Abstract
Background
Copper homeostasis imbalance has been implicated in tumor progression, aggressiveness, and treatment response. However, the precise roles of cuproptosis-related genes (CRGs) in hepatocellular carcinoma (HCC) remain poorly understood.
Methods
In this study, we employed a consensus clustering algorithm to identify distinct molecular subtypes. We then performed Kaplan–Meier analysis and univariate Cox regression analysis to identify prognostic differentially expressed genes. The expression of these genes was subsequently validated using qPCR on fresh-frozen tissues obtained from HCC patients. Moreover, leveraging the TCGA-HCC cohort, we constructed a CRGs-related risk prediction model using the LASSO and multivariate Cox regression analysis.
Results
By analyzing the data, we successfully established a CRGs risk prognostic model for HCC patients, comprising five differential genes (CAD, SGCB, TXNRD1, KDR, and MTND4P20). Cox regression analysis revealed that the CRGs risk score could serve as an independent prognostic factor for overall survival (hazard ratio [HR] = 1.308, 95% confidence interval [CI] = 1.200 − 1.426, P < 0.001). The area under the curve (AUC) values of the CRGs-score for predicting 1-year, 3-year, and 5-year survival rates were 0.785, 0.724, and 0.723, respectively. Notably, the expression levels of immune checkpoints (including PD-1, PD-L1, and CTLA4) significantly differed between the low- and high-risk score groups. Furthermore, the low-risk score group displayed increased sensitivity to sorafenib, cisplatin, cyclopamine, nilotinib, salubrinal, and gemcitabine, whereas the high-risk score group exhibited heightened sensitivity to lapatinib, erlotinib, and gefitinib.
Conclusions
Our findings highlight the potential of the CRGs risk score as an independent and promising biomarker for clinical prognosis and immunotherapy sensitivity in HCC patients.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00432-023-05099-x.
Keywords: Cuproptosis, Hepatocellular carcinoma, Tumor microenvironment, Prognostic model, Chemotherapy sensitivity
Introduction
Primary liver cancer, also known as hepatocellular carcinoma (HCC), is a heterogeneous malignant tumor with a poor prognosis (Llovet et al. 2021). Despite aggressive treatment regimens, the overall survival (OS) of HCC patients remains poor, with approximately 70% of liver cancers relapse within 5 years after resection or ablation (Roayaie et al. 2009; Morgan et al. 2013). In recent years, high-throughput analysis of large sample sizes has improved our understanding of the HCC and the identification of biomarkers (Nakagawa et al. 2019; Zucman-Rossi et al. 2015). However, the investigation of hub genes that can serve as biomarkers for the diagnosis and treatment of HCC is still limited. Currently, commonly used diagnostic strategies of HCC, such as imaging techniques and serological tumor markers, are insufficient to meet the clinical requirements. Therefore, establishing a reliable tumor risk prediction model for HCC patients is of great significance for prognosis and survival.
Copper is an indispensable trace metal that plays a crucial role in mitochondrial respiration, antioxidant defense, and biosynthesis. Many transcription factors and critical enzymes require copper for their activities (Daniel et al. 2004; Aggett and Fairweather-Tait 1998). Copper-driven redox chemistry is necessary for various biological processes, and dysregulation of copper homeostasis may trigger oxidative stress and cytotoxicity (Que et al. 2008). Clinical investigations have observed elevated serum and intra-tumoral copper levels in liver cancer patients (Ebara et al. 2000; Poo et al. 2003; Jie et al. 2007), suggesting that persistent intracellular copper accumulation impairs liver function. In clinical practice, copper chelators (trientine, tetrathiomolybdate, etc.) and copper ionophores (disulfiram, dithiocarbamates, elesclomol, etc.) have been applied for anticancer treatment (Brady et al. 2017). Furthermore, extensive research on both patients and animal models also supports the association between excessive copper accumulation and hepatobiliary malignancies (Huster 2014; Huster et al. 2007; Pfeiffenberger et al. 2015). Emerging evidence indicates that cuproptosis is a novel form of programmed cell death (Aubert et al. 2020; Tsvetkov et al. 2022; Dong et al. 2021; Ren et al. 2021; Polishchuk et al. 2019), but its role in HCC remains unclear. Therefore, conducting a preliminary study on the potential role of cuproptosis in HCC is necessary to further understand its effects on patient prognosis and drug therapy.
In this study, we intended to construct a risk score model based on cuproptosis-related genes (CRGs) to guide clinical treatment and predict prognosis. A total of 371 HCC samples from The Cancer Genome Atlas (TCGA) website (https://portal.gdc.cancer.gov/repository) were used to establish the risk score model. Subsequently, the patients were divided into three cuproptosis-related subtypes based on this risk score model. We then explored the clinical prognosis, immune infiltration, and response to immunotherapy among the different subtypes. Our findings indicate that the CRGs risk score is a promising marker for HCC prognosis and treatment.
Materials and methods
Data source and preprocessing
RNA-seq data with corresponding clinical information on HCC were obtained from TCGA website (https://portal.gdc.cancer.gov/repository). Patients without survival information were excluded from the analysis. The level-3 RNA-seq data (HTSeq-FPKM) was transformed into transcripts per million reads (TPM) for subsequent analyses. Additionally, somatic mutation and copy number variation (CNV) data for HCC were also downloaded from TCGA website. A total of 19 cuproptosis-related genes (CRGs) were identified from the previous reports (Aubert et al. 2020; Tsvetkov et al. 2022; Dong et al. 2021; Ren et al. 2021; Polishchuk et al. 2019), which included CDKN2A, FDX1, DLD, DLAT, LIAS, GLS, LIPT1, MTF1, PDHA1, PDHB, NFE2L2, NLRP3, ATP7B, ATP7A, LIPT2, DBT, GCSH, DLST, and SLC31A1. These genes were selected for further analysis based on their reported involvement in cuproptosis and relevant biological processes.
Consensus clustering analysis
The “ConsensusClusterPlus” R package was employed for cluster analysis in all cohorts (Wilkerson and Hayes 2010). The clustering criteria were as follows: (1) the cumulative distribution function (CDF) curve showed a gradual and smooth increase; (2) there were no small sample sizes within any group; (3) the intra-group correlation increased while the inter-group correlation decreased. Initially, based on the expression of the 19 CRGs, a consensus clustering algorithm was applied to stratify 371 cases with complete clinical information from TCGA into CRG subtypes. Subsequently, the overlapping differentially expressed genes (DEGs) between CRGs subtypes were analyzed using univariate Cox regression to identify DEGs associated with overall survival (OS) (P < 0.05). Finally, a new consensus cluster analysis was performed on DEGs related OS in order to identify gene cluster subtypes.
Functional and pathway enrichment analysis
The gene set variation analysis (GSVA) was performed to investigate the differences in biological processes among the different CRGs subtypes using the “GSVA” R packages (Hanzelmann et al. 2013). The hallmark gene set (c2.cp.kegg.v7.4.symbols.gmt) was obtained from the MSigDB database. A statistical significance level of adjusted P < 0.05 was considered. Differential expression analysis was conducted using the “limma” package in R (version 4.0.5) to identify DEGs (|log2FoldChange |> 0.5 and adjusted P < 0.05) between the different CRGs subtypes. The R package “clusterProfiler” was utilized for the functional enrichment analysis of the identified DEGs.
Tumor microenvironment and immune infiltration analysis
A single-sample gene set enrichment analysis (ssGSEA) algorithm was employed to evaluate the levels of immune cell infiltration in the HCC. The immune and stromal scores of each HCC patient were determined using ESTIMATE algorithm. Additionally, the relative proportion of 22 immune cells were evaluated by using CIBERSORT (https://cibersort.stanford.edu/).
Construction of CRGs risk score model for HCC prognosis
The overlapping DEGs between CRGs subtypes were first subjected to univariate Cox regression to obtain the OS-related DEGs. Followed by least absolute shrinkage and selection operator (LASSO) penalties regression, we identified the most powerful prognostic DEGs and their correlative coefficients. Meanwhile, TCGA-HCC cases (n = 371) were randomly divided into training sets and testing sets with a 1:1 ratio. Next, hub prognostic CRGs genes were identified using multivariate Cox regression analysis to construct a CRGs-related prognostic CRGs-score in the training set. The CRGs-score was calculated using the formula: CRGs-score = Σ (Expression * risk coefficient). Based on the median risk score, the training set and testing sets were stratified into low- and high-risk groups, respectively. Finally, survival analysis and receiver operating characteristic (ROC) curve analysis were performed for the two risk groups using the “survminer” and “survivalROC” R packages, respectively.
Drug susceptibility analysis
To explore the potential applicability of the CRGs-score in clinical treatment decisions, the “pRRophetic” R package was used to evaluate the susceptibility to commonly used chemotherapy or targeted drugs in the two risk groups (Geeleher et al. 2014).
Clinical sample collection, RNA isolation, and qPCR
Thirty-two pairs of fresh-frozen tissues (HCC tissues and adjacent tissues) were collected from the Zhongnan Hospital of Wuhan University. The study was approved by the ethics committee under Approval Number 2017058, and written informed consent was obtained from all participants. Complementary DNA (cDNA) was synthesized from total RNA using the Prime Script RT Reagent Kit (Vazyme, R333-01, China). Quantitative PCR (qPCR) was performed using the SYBR Prime Script RT-PCR kit (Vazyme, Q712-02, China) on a CFX96 instrument (Bio-Rad, America). The data were analyzed using the 2−ΔΔct method and normalized to beta-actin. The primer sequences of five prognostic genes in CRGs-score model were as follows: SGCB-F, 5′-ATGCGTGAGAAGGCTGTTGA-3′; SGCB-R, 5′-TCGAAGCAGGCCACTTTCAT-3′; TXNRD1-F, 5′-CGCCGTAGGTCAGCTAAAGAT-3′; TXNRD1-R, 5′-TTCACAAACACAACGGGCAG-3′; KDR-F, 5′-GTTGGAGCAATCCCTGTGGA-3′; KDR-R, 5′-AACTCCATGCCCTTAGCCAC-3′; CAD-F, 5′-CAGCTGAGGAGCCAAAGGAG-3′; CAD-R, 5′-TAATGAGTGCAGCAGGGGTG-3′; MTND4P20-F, 5′-CAGCCAAACCATACTACTAACAC-3′; MTND4P20-R, 5′- TCACATGGCTATCAGGGAG-3′.
Construction of a nomogram scoring system
The CRGs risk score and common clinical characteristics were utilized to construct a prognostic nomogram using the “rms” R package (Iasonos et al. 2008). The predictive performance of the nomogram can be assessed through the ROC curve and calibration diagram, which provide information about the accuracy and calibration of the predictive model, respectively.
Statistical analysis
All statistical analyses and graphical plotting were performed using R software (version 4.0.5.). Unless specified otherwise, a significance level of P < 0.05 (two-sided) was considered statistically significant.
Results
Genetic and transcriptional alterations of CRGs in HCC
Figure 1A provides a schematic diagram illustrating the analysis process. As depicted in Fig. 1B, out of the 371 HCC samples in the TCGA-HCC cohort, a total of 38 samples (10.24%) had somatic mutations in the 19 CRGs. Among these samples, CDKN2A had a relatively high mutation frequency of 3%, followed by NLE2L2 and NLRP3 with a frequency of 2%. Additionally, 12 CRGs (ATP7B, LIAS, PDHA1, PDHB, GLS, DLST, SLC31A1, FDX1, LIPT1, LIPT2, DLAT, and GCSH) did not have any mutations.
Fig. 1.
Genetic and transcriptional alterations of CRGs in HCC. A The schematic diagram for entire analytical process. B Mutation frequencies of 19 CRGs in 371 patients with HCC from the TCGA dataset. C Frequencies of CNV gain and loss among CRGs. D Locations of CNV alterations in CRGs on 23 chromosomes. E Expression distributions of 19 CRGs between tumor and normal tissues. CRGs cuproptosis-related genes, HCC hepatocellular cancer, CNV copy number variant, DEGs differentially expressed genes, TME tumor microenvironment. *P < 0.05; **P < 0.01; ***P < 0.001
Next, we further explored the copy number variation (CNV) in these CRGs and found extensive CNV alterations in all 20 CRGs (Fig. 1C). Among them, NLRP3, NFE2L2, LIAS, GLS, DLD, and LIPT2 had CNV increases, while CDKN2A, ATP7B, MTF1, DLAT, DLST, FDX1, PDHB, and GCSH showed CNV decreases. Figure 1D displays the positions of the CNV changes in the CRGs on their respective chromosomes. Furthermore, the differential expression analysis revealed that the mRNA expression of the most CRGs was positively related to CNV alterations. As shown in Fig. 1E and C, the expression of CRGs with CNV gain (ATP7A, LIAS, LIPT1, LIPT2, DLD, and GLS) was significantly elevated in HCC samples. Conversely, CRGs with CNV loss, namely SLC31A1 and DBT, exhibited lower expression levels in HCC samples. This observation suggests a potential association between CNV alterations and the expression patterns of these CRGs in HCC.
Identification of CRGs subtypes in HCC
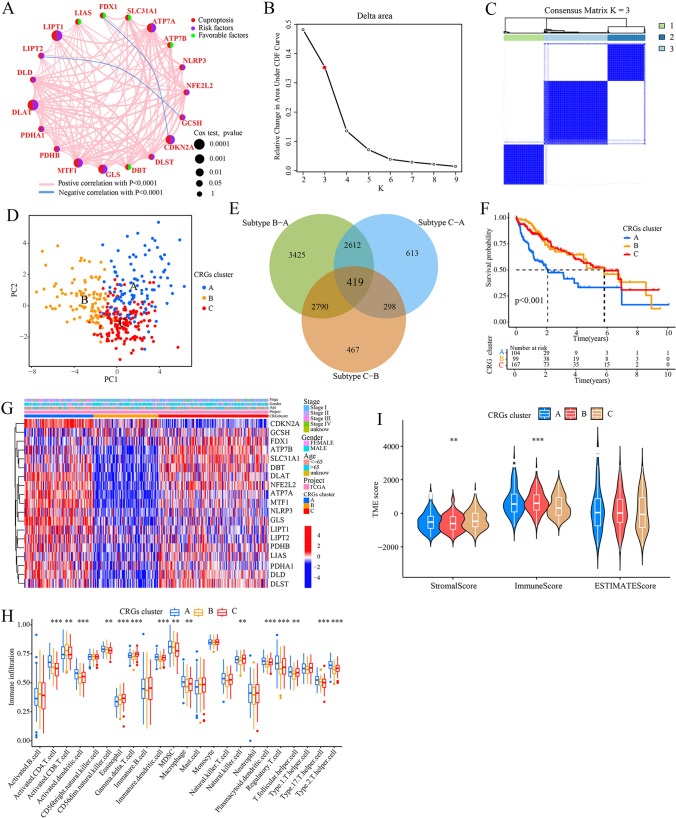
As shown in Fig. 2A, the cuproptosis network provides a comprehensive overview of the interactions, interconnections, and prognostic value of the 19 CRGs in HCC patients. Prognostic analysis using univariate Cox regression and Kaplan–Meier (K-M) analysis revealed that high expression levels of 13 out of the 19 CRGs were significantly associated with poor prognosis in HCC patients (P < 0.05, Supplementary Table S1 and Fig. S1). These 13 CRGs included CDKN2A, ATP7A, DLAT, DLD, DLST, GCSH, GLS, LIPT1, MTF1, NFE2L2, NLRP3, PDHA1, PDHB. In contrast, high expression of ATP7B, FDX1, and SCL31A1 was significantly associated with better prognosis. To further categorize the HCC patients, a consensus clustering algorithm was applied based on the expression profiles of the 19 CRGs. The cumulative distribution function (CDF) curves of the consensus score matrix indicated that dividing the HCC samples into three subtypes, namely subtypes A (n = 104), B (n = 99), and C (n = 167), was the optimal choice (Fig. 2B and C). Next, principal component analysis (PCA) confirmed distinct differences among the three subtypes (Fig. 2D), and Kaplan–Meier curves demonstrated that patients in subtype A had a shorter overall survival (OS) compared to patients in subtype B and C (P < 0.001; Fig. 2F). Furthermore, a total of 419 overlapping differentially expressed genes (DEGs) were identified among the three CRGs cluster subtypes (Fig. 2E and Supplementary Tables S2–S5), which were subsequently used for further analysis. Further analysis revealed relative differences in the expression of CRGs and clinicopathological features among the three CRGs cluster subtypes (Fig. 2G).
Fig. 2.
Identification of CRGs cluster subtypes. A Interactions among CRGs in HCC. The thickness of the connecting line indicates the strength of the association. B The change in area under the CDF curves of consensus matrix for each k. C The consensus score matrix of all samples when k = 3. D PCA analysis showing a remarkable difference in transcriptomes among CRGs cluster subtypes. E Venn diagram of the identified DEGs among the three CRGs cluster subtypes. F The Kaplan–Meier curve showing the difference in OS among the three CRGs clusters. G Differences in clinicopathological features and CRGs expression among the three CRGs cluster subtypes. H The distribution of 22 immune cell subsets infiltration between three CRGs cluster subtypes. I Correlation of CRGs clusters with immune scores and stromal scores. CRGs cuproptosis-related genes, OS overall survival, PCA principal components analysis. **P < 0.01; ***P < 0.001
We then used the CIBERSORT algorithm to investigate the correlation between CRGs cluster subtypes and 23 human immune cell subsets to evaluate the tumor microenvironment (TME) characteristics of different subtypes, and found that subtype A had a markedly significantly higher overall infiltration abundance compared to subtype B or C (Fig. 2H). This indicates that subtype A is associated with a more enriched and activated immune cell population within the tumor microenvironment. In addition, we again assessed the TME score of the three CRGs subtypes using the “ESTIMATE” package to verify the stability and robustness of the ssGSEA results and found that subtype A exhibited higher stromal and immune scores compared to subtypes B and C (Fig. 2I). This suggests that subtype A is characterized by increased infiltration and activity of stromal cells and immune cells in the tumor microenvironment. Overall, these results indicate that subtype A exhibits a distinct TME profile, with higher infiltration and activity of stromal and immune cells, suggesting a potentially more immunogenic and dynamic tumor microenvironment in this subtype.
Identification of gene subtypes based on 419 overlapping DEGs
Next, a functional analysis was conducted on the 419 overlapping differentially expressed genes (DEGs) among the three CRGs cluster subtypes. GO and KEGG enrichment analysis revealed significant enrichment of DEGs in the following biological processes: the extracellular matrix organization, extracellular structure organization, cell-substrate adhesion, response to the drug, and transmembrane receptor protein serine/threonine kinase signaling pathway (Fig. 3A and B). Furthermore, univariate Cox analysis showed that 320 of 419 DEGs were significantly associated with prognosis (Supplementary Table S6). Subsequently, based on these 320 prognostic DEGs, a new consensus clustering was performed using the “Consensus Cluster Plus” R package to explore potential regulatory mechanisms. Consequently, TCGA-HCC cohort samples were again categorized into three new gene subtypes (Fig. 3C and D). In addition, the K-M curve showed that gene subtype A had a worse overall survival (OS) compared to gene subtypes B or C (p < 0.001, Fig. 3E). In alignment with the expected patterns of the CRGs, notable differences in CRGs expressions were also observed among the three gene cluster subtypes (Fig. 3F). Figure 3G depicts the relative differences in TNM stage, age, gender, and CRGs cluster among gene subtypes A, B, and C.
Fig. 3.
Identification of gene subtypes based on DEGs. A, B GO and KEGG enrichment analyses of DEGs among the three CRGs cluster subtypes. C The change in area under the CDF curves of consensus matrix for each k. D The consensus score matrix of all samples when k = 3. E Kaplan–Meier curves for OS among the three gene clusters. F Differences in 19 CRGs expression among the three gene cluster subtypes. G Relationships between clinicopathologic features and the three gene cluster subtypes. ***P < 0.001
Construction and validation of the CRGs-score in the TCGA cohort
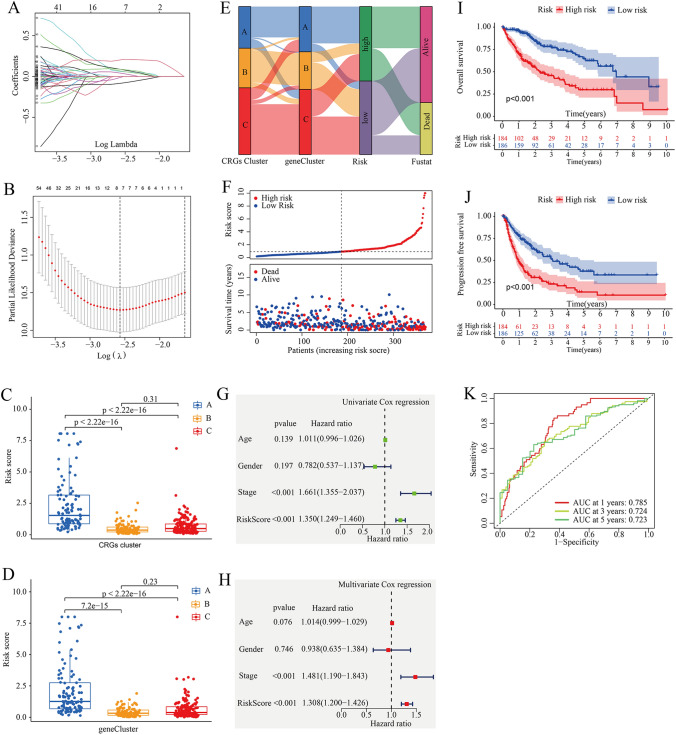
Next, we performed LASSO and multivariate Cox regression analysis for 320 prognostic DEGs to further select the optimum prognostic signature. Through LASSO analysis, eight prognostic DEGs related to OS time were retained based on the minimum partial likelihood deviance (Fig. 4A, B and Supplementary Table S7). Subsequently, the eight prognostic DEGs (PPAT, CAD, GCLM, SGCB, TXNRD1, KDR, JPT1, MTND4P20) underwent further analysis using multivariate Cox analysis, resulting in the retention of five genes (CAD, SGCB, TXNRD1, KDR, MTND4P20) based on the Akaike information criterion (AIC) value (Supplementary Table S8). Consequently, the CRGs-score model was constructed as follows: Risk score = (0.3385* expression of CAD) + (0.2609*expression of SGCB) + (0.2741* expression of TXNRD1) + (− 0.2514* expression of KDR) + (− 0.1806* expression of MTND4P20). Here, the expression levels of these five prognostic genes were determined through RT-qPCR. As shown in Supplementary Fig. S2, HCC tissues exhibited elevated CAD expression levels (P < 0.01), while KDR and SGCB expression levels (P < 0.01) were downregulated compared to adjacent tissues.
Fig. 4.
Construction and validation of the CRGs-score in the TCGA cohort. A, B LASSO regression analysis and partial likelihood deviance of the prognostic genes. C Differences in CRGs-score between gene subtypes. D Differences in CRGs-score between CRGs cluster subtypes. E Alluvial diagram of subtype distributions in groups with different CRGs-scores and survival outcomes. F The distribution plots of OS status, OS and risk score in the TCGA-HCC cohort. G, H Univariate and Multivariate Cox regression analysis for risk score in the TCGA-HCC cohort, respectively. I, J Kaplan–Meier analysis of the OS and PFS between the two groups, respectively. K AUC of ROC curves verified the prognostic performance of the risk score in the TCGA-HCC cohort
Figure 4C and D demonstrate a significant difference in risk score distribution among different subtypes. Compared to subtypes B and C, subtype A exhibited a significantly higher CRGs-score. TCGA-HCC case samples were stratified into high- and low-risk groups based on the median risk score. Figure 4E illustrates the distribution of HCC patients in terms of CRGs subtypes, gene subtypes, and CRGs-score groups. The scatter plot depicting risk distribution of CRGs-score revealed that survival times decreased while mortality increased with an increase in CRGs-score (Fig. 4F). Next, we also investigated the association between the CRGs-score and clinical characteristics by using univariate and multivariate Cox regression (Fig. 4G and H). The results indicated that CRGs-score could serve as an independent prognostic factor (HR = 1.308, 95% CI = 1.200 − 1.426, P < 0.001). Again, Kaplan–Meier (K–M) survival analysis revealed that HCC patients with low scores had a significantly favorable overall survival (OS, P < 0.0013) and progression free survival (PFS, P < 0.001) compared to those with high scores (Fig. 4I and J). The AUC values of CRGs-score for predicting 1-year, 3-year and 5-year survival rates were 0.785, 0.724 and 0.723, respectively (Fig. 4K). These findings indicate that a high CRGs-score is independently predictive of poor survival in HCC patients.
Evaluation of the checkpoints and immune cell abundance
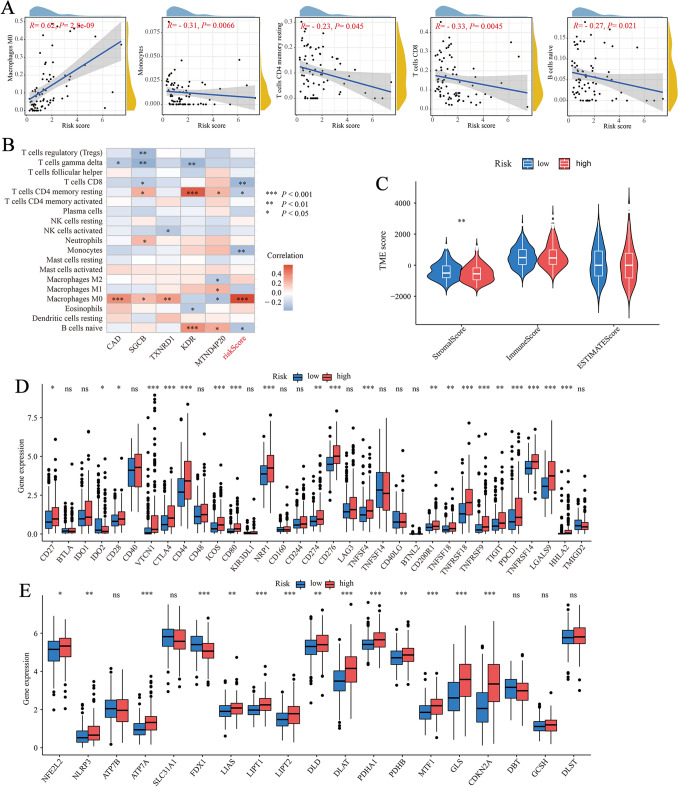
Here, the potential association between CRGs-scores and immune cell abundance was evaluated using CIBERSORT algorithm. As shown in Fig. 5A, CRGs-scores were positively correlated with M0 macrophages and negatively correlated with monocytes, CD8+ T cells, resting memory CD4+ T cells, and naive B cells. Moreover, five genes in the proposed risk model were also positively correlated with M0 macrophages, resting memory CD4+ T cells, and naive B cells (Fig. 5B). A higher CRGs score was associated with a higher stromal score, however, no significant association was found between CRGs-score and immune score (Fig. 5C). Next, we explored the associations between our risk model and 33 immune checkpoints (Fig. 5D) and found significant differences in the expression of immune checkpoints (such as PDCD1 (PD-1), CD274 (PD-L1), and CTLA4) in the high- and low-risk groups. There were also significant differences in CRGs expression between the two risk groups, which was consistent with the expected results (Fig. 5E).
Fig. 5.
Evaluation of the TME and checkpoints between the two groups. A Correlations between CRGs-score and immune cell types. B Correlations between the abundance of immune cells and five genes in the proposed model. C Correlations between CRGs-score and both immune and stromal scores. D Expression of immune checkpoints in the high and low-risk groups. E Expression of CRGs in the high and low-risk groups. TME, tumor microenvironment. *P < 0.05; **P < 0.01; ***P < 0.001; ns, no significance
Analysis of tumor mutation burden and drug susceptibility
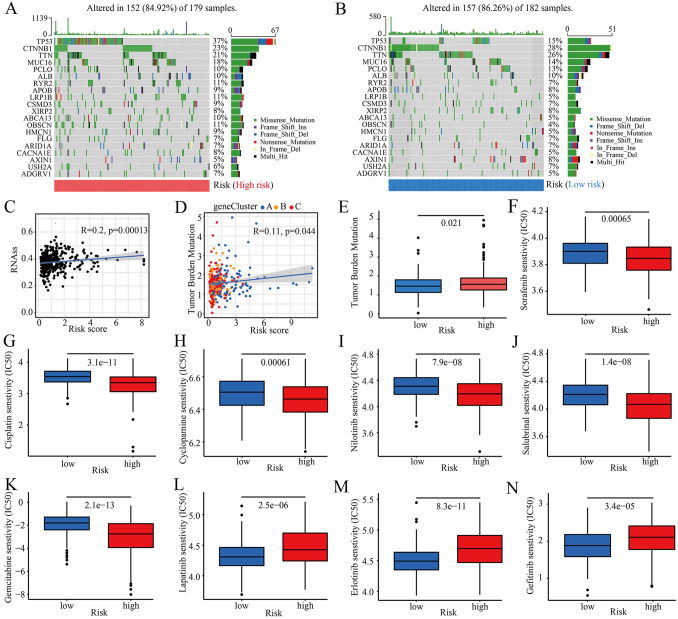
Increasing evidence shows that patients with a high tumor mutation burden (TMB) have a higher number of neoantigens and may benefit from immunotherapy. Here, we further analyzed the characteristics of somatic mutations between high- and low-CRGs-score groups. The top five mutated genes in the high- and low-risk groups were TP53, CTNNB1, TTN, MUC16, and PCLO (Fig. 6A and B). The mutation frequencies of TP53 and TTN in patients with high CRGs-score were significantly higher than those in patients with a low CRGs-score. However, the mutation levels of CTNNB1, MUC16, and PCLO were just the opposite (Fig. 6A and B). We then assessed the potential correlation between the CRGs-score and the cancer stem cell (CSC) index in HCC. As shown in Fig. 6C, a positive linear correlation between CRGs-score and CSC index was observed (R = 0.2, P < 0.001). The results suggested that HCC cells with a higher CRGs-score may have more pronounced stem cell characteristics and a lower degree of cell differentiation. Additionally, CRGs-score was negatively correlated with TMB (R = 0.11, P = 0.044; Fig. 6D), and TMB was higher in the high-CRGs-score group than in the low-CRGs-score group (Fig. 6E). As shown in Fig. 6F–N, we next assessed the sensitivities of patients in two different risk groups to chemotherapy drugs. We found that patients in the high-risk group had lower half inhibitory concentration (IC50) values for sorafenib, cisplatin, cyclopamine, nilotinib, salubrinal, gemcitabine, and higher IC50 values for lapatinib, erlotinib, and gefitinib. In conclusion, our analysis showed that CRGs-scores were associated with chemotherapy sensitivity.
Fig. 6.
Comprehensive analysis of the CRGs-score in HCC. A, B Waterfall plot of somatic mutation characteristics constructed based on CRGs-score. The upper bar plot showed TMB, and the right bar plot showed the proportion of each variant type. The number on the right indicated the mutation frequency in each gene. C Relationships between CRGs-score and cancer stem cell (CSC) index. D Relationships between CRGs-score and tumor mutation burden (TMB). E The tumor mutation burden (TMB) in different CRGs-score groups. F–N Relationships between CRGs-scores and chemotherapy sensitivity. IC50, half inhibitory concentration
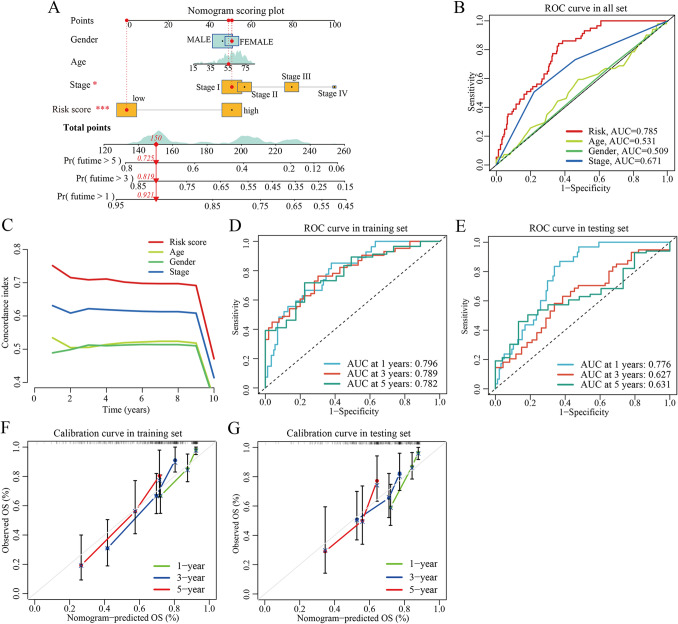
Construction and validation of nomogram
To facilitate the clinical feasibility of the CRGs risk score, a nomogram was constructed by integrating the CRGs risk score and clinicopathological features to predict overall survival (OS) at 1-, 3-, and 5- years (Fig. 7A). As shown in Fig. 7A, the predictors included the CRGs risk score and TNM stage. The Area Under the Curve (AUC) values of the risk model (0.785) were higher than that of the TNM stage (0.671), suggesting that the nomogram exhibited superior survival predictive ability compared to the TNM stage (Fig. 7B). Similarly, the C-index of the risk model was mostly above 0.7 (Fig. 7C), indicating a relatively good prognostic value of the nomogram. Besides, in the training set, the 1-, 3-, and 5-year AUC values of the nomogram were 0.796, 0.789, and 0.782, respectively (Fig. 7D). In the testing set, the 1-, 3-, and 5-year AUC values of the nomogram were 0.776, 0.627, and 0.631, respectively (Fig. 7E). Furthermore, the calibration plots indicated that the proposed nomogram had a similar performance in the training and testing sets (Fig. 7F and G).
Fig. 7.
Construction and validation of a nomogram. A Nomogram for predicting the 1-, 3-, and 5-year OS of HCC patients in all TCGA-HCC cohort. B ROC curve of clinical characteristics in the entire TCGA-HCC cohort. C Pooled concordance index for HCC death estimated in the entire TCGA-LIHC cohort. The C-index was estimated by truncating follow-up time from 1 to 10 years across the entire TCGA-LIHC cohort and plotted on the X-axis as truncated years. D, E ROC curves in the training and testing sets, respectively. F, G Calibration curves of the nomogram in terms of the agreement between predicted and observed outcomes in the training and testing sets. OS overall survival, HCC hepatocellular carcinoma, ROC receiver operating characteristic
Discussion
Hepatocellular carcinoma remains a global health challenge with a growing incidence worldwide (Llovet et al. 2016; Villanueva 2019). The existing prognostic staging system still has some limitations in evaluating clinical prognosis and individual treatment for HCC patients. Currently, gene expression profiling has emerged as the most promising high-throughput molecular method for identifying novel prognostic markers in cancer. The role of cuproptosis, the latest reported form of programmed cell death (Tsvetkov et al. 2022), in HCC remains unclear. Therefore, in this study, we comprehensively described 19 cancer-related genes (CRGs) by mining multi-omics data from 371 HCC patients in TCGA.
Most CRGs showed differentially expressed between tumor and tumor-adjacent tissues and were significantly associated with overall survival (OS) and progression-free survival (PFS), suggesting the potential value of CRGs in HCC prognosis. Based on the expression of 19 CRGs, we then identified three distinct molecular subtypes. Compared to patients with CRGs subtype B or C, patients with subtype A had more advanced immune cell infiltration and worse OS. Additionally, the features of the TME also differed significantly among CRGs subtypes, indicating that subtype A exhibited higher infiltration and activity of stromal cells and immunity. Furthermore, differences in mRNA transcriptomes between different CRGs subtypes were significantly associated with extracellular matrix organization, extracellular structure organization, cell-substrate adhesion, response to drug, and transmembrane receptor protein serine/threonine kinase signaling pathway. Using Lasso and Cox regression analysis, we constructed a risk-scoring model of five genes (CAD, SGCB, TXNRD1, KDR, MTND4P20) and verified its predictive ability. Our results indicated that the CRGs risk score performed well in predicting the diagnosis and prognosis of HCC in the TCGA cohort. Particularly, a high risk score was associated with a poor clinical outcome, high tumor mutation burden, and low drug sensitivity. In clinical practice, the TNM stage is a conventional reference for evaluating clinical outcomes and treatment decisions. Surprisingly, multi-Cox regression analysis further validated the superiority of the constructed CRGs risk score in predicting OS in HCC patients, independent of other clinical features such as age, gender, and TMN stage. Finally, by integrating the CRGs risk score and clinical features, we constructed a quantitative nomogram that enhances the clinical operability of CRGs scores. The prognostic model can be used for stratifying the prognosis of HCC patients and provides new ideas for targeted therapies.
In the CRGs risk score, high expression of CAD, SGCB, and TXNRD1 was associated with a poor outcome (multi-Cox coefficient > 0), indicating that these three genes are adverse factors for HCC patients. On the other hand, KDR and MTND4P20 were identified as protective factors. TXNRD1 is a selenocysteine-containing flavoenzyme that plays a key role in redox homeostasis. It reduces thioredoxins and other substrates, and regulates the redox signaling system by blocking reactive oxygen species (ROS) produced by DNA-damaging agents such as H2O2 and lipid peroxides. TXNRD1 is generally considered to exert antitumor effects (Tan et al. 2018; Rahmanto and Davies 2012). Some studies have reported that inhibitors of TXNRD1 can selectively induce carcinoma cell death by directly increasing the reactive oxygen species (ROS) level in carcinoma cells (Zou et al. 2016; Iverson et al. 2013; Chew et al. 2010; Zhang et al. 2017; Trachootham et al. 2009). Additionally, inactivation of the TXNRD1 gene may impair the function of P53, thereby increasing the likelihood of cancer (Maillet and Pervaiz 2012). The KDR gene encodes the protein VEGFR2, a receptor tyrosine kinase involved in angiogenesis (Rapisarda and Melillo 2012). Tumor growth and metastasis formation are dependent on an adequate blood supply, and VEGFR2 is involved in pathological vasculogenesis and angiogenesis. It is upregulated in endothelial cells of various solid tumors (Rapisarda and Melillo 2012; Smith et al. 2010; Miura et al. 1997). Some VEGFR antagonists, such as lenvatinib and motesanib, are currently being investigated for the treatment of various cancers. There is limited research on the role of SGCB and CAD in cancer, and further studies are needed to understand their significance in HCC.
With in-depth research on tumor immunology, immunotherapy has emerged as a promising approach in oncotherapy. Immune checkpoint inhibitor (ICI) therapy is currently the most successful and common immunotherapy strategy (Topalian et al. 2020; Ribas and Wolchok 2018). Currently, PD-1 monoclonal antibodies (nivolumab, pembrolizumab, and cemiplimab) and PD-L1 monoclonal antibodies (atezolizumab, avelumab, and durvalumab) have become important targeted drugs for a variety of tumor immunotherapy. Although clinical immunotherapy (such as anti-PD-1, anti-PD-L1, and anti-CTLA-4) for HCC has been widely carried out worldwide (Wu et al. 2019; Sangro et al. 2021), only a small number of patients benefited from immunotherapy. Thus, there is an urgent need for more effective biomarkers to assess whether patients with liver cancer benefit from tumor immunotherapy. PD-1 is an important checkpoint molecule leading to tumor immune escape. Blocking PD-1/PD-L1 can reactivate the killing effect of cytotoxic T cells on tumors. Increased PD-L1 expression in immune cells of TME is accompanied by an increase in tumor-infiltrating lymphocytes and effector T cells. CTLA-4 is an efficient immunomodulatory molecule that downregulates T cell activation and suppresses the antitumor immune response. In this study, our findings indicated that high-risk group appeared to coexist with higher levels of immune checkpoint molecules (including PD-1, PD-L1, and CTLA-4), which indirectly suggested that CRGs-score may have a good predictive performance for immunotherapy in HCC patients.
Besides, the AUC of the risk model was more than 0.7, indicating that CRGs-score may be a superior clinical predictor for disease occurrence. Thus, HCC patients with high-risk-score might be inclined to respond to immune checkpoint blockade. Finally, we screened the effective drugs for tumor immunotherapy by using the pRRophetic algorithm and explored drug sensitivity, and observed that high-risk patients had more sensitivity to most anticancer drugs. Moreover, our analysis also showed that the IC50 values of some anticancer drugs were positively correlated with CRGs-scores, implying that HCC patients with low CRGs-scores might have a better response to these drugs. However, the drug mechanisms and their impact on HCC progression need further study.
Conclusion
In our study, we constructed a risk score model for HCC survival prediction and treatment evaluation.
Findings from this study warranted an independent cohort verification and a large-scale prospective and experimental investigation in the future.
Supplementary Information
Below is the link to the electronic supplementary material.
Author contributions
SX, WC, and S-ML conceived and drafted the original manuscript; SX and YY conducted data analysis and bioinformatics analysis; KD, and RG performed the experiments. S-ML, YZ, and CL revised the manuscript for intellectual content. S-ML conduced project administration. All authors reviewed and approved the final version prior to its submission.
Funding
This work was supported by the National Natural Science Foundation of China (81772276) and Hubei Provincial Natural Science Fund for Creative Research Groups (2019CFA018).
Availability of data and materials
The raw data used in this article were retrieved from public databases (https://portal.gdc.cancer.gov/repository). All analyzed data are included in this published article and its supplementary information file, which are available from the corresponding author upon reasonable request.
Declarations
Conflict of interest
The authors declare no competing interests.
Ethical approval and consent to participate
The processing of clinical tissue samples is in strict compliance with the ethical standards of the Declaration of Helsinki. The present study was approved by the ethics committee of Zhongnan Hospital of Wuhan University (Approval number: 2017058). Written informed consent was obtained from all the participants.
Consent for publication
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Shaohua Xu and Kexin Dong are contributed equally to this work.
Contributor Information
Wei Chen, Email: 602400856@qq.com.
Song-Mei Liu, Email: smliu@whu.edu.cn.
References
- Aggett PJ, Fairweather-Tait S (1998) Adaptation to high and low copper intakes: its relevance to estimated safe and adequate daily dietary intakes. Am J Clin Nutr 67(5 Suppl):1061S-S1063. 10.1093/ajcn/67.5.1061 [DOI] [PubMed] [Google Scholar]
- Aubert L, Nandagopal N, Steinhart Z, Lavoie G, Nourreddine S, Berman J et al (2020) Copper bioavailability is a KRAS-specific vulnerability in colorectal cancer. Nat Commun 11(1):3701. 10.1038/s41467-020-17549-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady DC, Crowe MS, Greenberg DN, Counter CM (2017) Copper chelation inhibits BRAF(V600E)-driven melanomagenesis and counters resistance to BRAF(V600E) and MEK1/2 inhibitors. Cancer Res 77(22):6240–6252. 10.1158/0008-5472.CAN-16-1190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chew EH, Nagle AA, Zhang Y, Scarmagnani S, Palaniappan P, Bradshaw TD et al (2010) Cinnamaldehydes inhibit thioredoxin reductase and induce Nrf2: potential candidates for cancer therapy and chemoprevention. Free Radic Biol Med 48(1):98–111. 10.1016/j.freeradbiomed.2009.10.028 [DOI] [PubMed] [Google Scholar]
- Daniel KG, Harbach RH, Guida WC, Dou QP (2004) Copper storage diseases: Menkes, Wilsons, and cancer. Front Biosci 9:2652–2662. 10.2741/1424 [DOI] [PubMed] [Google Scholar]
- Dong J, Wang X, Xu C, Gao M, Wang S, Zhang J et al (2021) Inhibiting NLRP3 inflammasome activation prevents copper-induced neuropathology in a murine model of Wilson’s disease. Cell Death Dis 12(1):87. 10.1038/s41419-021-03397-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebara M, Fukuda H, Hatano R, Saisho H, Nagato Y, Suzuki K et al (2000) Relationship between copper, zinc and metallothionein in hepatocellular carcinoma and its surrounding liver parenchyma. J Hepatol 33(3):415–422. 10.1016/s0168-8278(00)80277-9 [DOI] [PubMed] [Google Scholar]
- Geeleher P, Cox N, Huang RS (2014) pRRophetic: an R package for prediction of clinical chemotherapeutic response from tumor gene expression levels. PLoS ONE 9(9):e107468. 10.1371/journal.pone.0107468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanzelmann S, Castelo R, Guinney J (2013) GSVA: gene set variation analysis for microarray and RNA-seq data. BMC Bioinformatics 14:7. 10.1186/1471-2105-14-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huster D (2014) Structural and metabolic changes in Atp7b-/-mouse liver and potential for new interventions in Wilson’s disease. Ann N Y Acad Sci 1315:37–44. 10.1111/nyas.12337 [DOI] [PubMed] [Google Scholar]
- Huster D, Purnat TD, Burkhead JL, Ralle M, Fiehn O, Stuckert F et al (2007) High copper selectively alters lipid metabolism and cell cycle machinery in the mouse model of Wilson disease. J Biol Chem 282(11):8343–8355. 10.1074/jbc.M607496200 [DOI] [PubMed] [Google Scholar]
- Iasonos A, Schrag D, Raj GV, Panageas KS (2008) How to build and interpret a nomogram for cancer prognosis. J Clin Oncol 26(8):1364–1370. 10.1200/JCO.2007.12.9791 [DOI] [PubMed] [Google Scholar]
- Iverson SV, Eriksson S, Xu J, Prigge JR, Talago EA, Meade TA et al (2013) A Txnrd1-dependent metabolic switch alters hepatic lipogenesis, glycogen storage, and detoxification. Free Radic Biol Med 63:369–380. 10.1016/j.freeradbiomed.2013.05.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jie J, Hao S, Hongxiu Y, Huiying Y, Jun M, Chenji W et al (2007) Evaluation of Cu in hepatocellular carcinoma by particle induced X-ray emission. J Trace Elem Med Biol 21(4):255–260. 10.1016/j.jtemb.2007.06.004 [DOI] [PubMed] [Google Scholar]
- Llovet JM, Zucman-Rossi J, Pikarsky E, Sangro B, Schwartz M, Sherman M (2016) Hepatocellular carcinoma. Nat Rev Dis Primers 2:16018. 10.1038/nrdp.2016.18 [DOI] [PubMed] [Google Scholar]
- Llovet JM, Kelley RK, Villanueva A, Singal AG, Pikarsky E, Roayaie S et al (2021) Hepatocellular carcinoma. Nat Rev Dis Primers 7(1):6. 10.1038/s41572-020-00240-3 [DOI] [PubMed] [Google Scholar]
- Maillet A, Pervaiz S (2012) Redox regulation of p53, redox effectors regulated by p53: a subtle balance. Antioxid Redox Signal 16(11):1285–1294. 10.1089/ars.2011.4434 [DOI] [PubMed] [Google Scholar]
- Miura H, Miyazaki T, Kuroda M, Oka T, Machinami R, Kodama T et al (1997) Increased expression of vascular endothelial growth factor in human hepatocellular carcinoma. J Hepatol 27(5):854–861. 10.1016/s0168-8278(97)80323-6 [DOI] [PubMed] [Google Scholar]
- Morgan RL, Baack B, Smith BD, Yartel A, Pitasi M, Falck-Ytter Y et al (2013) Eradication of hepatitis C virus infection and the development of hepatocellular carcinoma: a meta-analysis of observational studies. Ann Intern Med 158(5 Pt 1):329–337. 10.7326/0003-4819-158-5-201303050-00005 [DOI] [PubMed] [Google Scholar]
- Nakagawa H, Fujita M, Fujimoto A (2019) Genome sequencing analysis of liver cancer for precision medicine. Semin Cancer Biol 55:120–127. 10.1016/j.semcancer.2018.03.004 [DOI] [PubMed] [Google Scholar]
- Pfeiffenberger J, Mogler C, Gotthardt DN, Schulze-Bergkamen H, Litwin T, Reuner U et al (2015) Hepatobiliary malignancies in Wilson disease. Liver Int 35(5):1615–1622. 10.1111/liv.12727 [DOI] [PubMed] [Google Scholar]
- Polishchuk EV, Merolla A, Lichtmannegger J, Romano A, Indrieri A, Ilyechova EY et al (2019) Activation of autophagy, observed in liver tissues from patients with Wilson disease and from ATP7B-deficient animals, protects hepatocytes from copper-induced apoptosis. Gastroenterology 156(4):1173–1189. 10.1053/j.gastro.2018.11.032 [DOI] [PubMed] [Google Scholar]
- Poo JL, Rosas-Romero R, Montemayor AC, Isoard F, Uribe M (2003) Diagnostic value of the copper/zinc ratio in hepatocellular carcinoma: a case control study. J Gastroenterol 38(1):45–51. 10.1007/s005350300005 [DOI] [PubMed] [Google Scholar]
- Que EL, Domaille DW, Chang CJ (2008) Metals in neurobiology: probing their chemistry and biology with molecular imaging. Chem Rev 108(5):1517–1549. 10.1021/cr078203u [DOI] [PubMed] [Google Scholar]
- Rahmanto AS, Davies MJ (2012) Selenium-containing amino acids as direct and indirect antioxidants. IUBMB Life 64(11):863–871. 10.1002/iub.1084 [DOI] [PubMed] [Google Scholar]
- Rapisarda A, Melillo G (2012) Role of the VEGF/VEGFR axis in cancer biology and therapy. Adv Cancer Res 114:237–267. 10.1016/B978-0-12-386503-8.00006-5 [DOI] [PubMed] [Google Scholar]
- Ren X, Li Y, Zhou Y, Hu W, Yang C, Jing Q et al (2021) Overcoming the compensatory elevation of NRF2 renders hepatocellular carcinoma cells more vulnerable to disulfiram/copper-induced ferroptosis. Redox Biol 46:102122. 10.1016/j.redox.2021.102122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribas A, Wolchok JD (2018) Cancer immunotherapy using checkpoint blockade. Science 359(6382):1350–1355. 10.1126/science.aar4060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roayaie S, Blume IN, Thung SN, Guido M, Fiel MI, Hiotis S et al (2009) A system of classifying microvascular invasion to predict outcome after resection in patients with hepatocellular carcinoma. Gastroenterology 137(3):850–855. 10.1053/j.gastro.2009.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangro B, Sarobe P, Hervas-Stubbs S, Melero I (2021) Advances in immunotherapy for hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol 18(8):525–543. 10.1038/s41575-021-00438-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith NR, Baker D, James NH, Ratcliffe K, Jenkins M, Ashton SE et al (2010) Vascular endothelial growth factor receptors VEGFR-2 and VEGFR-3 are localized primarily to the vasculature in human primary solid cancers. Clin Cancer Res 16(14):3548–3561. 10.1158/1078-0432.CCR-09-2797 [DOI] [PubMed] [Google Scholar]
- Tan HW, Mo HY, Lau ATY, Xu YM (2018) Selenium species: current status and potentials in cancer prevention and therapy. Int J Mol Sci. 10.3390/ijms20010075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topalian SL, Taube JM, Pardoll DM (2020) Neoadjuvant checkpoint blockade for cancer immunotherapy. Science. 10.1126/science.aax0182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trachootham D, Alexandre J, Huang P (2009) Targeting cancer cells by ROS-mediated mechanisms: a radical therapeutic approach? Nat Rev Drug Discov 8(7):579–591. 10.1038/nrd2803 [DOI] [PubMed] [Google Scholar]
- Tsvetkov P, Coy S, Petrova B, Dreishpoon M, Verma A, Abdusamad M et al (2022) Copper induces cell death by targeting lipoylated TCA cycle proteins. Science 375(6586):1254–1261. 10.1126/science.abf0529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villanueva A (2019) Hepatocellular carcinoma. N Engl J Med 380(15):1450–1462. 10.1056/NEJMra1713263 [DOI] [PubMed] [Google Scholar]
- Wilkerson MD, Hayes DN (2010) ConsensusClusterPlus: a class discovery tool with confidence assessments and item tracking. Bioinformatics 26(12):1572–1573. 10.1093/bioinformatics/btq170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Gu Z, Chen Y, Chen B, Chen W, Weng L et al (2019) Application of PD-1 blockade in cancer immunotherapy. Comput Struct Biotechnol J 17:661–674. 10.1016/j.csbj.2019.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Yao J, Peng S, Li X, Fang J (2017) Securinine disturbs redox homeostasis and elicits oxidative stress-mediated apoptosis via targeting thioredoxin reductase. Biochim Biophys Acta Mol Basis Dis 1863(1):129–138. 10.1016/j.bbadis.2016.10.019 [DOI] [PubMed] [Google Scholar]
- Zou P, Xia Y, Ji J, Chen W, Zhang J, Chen X (2016) Piperlongumine as a direct TrxR1 inhibitor with suppressive activity against gastric cancer. Cancer Lett 375(1):114–126. 10.1016/j.canlet.2016.02.058 [DOI] [PubMed] [Google Scholar]
- Zucman-Rossi J, Villanueva A, Nault JC, Llovet JM (2015) Genetic landscape and biomarkers of hepatocellular carcinoma. Gastroenterology 149(5):1226–1239. 10.1053/j.gastro.2015.05.061. (e4) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data used in this article were retrieved from public databases (https://portal.gdc.cancer.gov/repository). All analyzed data are included in this published article and its supplementary information file, which are available from the corresponding author upon reasonable request.