Abstract
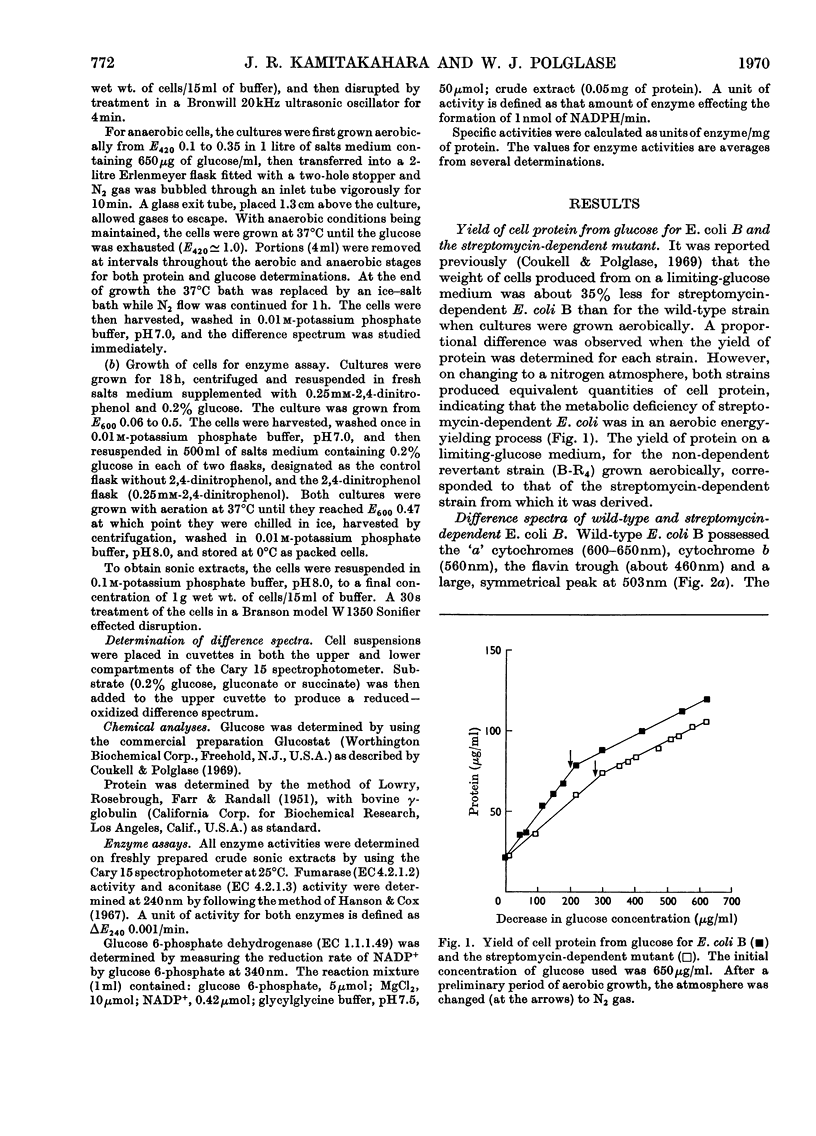
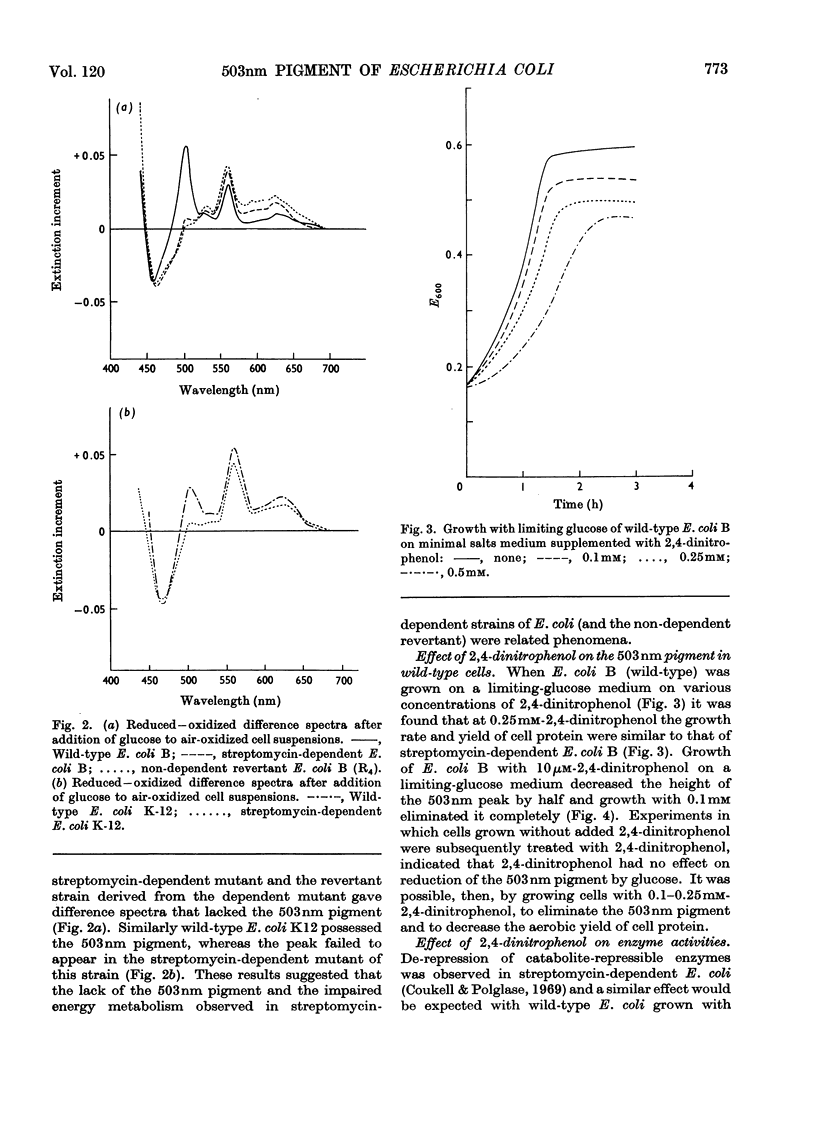
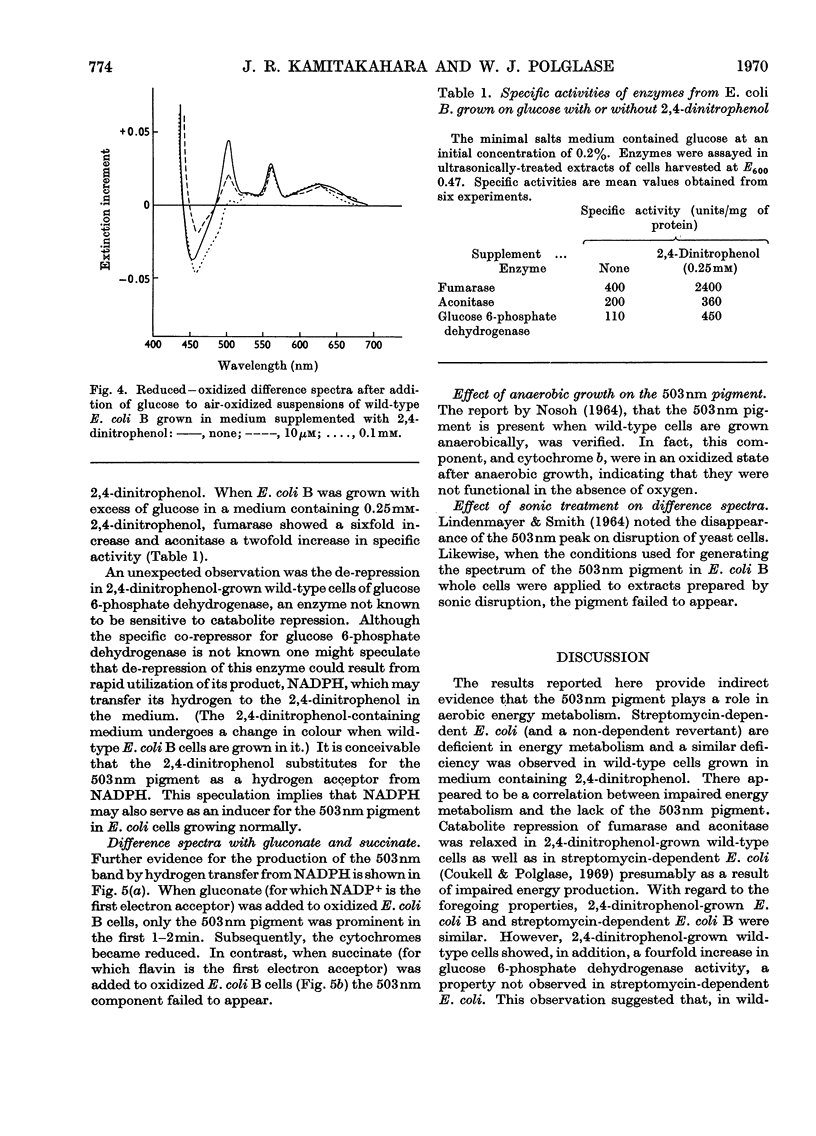
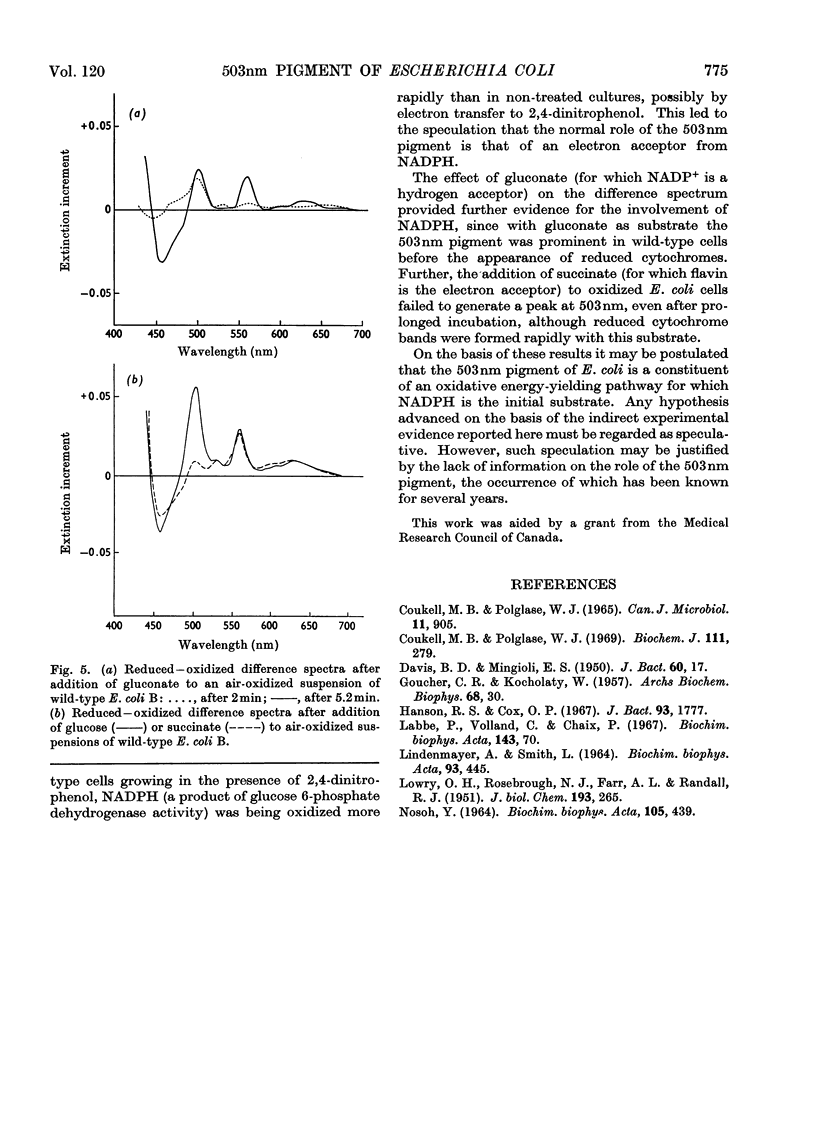
The yield of cell protein was one-third less for streptomycin-dependent Escherichia coli B than for the wild-type parent strain when both were grown aerobically on a medium with limiting glucose, but anaerobically the yield of protein was similar for both strains. The transient pigment absorbing at 503nm that is known to be present in E. coli and other organisms was not detectable in streptomycin-dependent mutants nor in a non-dependent (energy-deficient) revertant. When wild-type E. coli B was grown on limiting glucose–salts medium containing 2,4 dinitrophenol, the yield of cell protein was decreased and formation of the 503nm pigment was inhibited. Fumarase, aconitase and glucose 6-phosphate dehydrogenase were de-repressed in E. coli B cells grown with excess of glucose in a medium containing 2,4-dinitrophenol. In air-oxidized, wild-type E. coli B cells, the 503nm pigment appeared before reduced cytochromes when gluconate was the substrate but failed to appear when succinate was the substrate. The results provide evidence for a role of the 503nm pigment in aerobic energy metabolism, possibly as an electron acceptor from NADPH.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Coukell M. B., Polglase W. J. Acetolactate formation by streptomycin mutants of Escherichia coli. Can J Microbiol. 1965 Dec;11(6):905–911. doi: 10.1139/m65-120. [DOI] [PubMed] [Google Scholar]
- Coukell M. B., Polglase W. J. Relaxation of catabolite repression in streptomycin-dependent Escherichia coli. Biochem J. 1969 Feb;111(3):279–286. doi: 10.1042/bj1110279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. D., MINGIOLI E. S. Mutants of Escherichia coli requiring methionine or vitamin B12. J Bacteriol. 1950 Jul;60(1):17–28. doi: 10.1128/jb.60.1.17-28.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson R. S., Cox D. P. Effect of different nutritional conditions on the synthesis of tricarboxylic acid cycle enzymes. J Bacteriol. 1967 Jun;93(6):1777–1787. doi: 10.1128/jb.93.6.1777-1787.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LINDENMAYER A., SMITH L. CYTOCHROMES AND OTHER PIGMENTS OF BAKER'S YEAST GROWN AEROBICALLY AND ANAEROBICALLY. Biochim Biophys Acta. 1964 Dec 9;93:445–461. doi: 10.1016/0304-4165(64)90329-0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- NOSOH Y. ABSORPTION SPECTRUM OF ACTIVELY RESPIRING YEAST CELLS. Arch Biochem Biophys. 1964 May;105:439–445. doi: 10.1016/0003-9861(64)90028-1. [DOI] [PubMed] [Google Scholar]


