaluated in vitro by isobologram analyses, using K7 cells
Besides this, isobologram analysis was used to evaluate the possible synergistic interaction between sorafenib and doxorubicin. As shown in Fig. 2D, the combination of doxorubicin and sorafenib exhibited tiny synergistic effects in the Osteosarcoma cells, which is not consistent with the in vivo effectiveness.
Sorafenib could inhibit the upregulation of PD-L1 induced by doxorubicin in Osteosarcoma cells in vivo
To confirm whether sorafenib could inhibit the doxorubicin-induced upregulation of PD-L1 on Osteosarcoma cells in vivo, we used flow cytometry to analyze PD-L1 expression on the surfaces of tumor cells from the tumor tissues. Meanwhile, IHC analysis was performed using subcutaneous tumor tissue to analyze PD-L1 levels in the tumor bed.
As shown in Fig. 3A–C, compared with the control group, doxorubicin significantly increased PD-L1 expression on Osteosarcoma cells in vivo. PD-L1 expression was decreased in the sorafenib plus doxorubicin group compared with the doxorubicin group. In addition, sorafenib therapy alone did not alter PD-L1 expression compared with the control group.
Fig. 3.
Sorafenib could inhibit the upregulation of PD-L1 induced by doxorubicin in OS cells in vivo. PD-L1 expression was significantly increased in the doxorubicin group while decreased in the doxorubicin plus sorafenib group. A Flow cytometry analysis of PD-L1 expression on the surface of OS cells from subcutaneous tumors and B Quantification of the results. C. IHC analysis of PD-L1 expression in the tumor bed of subcutaneous tumors. Scale bar indicates 100 μm. All data are presented as the means ± SD. *p < 0.05
Proportion of CTLs among CD8 + T cells was significantly elevated in the combined group
PD-L1 has been shown to play critical roles in immune escape by interacting with PD-1, which is expressed on lymphocytes or other host immune cells (Francisco et al. 2009). The interaction between PD-L1 and PD-1 can lead to the apoptosis of lymphocytes including CTLs (Zhang et al. 2008). Our previous study has shown that doxorubicin-induced PD-L1 upregulation on Osteosarcoma cells could decrease the proportion of CTLs in Osteosarcoma tissue (Wang et al. 2019).
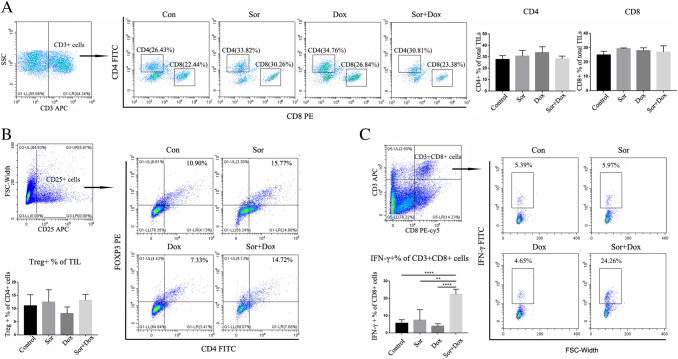
To analyze the T lymphocytes in the tumor tissue, cells from tumor tissues of the abovementioned four different groups were analyzed for the proportion of T lymphocytes, Tregs, and CTLs.
There was no significant difference among the four groups in the proportion of CD4 + , CD8 + T cells, and Tregs (Fig. 4A-B). Doxorubicin and sorafenib therapy alone did not significantly alter the proportion of CTLs among CD8 + T lymphocytes, while the proportion significantly increased in the sorafenib plus doxorubicin group (Fig. 4C).
Fig. 4.
Elevated Proportions of CTLs among CD8 + T cells was observed in the combined group. A Flow cytometry analysis of the proportion of CD4 + T cells and CD8 + T cells, B the proportion of Tregs among the overall TILs (TILs: tumor infiltrated lymphocytes) and C the proportion of CTLs among the overall CD8 + T cell population in the tumor tissues. All data were obtained from at least three independent experiments. All data are presented as the means ± SD. *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001
Sorafenib and doxorubicin regulate PD-L1 expression via the ERK pathway in Osteosarcoma cells
To investigate the mechanism by which sorafenib and doxorubicin regulated PD-L1 expression, we analyzed different pathways. As shown in Fig. 5A–D and supplemental Fig. 1, compared with the control group, doxorubicin activated phospho-ERK (p-ERK) in Osteosarcoma cell lines, while sorafenib decreased p-ERK levels. The change in ERK phosphorylation level was consistent with the regulation of PD-L1 expression. In addition, no significant association was observed between either the mTOR or Stat3 pathway and PD-L1 expression in Osteosarcoma cells.
Fig. 5.
Sorafenib and doxorubicin can regulate PD-L1 expression in OS cells via the ERK pathway. Western blotting analysis of phosphorylation level of ERK, mTOR, and Stat3 pathways was implemented in A MG63 and C K7 OS cell lines. Quantification of the results is shown in B and D, respectively. GAPDH was included as the loading control. All data are presented as the means ± SD. *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001
To confirm the relationship between the ERK pathway and PD-L1 expression, we used the ERK1/2-specific inhibitor PD98059. As shown in Fig. 6A–B, PD98059 significantly decreased the ERK phosphorylation. Western blot analysis indicated that basic PD-L1 expression in Osteosarcoma cells could not be decreased by PD98059, while doxorubicin-induced PD-L1 upregulation was inhibited by PD98059, which was consistent with the effect of sorafenib. Moreover, flow cytometry analysis of cell surface PD-L1 expression showed similar results (Fig. 6C–D). These data demonstrated that the ERK pathway participates in doxorubicin-induced PD-L1 upregulation on Osteosarcoma cells.
Fig. 6.
Sorafenib and doxorubicin regulate PD-L1 expression in OS cells via the ERK pathway. The ERK1/2-specific inhibitor PD98059 was used to confirm the correlation between the phosphorylation of ERK1/2 (Thr202/Tyr204) and PD-L1 expression. PD-L1 expression in OS cells and phosphorylation levels of the ERK1/2 (Thr202/Tyr204) in MG63 and K7 OS cells were analyzed by Western blotting (A) and flow cytometry (C, E). Quantification of western blotting results (B) and flow cytometry results (D, F) were shown, respectively. All data were obtained from at least three independent experiments. All data are presented as the means ± SD. *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001
Discussion
Owing to chemotherapy failure and the absence of novel effective therapeutic agents, there have been no major advances in the treatment for Osteosarcoma in the past 40 years (Jaffe 2014; Luetke et al. 2014). Elucidating the mechanism of chemotherapy failure and finding new therapeutic regimens that could increase therapeutic effectiveness is of great value. Previous studies highlighted the therapeutic value of sorafenib in combination with other conventional therapeutic agents for Osteosarcoma (Coventon 2017). Recently, one study found synergistic effects of sorafenib and doxorubicin on Osteosarcoma by inducing cell cycle arrest (Ya-Ting et al. 2022). However, little is known about the effect on immune microenvironment and the mechanism of improved effectiveness of sorafenib combined therapy in Osteosarcoma. In this study, we observed that sorafenib could modulate immunosuppressive microenvironment in Osteosarcoma by inhibiting doxorubicin-induced PD-L1 expression. Moreover, we elucidated that the ERK pathway participates in doxorubicin and sorafenib induced PD-L1 regulation in Osteosarcoma cells.
In the present study, a combination of sorafenib with doxorubicin was significantly effective for Osteosarcoma treatment. This result was consistent with that in HCC therapy (Zhang et al. 2016). It has been demonstrated that sorafenib has the capacity to inhibit angiogenesis and tumor growth (Coventon 2017), and doxorubicin can kill tumor cells directly (Jaffe 2014). In addition, an increasing number of studies highlight that the local immune microenvironment is important in cancer therapy; (Mantovani et al. 2008, Chen et al. 2013, Muraro et al. 2017, Park and Cheung 2020) as it can be altered by therapeutic agents and affect the therapeutic effectiveness of agents. Our previous study revealed conventional chemotherapy can upregulate the expression of PD-L1, thus causing immunosuppressive status in Osteosarcoma (Wang et al. 2019). Other studies also have suggested that doxorubicin exerts its immunosuppressive effect through the upregulation of PD-L1 expression on monocytic myeloid cells and bone marrow stromal cells (Ding et al. 2014; Yang et al. 2017). In the present study, we further demonstrated that sorafenib could modulate the local immune microenvironment of Osteosarcoma by altering PD-L1 expression in the tumor. These findings suggest a complicated immunological condition in the tumor site following treatments; and influences of different treatments on tumor microenvironment need to be clarified by further studies.
In this study, we found that doxorubicin upregulates PD-L1 expression while sorafenib inhibits doxorubicin-induced PD-L1 upregulation in Osteosarcoma cells. However, different tumors respond differently to the same anti-tumor agent. It was reported that doxorubicin can downregulate cell surface PD-L1 expression in breast cancer (Ghebeh et al. 2010). Meanwhile, sorafenib was observed could increase PD-L1 expression in human HCC, which participates in acquired resistance to sorafenib (Liu et al. 2017). These results revealed that the same anti-tumor agent could modulate the immune microenvironment differently in different tumors and tissues; thus, the specific effect of anti-tumor agent on the tumor immune microenvironment needs to be further investigated.
Our results have proven that combined therapy with doxorubicin and sorafenib is a better choice for Osteosarcoma treatment than doxorubicin alone, as this combination preserves the immunostimulatory effect of doxorubicin by inducing immunogenic tumor cell death while overcoming the immunosuppressive effect by blocking doxorubicin-induced PD-L1 overexpression. In the present study, despite doxorubicin alone inducing significant PD-L1 overexpression on tumor cells in vivo, the proportion of CD4, CD8 + T lymphocytes and activated cytotoxic T lymphocytes (CTLs) in the doxorubicin group were not significantly lower than that in the control group. This finding is consistent with the results of a study that found that paclitaxel-induced PD-L1 overexpression on ovarian cancer cells did not decrease CD8 + T-cell infiltration into the tumor site (Peng et al. 2015). Studies have indicated that anthracycline kills tumor cells, stimulating a therapeutic immune response in cancer patients and animal models (Zappasodi et al. 2010). Other studies also revealed that doxorubicin can enhance systemic anti-tumor characteristics by increasing the frequencies of CD4 + and CD8 + T lymphocytes (Hannesdóttir et al. 2013; Alizadeh et al. 2014). One study demonstrated that sorafenib alone could increase frequency of interferon-γ-secreting T lymphocytes (Kalathil et al. 2019) but we did not observe that in this study. However, we found a significant increase of interferon-γ-secreting CD8 + T lymphocytes in tumor tissue when combined doxorubicin with sorafenib. This result indicated a synergic effect of doxorubicin and sorafenib in modulating the microenvironment by increasing interferon-γ-secreting CD8 + T lymphocytes in the Osteosarcoma tumor site. Although significant increase of interferon-γ-secreting CD8 + T lymphocytes was observed in combined treatment, the frequencies of CD4 + , CD8 + T cells, and Tregs were not changed statistically. This indicated doxorubicin and sorafenib did not affect lymphocytes infiltration, however, it improved activation of CD8 + T lymphocytes. Since tumor-infiltrating T cells, especially CTLs, play critical roles in anti-tumor immunity, significantly increased interferon-γ-secreting CD8 + T lymphocytes after combined treatment of doxorubicin and sorafenib demonstrated this therapeutic strategy may exert a better effect than conventional chemotherapy alone or sorafenib alone. Higher numbers of tumor-infiltrating T cells and elevated PD-L1 expression in metastatic tumors compared with primary tumors in Osteosarcoma implied that theoretically metastatic Osteosarcoma may especially benefit from PD-L1 regulation therapy (Palmerini et al. 2017; Sundara et al. 2017). One clinical trial using sorafenib alone for relapsed or metastatic Osteosarcoma patients achieved a response rate of 14% (Grignani et al. 2012). Unfortunately, these encouraging results were short-lived, with 46% of patient progression free at 4 months and 29% progression free at 6 months (Grignani et al. 2012). Therefore, according to our results, a combination of sorafenib with conventional chemotherapy may be a more effective strategy for treatment of Osteosarcoma, but further investigation and clinical trials are needed.
With regard to the mechanism through which doxorubicin regulates PD-L1, we demonstrated that the ERK pathway participates in PD-L1 upregulation in Osteosarcoma. Previous studies indicated that the Stat3, mTOR, and ERK pathways could participate in PD-L1 regulation (Qin et al. 2010; Lastwika et al. 2016; Moore et al. 2016; Zheng et al. 2018). However, we observed that neither the Stat3 nor the mTOR pathways were correlated with the regulation of PD-L1 expression in Osteosarcoma. Nevertheless, we observed that the levels of p-ERK were consistent with PD-L1 expression in Osteosarcoma, and further investigation using a p-ERK inhibitor confirmed these findings. Doxorubicin increases the phosphorylation level of ERK, which was concomitant with the upregulation of PD-L1. Reductions in p-ERK levels could be induced by either sorafenib or an ERK pathway inhibitor. When combined with sorafenib or ERK pathway inhibitors, doxorubicin failed to upregulate PD-L1. These results confirmed that doxorubicin can upregulate PD-L1 through the ERK pathway. However, the downregulation of p-ERK levels in cultured Osteosarcoma cells did not induce PD-L1 downregulation. One of the possible reasons for this discrepancy was that several pathways contained ERK participation in the regulation of PD-L1. Further investigations are expected to elucidate the other mechanisms in the future.
Conclusions
This study clarifies the mechanism of chemotherapy-induced upregulation of PD-L1 in Osteosarcoma and highlights the therapeutic value of the combination of sorafenib and doxorubicin for the treatment of Osteosarcoma. Sorafenib could reduce the doxorubicin-mediated upregulation of PD-L1 expression via the ERK pathway to inhibit immunosuppression in Osteosarcoma; this finding may partially explain the superior effectiveness of the combination of sorafenib and doxorubicin. There remains great values in future research on rational drug combinations and studies on how to reduce immune resistance in cancer therapy and to promote anti-tumor immunity.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file1 Supplemental Fig. 1 Western blotting analysis of PD-L1, phosphorylation level of ERK, mTOR, and Stat3 pathways was implemented in 143b and U2 cell lines. (TIF 1827 KB)
Acknowledgements
Thanks to Dr Zhengdong Cai for generously supporting of mouse Osteosarcoma cell line K7. This work was supported by the National Nature Science Foundation of China (No.81702661).
Author contributions
JXW and QYB conceived and designed the experiment. JZW, FQH and PY performed the experiments and wrote the manuscript draft. JXW, JW and ZCL edited the paper. All the authors have read and approved the final manuscript and agree to be accountable for all aspects of the study.
Funding
This work was supported by the National Nature Science Foundation of China (No. 81702661).
Availability of data and materials
The data generated in this study are available upon request from the corresponding author.
Declarations
Competing interests
The authors declare no competing interests.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Ethical approval
This study was performed in line with the principles of the Declaration of Helsinki. The protocol was approved by the animal ethical committee of Ruijin Hospital, affiliated with Shanghai Jiaotong University School of Medicine.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jizhuang Wang, Fangqiong Hu, and Pei Yu have contributed equally.
References
- Alizadeh D, Trad M, Nt H, Cb L, Janikashvili N, Bonnotte B, Katsanis E, Larmonier N (2014) Doxorubicin eliminates myeloid-derived suppressor cells and enhances the efficacy of adoptive T-cell transfer in breast cancer. Can Res 74(1):104–118. 10.1158/0008-5472.can-13-1545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielack S, Carrle D, Casali PG, Working GEG (2009) Osteosarcoma: ESMO clinical recommendations for diagnosis, treatment and follow-up. Ann Oncol 20:137–139. 10.1093/annonc/mdp154 [DOI] [PubMed] [Google Scholar]
- Biermann JS, Adkins DR, Agulnik M, Benjamin RS, Brigman B, Butrynski JE, Cheong D, Chow W, Curry WT, Frassica DA, Frassica FJ, Hande KR, Hornicek FJ, Jones RL, Mayerson J, Mcgarry SV, Mcgrath B, Morris CD, O’donnell RJ, Randall RL, Santana VM, Satcher RL, Siegel HJ, Von Mehren M, Bergman MA, Sundar H (2013) Bone cancer clinical practice guidelines in oncology. J Natl Comphr Cancer Netw 11(6):688–723 [Google Scholar]
- Chen DS, Mellman I (2013) Oncology meets immunology: the cancer-immunity cycle. Immunity 39(1):1–10. 10.1016/j.immuni.2013.07.012 [DOI] [PubMed] [Google Scholar]
- Chen Y, Ma DG, Molyneux S, Mckee T, Waterhouse P, Jm P, Khokha R (2015) RANKL blockade prevents and treats aggressive osteosarcomas. Sci Transl Med 7(317):317ra197. 10.1126/scitranslmed.aad0295 [DOI] [PubMed] [Google Scholar]
- Chou AJ, Gorlick R (2006) Chemotherapy resistance in osteosarcoma: current challenges and future directions. Expert Rev Anticancer Ther 6(7):1075–1085. 10.1586/14737140.6.7.1075 [DOI] [PubMed] [Google Scholar]
- Coventon J (2017) A review of the mechanism of action and clinical applications of sorafenib in advanced osteosarcoma. J Bone Oncol 8:4–7. 10.1016/j.jbo.2017.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Zc LuX, Yu M, Lemos H, Huang L, Chandler P, Liu K, Walters M, Krasinski A, Mack M, Br B, Al M, Munn Dh, Zhou G (2014) Immunosuppressive myeloid cells induced by chemotherapy attenuate antitumor CD4+ T-cell responses through the PD-1-PD-L1 axis. Can Res 74(13):3441–3453. 10.1158/0008-5472.can-13-3596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francisco Lm Salinas Vh, Ke B, Vk V, Gj F, Vk K, Sharpe Ah (2009) PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J Exp Med 206(13):3015–3029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghebeh H, Lehe C, Barhoush E, Al-Romaih K, Tulbah A, Al-Alwan M, Hendrayani SF, Manogaran P, Alaiya A, Al-Tweigeri T, Aboussekhra A, Dermime S (2010) Doxorubicin downregulates cell surface B7–H1 expression and upregulates its nuclear expression in breast cancer cells: role of B7–H1 as an anti-apoptotic molecule. Breast Cancer Res 12(4):R48. 10.1186/bcr2605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grignani G, Palmerini E, Dileo P, Asaftei SD, D’ambrosio L, Pignochino Y, Mercuri M, Picci P, Fagioli F, Casali PG, Ferrari S, Aglietta M (2012) A phase II trial of sorafenib in relapsed and unresectable high-grade osteosarcoma after failure of standard multimodal therapy: an Italian Sarcoma Group study. Ann Oncol 23(2):508–516. 10.1093/annonc/mdr151 [DOI] [PubMed] [Google Scholar]
- Hannesdóttir L, Tymoszuk P, Parajuli N, Wasmer Mh, Philipp S, Daschil N, Datta S, Jb K, Tripp Ch, Stoitzner P, Müller-Holzner E, Gj W, Sexl V, Villunger A, Doppler W (2013) Lapatinib and doxorubicin enhance the Stat1-dependent antitumor immune response. Eur J Immunol 43(10):2718–2729. 10.1002/eji.201242505 [DOI] [PubMed] [Google Scholar]
- Hu C, Chen X, Wen J, Gong L, Liu Z, Wang J, Liang J, Hu F, Zhou Q, Wei L, Shen Y, Zhang W (2017) Antitumor effect of focal adhesion kinase inhibitor PF562271 against human osteosarcoma in vitro and in vivo. Cancer Sci 108(7):1347–1356. 10.1111/cas.13256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe N (2014) Historical perspective on the introduction and use of chemotherapy for the treatment of osteosarcoma. Curr Adv Osteosarcoma 804:1–30. 10.1007/978-3-319-04843-7_1 [DOI] [PubMed] [Google Scholar]
- Jayson GC, Kerbel R, Ellis LM, Harris AL (2016) Antiangiogenic therapy in oncology: current status and future directions. Lancet 388(10043):518–529 [DOI] [PubMed] [Google Scholar]
- Jeon DG, Song WS (2010) How can survival be improved in localized osteosarcoma? Expert Rev Anticancer Ther 10(8):1313–1325. 10.1586/era.10.79 [DOI] [PubMed] [Google Scholar]
- Kalathil SG, Hutson HA, Barbi J, Iyer R, Thanavala Y (2019) Augmentation of IFN-γ+ CD8+ T cell responses correlates with survival of HCC patients on sorafenib therapy. JCI Insight. 10.1172/jci.insight.130116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lastwika KJ, Wilson W 3rd, Li QK, Norris J, Xu H, Ghazarian SR, Kitagawa H, Kawabata S, Taube JM, Yao S, Liu LN, Gills JJ, Dennis PA (2016) Control of PD-L1 expression by oncogenic activation of the AKT-mTOR pathway in non-small cell lung cancer. Cancer Res 76(2):227–238. 10.1158/0008-5472.CAN-14-3362 [DOI] [PubMed] [Google Scholar]
- Liu J, Liu Y, Meng L, Liu K, Ji B (2017) Targeting the PD-L1/DNMT1 axis in acquired resistance to sorafenib in human hepatocellular carcinoma. Oncol Rep 38(2):899–907. 10.3892/or.2017.5722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Leng A, Li L, Yang B, Shen S, Chen H, Zhu E, Xu Q, Ma X, Shi P, Liu Y, Liu T, Li L, Li K, Zhang D, Xiao J (2022) AMTB, a TRPM8 antagonist, suppresses growth and metastasis of osteosarcoma through repressing the TGFβ signaling pathway. Cell Death Dis 13(3):288. 10.1038/s41419-022-04744-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luetke A, Meyers PA, Lewis I, Juergens H (2014) Osteosarcoma treatment—where do we stand? A state of the art review. Cancer Treat Rev 40(4):523–532. 10.1016/j.ctrv.2013.11.006 [DOI] [PubMed] [Google Scholar]
- Ma Q, Zhang Y, Liu T, Jiang K, Wen Y, Fan Q, Qiu X (2017) Hypoxia promotes chemotherapy resistance by down-regulating SKA1 gene expression in human osteosarcoma. Cancer Biol Ther 18(3):177–185. 10.1080/15384047.2017.1294285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantovani A, Allavena P, Sica A, Balkwill F (2008) Cancer-related inflammation. Nature 454(7203):436–444. 10.1038/nature07205 [DOI] [PubMed] [Google Scholar]
- Martin-Broto J, Redondo A, Valverde C, Ma V, Mora J, Garcia Del Muro X, Gutierrez A, Tous C, Carnero A, Marcilla D, Carranza A, Sancho P, Martinez-Trufero J, Diaz-Beveridge R, Cruz J, Encinas V, Taron M, Ds M, Luna P, Hindi N, Lopez-Pousa A (2017) Gemcitabine plus sirolimus for relapsed and progressing osteosarcoma patients after standard chemotherapy: a multicenter, single-arm phase II trial of Spanish Group for Research on Sarcoma (GEIS). Ann Oncol 28(12):2994–2999. 10.1093/annonc/mdx536 [DOI] [PubMed] [Google Scholar]
- Meltzer PS, Helman LJ (2021) New horizons in the treatment of osteosarcoma. N Engl J Med 385(22):2066–2076. 10.1056/NEJMra2103423 [DOI] [PubMed] [Google Scholar]
- Moore EC, Cash HA, Caruso AM, Uppaluri R, Hodge JW, Van Waes C, Allen CT (2016) Enhanced tumor control with combination mTOR and PD-L1 inhibition in syngeneic oral cavity cancers. Cancer Immunol Res 4(7):611–620. 10.1158/2326-6066.CIR-15-0252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muraro E, Furlan C, Avanzo M, Martorelli D, Comaro E, Rizzo A, Fae DA, Berretta M, Militello L, Del Conte A, Spazzapan S, Dolcetti R, Trovo M (2017) Local high-dose radiotherapy induces systemic immunomodulating effects of potential therapeutic relevance in oligometastatic breast cancer. Front Immunol 8:1476. 10.3389/fimmu.2017.01476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmerini E, Agostinelli C, Picci P, Pileri S, Marafioti T, Lollini PL, Scotlandi K, Longhi A, Benassi MS, Ferrari S (2017) Tumoral immune-infiltrate (IF), PD-L1 expression and role of CD8/TIA-1 lymphocytes in localized osteosarcoma patients treated within protocol ISG-OS1. Oncotarget 8(67):111836–111846. 10.18632/oncotarget.22912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JA, Cheung NV (2020) GD2 or HER2 targeting T cell engaging bispecific antibodies to treat osteosarcoma. J Hematol Oncol 13(1):172. 10.1186/s13045-020-01012-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng J, Hamanishi J, Matsumura N, Abiko K, Murat K, Baba T, Yamaguchi K, Horikawa N, Hosoe Y, Sk M, Konishi I, Mandai M (2015) Chemotherapy induces programmed cell death-ligand 1 overexpression via the nuclear factor-κB to foster an immunosuppressive tumor microenvironment in ovarian cancer. Can Res 75(23):5034–5045. 10.1158/0008-5472.can-14-3098 [DOI] [PubMed] [Google Scholar]
- Qin X, Liu C, Zhou Y, Wang G (2010) Cisplatin induces programmed death-1-ligand 1(PD-L1) over-expression in hepatoma H22 cells via Erk /MAPK signaling pathway. Cell Mol Biol 56:OL1366-1372 [PubMed] [Google Scholar]
- Sundara YT, Kostine M, Cleven AH, Bovee JV, Schilham MW, Cleton-Jansen AM (2017) Increased PD-L1 and T-cell infiltration in the presence of HLA class I expression in metastatic high-grade osteosarcoma: a rationale for T-cell-based immunotherapy. Cancer Immunol Immunother 66(1):119–128. 10.1007/s00262-016-1925-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemoto A, Takagi S, Ukaji T, Gyobu N, Kakino M, Takami M, Kobayashi A, Lebel M, Kawaguchi T, Sugawara M, Tsuji-Takayama K, Ichihara K, Funauchi Y, Ae K, Matsumoto S, Sugiura Y, Takeuchi K, Noda T, Katayama R, Fujita N (2022) Targeting podoplanin for the treatment of osteosarcoma. Clin Cancer Res. 10.1158/1078-0432.ccr-21-4509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Hu C, Wang J, Shen Y, Bao Q, He F, Wang H, Gong L, Liu Z, Hu F, Liang J, Zhou Q, Wei L, Wen J, Zhang W (2019) Checkpoint blockade in combination with doxorubicin augments tumor cell apoptosis in osteosarcoma. J Immunother 42(9):321–330. 10.1097/CJI.0000000000000281 [DOI] [PubMed] [Google Scholar]
- Wilhelm SM, Carter C, Tang L, Wilkie D, Mcnabola A, Rong H, Chen C, Zhang X, Vincent P, Mchugh M, Cao Y, Shujath J, Gawlak S, Eveleigh D, Rowley B, Liu L, Adnane L, Lynch M, Auclair D, Taylor I, Gedrich R, Voznesensky A, Riedl B, Post LE, Bollag G, Trail PA (2004) BAY 43–9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res 64(19):7099–7109. 10.1158/0008-5472.CAN-04-1443 [DOI] [PubMed] [Google Scholar]
- Yang M, Liu P, Wang K, Glorieux C, Hu Y, Wen S, Jiang W, Huang P (2017) Chemotherapy induces tumor immune evasion by upregulation of programmed cell death ligand 1 expression in bone marrow stromal cells. Mol Oncol 11(4):358–372. 10.1002/1878-0261.12032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang YT, Vilma YG (2022) Sorafenib and Doxorubicin Show Synergistic Effects in Human and Canine Osteosarcoma Cell Lines. Int J Mol Sci 23(16): 9345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zappasodi R, Sm P, Gc G, Bongarzone I, Magni M, Cabras Ad, Mp C, Carlo-Stella C, Am G, Di Nicola M (2010) Improved clinical outcome in indolent B-cell lymphoma patients vaccinated with autologous tumor cells experiencing immunogenic death. Can Res 70(22):9062–9072. 10.1158/0008-5472.can-10-1825 [DOI] [PubMed] [Google Scholar]
- Zhang P, Su DM, Liang M, Fu J (2008) Chemopreventive agents induce programmed death-1-ligand 1 (PD-L1) surface expression in breast cancer cells and promote PD-L1-mediated T cell apoptosis. Mol Immunol 45(5):1470–1476. 10.1016/j.molimm.2007.08.013 [DOI] [PubMed] [Google Scholar]
- Zhang J, Hu J, Chan HF, Skibba M, Liang G, Chen M (2016) iRGD decorated lipid-polymer hybrid nanoparticles for targeted co-delivery of doxorubicin and sorafenib to enhance anti-hepatocellular carcinoma efficacy. Nanomedicine 12(5):1303–1311. 10.1016/j.nano.2016.01.017 [DOI] [PubMed] [Google Scholar]
- Zheng B, Ren T, Huang Y, Guo W (2018) Apatinib inhibits migration and invasion as well as PD-L1 expression in osteosarcoma by targeting STAT3. Biochem Biophys Res Commun 495(2):1695–1701. 10.1016/j.bbrc.2017.12.032 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary file1 Supplemental Fig. 1 Western blotting analysis of PD-L1, phosphorylation level of ERK, mTOR, and Stat3 pathways was implemented in 143b and U2 cell lines. (TIF 1827 KB)
Data Availability Statement
The data generated in this study are available upon request from the corresponding author.