Abstract
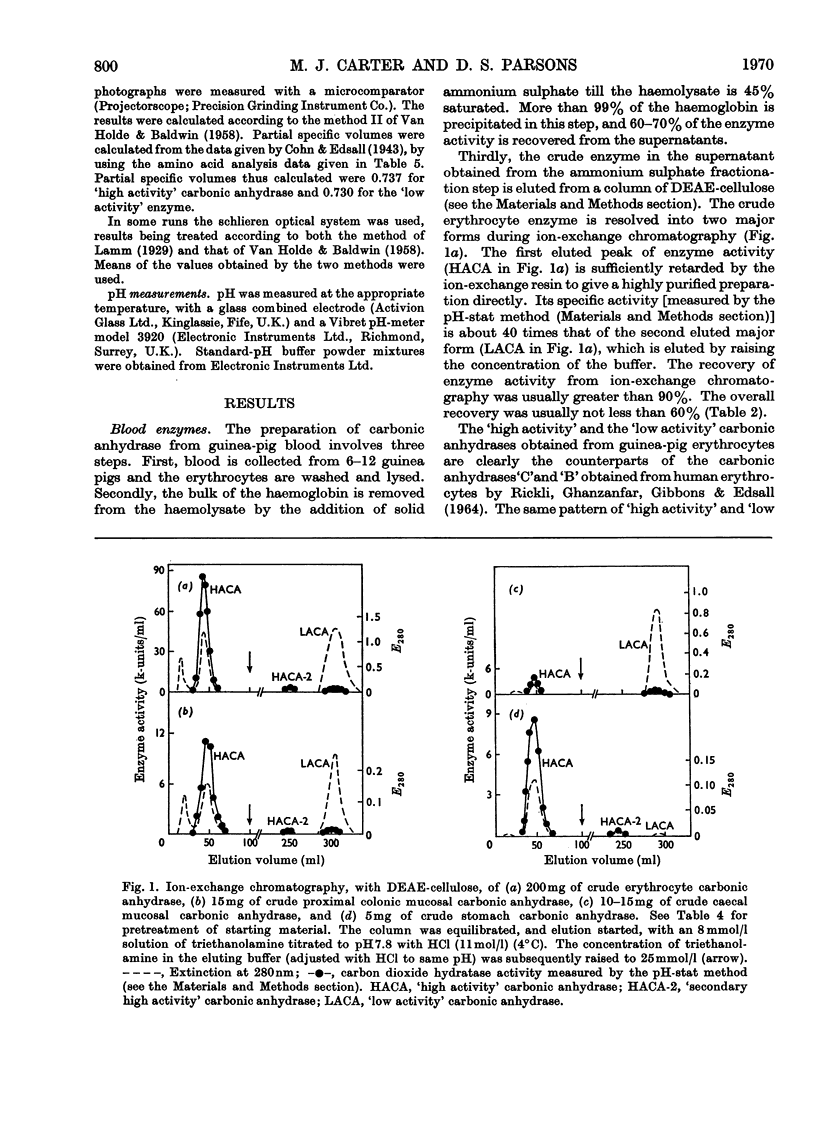
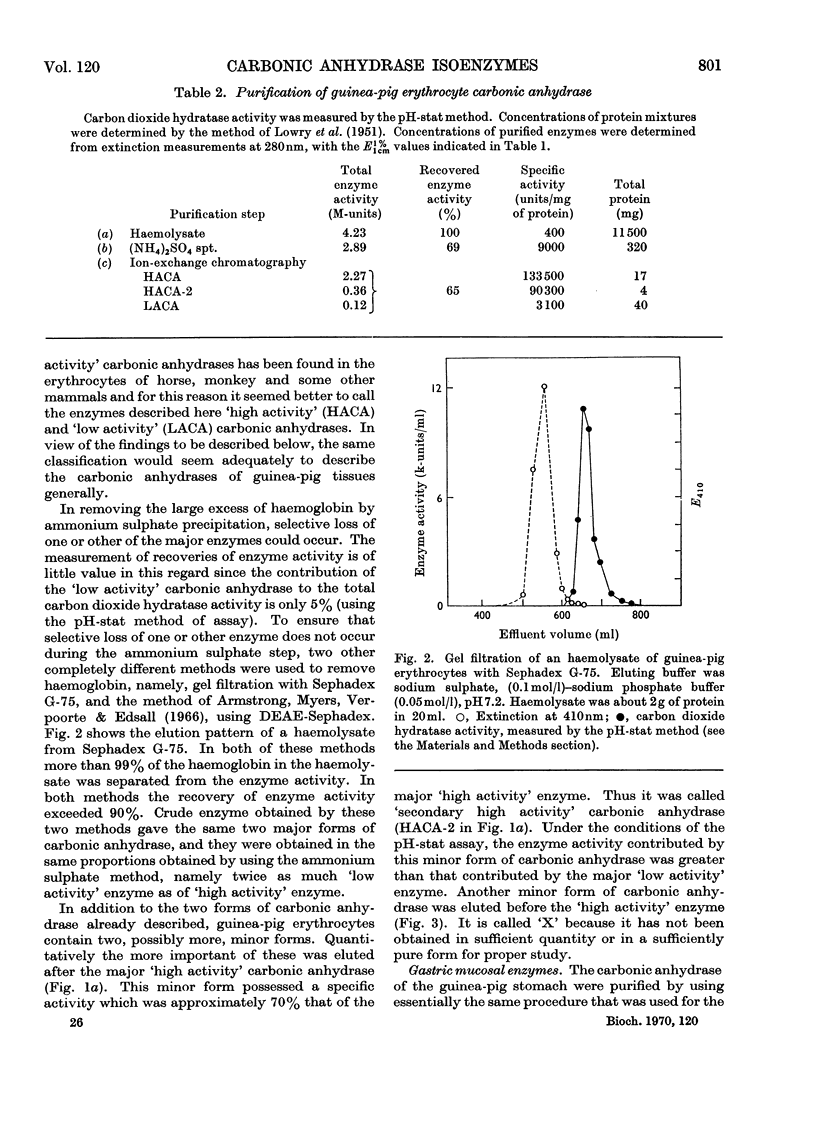
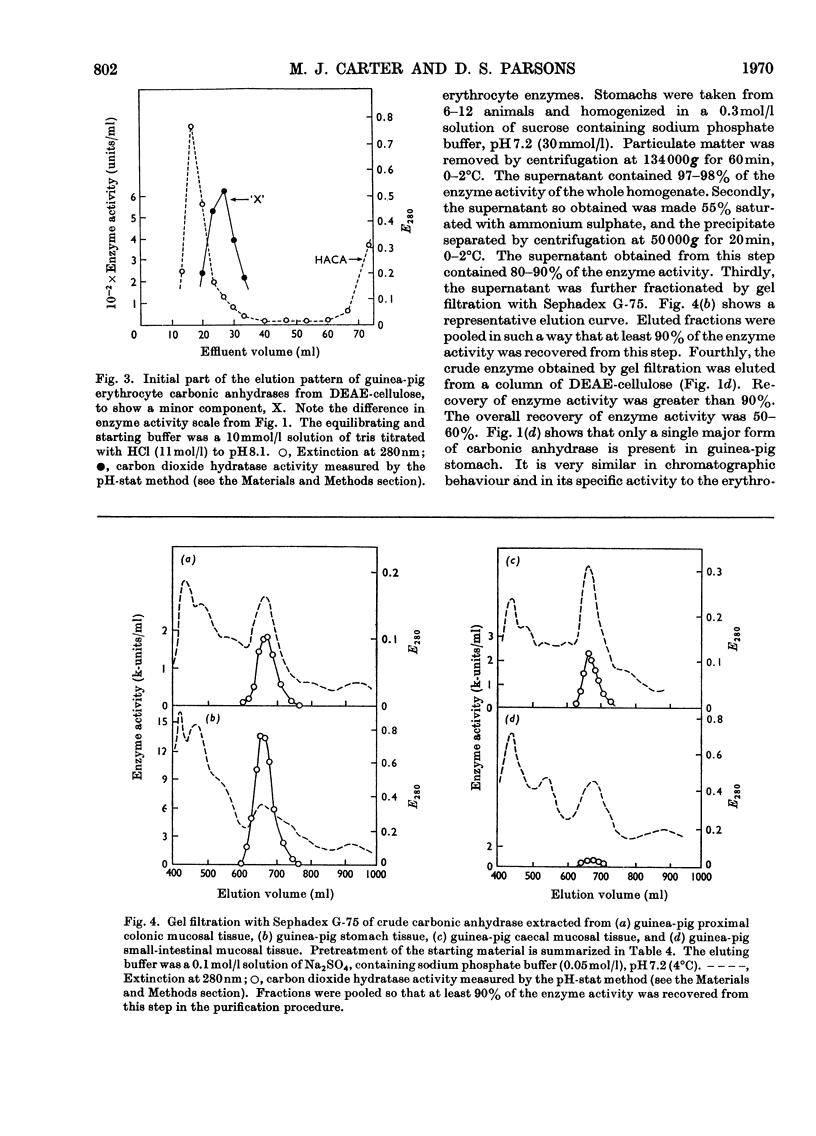
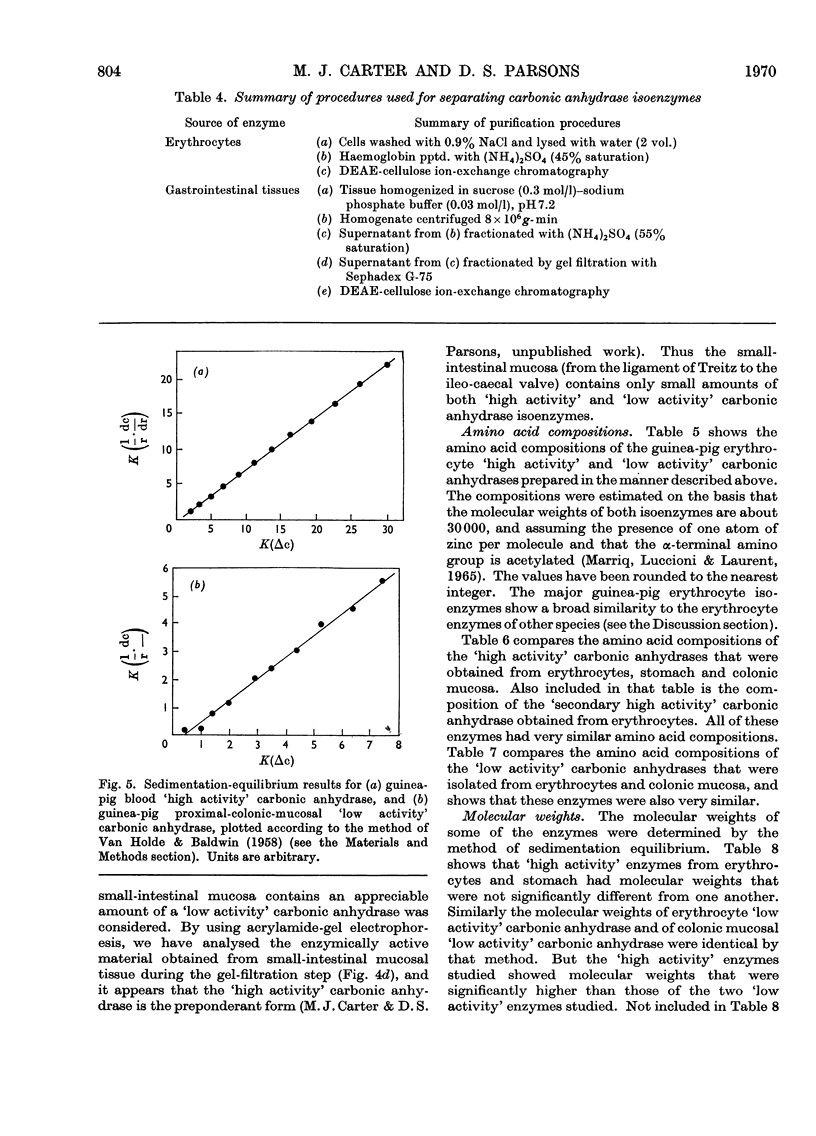
Procedures for isolating carbonic anhydrase (EC 4.2.1.1) enzymes from the erythrocytes and the mucosae of the gastrointestinal tract of guinea pigs are described. From a haemolysate, haemoglobin was removed by the addition of ammonium sulphate, and also by two other methods, namely by gel filtration or by adsorption on DEAE-Sephadex. The crude enzyme thus obtained was resolved into the different isoenzymes by chromatography with DEAE-cellulose. From particle-free supernatants of homogenates of some gastrointestinal tissues, carbonic anhydrases were purified by ammonium sulphate fractionation, gel filtration, and ion-exchange chromatography with DEAE-cellulose. The major isoenzymes from blood, stomach, proximal colonic mucosa and caecal mucosa were homogeneous during ion-exchange chromatography, acrylamide-gel electrophoresis, and centrifugal examination. From these tissues, carbonic anhydrase was isolated as two major isoenzymes. They resemble the pairs of isoenzymes discovered in the bloods of other species. The carbon dioxide hydratase activity of one isoenzyme (`high activity' carbonic anhydrase) was 40 times that of the other isoenzyme (`low activity' carbonic anhydrase), as measured at a single substrate concentration. Two other minor components of the enzyme are also found in guinea-pig erythrocytes. All of the enzymes isolated had molecular weights of nearly 30000 (sedimentation equilibrium). `High activity' carbonic anhydrases from blood and gastrointestinal tissues were indistinguishable according to some chemical, physical and kinetic measurements; similarly `low activity' carbonic anhydrases from those tissues were indistinguishable. `High activity' carbonic anhydrase was markedly different from the `low activity' carbonic anhydrase with respect to its amino acid composition, chromatographic behaviour and isoelectric pH value. Marked differences were also found in the tissue concentrations of the major isoenzymes. It is suggested that the characteristic and selective distribution of the different forms of carbonic anhydrase in the guinea-pig tissues is related to the specific and different physiological functions of the enzymes.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong J. M., Myers D. V., Verpoorte J. A., Edsall J. T. Purification and properties of human erythrocyte carbonic anhydrases. J Biol Chem. 1966 Nov 10;241(21):5137–5149. [PubMed] [Google Scholar]
- Byvoet P., Gotti A. Isolation and properties of carbonic anhydrase from dog kidney and erythrocytes. Mol Pharmacol. 1967 Mar;3(2):142–152. [PubMed] [Google Scholar]
- Carter M. J., Parsons D. S. Carbonic anhydrase activity of mucosa of small intestine and colon. Nature. 1968 Jul 13;219(5150):176–177. doi: 10.1038/219176a0. [DOI] [PubMed] [Google Scholar]
- Carter M. J., Parsons D. S. The carbonic anhydrases of some guinea-pig tissues. Biochim Biophys Acta. 1970 Apr 22;206(1):190–192. doi: 10.1016/0005-2744(70)90099-9. [DOI] [PubMed] [Google Scholar]
- Edmonds C. J. Transport of sodium and secretion of potassium and bicarbonate by the colon of normal and sodium-depleted rats. J Physiol. 1967 Dec;193(3):589–602. doi: 10.1113/jphysiol.1967.sp008380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edsall J. T. Multiple molecular forms of carbonic anhydrase in erythrocytes. Ann N Y Acad Sci. 1968 Jun 14;151(1):41–63. doi: 10.1111/j.1749-6632.1968.tb11877.x. [DOI] [PubMed] [Google Scholar]
- Epstein C. J., Schechter A. N. An approach to the problem of conformational isozymes. Ann N Y Acad Sci. 1968 Jun 14;151(1):85–101. doi: 10.1111/j.1749-6632.1968.tb11880.x. [DOI] [PubMed] [Google Scholar]
- Funakoshi S., Deutsch H. F. Human carbonic anhydrases. II. Some physicochemical properties of native isozymes and of similar isozymes generated in vitro. J Biol Chem. 1969 Jul 10;244(13):3438–3446. [PubMed] [Google Scholar]
- Furth A. J. Purification and properties of horse erythrocyte carbonic anhydrases. J Biol Chem. 1968 Sep 25;243(18):4832–4841. [PubMed] [Google Scholar]
- Goodwin T. W., Morton R. A. The spectrophotometric determination of tyrosine and tryptophan in proteins. Biochem J. 1946;40(5-6):628–632. doi: 10.1042/bj0400628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HIRS C. H. The oxidation of ribonuclease with performic acid. J Biol Chem. 1956 Apr;219(2):611–621. [PubMed] [Google Scholar]
- Keilin D., Mann T. Carbonic anhydrase. Purification and nature of the enzyme. Biochem J. 1940 Sep;34(8-9):1163–1176. doi: 10.1042/bj0341163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LINDSKOG S. Purification and properties of bovine erythrocyte carbonic anhydrase. Biochim Biophys Acta. 1960 Apr 8;39:218–226. doi: 10.1016/0006-3002(60)90156-6. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Marriq C., Luccioni F., Laurent G. Présence de radicaux acétyle dans les anhydrases carboniques érythrocytaires humaines et caractérisation d'un peptide N-terminal acétylé dans l'enzyme B. Biochim Biophys Acta. 1965 Sep 20;105(3):606–608. [PubMed] [Google Scholar]
- McIntosh J. E. Carbonic anhydrase isoenzymes in the erythrocytes and dorsolateral prostate of the rat. Biochem J. 1969 Sep;114(3):463–476. doi: 10.1042/bj1140463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meldrum N. U., Roughton F. J. Carbonic anhydrase. Its preparation and properties. J Physiol. 1933 Dec 5;80(2):113–142. doi: 10.1113/jphysiol.1933.sp003077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NYMAN P. O. Purification and properties of carbonic anhydrase from human erythrocytes. Biochim Biophys Acta. 1961 Sep 2;52:1–12. doi: 10.1016/0006-3002(61)90898-8. [DOI] [PubMed] [Google Scholar]
- NYMAN P., LINDSKOG S. AMINO ACID COMPOSITION OF VARIOUS FORMS OF BOVINE AND HUMAN ERYTHROCYTE CARBONIC ANHYDRASE. Biochim Biophys Acta. 1964 Apr 6;85:141–151. doi: 10.1016/0926-6569(64)90174-9. [DOI] [PubMed] [Google Scholar]
- RICKLI E. E., GHAZANFAR S. A., GIBBONS B. H., EDSALL J. T. CARBONIC ANHYDRASES FROM HUMAN ERYTHROCYTES. PREPARATION AND PROPERTIES OF TWO ENZYMES. J Biol Chem. 1964 Apr;239:1065–1078. [PubMed] [Google Scholar]
- SEN M., DRANCE S. M., WOODFORD V. R. Separation of bovine lens carbonic anhydrase into two components. Can J Biochem Physiol. 1963 May;41:1235–1241. [PubMed] [Google Scholar]
- SQUIRE P. G., STARMAN B., LI C. H. Studies of pituitary lactogenic hormone. XXII. Analysis of the state of aggregation of the ovine hormone by ultracentrifugation and exclusion chromatography. J Biol Chem. 1963 Apr;238:1389–1395. [PubMed] [Google Scholar]
- Swallow J. H., Code C. F. Intestinal transmucosal fluxes of bicarbonate. Am J Physiol. 1967 Mar;212(3):717–723. doi: 10.1152/ajplegacy.1967.212.3.717. [DOI] [PubMed] [Google Scholar]
- TAPPAN D. V., JACEY M. J., BOYDEN H. M. CARBONIC ANHYDRASE ISOZYMES OF NEONATAL AND ADULT HUMAN AND SOME ANIMAL ERYTHROCYTES. Ann N Y Acad Sci. 1964 Dec 28;121:589–599. doi: 10.1111/j.1749-6632.1964.tb14228.x. [DOI] [PubMed] [Google Scholar]



