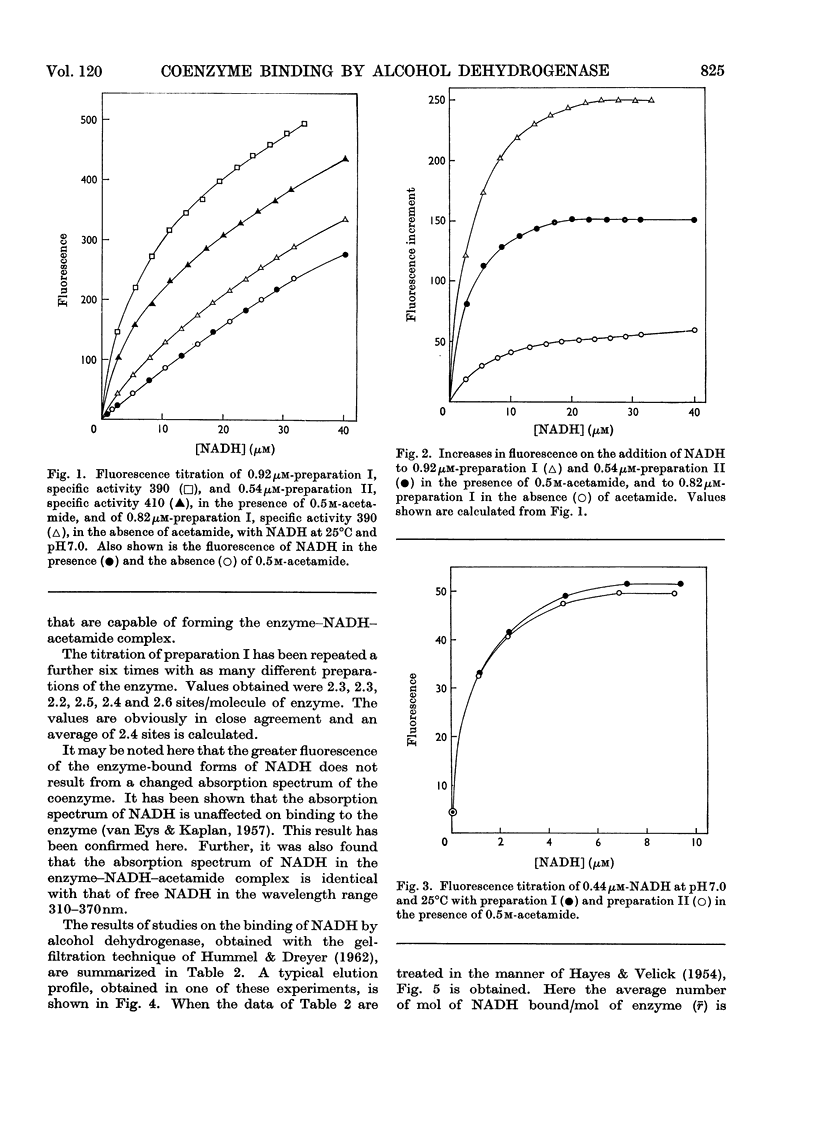
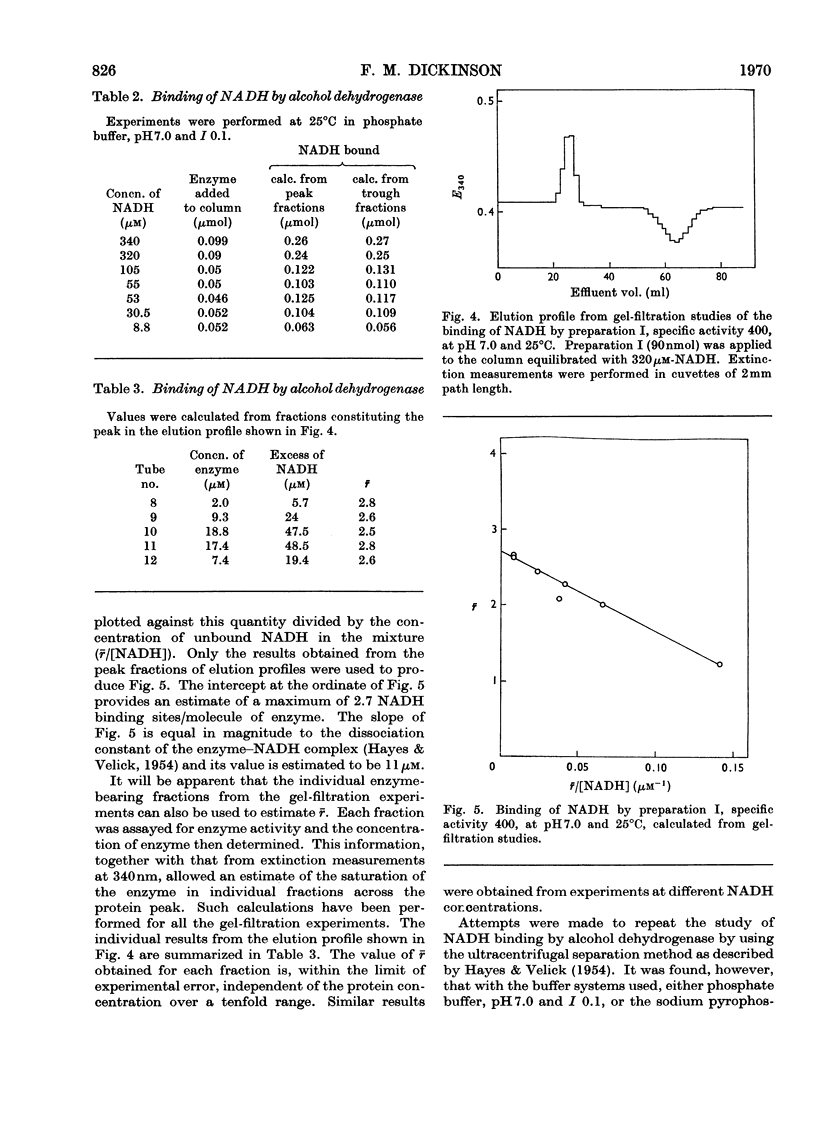
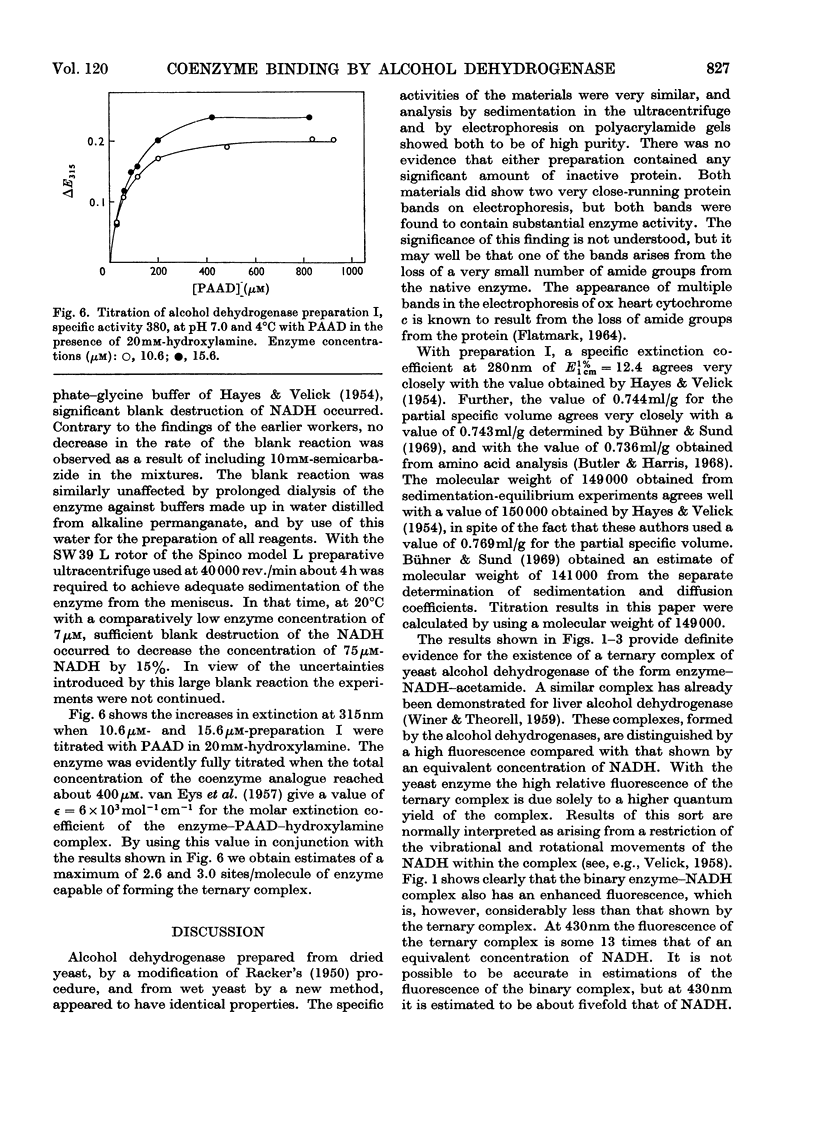
Abstract
1. Yeast alcohol dehydrogenase has been found to react with NADH in the presence of acetamide to form a highly fluorescent ternary complex. Titration of the enzyme to form this complex has provided a method for the estimation of the number of binding sites on the enzyme. 2. The binding of NADH by the enzyme has been studied independently, with a modified form of equilibrium dialysis, by using gel filtration. 3. A third method, depending upon the formation of a ternary complex of enzyme, hydroxylamine and pyridine-3-aldehyde–adenine dinucleotide, has also been used to titrate the enzyme. 4. Values obtained with all three methods are substantially in agreement that only three coenzyme-binding sites are available. This is in contrast with the established fact that the enzyme is composed of four identical subunits.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bühner M., Sund H. Yeast alcohol dehydrogenase: SH groups, disulfide groups, quaternary structure, and reactivation by reductive cleavage of disulfide groups. Eur J Biochem. 1969 Nov;11(1):73–79. doi: 10.1111/j.1432-1033.1969.tb00741.x. [DOI] [PubMed] [Google Scholar]
- CLARKE J. T. SIMPLIFIED "DISC" (POLYACRYLAMIDE GEL) ELECTROPHORESIS. Ann N Y Acad Sci. 1964 Dec 28;121:428–436. doi: 10.1111/j.1749-6632.1964.tb14214.x. [DOI] [PubMed] [Google Scholar]
- Conway A., Koshland D. E., Jr Negative cooperativity in enzyme action. The binding of diphosphopyridine nucleotide to glyceraldehyde 3-phosphate dehydrogenase. Biochemistry. 1968 Nov;7(11):4011–4023. doi: 10.1021/bi00851a031. [DOI] [PubMed] [Google Scholar]
- DALZIEL K. Kinetic studies of liver alcohol dehydrogenase. Biochem J. 1962 Aug;84:244–254. doi: 10.1042/bj0840244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DALZIEL K. Some observations on the preparation and properties of dihydronicotinamide-adenine dinucleotide. Biochem J. 1962 Aug;84:240–244. doi: 10.1042/bj0840240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalziel Keith, Engel Paul C. Antagonistic homotropic interactions as a possible explanation of coenzyme activation of glutamate dehydrogenase. FEBS Lett. 1968 Oct;1(5):349–352. doi: 10.1016/0014-5793(68)80153-x. [DOI] [PubMed] [Google Scholar]
- HARRIS I. STRUCTURE AND CATALYTIC ACTIVITY OF ALCOHOL DEHYDROGENASES. Nature. 1964 Jul 4;203:30–34. doi: 10.1038/203030a0. [DOI] [PubMed] [Google Scholar]
- HAYES J. E., Jr, VELICK S. F. Yeast alcohol dehydrogenase: molecular weight, coenzyme binding, and reaction equilibria. J Biol Chem. 1954 Mar;207(1):225–244. [PubMed] [Google Scholar]
- HUMMEL J. P., DREYER W. J. Measurement of protein-binding phenomena by gel filtration. Biochim Biophys Acta. 1962 Oct 8;63:530–532. doi: 10.1016/0006-3002(62)90124-5. [DOI] [PubMed] [Google Scholar]
- RACKER E. Crystalline alcohol dehydrogenase from baker's yeast. J Biol Chem. 1950 May;184(1):313–319. [PubMed] [Google Scholar]
- Rabin B. R., Cruz J. R., Watts D. C., Whitehead E. P. The reaction of yeast alcohol dehydrogenase with iodoacetamide as determined with a silver-silver iodide electrode. Biochem J. 1964 Mar;90(3):539–542. doi: 10.1042/bj0900539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VAN EYS J., CIOTTI M. M., KAPLAN N. O. Yeast alcohol dehydrogenase. II. Properties of the catalytically active site. Biochim Biophys Acta. 1957 Mar;23(3):581–587. doi: 10.1016/0006-3002(57)90380-3. [DOI] [PubMed] [Google Scholar]
- VAN EYS J., KAPLAN N. O. Yeasl alcohol dehydrogenase. I. The effect of pyridine derivatives on the reaction. Biochim Biophys Acta. 1957 Mar;23(3):574–581. doi: 10.1016/0006-3002(57)90379-7. [DOI] [PubMed] [Google Scholar]
- VELICK S. F., HAYES J. E., Jr, HARTING J. The binding of diphosphopyridine nucleotide by glyceraldehyde-3-phosphate dehydrogenase. J Biol Chem. 1953 Aug;203(2):527–544. [PubMed] [Google Scholar]
- VERLICK S. F. Fluorescence spectra and polarization of glyceraldehyde-3-phosphate and lactic dehydrogenase coenzyme complexes. J Biol Chem. 1958 Dec;233(6):1455–1467. [PubMed] [Google Scholar]
- Vallee B. L., Hoch F. L. ZINC, A COMPONENT OF YEAST ALCOHOL DEHYDROGENASE. Proc Natl Acad Sci U S A. 1955 Jun 15;41(6):327–338. doi: 10.1073/pnas.41.6.327. [DOI] [PMC free article] [PubMed] [Google Scholar]


