Abstract
Background
Cervical cancer is a gynecological malignant tumor and a serious threat to women's health. Although human papillomavirus (HPV) infection and the occurrence of cervical cancer are known to be closely related, the underlying carcinogenic mechanism of HPV is not fully understood. Extracellular vesicles (EVs) are found in a variety of body fluids and play an important role in both intercellular communication and cancer progression. Furthermore, the presence of EVs makes liquid biopsy of cervical cancer possible. The study of EVs in cervical cancer can provide clinical ideas for the diagnosis and treatment of the disease.
Objectives
The purpose of this article is to summarizes the role of EV contents in HPV-associated cervical cancer and discusses the possible clinical application of EVs in cervical cancer treatment.
Methods
The search terms included the following: HPV with cervical cancer and extracellular vesicles. The initial literature search ended on March 1, 2023.
Conclusions
In HPV-positive cervical cancer, EV contents are changed due to the presence of HPV. HPV-positive cervical cancer affects the cell microenvironment and other surrounding cells through the secretion of EVs.
Keywords: Extracellular vesicle, Cervical cancer, HPV, miRNA, E6/E7
Introduction
Cervical cancer (CC) is the second leading cause of cancer-related deaths in women aged 20–39 years. Its crude morbidity and mortality rates were 98.9 and 305 per 100,000 women, respectively, showing an upward trend in China (Chen et al. 2016). Screening for CC plays an important role in identifying high-grade intraepithelial neoplasia of the cervix and preventing it from developing into invasive carcinoma. Screening tests such as Pap smear and thin-layer cytology (TCT) can significantly reduce the incidence of CC and improve the 5-year survival rate (Smith et al. 2015). However, the diagnostic rates of Pap smears and TCT are still low, and these cytological tests vary widely between regions and hospitals and are not universally used in all parts of mainland China, especially in rural areas. Several factors limit the widespread use of Pap smears and TCT, such as personal beliefs and cultural factors (especially among women over 45 years of age or in rural areas), the risk of vaginal infection and bleeding, and the complexity and variability of the test procedure. Cervical biopsy, which is considered the gold standard for cervical lesion diagnosis, also has several shortcomings such as invasive, subjective, and incomplete sampling.
In recent years, extracellular vesicles (EVs) have garnered much attention, and the research on EVs related to CC has also gained much traction. EVs are vesicles released by cells into the extracellular matrix; they participate in cell communication, cell migration, angiogenesis, and tumor cell growth process, widely exist in various body fluids and cells in the supernatant, and are stable enough to carry some important signal molecules (Raposo and Stoorvogel 2013). The function of EVs has become a hot research topic as they are expected to play a role in the early diagnosis of many diseases, including cancer. EVs are divided into four subgroups based on their diameters and modes of occurrence: exosomes, (30–150 nm in diameter), microvesicles (100–1000 nm in diameter), apoptotic body (100–5000 nm in diameter), and oncosomes (1–10 μm in diameter) (Minciacchi et al. 2015). Although the characteristics of these EVs are different, their research and application directions are similar, and they are mainly applied in fields such as tissue repair, damage repair, diabetes, myocardial infarction, and antitumor. EVs can deliver rich genetic material such as DNA fragments, mRNA, long non-coding RNA, small RNA, proteins, and lipids, which are closely related to the development and progression of cancer (Wang et al. 2016). The role of EVs and their contents in human papillomavirus (HPV)-associated CC is complex and interesting. This report summarizes the recent findings on CC-related EVs and discusses their potential in clinical diagnostics and therapeutics.
Effects of HPV-derived components in EVs on cervical cancer
A virus is a parasitic non-cellular organism in the strict sense. It is composed of nucleic acid and a protein shell. After entering the host, the virus completes its proliferation through adsorption, penetration, uncoating, biosynthesis, assembly, release, and other processes. After entering the host, the virus plays a pathogenic role by invading susceptible cells or destroying or altering cell function. EVs have been shown to play a role in viral infection. Some viruses transport selected products to the neighboring cells via EV secretion pathways, thereby inducing biological changes (Khan and Ahmed 2017).
HPV oncogenes E6/E7 and EVs
Approximately, 99% of CCs are associated with HPV infection (Hu et al. 2023). Increasing evidence has revealed that EVs and their contents play an important role in HPV pathogenesis. Among patients with CC, HPV16 infection is a major cause, other high-risk HPV, such as HPV 18, HPV 45 and HPV 31, can also cause cervical cancer, and the main types of HPV infection vary by region (Boulenouar et al. 2010; Shalchimanesh et al. 2022). Many studies have shown that exosomes secreted by HPV-positive cells contain HPV oncogenes E6/E7 (Honegger et al. 2013).
E6 and E7 are two of the six early proteins expressed after HPV infection, and they play a carcinogenic role by promoting cell proliferation, inducing angiogenesis, activating cell invasion and migration, and inhibiting cell apoptosis (Basukala and Banks 2021; Pal and Kundu 2019). HPV-derived components, such as HPV E6 and E7 oncoproteins, have been shown to disrupt cellular pathways involved in cell cycle regulation, DNA damage response, and apoptosis, leading to genomic instability and cellular transformation. Recent studies have shown that HPV-derived components can also be packaged in exosomes and released from infected cells, which may contribute to the pathogenesis of cervical cancer (Acevedo-Sánchez et al. 2021). Moreover, HPV E6/E7 oncoproteins also affect the tumor microenvironment and immune function by promoting the synthesis and release of pro-inflammatory cytokines and chemokines (Iuliano et al. 2018).
Exosomes can communicate with other cells and tissues by transferring their cargo, thereby modulating various cellular processes (Zaborowski et al. 2015). Exosomes derived from HPV-infected cells have been shown to contain viral DNA and oncoproteins, and can be taken up by recipient cells, including cervical epithelial cells and immune cells (Acevedo-Sánchez et al. 2021). The uptake of HPV oncoproteins via exosomes can affect signaling pathways and downstream target genes in recipient cells, which can potentially contribute to the development and progression of cervical cancer. In HPV-associated CC, viral E6 and E7 oncoproteins are essential for the induction of HPV-associated transformation and maintenance of the tumorigenic phenotype of the HPV-positive CC cells (McLaughlin-Drubin and Münger 2009; von Knebel Doeberitz et al. 1988). Multiple studies have shown that the inhibition of E6 expression mainly leads to cell apoptosis (Butz et al. 2000, 2003; Griffin et al. 2006; Yamato et al. 2008), while the combined inhibition of E6 and E7 expression leads to cell growth arrest and cell senescence (Hall and Alexander 2003). The reversible phenotype of HPV-positive tumor cells makes it possible to use these oncoproteins as a reasonable basis for the treatment and intervention of cervical lesions. Honegger et al. (2013) found that in HeLa cells of CC, silencing HPV E6/E7 expression could promote EV secretion and affect intercellular communication by reinducing p53 and stimulating the expression of its target genes TSAP6 and CHMP4C. Sustained expression of E6/E7 translates HPV-induced intracellular changes into visible regulation of EV contents by affecting the protein levels secreted by the CC cells via EVs.
A novel tumor dynamic marker HPV DNA, which can be transmitted by EVs, has been found in plasma and cervical samples of patients with CC, but its role in EVs in the transformation and progression of CC remains unclear (Mata-Rocha et al. 2020). The carcinogenic effect of HPV is realized by inhibiting the expression of p53, p21, and Rb inhibitory genes in cancer and neighboring tissues as well as M2 macrophage polarization (Ambrosio et al. 2019). However, HPV DNA, including oncogenes E6 and E7, exist in the EVs secreted by CC cells (Mata-Rocha et al. 2020). In the EVs, from patients with CC, HPV DNA transported into the tumor microenvironment can promote tumor occurrence, transfer carcinogenic potential to the surrounding uninfected cells, and enhance the carcinogenic effect of the virus in the tumor microenvironment (Chiantore et al. 2016).
Overall, the packaging of HPV-derived components in exosomes can contribute to cervical cancer pathogenesis through the alteration of cellular signaling pathways and downstream gene expression.
EVs derived from HPV-infected cells affect the cellular microenvironment
There is increasing evidence that cancer-derived EVs may contribute to the recruitment and remodeling of the tumor microenvironment to form an environment conducive to cancer cell growth (Ge et al. 2012; Kahlert and Kalluri 2013). EVs can carry proteins and RNA, suggesting a different mechanism by which EVs influence the microenvironment to promote disease progression (Webber et al. 2015). In particular, miRNAs secreted by EVs in the microenvironment can regulate cancer cell proliferation, migration, intercellular communication, and matrix modification, thus promoting tumor growth and progression (Arroyo et al. 2011; Muralidharan-Chari et al. 2010). However, HPV-infected cells can transfer certain molecules through EVs, change the microenvironment of cell growth, and transfer its carcinogenic potential to the surrounding uninfected cells (Chiantore et al. 2016). Some researchers have pointed out that cancer development may be driven by a specific combination of inflammatory mediators (including cytokines, chemokines, and enzymes) in the tumor microenvironment (Coussens and Werb 2002). Furthermore, virus-induced tumors, such as HPV-induced squamous cell carcinoma, represent an example of the interaction between inflammation and malignant transformation. HPV E6 and E7 oncoproteins can regulate the expression of immune mediators in HPV-infected cells and affect their levels (mainly chemokines) in the cell microenvironment (Iuliano et al. 2018). Luliano et al. proved that E6 and E7 oncoproteins modulate the expression of inflammatory factors, in which IL-1α, IL-1β and IL-6 are down-regulated, while TNF-α is up-regulated (Iuliano et al. 2018). The upregulation of TNF-α may be related to the ability of HPV to activate the Erk MAPKs pathway (Branca et al. 2004). In addition, E6/E7 can also alter the production of chemokines, of which CCL20/MIP-3α is the main target, CCL2/MCP-1, CXCL8/IL-8, are also modulated by E6/E7 (Iuliano et al. 2018).
Toll-like receptors (TLRs) can induce anti-tumor and pro-tumor pathways, thereby affecting the tumor microenvironment (Ridnour et al. 2013). Various members of the TLR family are inhibited by some viruses such as Epstein–Barr virus (EBV), hepatitis B virus (HBV), and HPV (Fathallah et al. 2010; Hasan et al. 2007; Vincent et al. 2011). For example, HPV 16 can reduce the expression and function of TLR9. Persistent infection with HPV can lead to the alteration of specific cellular pathways that promote tumor development. Furthermore, sustained out-of-control immune responses can produce severe systemic and tissue damage, impair tumor monitoring, and eventually develop metastatic phenotypes and chemotherapy-resistant cancers (Huang et al. 2005). Gene rearrangement, proto-oncogene activation, and other events associated with genomic instability are necessary for HPV infection-induced tumor transformation. The E6 and E7 oncoproteins in HPV inhibit the NF-κB signaling pathway, which plays a key role in immune escape and transformation (Iuliano et al. 2018). In studies with HPV-positive cells, increased secretion of Angiogenin and TIMP-1 was observed in the cell supernatant, which is consistent with the increase in pro-angiogenesis and pro-metastasis activities regulated by E6 and E7 oncoproteins. It has been reported that tumors can influence the microenvironment by controlling the release and content of EVs (Minciacchi et al. 2015). Likewise, reports suggest that EVs derived from HPV positive cells have the capability to transport microRNAs and cellular proteins to both the microenvironment and recipient cells (Chiantore et al. 2016; Harden and Munger 2017; Harden et al. 2017; Honegger et al. 2013; Honegger et al. 2015).
Overall, the presence of HPV E6 and E7 in a cancerous microenvironment leads to modifications in the surrounding non-transformed cells, characterized by immunosuppression, evasion, and control over gene expression (Chiantore et al. 2016). Additionally, alterations in the composition of extracellular vesicles due to E6/E7 contribute to the disruption of the extracellular environment and regulate the functions of nearby non-transformed acceptor cells and immune cells through the transfer of vesicle content (Minciacchi et al. 2015).
Cervical cancer and miRNAs in EVs
Recently, it has been found that miRNAs show differential expression levels in different body fluids of healthy and disease states as well as in tumors, and these variations in expression are related to tumor occurrence, metastasis, disease progression, and drug resistance (Kosaka et al. 2010). The HPV-related oncoproteins in these genotypes directly or indirectly regulate the expression of multiple host miRNAs, leading to the regulation of the virus life cycle and disease progression (Gocze et al. 2015). Thus, miRNAs appear to be useful as cancer diagnostic biomarkers. Some miRNAs are released by exosomes and microvesicles into all body fluids of humans for their function. Additionally, Chiantore et al. (2016), Honegger et al. (2015), and Harden and Munger (2017) found that HPV E6/E7 oncoprotein expression could affect the tumor microenvironment and promote tumor cell proliferation by regulating the number and content of miRNAs carried by EVs.
When comparing EVs released from cancer cells and non-malignant cells, Muller et al. found that cancer cells are more likely to produce EVs (Muller et al. 2016). In cancer cases, the cellular molecules transported by EVs can lead to corresponding induced changes in recipient cells, further leading to tumor growth, metastasis, and angiogenesis (Yang and Robbins 2011). The contents of the EVs depend on cell type, extracellular environment, and host health, and can change with time. Nonetheless, the main content types remain the same and include DNA, mRNA, miRNA, viral microRNA (vmiRNA), viral genome, and protein (Chahar et al. 2015; Théry et al. 2001). Tumor-derived EVs and the miRNAs that they contain play an important role in the communication between cancer cells and their environment. Recently, several studies have emphasized the important role of cancer-related miRNAs in proliferation, differentiation, apoptosis, invasion, and resistance to chemotherapy (Zheng et al. 2010).Some CC-related miRNAs, including miR-10a, miR-21, miR-19, and miR-146a, have been reported to play an important role in cell growth, migration, and invasion, while some miRNAs, such as miR-372, miR-214, and miR-218, have been related to tumor inhibition (Long et al. 2012; Qiang et al. 2011; Tian et al. 2011; Yao et al. 2009; Yu et al. 2012).
One such miRNA is miR-21, which is upregulated in HPV-positive cervical cancer cells. It targets numerous tumor suppressor genes, including PTEN, PDCD4, and TPM1, thereby promoting tumor growth and proliferation (Yao et al. 2009). miR-143 and miR-146a are also dysregulated in HPV-positive cervical cancer cells. miR-143 targets KRAS (Qin et al. 2016) and ERK5 (Zheng et al. 2016), which are involved in cell proliferation and survival. Overexpression of miR-143 inhibits cell proliferation and induces apoptosis. In contrast, miR-146a targets WWC2, a protein that is involved in the Hippo-YAP pathway, which is known to promote inflammation and cancer (Wang et al. 2022). miR-146a overexpression inhibits inflammation and tumor growth. miR-146a-5p has been found to target the oncogene c-MYC (Peta et al. 2018), which promotes the cell cycle and proliferation. The downregulation of these genes by miR-146a-5p may contribute to the anti-proliferative and pro-apoptotic effects observed in cervical cancer cells. We summarize the role of several miRNAs of EVs in HPV-associated cervical cancer in Table 1.
Table 1.
The role of the miRNAs of EVs in HPV-associated cervical cancer
| miRNAs | Targeted gene | The role | References |
|---|---|---|---|
| miR-21 | PTEN、PDCD4 and TPM1 | Promote tumor growth and proliferation | Yao et al. (2009) |
| miR-143 | KRAS and ERK5 | Inhibit cell proliferation and induce apoptosis | Qin et al. (2016), Zheng et al. (2016) |
| miR-146a | WWC2 | Inhibit inflammation and tumor growth | Wang et al. (2022) |
| miR-146a-5p | c-MYC | Anti-proliferative and pro-apoptotic | Peta et al. (2018) |
Based on the degree of the cervical lesions, different miRNAs show differential expression levels. miRNAs may influence tumor formation by targeting the 3′-untranslated region (3′-UTR) of their corresponding mRNA and post-transcriptionally regulating subsequent gene expression. However, many studies have shown that miRNAs may act as therapeutic factors by influencing the occurrence of various cancers (Banno et al. 2014; Hayes et al. 2014). Therefore, the silencing of up-regulated miRNAs that promote tumorigenesis and inhibit apoptosis, or the use of tumor-suppressor miRNAs, may be useful approaches for CC therapy. Zheng et al. (2019) demonstrated that, in CC, EV-derived miRNAs can serve not only as potential diagnostic biomarkers but also as potential anticancer drug targets, because they are functionally involved in the occurrence and progression of CC. Compared with cervical biopsy, the accuracy of cytological examination is relatively low at present, and there is still much room for improvement in terms of screening (Zheng et al. 2019). Compared to the complex mechanism of long non-coding RNAs, the absence of cellular DNA heteromutation sites, and the unstable characteristics of mRNA, exosomal miRNAs are stable and relatively non-degradable and their detection methods are relatively mature (Zheng et al. 2019); hence, exosomal miRNAs are promising biomarkers for the diagnosis of cancer and other complex diseases (Taylor and Gercel-Taylor 2008). Indeed, recent studies have shown that exosomal miRNAs have the potential to be highly effective biomarkers for cancer screening, diagnosis, and surveillance. For example, miR-122, miR-192, miR-17-5p, and miR-25-3p are differentially expressed in different cancer tissues and are highly enriched in tumor-derived exosomes (Wang et al. 2009). The detection of EV-derived miRNAs in serum is more convenient than conducting TCT or Pap smear and is associated with a lower risk of vaginal/cervical infection. Additionally, miRNAs in plasma EVs are relatively stable and resistant to physical degradation, making them promising diagnostic biomarkers for screening CC. Therefore, miRNA detection can be included in routine blood tests; this would greatly reduce the testing time for patients and clinicians.
Role of EVs secretion in cervical cancer
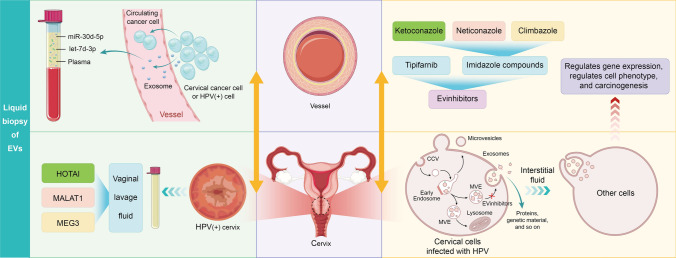
EVs play an important role in intercellular communication and have been found to be functional mediators in the progression of many serious diseases, including various cancer types. In some studies, tumor-derived EVs have been related to tumor progression, angiogenesis, and altered tumor microenvironment (Milane et al. 2015; Spugnini et al. 2018). Inhibition of EV release has been shown to slow the progression of some cancers (Zhang et al. 2020), making EVs a powerful target for cancer therapy. Amrita et al. (Datta et al. 2018) identified four inhibitors that block EV function by selectively inhibiting the biosynthesis and secretion of the EVs, thus slowing down the progression of cancer. The endosomal sorting complex required for transport (ESCRT) machinery is essential for exosome formation, sorting and release (Stoorvogel 2015). Additionally, alternative pathways such as lipid-mediated and tetraspanin-mediated processes are also significantly involved (Colombo et al. 2014). The exosome inhibitory effects of tipifarnib can happen through various pathways, such as the inhibition of exosome production and release, disruption of Ras/Raf/ERK signaling pathways, and suppression of both ESCRT-dependent and ESCRT-independent pathways (Datta et al. 2018). The exosome-inhibitory effects of imidazole compounds (ketoconazole, neticonazole, and climbazole) are triggered through ESCRT-independent and ESCRT-dependent pathways, as well as the Ras/ERK signaling pathways, as shown in Fig. 1 (Datta et al. 2018).During cancer development, the synthesis, secretion, and "cargo" content of EVs are greatly regulated, and there are many differences between normal and cancer cells in terms of the quantity and quality of the EVs released (Allenson et al. 2017; Chin and Wang 2016; Di Vizio et al. 2012; Isola et al. 2016; Yu et al. 2005).
Fig. 1.
The role of extracellular vesicles in HPV-associated cervical cancer. Some of the clinical applications of exosomes are shown on the left side, including blood testing and testing of vaginal lavage fluid. The right side of the Fig. 1 describes exosome production and how it functions between cells, while also explaining various exosome secretion inhibitors
Some studies have reported that cancer progression is due to the continuous exchange of information between tumor cells and their stromal microenvironment (Zhang et al. 2020). EVs can both induce and promote the pre-tumor microenvironment and regulate the immune response to initial tumor progression and survival by promoting angiogenesis, metastasis, and drug resistance (De Toro et al. 2015; Lobb et al. 2017). Clinical studies have shown that, as potential diagnostic biomarkers of tumors, elevated EV levels may be a marker of malignant cancer cells, and can be used as an indicator to evaluate a patient’s clinical status (Zocco et al. 2014). Based on extensive studies on EV-associated proteins and their mechanisms, a large number of drugs have been developed to reduce the exosome secretion by cancer cells or their absorption by recipient cells (Hessvik and Llorente 2018; Zhang et al. 2019). Notably, some of these compounds, such as Tiffany and ketoconazole, only affect EVs released by tumor cells and not those secreted by normal cells. The research on EV-based therapy for patients with CC is a current hot research topic. As the important role of EVs is widely understood, EV inhibitors may be used as synergists in the treatment of CC in the future.
Liquid biopsy of EVs in cervical cancer
Liquid biopsies are considered revolutionary techniques in precision medicine because they are non-invasive. Exosomes are considered important biomarkers in liquid biopsies because they play an important role in cell–cell communication and are closely related to the pathogenesis of most human malignancies (Lin et al. 2021). The traditional assessment of cancer is usually achieved through the surgical acquisition of cancer tissue biopsy. However, tissue biopsy has several limitations, such as the limited method of sample collection, the inability to collect deep tumor tissue, heterogeneity within the tumor, and invasiveness (Vaidyanathan et al. 2019). Unlike tissue biopsy, liquid biopsy is a non-invasive technique that can be used to analyze disease biomarkers in a variety of biological fluids such as blood, urine, saliva, ascites, and breast milk (Junqueira-Neto et al. 2019). Liquid biopsies are one of the most innovative technologies as they can also be used to monitor treatment responses, allowing for required treatment adjustments with time.
The main components detected by liquid biopsy are circulating tumor cells (CTCS) and circulating tumor DNA, as well as circulating exosomes released from primary tumors and metastases (Wang et al. 2017). Exosomes are promising biomarkers for tumor detection and screening, offering advantages over other liquid biopsy targets in several respects. First, as a subtype of EVs, exosomes are secreted by almost all cells and are present in most body fluids, including human plasma, saliva, and urine (Lin et al. 2021). Compared with the rarity of CTCS in the blood (< 100 CTCS cells/mL), the concentration of exosomes is relatively abundant, reaching more than 109 exosomes/mL (Crowley et al. 2013). Second, exosomes transport a variety of bioactive molecules involved in intercellular communication; hence, we can obtain a large amount of information reflecting genetic and epigenetic changes in the original cell. Third, the liposome-like membrane structure of exosomes exhibits inherent stability that prevents enzymatic degradation and preserves the integrity of the content molecules. This stability and integrity, which are desirable properties for biomarkers, are important reasons for the increasing popularity of exosomes in liquid biopsy of CC.
Exosomes can be released by cervical cells with varying degrees of pathology in cervical cancer and are present in abundance in vaginal lavage fluid and plasma, playing a vital role in the different stages of cervical cancer formation and development (Tomasetti et al. 2017; Wang et al. 2020; Zhang et al. 2022). Zheng et al. conducted miRNA sequencing on 121 plasma exosomes from healthy volunteers, cervical cancer patients and cervical intraepithelial neoplasia patients, and found that miR-30 d-5p and let-7 d-3p in plasma exosomes were potential diagnostic biomarkers for non-invasive screening of cervical cancer and its precancerous lesions (Zheng et al. 2019). Zhang et al. (2016) collected cervicovaginal lavage fluid from 30 cervical cancer patients and 60 normal control healthy people, and analyzed the hox transcript antisense intergenic RNA (HOTAI), metastasis associated lung adenocarcinoma transcript 1 (MALAT1) and maternally expressed gene 3 (MEG3) in exosomes by fluorescence quantitative PCR. The researchers discovered that all three lncRNAs were enriched in cervical cancer exosomes and had distinct expression patterns between cervical cancer patients and healthy individuals, indicating their potential to identify cervical cancer in healthy people, as shown in Fig. 1 (Zhang et al. 2016).
In general, blood and vaginal lavage fluid are the most suitable body fluids for exosome liquid biopsy of cervical cancer. Nevertheless, the clinical detection methods for cervical cancer exosomes are still relatively uncommon. Fortunately, the continual emergence of novel detection technologies offers valuable guidance and direction for the advancement of related techniques.
Conclusion
HPV infection is closely related to CC, and there are a variety of complex and interesting changes through which HPV causes cancer. Among these, EVs and their contents play an important and irreplaceable role. EVs can transmit a variety of genetic material as well as a variety of cytokines associated with the production and progression of cancer. EVs of tumor origin and the miRNAs transmitted by the EVs play an important role in cancer cell communication. Compared with the complex mechanism and unstable properties of other RNA types, miRNAs from EVs are stable and relatively non-degradable, and their detection methods are relatively mature. Therefore, they are expected to become a new diagnostic marker for CC.
As the gold standard for CC diagnosis, cervical tissue biopsy has some shortcomings, such as invasiveness, limited sampling methods, inability to collect deep tumor tissue, and heterogeneity within the tumor. The recent emerging technology of liquid biopsy is considered to be revolutionary in precision medicine, and exosomes are considered to be important biomarkers in liquid biopsy. Sustained expression of E6/E7 in turn translates HPV-induced intracellular changes into visible regulation of EV contents by affecting the protein levels secreted by the CC cells. By regulating EV secretion from HPV-positive CC cells via affecting the expression of E6/E7 or inhibitors, it is possible to slow down the progression of CC and thereby have an impact on CC development. This direction is quite significant for future experimental research on CC treatment.
CC causes physical and financial distress for many women. The diagnosis and recognition of CC are evolving. EVs exist widely in almost all body fluids and cell supernatants, and their biological functions are being gradually understood, with their application in the diagnosis and treatment of CC being investigated. Understanding the various roles of EV contents in the development of CC is helpful to understand the biological function of EVs more comprehensively and objectively, and can provide a new clinical idea for the diagnosis and treatment of CC. Finally, we briefly summarize the role of EVs in HPV-associated cervical cancer in Fig. 1.
Acknowledgements
We would like to thank Bullet Edits Limited for the linguistic editing and proofreading of the manuscript.
Author contributions
SD: Writing—Original Draft, YZ: Supervision, YW: Supervision; All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability
The corresponding author can provide the datasets used and/or analyzed during the current study upon reasonable request.
Declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Yan Zhang, Email: qdzhangyan01@163.com.
Yankui Wang, Email: qdwangyk@163.com.
References
- Acevedo-Sánchez V, Rodríguez-Hernández RM, Aguilar-Ruíz SR, Torres-Aguilar H, Romero-Tlalolini MLA (2021) Extracellular vesicles in cervical cancer and HPV infection. Membranes (Basel). 10.3390/membranes11060453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allenson K, Castillo J, San Lucas FA, Scelo G, Kim DU, Bernard V, Davis G, Kumar T, Katz M, Overman MJ, Foretova L, Fabianova E, Holcatova I, Janout V, Meric-Bernstam F, Gascoyne P, Wistuba I, Varadhachary G, Brennan P, Hanash S, Li D, Maitra A, Alvarez H (2017) High prevalence of mutant KRAs in circulating exosome-derived DNA from early-stage pancreatic cancer patients. Ann Oncol 28(4):741–747. 10.1093/annonc/mdx004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambrosio MR, Vernillo R, De Carolis S, Carducci A, Mundo L, Ginori A, Rocca BJ, Nardone V, Lucenti Fei A, Carfagno T, Lazzi S, Cricca M, Tosi P (2019) Putative role of circulating human papillomavirus DNA in the development of primary squamous cell carcinoma of the middle rectum: a case report. Front Oncol 9:93. 10.3389/fonc.2019.00093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arroyo JD, Chevillet JR, Kroh EM, Ruf IK, Pritchard CC, Gibson DF, Mitchell PS, Bennett CF, Pogosova-Agadjanyan EL, Stirewalt DL, Tait JF, Tewari M (2011) Argonaute2 complexes carry a population of circulating microRNAs independent of vesicles in human plasma. Proc Natl Acad Sci USA 108(12):5003–5008. 10.1073/pnas.1019055108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banno K, Iida M, Yanokura M, Kisu I, Iwata T, Tominaga E, Tanaka K, Aoki D (2014) MicroRNA in cervical cancer: oncomiRs and tumor suppressor miRs in diagnosis and treatment. Sci World J 2014:178075. 10.1155/2014/178075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basukala O, Banks L (2021) The not-so-good, the bad and the ugly: HPV E5, E6 and E7 oncoproteins in the orchestration of carcinogenesis. Viruses. 10.3390/v13101892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulenouar S, Weyn C, Van Noppen M, Moussa Ali M, Favre M, Delvenne PO, Bex F, Noël A, Englert Y, Fontaine V (2010) Effects of HPV-16 E5, E6 and E7 proteins on survival, adhesion, migration and invasion of trophoblastic cells. Carcinogenesis 31(3):473–480. 10.1093/carcin/bgp281 [DOI] [PubMed] [Google Scholar]
- Branca M, Ciotti M, Santini D, Bonito LD, Benedetto A, Giorgi C, Paba P, Favalli C, Costa S, Agarossi A, Alderisio M, Syrjänen K (2004) Activation of the ERK/MAP kinase pathway in cervical intraepithelial neoplasia is related to grade of the lesion but not to high-risk human papillomavirus, virus clearance, or prognosis in cervical cancer. Am J Clin Pathol 122(6):902–911. 10.1309/vqxf-t880-jxc7-qd2w [DOI] [PubMed] [Google Scholar]
- Butz K, Denk C, Ullmann A, Scheffner M, Hoppe-Seyler F (2000) Induction of apoptosis in human papillomaviruspositive cancer cells by peptide aptamers targeting the viral E6 oncoprotein. Proc Natl Acad Sci USA 97(12):6693–6697. 10.1073/pnas.110538897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butz K, Ristriani T, Hengstermann A, Denk C, Scheffner M, Hoppe-Seyler F (2003) siRNA targeting of the viral E6 oncogene efficiently kills human papillomavirus-positive cancer cells. Oncogene 22(38):5938–5945. 10.1038/sj.onc.1206894 [DOI] [PubMed] [Google Scholar]
- Chahar HS, Bao X, Casola A (2015) Exosomes and their role in the life cycle and pathogenesis of RNA viruses. Viruses 7(6):3204–3225. 10.3390/v7062770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J (2016) Cancer statistics in china, 2015. CA Cancer J Clin 66(2):115–132. 10.3322/caac.21338 [DOI] [PubMed] [Google Scholar]
- Chiantore MV, Mangino G, Iuliano M, Zangrillo MS, De Lillis I, Vaccari G, Accardi R, Tommasino M, Columba Cabezas S, Federico M, Fiorucci G, Romeo G (2016) Human papillomavirus E6 and E7 oncoproteins affect the expression of cancer-related microRNAs: additional evidence in HPV-induced tumorigenesis. J Cancer Res Clin Oncol 142(8):1751–1763. 10.1007/s00432-016-2189-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin AR, Wang SE (2016) Cancer-derived extracellular vesicles: the “soil conditioner” in breast cancer metastasis? Cancer Metastasis Rev 35(4):669–676. 10.1007/s10555-016-9639-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombo M, Raposo G, Théry C (2014) Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol 30:255–289. 10.1146/annurev-cellbio-101512-122326 [DOI] [PubMed] [Google Scholar]
- Coussens LM, Werb Z (2002) Inflammation and cancer. Nature 420(6917):860–867. 10.1038/nature01322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowley E, Di Nicolantonio F, Loupakis F, Bardelli A (2013) Liquid biopsy: monitoring cancer-genetics in the blood. Nat Rev Clin Oncol 10(8):472–484. 10.1038/nrclinonc.2013.110 [DOI] [PubMed] [Google Scholar]
- Datta A, Kim H, McGee L, Johnson AE, Talwar S, Marugan J, Southall N, Hu X, Lal M, Mondal D, Ferrer M, Abdel-Mageed AB (2018) High-throughput screening identified selective inhibitors of exosome biogenesis and secretion: a drug repurposing strategy for advanced cancer. Sci Rep 8(1):8161. 10.1038/s41598-018-26411-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Toro J, Herschlik L, Waldner C, Mongini C (2015) Emerging roles of exosomes in normal and pathological conditions: new insights for diagnosis and therapeutic applications. Front Immunol 6:203. 10.3389/fimmu.2015.00203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Vizio D, Morello M, Dudley AC, Schow PW, Adam RM, Morley S, Mulholland D, Rotinen M, Hager MH, Insabato L, Moses MA, Demichelis F, Lisanti MP, Wu H, Klagsbrun M, Bhowmick NA, Rubin MA, D’Souza-Schorey C, Freeman MR (2012) Large oncosomes in human prostate cancer tissues and in the circulation of mice with metastatic disease. Am J Pathol 181(5):1573–1584. 10.1016/j.ajpath.2012.07.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fathallah I, Parroche P, Gruffat H, Zannetti C, Johansson H, Yue J, Manet E, Tommasino M, Sylla BS, Hasan UA (2010) EBV latent membrane protein 1 is a negative regulator of TLR9. J Immunol 185(11):6439–6447. 10.4049/jimmunol.0903459 [DOI] [PubMed] [Google Scholar]
- Ge R, Tan E, Sharghi-Namini S, Asada HH (2012) Exosomes in cancer microenvironment and beyond: have we overlooked these extracellular messengers? Cancer Microenviron 5(3):323–332. 10.1007/s12307-012-0110-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gocze K, Gombos K, Kovacs K, Juhasz K, Gocze P, Kiss I (2015) MicroRNA expressions in HPV-induced cervical dysplasia and cancer. Anticancer Res 35(1):523–530 [PubMed] [Google Scholar]
- Griffin H, Elston R, Jackson D, Ansell K, Coleman M, Winter G, Doorbar J (2006) Inhibition of papillomavirus protein function in cervical cancer cells by intrabody targeting. J Mol Biol 355(3):360–378. 10.1016/j.jmb.2005.10.077 [DOI] [PubMed] [Google Scholar]
- Hall AH, Alexander KA (2003) RNA interference of human papillomavirus type 18 E6 and E7 induces senescence in HeLa cells. J Virol 77(10):6066–6069. 10.1128/jvi.77.10.6066-6069.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harden ME, Munger K (2017) Human papillomavirus 16 E6 and E7 oncoprotein expression alters microRNA expression in extracellular vesicles. Virology 508:63–69. 10.1016/j.virol.2017.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harden ME, Prasad N, Griffiths A, Munger K (2017) Modulation of microRNA-mRNA target pairs by human papillomavirus 16 oncoproteins. Mbio. 10.1128/mBio.02170-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasan UA, Bates E, Takeshita F, Biliato A, Accardi R, Bouvard V, Mansour M, Vincent I, Gissmann L, Iftner T, Sideri M, Stubenrauch F, Tommasino M (2007) TLR9 expression and function is abolished by the cervical cancer-associated human papillomavirus type 16. J Immunol 178(5):3186–3197. 10.4049/jimmunol.178.5.3186 [DOI] [PubMed] [Google Scholar]
- Hayes J, Peruzzi PP, Lawler S (2014) MicroRNAs in cancer: biomarkers, functions and therapy. Trends Mol Med 20(8):460–469. 10.1016/j.molmed.2014.06.005 [DOI] [PubMed] [Google Scholar]
- Hessvik NP, Llorente A (2018) Current knowledge on exosome biogenesis and release. Cell Mol Life Sci 75(2):193–208. 10.1007/s00018-017-2595-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honegger A, Leitz J, Bulkescher J, Hoppe-Seyler K, Hoppe-Seyler F (2013) Silencing of human papillomavirus (HPV) E6/E7 oncogene expression affects both the contents and the amounts of extracellular microvesicles released from HPV-positive cancer cells. Int J Cancer 133(7):1631–1642. 10.1002/ijc.28164 [DOI] [PubMed] [Google Scholar]
- Honegger A, Schilling D, Bastian S, Sponagel J, Kuryshev V, Sültmann H, Scheffner M, Hoppe-Seyler K, Hoppe-Seyler F (2015) Dependence of intracellular and exosomal microRNAs on viral E6/E7 oncogene expression in HPV-positive tumor cells. PLoS Pathog 11(3):e1004712. 10.1371/journal.ppat.1004712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J, Liao D, Sun Z, Ren W, Zhao L, Fang Y, Hu K, Yu H, Liu S, Zhou L, He T, Zhang Y (2023) The HPV16 E6, E7/miR-23b-3p/ICAT signaling axis promotes proliferation, migration, invasion and EMT of cervical cancer cells. Carcinogenesis 44(3):221–231. 10.1093/carcin/bgad008 [DOI] [PubMed] [Google Scholar]
- Huang B, Zhao J, Li H, He KL, Chen Y, Chen SH, Mayer L, Unkeless JC, Xiong H (2005) Toll-like receptors on tumor cells facilitate evasion of immune surveillance. Cancer Res 65(12):5009–5014. 10.1158/0008-5472.can-05-0784 [DOI] [PubMed] [Google Scholar]
- Isola AL, Eddy K, Chen S (2016) Biology, therapy and implications of tumor exosomes in the progression of melanoma. Cancers (Basel). 10.3390/cancers8120110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iuliano M, Mangino G, Chiantore MV, Zangrillo MS, Accardi R, Tommasino M, Fiorucci G, Romeo G (2018) Human papillomavirus E6 and E7 oncoproteins affect the cell microenvironment by classical secretion and extracellular vesicles delivery of inflammatory mediators. Cytokine 106:182–189. 10.1016/j.cyto.2017.11.003 [DOI] [PubMed] [Google Scholar]
- Junqueira-Neto S, Batista IA, Costa JL, Melo SA (2019) Liquid biopsy beyond circulating tumor cells and cell-free DNA. Acta Cytol 63(6):479–488. 10.1159/000493969 [DOI] [PubMed] [Google Scholar]
- Kahlert C, Kalluri R (2013) Exosomes in tumor microenvironment influence cancer progression and metastasis. J Mol Med (Berl) 91(4):431–437. 10.1007/s00109-013-1020-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan G, Ahmed W (2017) Isolation and characterization of exosomes released by EBV-immortalized cells. Methods Mol Biol 1532:147–158. 10.1007/978-1-4939-6655-4_10 [DOI] [PubMed] [Google Scholar]
- Kosaka N, Iguchi H, Ochiya T (2010) Circulating microRNA in body fluid: a new potential biomarker for cancer diagnosis and prognosis. Cancer Sci 101(10):2087–92. 10.1111/j.1349-7006.2010.01650.x [DOI] [PMC free article] [PubMed]
- Lin B, Lei Y, Wang J, Zhu L, Wu Y, Zhang H, Wu L, Zhang P, Yang C (2021) Microfluidic-based exosome analysis for liquid biopsy. Small Methods 5(3):e2001131. 10.1002/smtd.202001131 [DOI] [PubMed] [Google Scholar]
- Lobb RJ, Lima LG, Möller A (2017) Exosomes: key mediators of metastasis and pre-metastatic niche formation. Semin Cell Dev Biol 67:3–10. 10.1016/j.semcdb.2017.01.004 [DOI] [PubMed] [Google Scholar]
- Long MJ, Wu FX, Li P, Liu M, Li X, Tang H (2012) MicroRNA-10a targets chl1 and promotes cell growth, migration and invasion in human cervical cancer cells. Cancer Lett 324(2):186–196. 10.1016/j.canlet.2012.05.022 [DOI] [PubMed] [Google Scholar]
- Mata-Rocha M, Rodriguez-Hernandez RM, Chavez-Olmos P, Garrido E, Robles-Vazquez C, Aguilar-Ruiz S, Torres-Aguilar H, Gonzalez-Torres C, Gaytan-Cervantes J, Mejia-Arangure JM, Romero-Tlalolini MLA (2020) Presence of HPV DNA in extracellular vesicles from HeLa cells and cervical samples. Enferm Infecc Microbiol Clin (Engl Ed) 38(4):159–165. 10.1016/j.eimc.2019.06.011 [DOI] [PubMed] [Google Scholar]
- McLaughlin-Drubin ME, Münger K (2009) Oncogenic activities of human papillomaviruses. Virus Res 143(2):195–208. 10.1016/j.virusres.2009.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milane L, Singh A, Mattheolabakis G, Suresh M, Amiji MM (2015) Exosome mediated communication within the tumor microenvironment. J Control Release 219:278–294. 10.1016/j.jconrel.2015.06.029 [DOI] [PubMed] [Google Scholar]
- Minciacchi VR, Freeman MR, Di Vizio D (2015) Extracellular vesicles in cancer: exosomes, microvesicles and the emerging role of large oncosomes. Semin Cell Dev Biol 40:41–51. 10.1016/j.semcdb.2015.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller L, Mitsuhashi M, Simms P, Gooding WE, Whiteside TL (2016) Tumor-derived exosomes regulate expression of immune function-related genes in human T cell subsets. Sci Rep 6:20254. 10.1038/srep20254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muralidharan-Chari V, Clancy JW, Sedgwick A, D’Souza-Schorey C (2010) Microvesicles: mediators of extracellular communication during cancer progression. J Cell Sci 123(Pt 10):1603–1611. 10.1242/jcs.064386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal A, Kundu R (2019) Human papillomavirus E6 and E7: the cervical cancer hallmarks and targets for therapy. Front Microbiol 10:3116. 10.3389/fmicb.2019.03116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peta E, Sinigaglia A, Masi G, Di Camillo B, Grassi A, Trevisan M, Messa L, Loregian A, Manfrin E, Brunelli M, Martignoni G, Palù G, Barzon L (2018) HPV16 E6 and E7 upregulate the histone lysine demethylase KDM2B through the c-MYC/miR-146a-5p axys. Oncogene 37(12):1654–1668. 10.1038/s41388-017-0083-1 [DOI] [PubMed] [Google Scholar]
- Qiang R, Wang F, Shi LY, Liu M, Chen S, Wan HY, Li YX, Li X, Gao SY, Sun BC, Tang H (2011) Plexin-B1 is a target of miR-214 in cervical cancer and promotes the growth and invasion of HeLa cells. Int J Biochem Cell Biol 43(4):632–641. 10.1016/j.biocel.2011.01.002 [DOI] [PubMed] [Google Scholar]
- Qin HX, Cui HK, Pan Y, Hu RL, Zhu LH, Wang SJ (2016) miR-143 inhibits cell proliferation through targeted regulating the expression of k-RAS gene in HeLa cells. Zhonghua Zhong Liu Za Zhi 38(12):893–897. 10.3760/cma.j.issn.0253-3766.2016.12.003 [DOI] [PubMed] [Google Scholar]
- Raposo G, Stoorvogel W (2013) Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol 200(4):373–383. 10.1083/jcb.201211138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridnour LA, Cheng RY, Switzer CH, Heinecke JL, Ambs S, Glynn S, Young HA, Trinchieri G, Wink DA (2013) Molecular pathways: toll-like receptors in the tumor microenvironment–poor prognosis or new therapeutic opportunity. Clin Cancer Res 19(6):1340–1346. 10.1158/1078-0432.ccr-12-0408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalchimanesh Z, Ghane M, Kalantar E (2022) Prevalence of human papillomavirus genotypes in Tehran, Iran. J Res Health Sci 22(3):e00553. 10.34172/jrhs.2022.88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RA, Manassaram-Baptiste D, Brooks D, Doroshenk M, Fedewa S, Saslow D, Brawley OW, Wender R (2015) Cancer screening in the United States, 2015: a review of current American Cancer Society guidelines and current issues in cancer screening. CA Cancer J Clin 65(1):30–54. 10.3322/caac.21261 [DOI] [PubMed] [Google Scholar]
- Spugnini EP, Logozzi M, Di Raimo R, Mizzoni D, Fais S (2018) A role of tumor-released exosomes in paracrine dissemination and metastasis. Int J Mol Sci. 10.3390/ijms19123968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoorvogel W (2015) Resolving sorting mechanisms into exosomes. Cell Res 25(5):531–532. 10.1038/cr.2015.39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor DD, Gercel-Taylor C (2008) MicroRNA signatures of tumor-derived exosomes as diagnostic biomarkers of ovarian cancer. Gynecol Oncol 110(1):13–21. 10.1016/j.ygyno.2008.04.033 [DOI] [PubMed] [Google Scholar]
- Théry C, Boussac M, Véron P, Ricciardi-Castagnoli P, Raposo G, Garin J, Amigorena S (2001) Proteomic analysis of dendritic cell-derived exosomes: a secreted subcellular compartment distinct from apoptotic vesicles. J Immunol 166(12):7309–7318. 10.4049/jimmunol.166.12.7309 [DOI] [PubMed] [Google Scholar]
- Tian RQ, Wang XH, Hou LJ, Jia WH, Yang Q, Li YX, Liu M, Li X, Tang H (2011) MicroRNA-372 is down-regulated and targets cyclin-dependent kinase 2 (CDK2) and cyclin a1 in human cervical cancer, which may contribute to tumorigenesis. J Biol Chem 286(29):25556–25563. 10.1074/jbc.M111.221564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasetti M, Lee W, Santarelli L, Neuzil J (2017) Exosome-derived microRNAs in cancer metabolism: possible implications in cancer diagnostics and therapy. Exp Mol Med 49(1):e285. 10.1038/emm.2016.153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaidyanathan R, Soon RH, Zhang P, Jiang K, Lim CT (2019) Cancer diagnosis: from tumor to liquid biopsy and beyond. Lab Chip 19(1):11–34. 10.1039/c8lc00684a [DOI] [PubMed] [Google Scholar]
- Vincent IE, Zannetti C, Lucifora J, Norder H, Protzer U, Hainaut P, Zoulim F, Tommasino M, Trépo C, Hasan U, Chemin I (2011) Hepatitis B virus impairs TLR9 expression and function in plasmacytoid dendritic cells. PLoS ONE 6(10):e26315. 10.1371/journal.pone.0026315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Knebel DM, Oltersdorf T, Schwarz E, Gissmann L (1988) Correlation of modified human papilloma virus early gene expression with altered growth properties in C4-1 cervical carcinoma cells. Cancer Res 48(13):3780–3786 [PubMed] [Google Scholar]
- Wang K, Zhang S, Marzolf B, Troisch P, Brightman A, Hu Z, Hood LE, Galas DJ (2009) Circulating microRNAs, potential biomarkers for drug-induced liver injury. Proc Natl Acad Sci USA 106(11):4402–4407. 10.1073/pnas.0813371106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Chen JQ, Liu JL, Tian L (2016) Exosomes in tumor microenvironment: novel transporters and biomarkers. J Transl Med 14(1):297. 10.1186/s12967-016-1056-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JY, Chang S, Li GC, Sun YL (2017) Application of liquid biopsy in precision medicine: opportunities and challenges. Front Med 11(4):522–527. 10.1007/s11684-017-0526-7 [DOI] [PubMed] [Google Scholar]
- Wang W, Han Y, Jo HA, Lee J, Song YS (2020) Non-coding RNAs shuttled via exosomes reshape the hypoxic tumor microenvironment. J Hematol Oncol 13(1):67. 10.1186/s13045-020-00893-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Wu L, Tian J, Yan W, Qi C, Liu W, Xuan S, Shang A (2022) Cervical cancer cells-derived extracellular vesicles containing microRNA-146a-5p affect actin dynamics to promote cervical cancer metastasis by activating the Hippo-YAP signaling pathway via WWC2. J Oncol 2022:4499876. 10.1155/2022/4499876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webber J, Yeung V, Clayton A (2015) Extracellular vesicles as modulators of the cancer microenvironment. Semin Cell Dev Biol 40:27–34. 10.1016/j.semcdb.2015.01.013 [DOI] [PubMed] [Google Scholar]
- Yamato K, Yamada T, Kizaki M, Ui-Tei K, Natori Y, Fujino M, Nishihara T, Ikeda Y, Nasu Y, Saigo K, Yoshinouchi M (2008) New highly potent and specific E6 and E7 siRNAs for treatment of HPV16 positive cervical cancer. Cancer Gene Ther 15(3):140–153. 10.1038/sj.cgt.7701118 [DOI] [PubMed] [Google Scholar]
- Yang C, Robbins PD (2011) The roles of tumor-derived exosomes in cancer pathogenesis. Clin Dev Immunol 2011:842849. 10.1155/2011/842849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Q, Xu H, Zhang QQ, Zhou H, Qu LH (2009) MicroRNA-21 promotes cell proliferation and down-regulates the expression of programmed cell death 4 (PDCD4) in HeLa cervical carcinoma cells. Biochem Biophys Res Commun 388(3):539–542. 10.1016/j.bbrc.2009.08.044 [DOI] [PubMed] [Google Scholar]
- Yu JL, May L, Lhotak V, Shahrzad S, Shirasawa S, Weitz JI, Coomber BL, Mackman N, Rak JW (2005) Oncogenic events regulate tissue factor expression in colorectal cancer cells: implications for tumor progression and angiogenesis. Blood 105(4):1734–1741. 10.1182/blood-2004-05-2042 [DOI] [PubMed] [Google Scholar]
- Yu J, Wang Y, Dong R, Huang X, Ding S, Qiu H (2012) Circulating microRNA-218 was reduced in cervical cancer and correlated with tumor invasion. J Cancer Res Clin Oncol 138(4):671–674. 10.1007/s00432-012-1147-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaborowski MP, Balaj L, Breakefield XO, Lai CP (2015) Extracellular vesicles: composition, biological relevance, and methods of study. Bioscience 65(8):783–797. 10.1093/biosci/biv084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Liu SC, Luo XH, Tao GX, Guan M, Yuan H, Hu DK (2016) Exosomal long noncoding RNAs are differentially expressed in the cervicovaginal lavage samples of cervical cancer patients. J Clin Lab Anal 30(6):1116–1121. 10.1002/jcla.21990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Liu Y, Liu H, Tang WH (2019) Exosomes: biogenesis, biologic function and clinical potential. Cell Biosci. 10.1186/s13578-019-0282-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Lu J, Liu J, Zhang G, Lu A (2020) Advances in the discovery of exosome inhibitors in cancer. J Enzyme Inhib Med Chem 35(1):1322–1330. 10.1080/14756366.2020.1754814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R, Zou Y, Luo J (2022) Application of extracellular vesicles in gynecologic cancer treatment. Bioengineering (Basel). 10.3390/bioengineering9120740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng T, Wang J, Chen X, Liu L (2010) Role of microRNA in anticancer drug resistance. Int J Cancer 126(1):2–10. 10.1002/ijc.24782 [DOI] [PubMed] [Google Scholar]
- Zheng F, Zhang J, Luo S, Yi J, Wang P, Zheng Q, Wen Y (2016) miR-143 is associated with proliferation and apoptosis involving ERK5 in HeLa cells. Oncol Lett 12(4):3021–3027. 10.3892/ol.2016.5016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng M, Hou L, Ma Y, Zhou L, Wang F, Cheng B, Wang W, Lu B, Liu P, Lu W, Lu Y (2019) Exosomal let-7d-3p and miR-30d-5p as diagnostic biomarkers for non-invasive screening of cervical cancer and its precursors. Mol Cancer 18(1):76. 10.1186/s12943-019-0999-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zocco D, Ferruzzi P, Cappello F, Kuo WP, Fais S (2014) Extracellular vesicles as shuttles of tumor biomarkers and anti-tumor drugs. Front Oncol 4:267. 10.3389/fonc.2014.00267 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The corresponding author can provide the datasets used and/or analyzed during the current study upon reasonable request.