Abstract
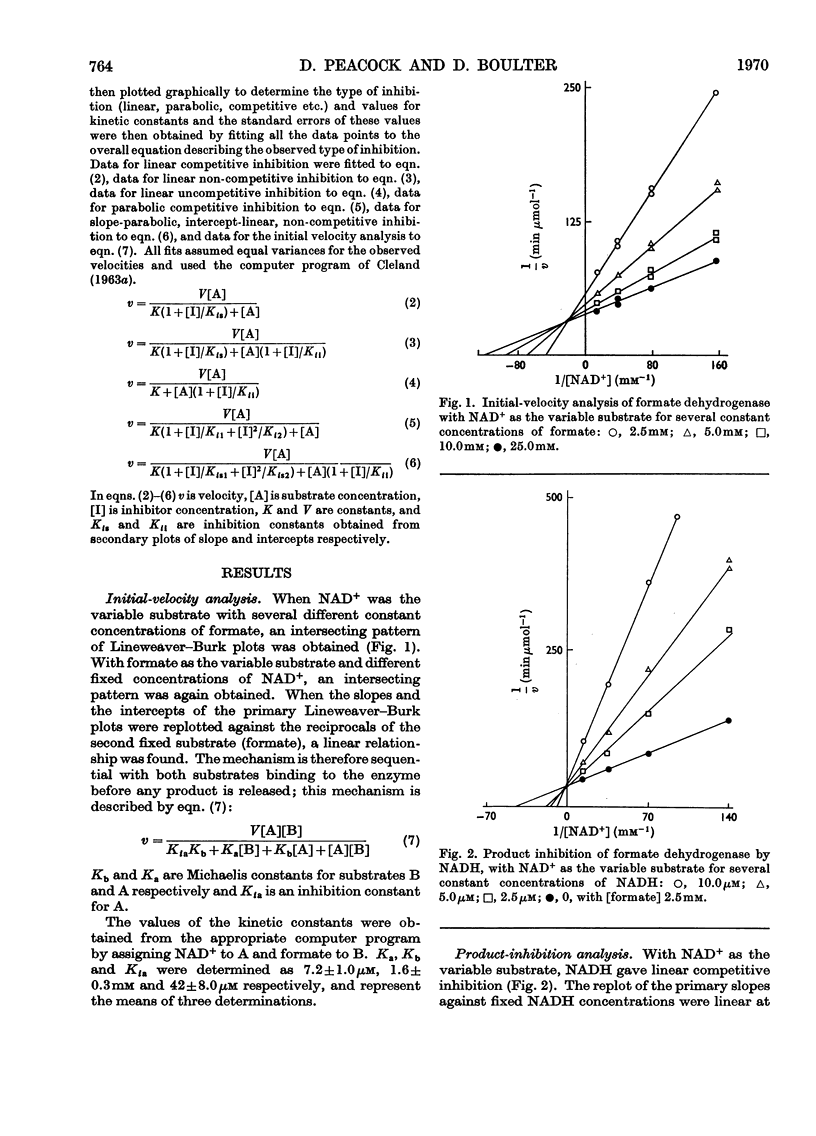
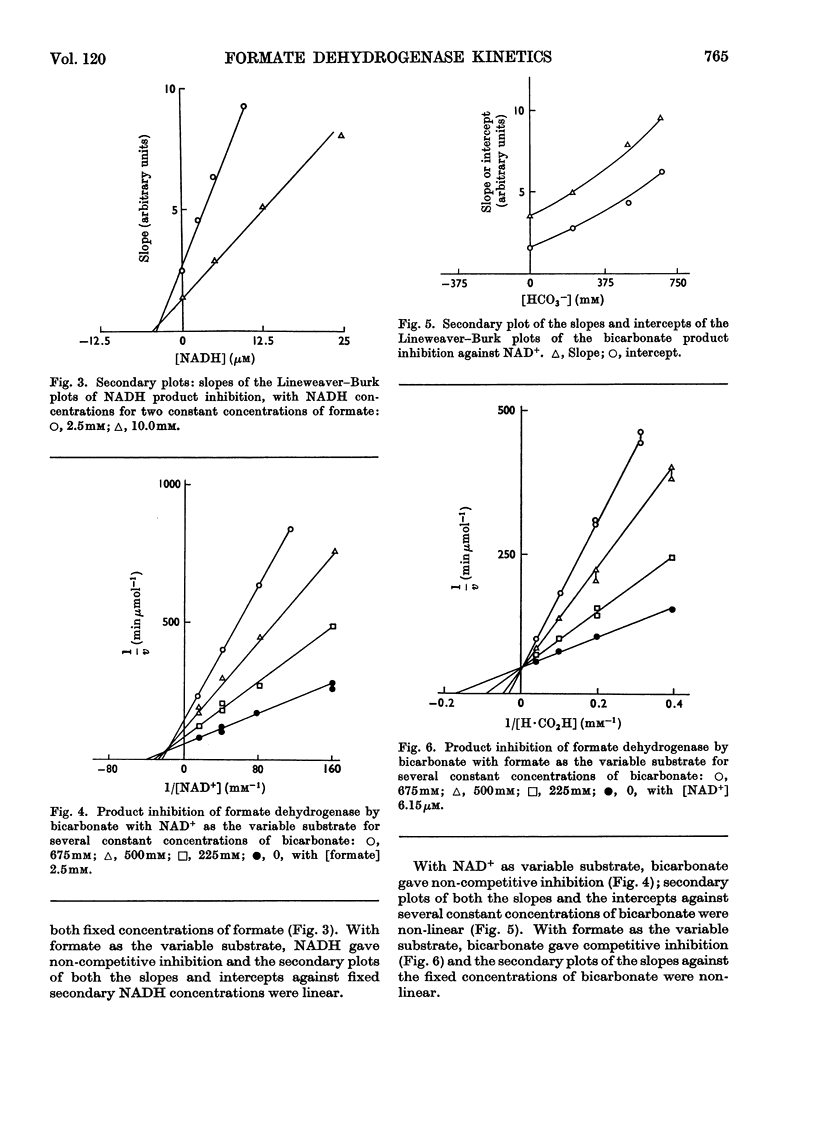
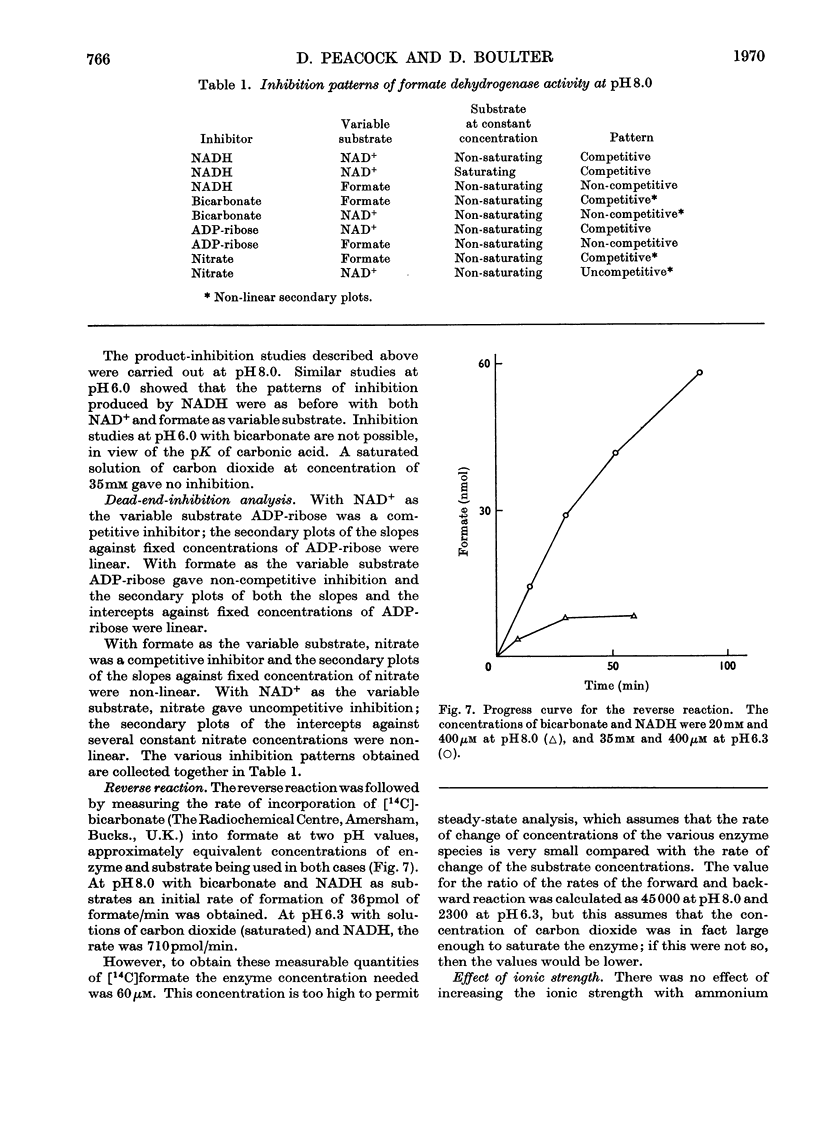
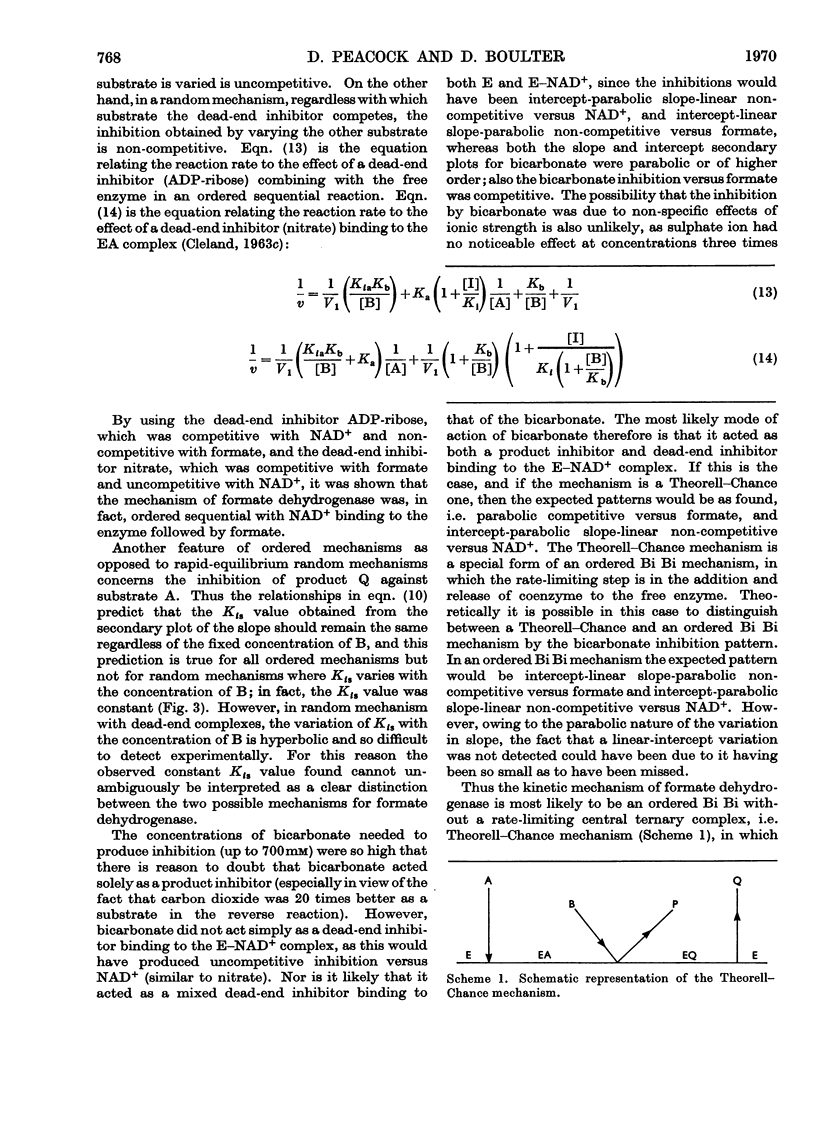
1. The kinetic mechanism of formate dehydrogenase is a sequential pathway. 2. The binding of the substrates proceeds in an obligatory order, NAD+ binding first, followed by formate. 3. It seems most likely that the interconversion of the central ternary complex is extremely rapid, and that the rate-limiting step is the formation or possible isomerization of the enzyme–coenzyme complexes. 4. The secondary plots of the inhibitions with HCO3− and NO3− are non-linear, which suggests that more than one molecule of each species is able to bind to the same enzyme form. 5. The rate of the reverse reaction with carbon dioxide at pH6.0 is 20 times that with bicarbonate at pH8.0, although no product inhibition could be detected with carbon dioxide. The low rate of the reverse reaction precluded any steady-state analysis as the enzyme concentrations needed to obtain a measurable rate are of the same order as the Km values for NAD+ and NADH.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CLELAND W. W. Computer programmes for processing enzyme kinetic data. Nature. 1963 May 4;198:463–465. doi: 10.1038/198463a0. [DOI] [PubMed] [Google Scholar]
- CLELAND W. W. The kinetics of enzyme-catalyzed reactions with two or more substrates or products. I. Nomenclature and rate equations. Biochim Biophys Acta. 1963 Jan 8;67:104–137. doi: 10.1016/0006-3002(63)91800-6. [DOI] [PubMed] [Google Scholar]
- CLELAND W. W. The kinetics of enzyme-catalyzed reactions with two or more substrates or products. II. Inhibition: nomenclature and theory. Biochim Biophys Acta. 1963 Feb 12;67:173–187. doi: 10.1016/0006-3002(63)91815-8. [DOI] [PubMed] [Google Scholar]
- DALZIEL K. KINETIC STUDIES OF LIVER ALCOHOL DEHYDROGENASE AND PH EFFECTS WITH COENZYME PREPARATIONS OF HIGH PURITY. J Biol Chem. 1963 Aug;238:2850–2858. [PubMed] [Google Scholar]
- DALZIEL K. The purification of nicotinamide adenine dinucleotide and kinetic effects of nucleotide impurities. J Biol Chem. 1963 Apr;238:1538–1543. [PubMed] [Google Scholar]
- DAVISON D. C. Studies on plant formic dehydrogenase. Biochem J. 1951 Sep;49(4):520–526. doi: 10.1042/bj0490520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale E. F. Formic dehydrogenase of Bacterium coli: its inactivation by oxygen and its protection in the bacterial cell. Biochem J. 1939 Jun;33(6):1012–1027. doi: 10.1042/bj0331012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MATHEWS M. B., VENNESLAND B. Enzymic oxidation of formic acid. J Biol Chem. 1950 Oct;186(2):667–682. [PubMed] [Google Scholar]
- WILKINSON G. N. Statistical estimations in enzyme kinetics. Biochem J. 1961 Aug;80:324–332. doi: 10.1042/bj0800324. [DOI] [PMC free article] [PubMed] [Google Scholar]


