Abstract
Background
With the update and release of the newest version of WHO classification (2019) for neuroendocrine neoplasm, the clinical features, risk factors of prognosis and the effect of surgical treatment on newly classified pancreatic neuroendocrine carcinoma (pNEC) patients with liver metastasis were not deeply analyzed. In the present study, we tried to reveal the clinical features, and prognostic factors of pNEC patients with liver metastasis with the newest definition of WHO 2019, and explore whether primary tumor resection (PTR), chemotherapy and radiotherapy affect overall survival (OS) and cancer-specific survival (CSS) in those patients.
Methods
We collected data from pNEC patients with liver metastasis from the Surveillance, Epidemiology, and End Results (SEER) database who were diagnosed between 2010 and 2019. We strictly selected pNEC patients according to the 2019 WHO classification criteria. The univariate and multivariate Cox regression analysis were used to determine independent predictors of the survival of these patients. The forest plots map was drawn by R-4.2.2 software to display the results of the multivariate analysis visually. Kaplan–Meier method was used to estimate the OS and CSS. Based on the multivariate analysis outcomes, we established the predictable nomogram model to predict the prognosis of pNEC patients with liver metastasis. The calibration plots were shown to prove the predictive value of the nomogram predictable model.
Results
We identified 205 eligible pNEC patients with liver metastasis. According to the multivariable Cox regression analysis in this study, we found that PTR, chemotherapy, primary tumor size and diagnosis to treatment time were independent prognostic factors for both OS and CSS. Kaplan–Meier survival curves demonstrated that PTR and chemotherapy were correlated with increased survival for pNEC patients with liver metastasis. The accuracy of the nomogram model was visually proved by the calibration plot with acceptable predictive performance.
Conclusion
Four independent predictors of prognosis in pNEC patients with liver metastasis were identified in this study, including PTR, chemotherapy, tumor size and diagnosis to treatment time. PTR and chemotherapy for pNEC with liver metastasis could lead to a better prognosis, which may provide inspiration for practical clinical guidelines.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00432-023-04847-3.
Keywords: Pancreatic neuroendocrine carcinoma, Liver metastasis, Primary tumor resection, WHO classification, SEER program
Introduction
Pancreatic neuroendocrine carcinomas (pNEC) are sporadic malignancies, accounting for less than 2% of primary pancreatic tumors (Dasari et al. 2017). The clinical manifestations of pNEC are diverse, while the diagnosis is unambiguous, and treatment measures are relatively complex. The classical 2010 World Health Organization (WHO) classification of pancreatic neuroendocrine neoplasms (NEN) divided into well-differentiated low-grade neuroendocrine tumors (NET) (G1-G2) or poorly differentiated high-grade neuroendocrine carcinomas (NEC) (G3). The 2017 WHO classification criteria updated the definition with a new classification of well-differentiated G3 pancreatic tumors (NET G3) to distinguish poorly differentiated NECs (Rindi et al. 2018). The 2019 WHO classification showed that NECs are no longer graded, just including large cell neuroendocrine carcinoma and small cell neuroendocrine carcinoma (Nagtegaal et al. 2020) (Supplementary Table 1).pNET which is well differentiated, and pNEC which is poorly differentiated, are both belonging to pNEN. Although both have similar neuroendocrine markers, the prognosis and invasive behavior are totally different. With the update of the newest version of WHO classification, the once blurred boundary between pNET and pNEC has become obviously clear. A recent study published in JAMA Oncology showed that the incidence and prevalence of NETs are steadily rising, mainly due to the development of diagnostic detection methods, and survival for all NETs has improved over time (Dasari et al. 2017). However, it also stated that, compared with other digestive system NETs, pNET has a poor prognosis.
NEC is a kind of highly malignant, rare, and poorly differentiated tumor, which can occur in all parts of the body, but is common in the lung and digestive tract (Dasari et al. 2018). Compared with other NEC, pNEC has a poorer prognosis (Dasari et al. 2018). Finding an appropriate therapeutic method for pNEC patients is challenging owing to the lack of high-quality data. Moreover, distant metastasis is proved to be a strong predictor of poor prognosis for patients with pNEN, including pNEC (Halfdanarson et al. 2008). A recent study demonstrated that NEC patients have a higher liver organotrophic metastasis rate than non-NEC patients (Park and Kwon 2023). Approximately 70% of NECs patients were found with metastasis at the time of diagnosis, and most individuals are unresectable (Umetsu et al. 2023). Another recent study showed that pNECs patients are common to be found with liver metastases, which predicted a poor prognosis (Dasari et al. 2018). Since liver metastasis is a late event in the progression of malignant diseases, it is of great significance to find effective treatment methods.
Therapeutic surgery is usually applied in local diseases, while metastatic resection is not commonly recommended (Garcia-Carbonero et al. 2016). Moreover, the effect of surgery, chemotherapy or radiotherapy on pNEC patients with liver metastasis is often controversial without large prospective studies. Feng et al. proved that patients with advanced stage pNEC might benefit from primary tumor resection (PTR), but a number of patients cannot be diagnosed with pNEC according to the newest definition of WHO 2019 classification (Feng et al. 2019). Therefore, more clinical studies and new data are required for a solid conclusion. Currently, relative factors for treatment benefits on pNEC patients with liver metastasis are scarce, and few relevant studies are available.
This study was to investigate the clinical features, and prognostic factors of pNEC patients with liver metastasis with the newest definition of WHO 2019, and explore whether the survival rate of pNEC patients with liver metastasis was improved after PTR, radiotherapy or chemotherapy according to the surveillance, epidemiology and final outcome (SEER) database.
Methods
Study design, patients and data selection
The SEER database is a large clinical dataset supported by the US National Cancer Institute, providing free data on the incidence, treatment, prognosis, and other information about cancer patients since 1973. We retrieved the data of pNEC patients with liver metastasis between 2010 and 2019 from the SEER database (version 8.4.0.1). We chose the latest and most comprehensive data in the current database “The incidence SEER Research Plus Data, 17 Registries, Nov 2021, Sub (2000–2019)” as the data source. To match with the newest definitions for pNEC patients, the “SEER Site Recode” including “pancreas”, and ICD-0–3 histology codes including “8013 (Large cell neuroendocrine carcinoma), 8041 (Small cell carcinoma, NOS)” were selected. The SEER Combined Mets at DX-liver (2010 +) was selected with the “YES” choice. The diagnostic confirmation should be “positive histology”. The baseline information of pNEC patients with liver metastasis, such as gender, race, age at diagnosis, primary site, tumor size, therapy, metastasis details, and follow-up information, were included. Those who had incomplete or unknown survival information were excluded. The flowchart of the selection procedure for this study is shown in Fig. 1.
Fig. 1.
The flow chart for patients selection
Statistical methods
Overall survival (OS) means the time from randomization to death for any reason. Cancer-specific survival (CSS) means the time from randomization to death due to specific cancer. Survival and death due to other reasons are considered as survival in the CSS concept.
The independent predictors of OS and CSS were determined by univariate and multivariable Cox regression models respectively, with the presentation of hazard ratio (HR) and 95% confidence interval (CIs) outcomes. We used Kaplan–Meier method to estimate the OS and CSS between the two groups. Nomogram was applied based on the positive independent prognostic factors from multivariate Cox analysis to predict the CSS. A practical survival prediction table predicting 6-month, 1- and 2-year OS and CSS was established for clinical references.
Survival data and corresponding figures were completed by R-4.2.2 software. The results of multivariate analysis were visually shown with forest plots by R-4.2.2 software. P value less than 0.05 was considered statistically significant by two-sided hypothesis tests.
Results
Patients clinicopathologic characteristics
Two hundred and five eligible patients with pNEC liver metastasis were selected. Table 1 demonstrates the baseline characteristics of included patients. The continuous variables such as age, tumor size and diagnosis to treatment time did not conform to normal distribution according to the normality and lognormality tests. The median and interquartile range was used to estimate those variables. The median age at diagnosis was 67 years old. The median tumor size and median diagnosis to treatment time were 4.6 cm and 1 month, respectively. Patients with age ≤ 70 years were slightly more than those aged > 70 (60.5% vs 39.5%). More than 70% of patients were white. 54.1% of patients were males, while 45.9% of patients were females. As for the primary tumor site, 42.9% of patients are located in the head of the pancreas, while 10.7% of patients are located in the body of the pancreas, and 14.6% are in the tail of the pancreas. The large cell neuroendocrine carcinoma accounted for 32.2%, while small cell carcinoma accounted for 67.8%. Tumor size ≤ 5 cm was found in 33.2% of patients, while tumor size < 5 cm was detected in 22.9% of patients.
Table 1.
Characteristics of 205 patients with pancreatic neuroendocrine carcinoma liver metastasis
| Variable | Value |
|---|---|
| Race | |
| White | 158 (77.1%) |
| Black | 27 (13.2%) |
| Others | 20 (9.7%) |
| Gender | |
| Female | 94 (45.9%) |
| Male | 111 (54.1%) |
| Age (years) | |
| ≤ 70 | 124 (60.5%) |
| > 70 | 81 (39.5%) |
| Median, IQR | 67, 15 |
| Primary site | |
| Head of pancreas | 88 (42.9%) |
| Body of pancreas | 22 (10.7%) |
| Tail of pancreas | 30 (14.6%) |
| Others | 65 (31.8%) |
| Histology type | |
| Large cell neuroendocrine carcinoma | 66 (32.2%) |
| Small cell carcinoma | 139 (67.8%) |
| Tumor size (cm) | |
| ≤ 5 | 68 (33.2%) |
| > 5 | 47 (22.9%) |
| Unknown | 90 (43.9%) |
| Median, IQR | 4.6, 2.9 |
| Surgery | |
| Yes | 8 (3.9%) |
| No | 197 (96.1%) |
| Radiotherapy | |
| Yes | 16 (7.8%) |
| No | 189 (92.2%) |
| Chemotherapy | |
| Yes | 120 (58.5%) |
| No | 85 (41.7%) |
| Brain metastasis | |
| No | 188 (91.7%) |
| Yes | 8 (3.9%) |
| Unknown | 9 (4.4%) |
| Lung metastasis | |
| No | 166 (81.0%) |
| Yes | 32 (15.6%) |
| Unknown | 7 (3.4%) |
| Bone metastasis | |
| No | 173 (84.4%) |
| Yes | 25 (12.2%) |
| Unknown | 7 (3.4%) |
| Diagnose to treatment (months) | |
| Median, IQR | 1, 0 |
| 1, 3, 5 year OS rate | 17.4%, 2.4%, 1.6% |
| 1, 3, 5 year CSS rate | 20.2%, 3.2%, 2.1% |
Regarding the treatment, 3.9% of patients received PTR, while 96.1% of patients did not. 7.8% of patients accepted radiotherapy, and 58.5% of patients accepted chemotherapy, respectively. As for visceral metastasis, all included patients presented with liver metastasis, while only 3.9% of patients combined with brain metastasis, and 15.6%, 12.2% of patients diagnosed with lung and bone metastasis, respectively. 1-, 3-, 5-year OS rate are 17.4%, 2.4%, 1.6% while the 1-, 3-, 5-year CSS rate are 20.2%, 3.2%, 2.1%.
Univariate cox regression analysis
Table 2 summarizes the OS and CSS for each potential risk factor by univariate analysis. We found that age (> 70 vs. ≤ 70, OS: HR 1.51; 95% CI 1.122–2.032; p = 0.007; CSS: HR 1.368; 95% CI 1.000–1.870; p = 0.0497), tumor size (OS: HR 1.006; 95% CI 1–1.013; p = 0.045; CSS:HR 1.008; 95% CI 1.001–1.014; p = 0.021), chemotherapy (Yes vs. No, OS: HR 0.285; 95% CI 0.210–0.388; p < 0.001; CSS:HR 0.291; 95% CI 0.211–0.401; p < 0.001) and PTR (Yes vs. No, OS: HR 0.347; 95% CI 0.142–0.850; p = 0.021; CSS:HR 0.303; 95% CI 0.112–0.821; p = 0.019) were significant predictors for both OS and CSS. Diagnose to treatment time (OS: HR 0.863; 95% CI 0.717–1.037; p = 0.116; CSS:HR 0.720; 95% CI 0.566–0.918; p = 0.008) was significant predictor for CSS.
Table 2.
Univariate Cox analysis of variables in included patients
| Variable | OS | CSS | ||
|---|---|---|---|---|
| HR | P | HR | P | |
| Race | ||||
| White | Reference | Reference | ||
| Black | 0.951 (0.620–1.459) | 0.819 | 0.977 (0.625–1.527) | 0.918 |
| Others | 0.738 (0.450–1.212) | 0.230 | 0.820 (0.497–1.350) | 0.435 |
| Gender | ||||
| Female | Reference | Reference | ||
| Male | 0.916 (0.682–1.230) | 0.559 | 0.942 (0.692–1.281) | 0.703 |
| Age(years) | 1.027 (1.013–1.040) | < 0.001*** | 1.024 (1.010–1.038) | < 0.001*** |
| ≤ 70 | Reference | Reference | ||
| > 70 | 1.51 (1.122–2.032) | 0.007** | 1.368(1.000–1.870) | 0.0497* |
| Primary site | ||||
| Head of pancreas | Reference | Reference | ||
| Body of pancreas | 1.204 (0.728–1.990) | 0.469 | 1.167 (0.687–1.981) | 0.568 |
| Tail of pancreas | 0.643 (0.401–1.032) | 0.067 | 0.658 (0.405–1.069) | 0.091 |
| Others* | 1.234 (0.878–1.735) | 0.225 | 1.183 (0.828–1.691) | 0.356 |
| Histologic type | ||||
| Large cell neuroendocrine carcinoma | Reference | Reference | ||
| Small cell carcinoma | 1.359 (0.991–1.864) | 0.057 | 1.221 (0.883–1.689) | 0.228 |
| Tumor size (cm) | 1.006 (1–1.013) | 0.045* | 1.008 (1.001–1.014) | 0.021* |
| ≤ 5 | Reference | Reference | ||
| > 5 | 1.445 (0.979–2.133) | 0.064 | 1.46 (0.971–2.195) | 0.069 |
| Surgery | ||||
| No | Reference | Reference | ||
| Yes | 0.347 (0.142–0.850) | 0.021* | 0.303 (0.112–0.821) | 0.019* |
| Radiotherapy | ||||
| No | Reference | Reference | ||
| Yes | 0.588 (0.327–1.059) | 0.077 | 0.585 (0.317–1.081) | 0.087 |
| Chemotherapy | ||||
| No | Reference | Reference | ||
| Yes | 0.285 (0.210–0.388) | < 0.001*** | 0.291 (0.211–0.401) | < 0.001*** |
| Brain metastasis | ||||
| No | Reference | Reference | ||
| Yes | 1.36 (0.636–2.91) | 0.428 | 1.261 (0.555–2.862) | 0.580 |
| Lung metastasis | ||||
| No | Reference | Reference | ||
| Yes | 1.481 (0.997–2.201) | 0.052 | 1.394 (0.913–2.128) | 0.124 |
| Bone metastasis | ||||
| No | Reference | Reference | ||
| Yes | 0.981 (0.620–1.552) | 0.933 | 0.969 (0.598–1.569) | 0.897 |
| Diagnose to treatment (months) | 0.863 (0.717–1.037) | 0.116 | 0.720 (0.566–0.918) | 0.008** |
Significantly difference: *P < 0.05; **P < 0.01; ***P < 0.001
Multivariate cox regression analysis
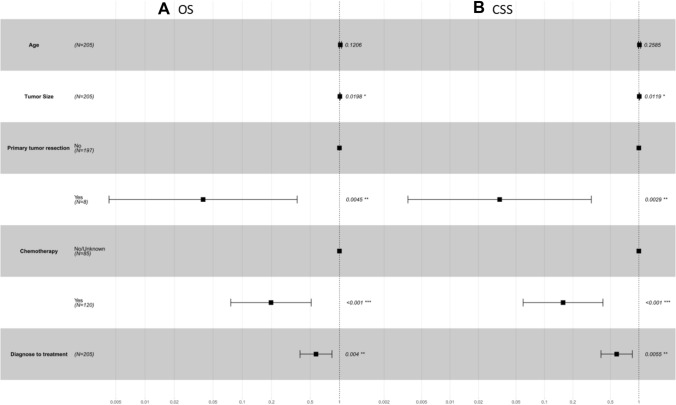
Table 3 demonstrates the OS and CSS outcomes among patients with pNEC patients liver metastasis by multivariate Cox regression analysis. The multivariate Cox regression analysis was visually presented via forest plots (Fig. 2). Multivariable Cox regression analysis indicated that tumor size(OS: HR 1.011; 95% CI 1.002–1.020; p = 0.020; CSS:HR 1.012; 95% CI 1.002–1.021; p = 0.012), PTR (Yes vs. No, OS: HR 0.040; 95% CI 0.004–0.367; p = 0.004; CSS:HR 0.034; 95% CI 0.004–0.314; p = 0.003), chemotherapy (Yes vs. No, OS: HR 0.198; 95% CI 0.076–0.513; p < 0.001; CSS:HR 0.158; 95% CI 0.059–0.417; p < 0.001) as well as diagnose to treatment time (OS: HR 0.574; 95% CI 0.393–0.837; p < 0.001; CSS:HR 0.582; 95% CI 0.397–0.853; p < 0.001) were independent risk factors.
Table 3.
Multivariate Cox analysis of included patients
| Variable | OS | CSS | ||
|---|---|---|---|---|
| HR | P | HR | P | |
| Age (years) | 1.021 (0.995–1.048) | 0.121 | 1.015 (0.989–1.041) | 0.259 |
| Tumor size | 1.011 (1.002–1.020) | 0.02* | 1.012 (1.002–1.021) | 0.012* |
| Surgery | ||||
| No | Reference | Reference | ||
| Yes | 0.040 (0.004–0.367) | 0.004** | 0.034 (0.004–0.314) | 0.003** |
| Unknown | ||||
| Chemotherapy | ||||
| No | Reference | Reference | ||
| Yes | 0.198 (0.076–0.513) | < 0.001*** | 0.158 (0.059–0.417) | < 0.001*** |
| Diagnose to treatment (months) | 0.574 (0.393–0.837) | < 0.001*** | 0.582 (0.397–0.853) | 0.005** |
Significantly difference: *P < 0.05; **P < 0.01; ***P < 0.001
Fig. 2.
Forest plots of the predictors of OS (A) and CSS (B) of included patients. OS overall survival, CSS cancer-specific survival
Survival analysis
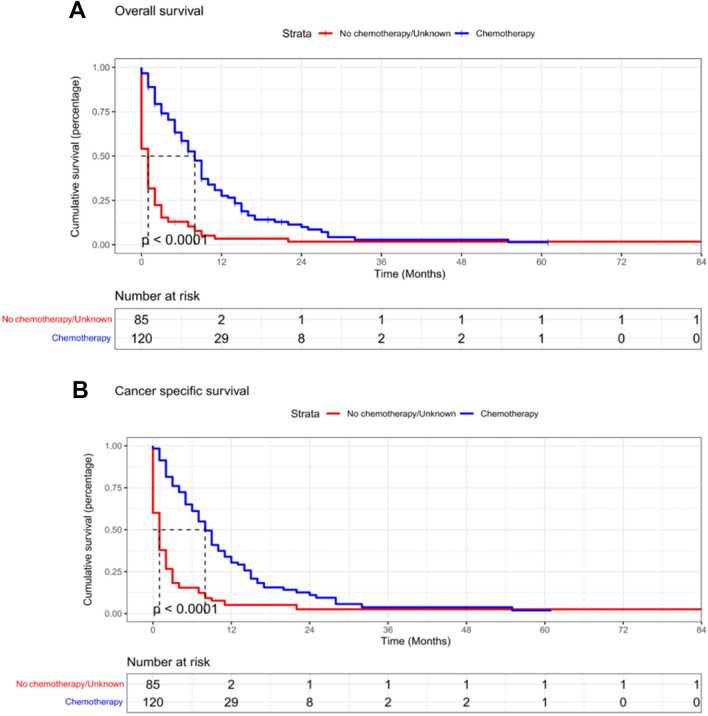
Kaplan–Meier survival curves were completed by R using R-4.2.2 software to draw the independent prognostic factors detected by the above analysis visually. PTR and chemotherapy had a positive effect on better OS and CSS of patients (Figs. 3, 4).
Fig. 3.
Kaplan–Meier survival curves for estimating OS (A) and CSS (B) of included patients based on chemotherapy
Fig. 4.
Kaplan–Meier survival curves for estimating OS (A) and CSS (B) of included patients based on PTR
The predictable model for OS and CSS
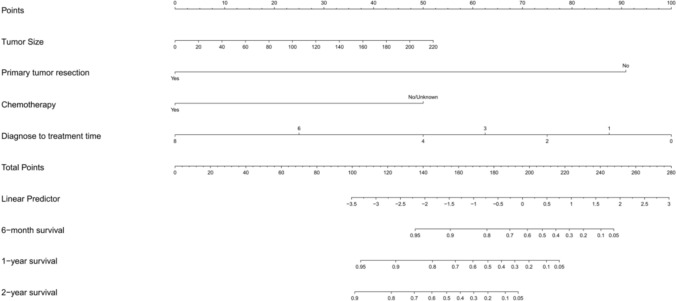
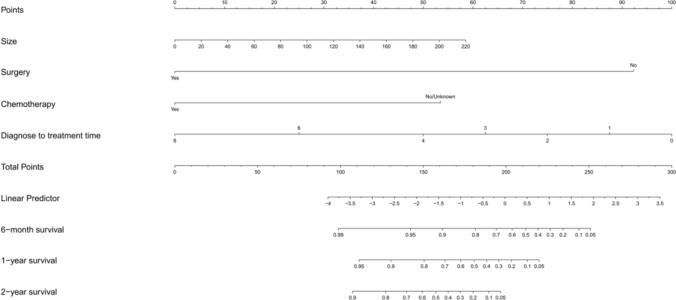
We constructed a nomogram to predict the probabilities of OS and CSS at 6 months, 1 and 2 years based on the independent prognostic factors from the multivariable Cox regression analysis (Figs. 5, 6). A predictable table (Table 4) was established by the results of the nomogram model for clinical references. By observing the calibration plots, the nomogram predictions are relatively accurate and consistent with observed survival (Fig. 7). In Fig. 7, the x axis represents the nomogram-predicted survival rates, whereas the y axis represents the actual survival rates.
Fig. 5.
The prediction nomogram of OS rates of 6 months, 1 and 2 years of pNEC patients with liver metastasis. Total points correspond to corresponding survival rates
Fig. 6.
The prediction nomogram of CSS rates of 6 months, 1 and 2 years of pNEC patients with liver metastasis. Total points correspond to corresponding survival rates
Table 4.
The predictable and referable table for survival
| OS | CSS | |
|---|---|---|
| Size(cm) | Points | Points |
| 0 | 0 | 0 |
| 20 | 5 | 5 |
| 40 | 9 | 11 |
| 60 | 14 | 16 |
| 80 | 19 | 21 |
| 100 | 24 | 27 |
| 120 | 28 | 32 |
| 140 | 33 | 37 |
| 160 | 38 | 43 |
| 180 | 43 | 48 |
| 200 | 47 | 53 |
| 220 | 52 | 59 |
| Surgery | ||
| Yes | 0 | 0 |
| No | 91 | 92 |
| Chemotherapy | ||
| Yes | 0 | 0 |
| No | 50 | 53 |
| Diagnosis to treatment (month) | ||
| 0 | 100 | 100 |
| 1 | 88 | 88 |
| 2 | 75 | 75 |
| 3 | 62 | 62 |
| 4 | 50 | 50 |
| 6 | 25 | 25 |
| 8 | 0 | 0 |
| Total points 6-month survival | ||
| 0.05 | 248 | 251 |
| 0.1 | 240 | 244 |
| 0.2 | 230 | 234 |
| 0.3 | 222 | 227 |
| 0.4 | 215 | 219 |
| 0.5 | 207 | 212 |
| 0.6 | 199 | 204 |
| 0.7 | 189 | 194 |
| 0.8 | 176 | 182 |
| 0.9 | 155 | 162 |
| 0.95 | 136 | 142 |
| 0.99 | 99 | |
| Total points 1-year survival | ||
| 0.05 | 217 | 220 |
| 0.1 | 210 | 213 |
| 0.2 | 200 | 203 |
| 0.3 | 192 | 196 |
| 0.4 | 184 | 188 |
| 0.5 | 177 | 181 |
| 0.6 | 168 | 173 |
| 0.7 | 158 | 163 |
| 0.8 | 145 | 151 |
| 0.9 | 125 | 130 |
| 0.95 | 105 | 111 |
| Total points 2-year survival | ||
| 0.05 | 194 | 197 |
| 0.1 | 186 | 190 |
| 0.2 | 177 | 180 |
| 0.3 | 169 | 172 |
| 0.4 | 161 | 165 |
| 0.5 | 153 | 158 |
| 0.6 | 145 | 149 |
| 0.7 | 135 | 140 |
| 0.8 | 122 | 127 |
| 0.9 | 101 | 107 |
| Points per unit of linear predictor | 27.560 | 26.720 |
| Linear predictor units per point | 0.036 | 0.037 |
Fig. 7.
Calibrations of the nomograms for predicting survival rates. Calibration of the nomogram for predicting (A) the 6-month OS rate. B the 6-month CSS rate. C the 1-year OS rate. D the 1-year CSS rate
Discussion
Currently, this research provided the first comprehensive analysis based on the newest definition of WHO classification 2019 to explore the effect of mainstream therapy methods such as PTR, chemotherapy and radiotherapy on pNEC patients with liver metastasis. Meanwhile, by far, this is the most extensive study probing the clinical features, prognosis and survival of pNEC patients with liver metastasis with a brand new pNEC definition. Previous studies were not based on the latest definition of WHO and were limited by small sample sizes.
As we know, extra-pulmonary NEC is poorly studied, especially pNEC with distant metastasis. High-quality data with a large sample of pNEC patients with liver metastasis are extremely lacking due to their rare incidence. From the multivariate Cox regression analysis above, we found that PTR and chemotherapy are closely associated with better OS and CSS in pNEC patients with liver metastasis. In our study, PTR and chemotherapy does improve the OS or CSS rate of pNEC patients with liver metastasis, supporting the value of PTR and chemotherapy in those patients. As demonstrated in this research, pNEC patients with liver metastasis had a relatively poor 5-year OS and CSS rates of less than 3%.
As we know above, the survival rate of patients with pNEC liver metastasis is relatively low, with relatively poor prognosis. Surgery for pNEC patients with liver metastasis is not commonly recommended. National Comprehensive Cancer Network (NCCN) Clinical practice guidelines in oncology regarding neuroendocrine and adrenal tumors recommend PTR for resectable metastatic disease with favorable biology, but do not recommend PTR for unresectable metastatic tumors or symptomatic with low tumor burden patients (Shah et al. 2021). The purpose of primary surgical resection is either to radically cure cancers or to reduce the tumor load to minimize the impact of the tumor on patients. However, if the cancer of patients has aggressive and invasive biological behaviors, the risk of surgery outweighs its benefits, which means surgery is unsuitable for those patients. In this study, we proved that even though pNEC with liver metastasis has aggressive biological behaviors, PTR remains the vital modality for treating pNEC patients with liver metastasis. The deeper mechanism of the positive impact of PTR on better survival outcomes is still unknown. One possible reason may be the development of surgical techniques, with increasing experiences of surgeons and decreasing complications after surgery in recent years, which means the risk of surgery falls. Therefore, PTR still is an inevitable component of comprehensive multidisciplinary methods for the treatment of pNEC patients with liver metastasis.
As for chemotherapy, guidelines from the North American Neuroendocrine Society and European Neuroendocrine Tumor Society did not recommend a specific chemotherapy regimen for pNEC patients with liver metastasis. NCCN guidelines suggested Cisplatin and Etoposide to treat the pNEC patients with metastasis10. Carboplatin–etoposide is a first-line option for patients with advanced extra-pulmonary, poorly differentiated NEC. Previous retrospective study has demonstrated that Carboplatin–etoposide benefits a lot in patients with advanced and metastatic NECs (Frizziero et al. 2019). In this study, we collected 205 patients between 2010 and 2019 years, and showed that chemotherapy could significantly improve the survival outcomes of pNEC patients with liver metastasis. More prospective studies are needed to explore the specific chemotherapy regimen for pNEC patients with liver metastasis.
Radiotherapy was not the independent prognostic factor for pNEC patients with liver metastasis. NCCN guidelines did not mention or recommend the mono-application of radiotherapy in pNEC patients with distant metastasis. Concurrent or sequential radiotherapy in combination with chemotherapy was recommended in unresectable locoregional pNEC, but not mentioned in pNEC with liver metastasis (Shah et al. 2021). Besides surgery, chemotherapy and radiotherapy, novel therapeutic strategies such as combined immunotherapy still need to be focused on (Klein et al. 2020). However, limited data about immunotherapy or other novel strategy was found in the current SEER database.
In addition, we showed that tumor size and diagnosis to treatment time are independent prognostic factors for pNEC patients with liver metastasis, which means the earlier we confirm the diagnosis and put effective treatment into action, the better prognostic outcomes patients will benefit.
A recent study showed that patients whose primary tumor locates in the pancreatic body or tail had better survival than those in the pancreatic head (Hua et al. 2017). Because tumors in the body or tail are less likely to invade the other visceral organs or vessels compared with tumors in the head of the pancreas. However, in this study, we found that for pNEC patients with liver metastasis, the primary tumor site was not correlated with OS or CSS. The reason may be that pNEC patients with liver metastasis already have distant metastasis, which means the potential risk has exceeded the risk of the primary tumor site invading other visceral organs or vessels.
Furthermore, we should not ignore the important reminder of those negative results. For example, there is no significant difference in survival or prognosis outcomes no matter what is your age, your race, your gender or your combined metastasis. We can learn from it that pNEC with liver metastasis is not age oriented, gender oriented or race oriented. The combined metastasis may not affect the prognostic outcomes of pNEC patients with liver metastasis.
Several limitations exist in this study. First, the retrospective study itself, and patients in the SEER database are mostly white race, which may cause the possible selective bias. Second, some potential factors, such as immunotherapy, were not added in this study to be adjusted for multivariate analysis because we cannot acquire relevant information from the database. Lastly, although we found better survival outcomes in patients after PTR or chemotherapy, no detailed surgical manner or clear chemotherapy program was suggested in this study. To overcome the obstacles from those limitations above, further multi-center prospective studies still need to be completed to become the references for consensus guidance and clinics.
Conclusion
In this study, we analyzed the clinical features, prognosis and survival of a large sample of pNEC patients with liver metastasis, and attached great importance to the effect of PTR. We demonstrated that PTR or chemotherapy may improve survival in pNEC patients with liver metastasis, which may become a significant inspiration for the following clinical practice guidelines. Additionally, tumor size and diagnosis to treatment time were independent prognostic factors for pNEC patients with liver metastasis, emphasizing the significance of early diagnosis and timely treatment.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We acknowledge all the SEER database founders and data providers who made such studies possible. Thank you so much for making the SEER database available to us. We acknowledge the clinic staff and managers of Peking Union Medical College Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College for their valuable contributions to this research.
Author contributions
LWH conceived the original idea, collected the data, analyzed the data and wrote the manuscript. ZTP supervised the project. All authors reviewed the manuscript.
Funding
Research grant supports were provided by the National Key R&D Program of China (2018YFE0118600); National Natural Science Foundation of China (No.81972258, No.81974376, No. 82103016, No. 82172836, No.82272917, No.82203158); CAMS Innovation Fund for Medical Sciences (CIFMS) (2021-1-I2M-002); China Postdoctoral Science Foundation (2021T140071 and 2021M690462); Youth Research Fund of Peking Union Medical College Hospital (pumch201911710, pumch201910819); National High Level Hospital Clinical Research Funding (2022-PUMCH-A-056; 2022-PUMCH-A-133; 2022-PUMCH-A-245); National Multidisciplinary Cooperative Diagnosis and Treatment Capacity Building Project for Major Diseases.
Data availability
All data were extracted from the SEER database (US National Cancer Institute).
Declarations
Conflict of interest
Not applicable.
Ethical approval
All data were extracted from the SEER database (US National Cancer Institute) in this study. No relevant approval was required for the study according to the policy of Peking Union Medical College Hospital.
Consent for publication
We sincerely hope that this article can be accepted.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Dasari A et al (2017) Trends in the incidence, prevalence, and survival outcomes in patients with neuroendocrine tumors in the United States. JAMA Oncol 3:1335–1342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasari A, Mehta K, Byers LA, Sorbye H, Yao JC (2018) Comparative study of lung and extrapulmonary poorly differentiated neuroendocrine carcinomas: A SEER database analysis of 162,983 cases. Cancer 124:807–815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng T et al (2019) Surgical resection of the primary tumor leads to prolonged survival in metastatic pancreatic neuroendocrine carcinoma. World J Surg Oncol 17:54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frizziero M et al (2019) Carboplatin in combination with oral or intravenous etoposide for extra-pulmonary, poorly-differentiated neuroendocrine carcinomas. Neuroendocrinology 109:100–112 [DOI] [PubMed] [Google Scholar]
- Garcia-Carbonero R et al (2016) ENETS consensus guidelines for high-grade gastroenteropancreatic neuroendocrine tumors and neuroendocrine carcinomas. Neuroendocrinology 103:186–194 [DOI] [PubMed] [Google Scholar]
- Halfdanarson TR, Rabe KG, Rubin J, Petersen GM (2008) Pancreatic neuroendocrine tumors (PNETs): incidence, prognosis and recent trend toward improved survival. Ann Oncol 19:1727–1733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua Y-Q et al (2017) Radiofrequency ablation for hepatic oligometastatic pancreatic cancer: an analysis of safety and efficacy. Pancreatology 17:967–973 [DOI] [PubMed] [Google Scholar]
- Klein O et al (2020) Immunotherapy of ipilimumab and nivolumab in patients with advanced neuroendocrine tumors: a subgroup analysis of the CA209-538 clinical trial for rare cancers. Clin Cancer Res 26:4454–4459 [DOI] [PubMed] [Google Scholar]
- Nagtegaal ID et al (2020) The 2019 WHO classification of tumours of the digestive system. Histopathology 76:182–188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park HK, Kwon GY (2023) Comparison of metastatic patterns among neuroendocrine tumors, neuroendocrine carcinomas, and nonneuroendocrine carcinomas of various primary organs. J Korean Med Sci 38:e85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rindi G et al (2018) A common classification framework for neuroendocrine neoplasms: an international agency for research on cancer (IARC) and world health organization (WHO) expert consensus proposal. Mod Pathol 31:1770–1786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah MH et al (2021) Neuroendocrine and adrenal tumors, version 2.2021, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 19:839–868 [DOI] [PubMed] [Google Scholar]
- Umetsu SE et al (2023) Integrated genomic and clinicopathologic approach distinguishes pancreatic grade 3 neuroendocrine tumor from neuroendocrine carcinoma and identifies a subset with molecular overlap. Mod Pathol 36:100065 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data were extracted from the SEER database (US National Cancer Institute).