Abstract
In maize (Zea mays) and other grasses, changes in orientation of stems are perceived by pulvinal tissue, which responds to the stimulus by differential growth resulting in upward bending of the stem. The amyloplast-containing bundle sheath cells are the sites of gravity perception, although the initial steps of gravity perception and transmission remain unclear. In columella cells of Arabidopsis roots, we previously found that cytoplasmic pH (pHc) is a mediator in early gravitropic signaling (A.C. Scott, N.S. Allen [1999] Plant Physiol 121: 1291–1298). The question arises whether pHc has a more general role in signaling gravity vector changes. Using confocal ratiometric imaging and the fluorescent pH indicator carboxy seminaphtorhodafluor acetoxymethyl ester acetate, we measured pHc in the cells composing the maize pulvinus. When stem slices were gravistimulated and imaged on a horizontally mounted confocal microscope, pHc changes were only apparent within the bundle sheath cells, and not in the parenchyma cells. After turning, cytoplasmic acidification was observed at the sides of the cells, whereas the cytoplasm at the base of the cells where plastids slowly accumulated became more basic. These changes were most apparent in cells exhibiting net amyloplast sedimentation. Parenchyma cells and isolated bundle sheath cells did not show any gravity-induced pHc changes although all cell types responded to external stimuli in the predicted way: Propionic acid and auxin treatments induced acidification, whereas raising the external pH caused alkalinization. The results suggest that pHc has an important role in the early signaling pathways of maize stem gravitropism.
The vector of the gravitational force is one of the main cues that determines the spatial orientation of plant organs (Masson, 1995; Tasaka et al., 1999; Kiss, 2000). A plant's ability to respond to the direction of gravity, the process of gravitropism, ensures the correct positioning of the seedling after germination and also enables mature plants to correct their position after a forced reorientation, e.g. by strong wind. In most cases, gravity perception occurs in specialized cells that contain dense particles, such as starch-filled plastids (amyloplasts; Sack, 1997; Tasaka et al., 1999). These shift their location within the cell when the normal plant orientation gets disturbed, thereby generating a cellular signal that sets up a chemical gradient between top and bottom of the plant organ resulting in differential growth.
Whereas the gravity-induced growth response in plants is well documented, little is known about the mechanism of gravity perception and the nature of the early steps of the signaling cascade. Research has focused on gravity perception in roots that occurs in amyloplast-containing root cap cells. Some of the earliest measurable responses induced by gravistimulation occur in and around these cells, and include complex changes in cytosolic and apoplastic pH (Scott and Allen, 1999; Fasano et al., 2001), changes in plasma membrane potential (Sievers et al., 1995), and the induction of ion flux changes around the root cap (Behrens et al., 1985; Björkman and Leopold, 1987). It is intriguing that in Arabidopsis, cytoplasmic pH (pHc) changes occur only in the inner collumella cells of the root cap (Scott and Allen, 1999; Fasano et al., 2001), which are the most competent in gravity perception (Blancaflor et al., 1998). Furthermore, artificial modification of pHc alters the gravitropic response (Scott and Allen, 1999; Fasano et al., 2001). This suggests that pHc changes play a key role in gravity-induced signaling.
Induced changes in pHc occur in response to a wide array of stimuli, apart from gravitropism, including phytohormones (Felle, 1988a; Gehring et al., 1990a, 1994; Beffagna et al., 1994), light (Felle and Bertl, 1986; Okazaki et al., 1994), Nod factors (Allen et al., 1994; Felle et al., 1996), and other elicitors (Mathieu et al., 1996). Furthermore, protons are implicated as a mediator in plant signal transduction (Felle, 1989; Guern et al., 1992; Roos et al., 1998; Zhou et al., 2000). Whether the gravity-induced pHc changes measured in roots have a general role in gravity perception in plants is still unknown because pHc changes in shoots have only been linked to the later phases of the gravitropic response (Gehring et al., 1990b). The objective of this study was to elucidate whether gravity-induced changes in pHc occur in shoot tissues that are specialized in gravity perception such as the maize (Zea mays) pulvinus.
The stem pulvinus of maize is a disc-shaped tissue that is located above each stem node and has a specific role in both gravity perception and the bending response (Collings et al., 1998; for review, see Kaufman et al., 1987, 1995). When the stem is placed horizontally, amyloplast sedimentation occurs in bundle sheath cells, and a growth response follows characterized by cell elongation specifically within the pulvinal cells, causing the stem to bend upwards. Pulvinal cells retain the capacity to elongate in the presence of an appropriate stimulus even after the surrounding tissue has fully differentiated, allowing the normal growth responses to be spatially and temporally separated from those induced by changes in the gravity vector (Collings et al., 1998). These properties make the maize pulvinus an ideal system to investigate biochemical (Winter et al., 1997; Perera et al., 1999), structural, and physiological changes at the cellular level (Collings et al., 1998) during gravitropism.
In this study, we focused on early gravity-induced responses and monitored pHc changes in pulvinal cell regions after rotation on a horizontally mounted confocal microscope using the ratiometric pH indicator carboxy seminaphtorhodafluor acetoxymethyl ester acetate (SNARF-1 AM). We compared the responses of amyloplast-containing bundle sheath cells with those of parenchyma cells to find out whether pHc changes similar to those found in Arabidopsis root cells occur and whether they are associated with the cells that perceive the gravity stimulus. Furthermore, we dissected the pHc responses of base and side regions (relative to the gravity vector) of the stimulated cells and compared cells that exhibited amyloplast sedimentation with those that did not. That pHc changes were most pronounced in cells in which there was net amyloplast sedimentation, with these changes confined to specific sites within these cells, is discussed in light of current models for the mechanism of gravity perception and the rising importance of pHc as a messenger in cellular signaling.
RESULTS
Specimen Preparation and Dye Loading
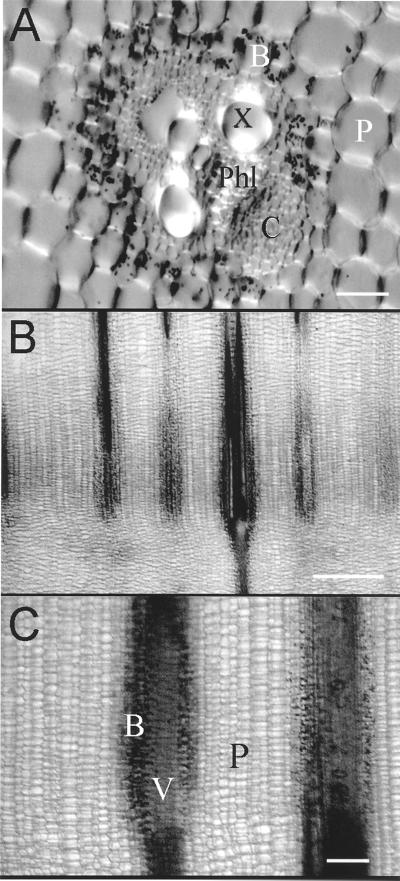
The pulvinus is a region of cells found between the differentiated node and elongated cells of the internode. The unelongated pulvinal cells surrounding the vascular strands are of two types. Several layers of bundle sheath cells occur immediately adjacent to the vascular tissue. Potassium iodide staining confirms that these cells contain starch-filled amyloplasts, and that these plastids are effectively confined to the pulvinus (Fig. 1, A–C). Surrounding the bundle sheath are numerous files of ground parenchyma.
Figure 1.
Cellular organization of the pulvinus. The image in A was taken of a hand cross section on an upright microscope (Zeiss, Thornwood, NJ) equipped with a CCD camera (Hamamatsu, Bridgewater, NJ) and the Image 1 software system (Universal Imaging, West Chester, PA), whereas the images shown in B and C were taken of longitudinal hand sections using a dissecting microscope (Leica, Wetzlar, Germany) and a Hamamatsu color CCD camera. Bars in A through C represent 50, 1,000, and 200 μm, respectively. B, Bundle sheath cells with amyloplasts (stained with 0.2% [w/v] iodine in 5% [w/v] KI). C, Collenchyma, P, parenchyma; Phl, phloem; V, vascular bundle; X, xylem.
To measure cytosolic pH (pHc) in maize pulvinal cells, both before and after gravistimulation, we investigated several specimen preparation protocols. Two methods proved satisfactory for imaging on a sideways-mounted microscope. In the first method, mild enzymatic digestion of cell wall material resulted in the isolation of long files of healthy cells that showed vigorous cytoplasmic streaming. These files could either be parenchymal cells or amyloplast-containing bundle sheath cells. When files of bundle sheath cells were incubated in a vertical position for 30 min and then gravistimulated, net amyloplast sedimentation occurred over the next several minutes (Fig. 2, A–C; for video sequence, see www.plantphysiol.org). In these cells, the average rate of plastid sedimentation was visibly slower than the streaming velocities for individual amyloplasts. However, amyloplast sedimentation in files of bundle sheath cells, as shown in Figure 2, A through C, was a relatively rare event, seen in only two out of 15 independent rotations. The second specimen preparation method involved taking 0.3- to 0.5-mm thick longitudinal sections through the pulvinus. Cells in these preparation also showed vigorous cytoplasmic streaming, but it is significant that sections were more likely to show amyloplast sedimentation following gravistimulation (Fig. 2, D–F; for video sequence, see www.plantphysiol.org), with this being seen in 67% of sections (n = 12).
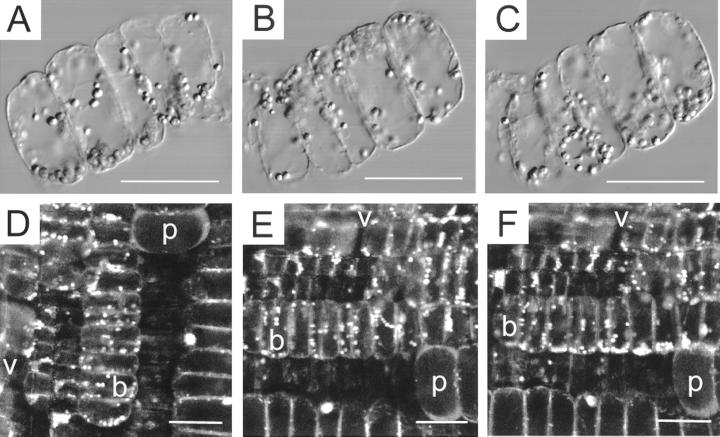
Figure 2.
Dynamics of amyloplast sedimentation. A through C, Amyloplast sedimentation in isolated files of bundle sheath cells. Amyloplasts follow the tracks of intracellular particle movement as well as the path predicted by physical parameters such as plastid density and cytosolic viscosity leading to net sedimentation of plastids to the new bottom of the cell. A, Thirty seconds before rotation of cell files through 180°. QuickTime movie located at www.plantphysiol.org. B, Thirty seconds after rotation. C, Seven minutes after rotation. D through E, Plastid sedimentation in bundle sheath cells of maize. Ratio images E2 (620–670 nm)/E1 (550–600 nm) of SNARF-1 AM-loaded longitudinal pulvinal sections, excitation 514 nm. D, Before rotation; E, 2 min after rotation by 90°. QuickTime movie located at www.plantphysiol.org. F, 12 Minutes after rotation, bar = 50 μm; v, vascular tissue; b, bundle sheath cells; p, parenchyma cells.
To measure pHc, both before and after gravistimulation, we also investigated numerous ratiometric pH indicators. The most promising dye was carboxy seminaphtorhodafluor (SNARF-1) because of a range of technical issues, and because when excited at 514 nm, pulvinal cells showed little autofluorescence at the emission peaks of SNARF's acidic and basic forms, recorded at 550 to 600 and 620 to 670 nm, respectively. After 1 h of incubation in the membrane permeant AM ester form of SNARF (10 μm), dye loaded predominantly into the cytoplasm and was largely excluded from the vacuole. However, SNARF fluorescence was also observed within amyloplasts. SNARF loaded rapidly into isolated file cells, whereas in tissue sections the dye loaded first into the vascular tissue then proceeded into bundle sheath cells and finally was found in the parenchyma. Attempts to load SNARF via the vascular bundles into excised pulvini without prior sectioning failed.
pHc Measurements in Maize Pulvinal Cells: Effects of Weak Acids, External pH Changes, and Indole Acetic Acid (IAA)
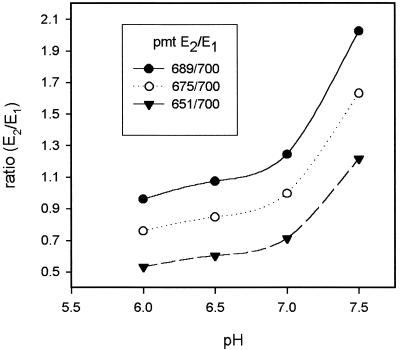
To determine the reliability of our confocal ratiometric imaging system, we measured pHc with SNARF-1 in non-gravistimulated file cells and tissue slices, observing both bundle sheath and parenchyma cells, and determined whether these cells responded to known stimuli in the normal way. Ratios of the two emission intensities (620–670 nm/550–600 nm) were generally stable in non-stimulated cells for the duration of experiments. In vitro calibrations with dextran-linked SNARF (10 kD) were carried out after each experiment. In most cases, these in vitro calibrations gave calculated pHc values of about pH 6.6 (or lower) that were more acidic than the pHc values reported in earlier studies (Smith and Raven, 1979; Kurkdjian and Guern, 1989; Guern et al., 1991, 1992). However, the cells appeared healthy, showing normal intracellular particle movement, and could react to weak acid and other treatments with similar ratio shifts as those cells whose calibrations reported a resting pHc around 7. Thus, results are presented in this paper as changes in ratio rather than changes in pHc, with an increase in ratio reflecting alkalinization and a decrease in ratio reflecting acidification. Adjustments in the photomultiplier settings for the two emission windows led mainly to a parallel ratio shift in the calibration curve and only had a small effect on the slope of the pH dependence (Fig. 3). Therefore, the change in ratio obtained from data sets with different starting ratios can be compared even though the pH dependence is not linear. It should, however, be noted that the bulk of the data were obtained within a more narrow range of photomultiplier adjustments than those depicted in Figure 3.
Figure 3.
In vitro calibrations with 50 μm SNARF-1 dextran (10 kD): pH dependence of emission intensity ratio E2 (620–670 nm)/E1 (550–600 nm) for different photomultiplier settings. An increase in the photomultiplier setting for E2 leads to an approximately parallel shift in the ratio values with only a slight increase in slope.
In vivo calibrations with nigericin in the presence of high K+ (Vercesi et al., 1994) are not reported in this paper because such experiments did not lead to sustained ratio changes and required complex solution changing that could not be performed for the majority of measurements that were made with a sideways-mounted microscope.
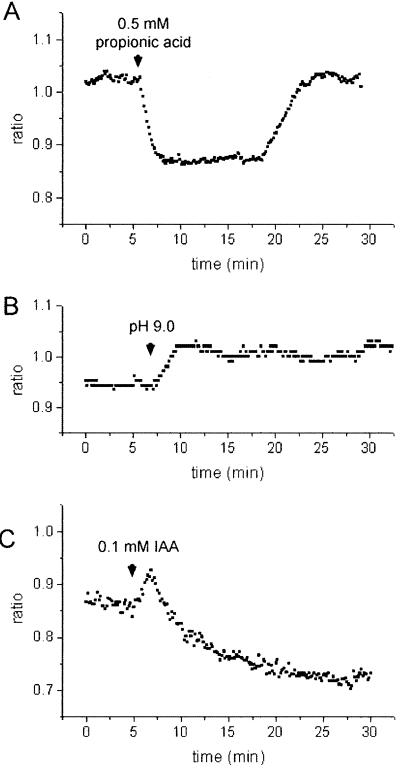
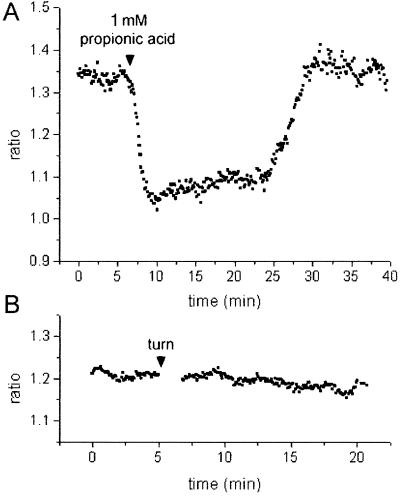
To test whether SNARF-1 AM can be used reliably to monitor pHc changes in maize pulvinal cells, a range of conditions were applied that are known to increase or decrease pHc in a large variety of plant cells. When subjected to 0.5 mm propionic acid applied at an external pH of 5.5, bundle sheath cells from tissue sections responded with a drop in ratio by Δr = −0.171 ± 0.009 (n = 16; acidification, Fig. 4A). Streaming remained visibly unaffected in these cells. After removal of the weak acid, the ratio returned to the resting value, and occasionally showed a transient overshoot to more positive values (alkalinization). The kinetics with which these ratio changes occurred are comparable to the weak acid-induced pHc changes measured with ion-selective microelectrodes (Felle, 1987; Frachisse et al., 1988) and 31P NMR spectroscopy (Guern et al., 1986) in a variety of other plant cells. Conditions that evoke alkalinizations were also tested. It is known from previous reports that changes in external pH lead to corresponding changes in pHc by about 0.1 pHc unit per pH unit change in external pH (for review, see Smith and Raven, 1979; Felle, 1988b). Figure 4B shows the response of pulvinal bundle sheath cells to a change in external pH from 5.5 to 9.0 that caused a rise in the ratio (alkalinization) by Δr = 0.077 ± 0.008 (n = 18).
Figure 4.
Ratio changes E2 (620–670 nm)/E1 (550–600 nm) in maize pulvinal cells from tissue slices following: A, application of 0.5 mm propionic acid; B, a change in external pH from pH 5.5 to 9.0; and C, addition of 0.1 mm IAA. Representative measurements are shown and statistics are given in the text. Substances were applied in buffer C (pH 5.5). Increase in ratio reflects alkalinization, decrease in ratio reflects acidification.
The phytohormone auxin (e.g. IAA), which plays an important role in the gravity-induced differential growth response (Evans, 1991; Estelle, 1996), also evoked ratio changes in maize pulvinal cells (Fig. 4C). Application of 0.1 mm IAA evoked a transient rise in the ratio (alkalinization) by Δr = 0.027 ± 0.004 (n = 12), which was apparent in 72% of analyzed recordings. This was followed by a drop in Δr (acidification) by −0.175 ± 0.015 (n = 18). IAA concentrations of 10 μm IAA caused a rise in Δr (alkalinization) by 0.040 ± 0.002 (n = 3, one data set), which returned to the resting level within about 10 min (data not shown). Although the acidification evoked by higher IAA concentrations might in part be attributable to a weak acid effect, the initial alkalinization could reflect a change in membrane transport activity. Previous studies concerning pHc changes in maize coleoptiles induced by auxins (1 μm) showed either an oscillatory pattern of pHc changes (Felle, 1988a) or a sustained acidification by 0.1 to 0.2 pH units (Gehring et al., 1990a).
Although the data shown in Figure 4 relate to inducible pHc changes in bundle sheath cells from tissue slices, we observed similar changes in the emission ratio in isolated files of bundle sheath cells (Fig. 5A), and in parenchyma cells in both slices and isolated files (data not shown).
Figure 5.
Ratio changes E2 (620–670 nm)/E1 (550–600 nm) in isolated pulvinal cell files following: A, application of 1 mm propionic acid; and B, after rotation by 90°. Representative measurements are shown. Substances were applied in buffer B (pH 5.5). The average ratio change was: A, −0.214 ± 0.019 (n = 10) in response to propionic acid; and B, 0.003 ± 0.005 (n = 10) after rotation.
Gravistimulation Experiments Demonstrate pHc Changes in Bundle Sheath Cells
Protocols for isolation of bundle sheath cell files were developed because these cells provide a much more favorable system for high resolution imaging than pulvinal slices. Although it was possible to measure pHc changes in this system in response to propionic acid (Fig. 5A), gravistimulation never led to any visible change in the ratio (Fig. 5B). Because plastid sedimentation was only rarely retained in isolated cell files, it might be argued that the lengthy isolation and dye loading procedure, the potential for cytoskeletal reorganization, or the loss of cell wall material and cell positioning information, led to disruption in cell function that might have affected early steps in the signaling cascade.
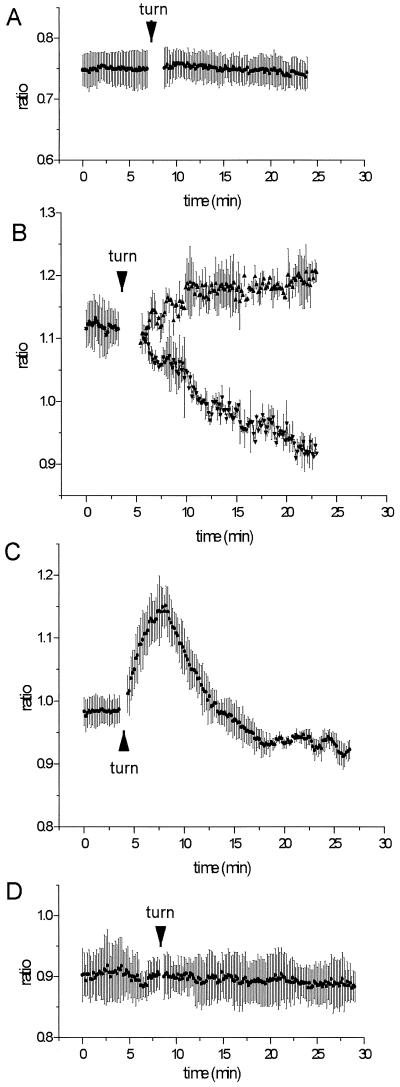
In longitudinal stem sections that were loaded with SNARF-1 AM, rotation by 90° resulted in significant ratio changes, indicative of pHc changes, in cells that contained sedimentable amyloplasts, as depicted in Figure 6, B and C, and summarized in Table I. Parenchyma cells that lack amyloplasts provided an ideal control for these experiments because they did not exhibit any notable change in the emission ratio after turning (Fig. 6A) either at the base of the cell or at the side of the cell (Table I). In bundle sheath cells that showed amyloplast sedimentation, cytoplasmic regions at the sides of the cells often responded with a drop in ratio (acidification), whereas regions at the base of cells showed a rise in ratio (alkalinization; Figs. 6B and 7). When cytoplasmic areas of whole cells were measured, the acidification often prevailed. In about 12% of cases, a transient alkalinization was followed by an acidification at the sides of cells but not at the base (Fig. 6C). Rotation by 360° (no gravistimulation) did not elicit any significant changes in the pHc of bundle sheath cells, suggesting that touch responses that might occur during rotation do not contribute to the ratio change (Fig. 6D).
Figure 6.
Ratio changes E2 (620–670 nm)/E1 (550–600 nm) in maize pulvinal cells from tissue slices before and after rotation by 90° (A–C) and 360° (D). A, Response of parenchyma cells (no amyloplasts); B, typical response of bundle sheath cells with amyloplast sedimentation base region of cells (▴) and side regions of cells (▾); C, transient response measured in side regions of bundle sheath cells showing amyloplast sedimentation, observed in 12% of recordings; D, response of bundle sheath cells to a full 360° rotation. Average values ± ses of representative data sets are shown and statistics are given in Table I.
Table I.
pH ratio changes in stem slices following gravistimulation
| Cell Type | Region | Cells | Average Rate Δr ± se | Significance Levels for Comparisons
between Different Changes in Ratioa
|
|||||
|---|---|---|---|---|---|---|---|---|---|
| Compared with parenchyma
|
Compared with
bundle sheath
|
Compared with bundle sheath, no amyloplast
sedimentation
|
|||||||
| Base | Side | Base | Side | Base | Side | ||||
| min−1b | |||||||||
| Parenchyma | Base | 25 | −0.0004 ± 0.0003 | – | t = −0.845P = 0.4022 | t = 2.3691P = 0.0208 | nd | t = −1.442P = 0.1610 | nd |
| Side | 27 | −0.0007 ± 0.0003 | t = −0.845P = 0.4022 | – | nd | t = −5.0049P = 1.9 10−6 | nd | t = −5.3568P = 1.3 10−6 | |
| Bundle sheath | Base | 42 | 0.0020 ± 0.0002 | t = 2.3691P = 0.0208 | nd | – | t = −8.2333P = 1.4 10−13 | t = −2.9415P = 0.0045 | nd |
| Side | 95 | −0.0049 ± 0.0004 | nd | t = −5.0049P = 1.9 10−6 | t = −8.2333P = 1.4 10−13 | – | nd | t = 1.6647P = 0.0984 | |
| Bundle sheath, no amyloplast sedimentation | Base | 24 | −0.0012 ± 0.0005 | t = −1.442P = 0.1610 | nd | t = −2.9415P = 0.0045 | nd | – | t = −3.6191P = 6.1 10−4 |
| Side | 37 | −0.0036 ± 0.0004 | nd | t = −5.3568P = 1.3 10−6 | nd | t = 1.6647P = 0.0984 | t = −3.6191P = 6.1 10−4 | – | |
| Bundle sheath turned 360° | Base | 21 | −0.0005 ± 0.0005 | t = −0.2289P = 0.8200 | nd | t = 2.2138P = 0.0306 | nd | t = −0.9603P = 0.3423 | nd |
| Side | 31 | 0.0005 ± 0.0004 | nd | t = 2.4002P = 0.0197 | nd | t = −6.6400P = 8.8 10−10 | t = −6.7702P = 4.1 10−9 | ||
t, Student's t distribution; P, significance level; nd, not determined as invalid comparison.
Student's t tests for two independent populations were performed to compare the responses. Significant values at P < 0.05 are shown in bold.
Emission ratio changes in response to rotation by 90° (or 360° as indicated) measured in cytoplasmic regions at different locations within cells, presented as the rate of change in the ratio per minute. Negative values indicate acidification; positive values indicate alkalinization.
Figure 7.
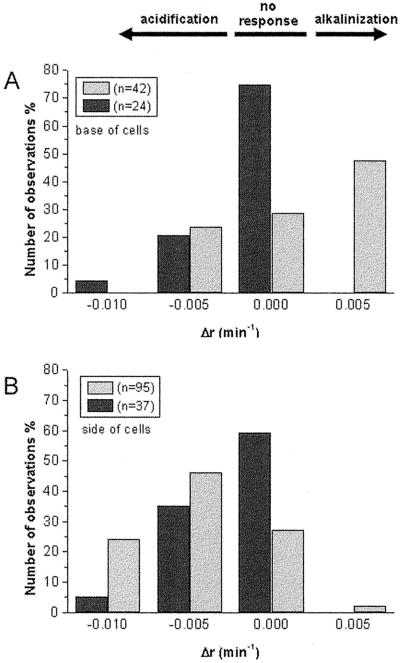
Histograms summarizing data represented in Figure 6B. The rate of ratio change after rotation was determined over a 15- to 20-min period using linear regression and least-square fit. Data obtained from bundle sheath cells showing amyloplast sedimentation (light shaded bars) were compared with those in which no amyloplast sedimentation occurred (dark shaded bars). A, Ratio changes in base regions; B, ratio changes in side regions. The maximum rates of ratio changes observed in data sets obtained from parenchyma cells (exemplified in Fig. 6A) fell within the range of Δr = ±0.0025 min−1 and were designated as no response. The bin width was Δr = 0.005 min−1; however, rates of acidification larger than Δr = −0.0125 min−1 were included in the left column. Statistics are given in Table I.
In contrast to these observations of changes in pHc in bundle sheath cells that showed amyloplast sedimentation, bundle sheath cells that failed to show amyloplast sedimentation within 30 min after rotation lacked pronounced changes in pHc. The results are summarized in the histograms presented in Figure 7 and the statistics in Table I. In bundle sheath cells not exhibiting plastid sedimentation, a higher percentage of cells showed no pHc response compared with cells that showed sedimentation; furthermore, alkalinization did not occur at the base of cells. The magnitude and rate of acidification measured in side regions was also markedly lower than that measured in cells with sedimenting plastids (at the 90% confidence interval; Fig. 7B, Table I).
In Table I, the rate of ratio change after rotation was compared statistically (Student's t test for two independent samples) for data sets obtained from side and base regions of parenchyma cells, and bundle sheath cells with and without plastid sedimentation. Bundle sheath cells showed a significantly different response than parenchyma cells (control), except for base regions of bundle sheath cells not exhibiting plastid sedimentation, which responded in a manner similar to the control. When cells with and without plastid sedimentation were compared the responses measured in the base regions were significantly different (95% confidence interval) between the two data sets, whereas those measured at the sides of the cells were different at the 90% confidence interval. Bundle sheath cells that were rotated 360° did not exhibit significant ratio changes (Fig. 6D) and their response was significantly different from bundle sheath cells that were rotated 90°.
DISCUSSION
pHc Measurements in Pulvinal Cells with SNARF-1 AM
Lipophilic derivatives of ion sensitive fluorescent dyes such as SNARF-1 AM can permeate intracellular organelles and cause errors in the determination of cytoplasmic ion concentrations (Fricker et al., 1993; Williams et al., 1993; Roos, 2000). In pulvinal bundle sheath cells, dye accumulation into the central vacuole is minimal during the first 60 to 90 min following SNARF-1 AM incubation. However, a large amount of dye accumulates in amyloplasts, an observation previously noted in barley (Hordeum vulgare) pulvinal cells where amyloplasts accumulate fluorescence following esterase cleavage of fluorescein diacetate (Dayanandan et al., 1982). The result of this dye accumulation is frequent signal saturation from amyloplasts under the conditions used for our experiments. To minimize errors in the pHc measurements arising from SNARF-1 AM-loaded amyloplasts, we used the following precautions. If possible, cytoplasmic regions free of amyloplasts were selected for measurement, and a thresholding method was used to determine the emission ratio eliminating most of the amyloplast-derived signal.
Quantification of Changes in the Emission Ratio
Because in vitro calibrations did not report reliable pHc values, it was difficult to quantify the observed ratio changes. However, taking into account measurements that reported normal pHc values when compared with in vitro calibration, and by comparing our results obtained with weak acids and external pH changes with those obtained by other investigators, we can estimate the range within which the gravity-induced pHc changes might fall. A lipohilic weak acid, propionic acid, was used to induce a cytosolic acidification. In the absence of a transporter, lipophilic weak acids enter the cell solely in their undissociated form, and it is this concentration that determines the magnitude of the pH change in a particular system (Frachisse et al., 1988; Felle and Johannes, 1991). The responses to lipophilic weak acids show variability among different plant species that could be due to variation in the buffer capacity of the cytoplasm and the ability of the plant to regulate its pHc (Smith and Raven, 1979; Kurkdjian and Guern, 1989; Guern et al., 1991, 1992).
Bundle sheath cells from tissue slices responded to 0.5 mm propionic acid (0.1 mm undissociated acid) with a drop in ratio (acidification) by Δr = −0.171 ± 0.009 (n = 16; Fig. 4A). Compared with in vitro calibrations that showed reliable pHc values, these changes fell within a range of 0.4 to 0.5 pH units and were similar to those measured in Riccia fluitans after addition of the same concentration of weak acids (Frachisse et al., 1988). Changes in external pH are known to evoke corresponding changes in pHc by about 0.1 unit per 1 unit change in extracellular pH in a large variety of plants (for review, see Felle, 1988b). In pulvinal bundle sheath cells, a change in external pH from 5.5 to 9 evoked a rise in the emission ratio by Δr = 0.077 ± 0.035 (Fig. 4B) that reflects an alkalinization by approximately 0.35 pH unit.
Amyloplast Redistribution following Gravistimulation Induces pHc Changes
We argue that the observed changes in emission ratio seen following gravistimulation indicate that pHc changes are a genuine consequence of amyloplast relocation following gravistimulation. Although measurement errors cannot be excluded, we base this contention on the following observations. Bundle sheath and parenchyma cells in both tissue slices and isolated files respond similarly to propionic acid and IAA, yet ratio changes following turning were only seen in bundle sheath cells in tissue slices. Furthermore, gravity-induced changes strongly correlated with amyloplast sedimentation. In 25% of bundle sheath cells exhibiting amyloplast sedimentation, no change in ratio was observed although other cells within the same preparation did show a response. This indicates that sedimentation per se does not change the emission ratio. In cells that did respond to gravistimulation, the ratio change persisted over the measuring period after most plastids had settled to the bottom of the cell. Again, this supports our view that the measured ratio changes reflect pHc changes induced by gravity.
Gravity-Induced pHc Changes: An Early Step in Gravity Perception?
The present investigation shows that gravity induces pHc changes in the amyloplast-containing bundle sheath cells of the maize pulvinus, indicating that pHc changes might have a generally important role in early gravity-induced signaling. Average ratio changes evoked in bundle sheath cells (with plastid sedimentation) within 30 min following rotation were about Δr = 0.06 (alkalinization) in bottom regions and Δr = −0.15 (acidification) in side regions (see Table I and Fig. 6B). Taking into account the considerations discussed in the previous section, the gravity-induced ratio changes are likely to reflect pHc changes in the range of 0.3 to 0.5 pH units. These changes are similar to stimulus-induced pHc changes reported in other plant systems where changes in pHc have a wide array of physiological effects in plant cells and can act as mediators in many signal transduction pathways (Felle, 1989; Guern et al., 1992; Putnam, 1998). Changes in pHc affect proton translocation by pumps and carriers (Felle and Johannes, 1991; Portillo, 2000), and can modulate the activity of various anion (Schulz-Lessdorf et al., 1996; Johannes et al., 1998) and cation (Grabov and Blatt, 1997; Lacombe et al., 2000) channels.
The sequence of signaling events that follow gravistimulation, and the involvement of pHc, remain largely unknown. However, in root gravitropism, many of the earliest measurable gravity-induced effects involve ionic fluxes in and around the root cap. These include membrane potential and ion flux measurements that showed gravity-induced hyperpolarizations and proton fluxes indicative of stimulation of the plasma membrane H+ ATPase (Behrens et al., 1985; Björkman and Leopold, 1987; Sievers et al., 1995). Fasano et al. (2001) also observed a uniform acidification of the Arabidopsis root cap apoplast from pH 5.5 to 4.5 within 2 min of gravistimulation. However, because the plasma membrane H+ pump can effect pHc changes as well as respond to them, it is difficult to tell which of these two early responses might come first.
Early Events in Gravisignaling and the Role of pHc
Three investigations have now shown that pHc changes are a key player in early gravity-induced signaling in root caps. First, Scott and Allen (1999) showed that microinjection of the pH-sensitive dye 1′,7′-bis-(2-carboxymethyl)-5-(and-6)-carboxyfluorescein into gravity-perceiving root cap cells of Arabidopsis revealed a rapid alkalinization in tier 2 cells and a slower acidification in tier 3 cells (Scott and Allen, 1999), the sites with the greatest competence for gravity perception (Blancaflor et al., 1998). When pHc changes were induced solely within the root cap by various pHc modifiers, the gravitropic response was also altered, with acidification of the columella causing enhanced bending, whereas alkalinization resulted in inhibition of bending. These observations were expanded by Fasano et al. (2001) who demonstrated, using both dye 1′,7′-bis-(2-carboxymethyl)-5-(and-6)-carboxyfluorescein-dextran microinjection and the constitutive expression of a pH-sensitive green fluorescent protein, that rapid alkalinization occurred in the Arabidopsis columella following gravistimulation. Furthermore, in a starchless Arabidopsis mutant, where plastid sedimentation and bending were reduced, pHc changes were also markedly smaller (Fasano et al., 2001). Taken together with our observations in the pulvinus, these results indicate that pHc changes might have a generally important role in early gravity-induced signaling.
Concurrent with rapid pHc changes in the pulvinus, gravity also induces rapid changes in the inositol phosphate metabolism in pulvini of maize and oats (Avena sativa) beginning within minutes of gravistimulation and resulting in oscillations of phosphatidylinositol 4,5-bisphosphate and inositol 1,4,5-trisphosphate (IP3; Perera et al., 1999, 2001). Increases in the second messenger IP3 are known to open Ca2+ channels in the vacuolar membrane of plant cells (Alexandre et al., 1990; Allen et al., 1995), and suggest a role of cytosolic free Ca2+ ([Ca2+]c) in the gravity-induced signaling cascade. Although direct measurements of [Ca2+]c in gravity-perceiving cells in the root cap of Arabidopsis failed to reveal such changes in response to gravistimulation (Legué et al., 1997), a role for [Ca2+]c has long been implicated by indirect experimental evidence (for review, see Chen et al., 1999). It is conceivable that [Ca2+]c changes occur and act locally in cellular microdomains and that high resolution imaging techniques in conjunction with more sensitive Ca2+ dyes are required to make them detectable.
However, several significant questions remain unclear. First, how does amyloplast sedimentation cause changes in pHc, phophatidylinositol 4,5-bisphosphate/IP3 levels, and possibly also [Ca2+]c, and how are these changes linked? Second, how is this signal integrated from the cellular to the tissue level, such that pHc changes visible in the majority of cells with amyloplast sedimentation can generate a chemical gradient between the top and bottom of the plant organ that results in differential growth and the bending response? And third, how does auxin, thought to be a key regulator of the bending response (Evans, 1991; Estelle, 1996), fit into this system? One possible integrating system that could satisfy all these questions would be the actin cytoskeleton.
Although subject to some debate (for review, see Baluška and Hasenstein, 1997), it has now been shown that the amyloplasts in the columella cells of the root cap from various plant species are surrounded by endoplasmic reticulum and actin filaments (Collings et al., 2001). In a similar manner, the dynamics of amyloplast streaming in the maize pulvinus (this study), and amyloplast motility in other gravitropic tissues (Sack and Leopold, 1985), indicate extensive interactions between amyloplasts and the actin cytoskeleton. Thus, it is possible that while moving to the new cell bottom, amyloplasts might interact with actin and/or membranes, thereby eliciting pHc changes in cellular microdomains. The actin cytoskeleton has several features that make it suitable for regulating gravitropic signaling and response generation. Actin-binding proteins that regulate the equilibrium between F actin and G actin, such as actin-depolymerizing factor, are modulated by pHc with depolymerization occurring at higher pHc values (Gungabissoon et al., 1998; Kovar and Staiger, 2000). Various actin-binding proteins, including actin-depolymerizing factor and profilin (Gibbon and Staiger, 2000; Kovar and Staiger, 2000), also interact extensively with signaling by phospholipids. Furthermore, actin modulates auxin transport through its regulation of the auxin efflux carrier (Muday, 2000).
The current state of knowledge suggests that changes in membrane transport (Behrens et al., 1985; Björkman and Leopold, 1987; Sievers and Busch, 1992; Sievers et al., 1995), actin dynamics (Collings et al., 2001), and phophoinositol metabolism (Perera et al., 1999, 2001) are early responses to a change in the gravity vector. It is likely that the observed pHc changes have an integral role in the gravity-induced signaling cascade because pHc alone or in conjunction with [Ca2+]c modulates the activity of membrane transport proteins (Felle and Johannes, 1991; Schulz-Lessdorf et al., 1996; Grabov and Blatt, 1997; Johannes et al., 1998; Lacombe et al., 2000; Portillo, 2000), cytoskeletal polymerization (Gungabissoon et al., 1998; Kovar and Staiger, 2000), organization of the endoplasmic reticulum (Quader and Fast, 1990), and enzymatic reactions (Putnam, 1998; Paterson and Nimmo, 2000), all of which are likely to play a role in the initial steps of gravity-induced signal transduction. It will be a challenge for future investigators to elucidate how the gravity signal is integrated and to dissect the sequence of events leading from gravity perception to the gravitropic response. Progress in this field will be aided by the availability of mutants impaired in perception or transduction of the gravity signal (Sedbrook et al., 1999; Firn et al., 2000) and through the use of more refined techniques for visualization of ionic changes in cellular microdomains using pH-sensitive green fluorescent protein constructs that can be targeted to specific cellular compartments (Miesenböck et al., 1998).
MATERIALS AND METHODS
Plant Material
Maize (Zea mays) plants (line 3183; Pioneer, Des Moines, IA) were grown in greenhouses (22°C –27°C), three plants per 22-cm pot. Pulvini 2 and 3 (the second and third pulvinus from the base of the stem) from 5- to 6-week-old plants were used for all experiments. These pulvini exhibit the strongest response to gravitistimulation, and lie adjacent to internodes that have ceased elongation (Collings et al., 1998).
Isolation of Stem Slices
Longitudinal hand sections, approximately 0.2 to 0.5 mm thick, were made with a razor blade through the pulvinal regions of pulvini 2 and 3. Replicate sections were stained to reveal and confirm the location of starch-containing amyloplasts with 0.2% (w/v) iodine in 5% (w/v) KI (10 min).
Isolation of Cell Files
Pulvini 2 and 3 from one or several maize plants were harvested, chopped in 1- to 2-mm3 pieces, and incubated for 40 to 50 min in cell wall-digesting enzymes (1% [w/v] Cellulase YC, 0.1% [w/v] Pectolyase Y23, 0.5% [w/v] bovine serum albumin, and 0.3 m mannitol in Murashige and Skoog medium, pH 5.3). Following enzyme digestion, the tissue was mechanically disrupted by gentle stirring and tapping at the tissue pieces with a glass rod to release files of cells. Buffer A {100 mm KCl, 0.285 m mannitol, and 10 mm PIPES [piperazine-N,N′-bis(2-ethanesulfonic acid)]/KOH, pH 6.8} was added and the suspension was allowed to sediment for 30 to 45 min at 1g (4°C), and this fast-sedimenting debris removed. Files of cells were recovered by further sedimentation of the suspension at 1g (4°C) over the next several hours. The pelleted cell files then were resuspended in buffer A to remove residual enzyme and again pelleted at 1g (4°C) for several hours. Centrifugation to accelerate the recovery of cell files was not feasible because low-speed centrifugation (<80g) ruptured the amyloplast-containing bundle sheath cells.
Ester Loading of SNARF1-AM and Mounting of Cells
Cell files were incubated for 60 min in 10 μm SNARF1-AM (0.8 mL of buffer A and 1% [v/v] dimethyl sulfoxide, 22°C). Cell files settled at the bottom of the Eppendorf tube and were resuspended in the recording buffer B {5 mm KCl, 0.475 m mannitol, 0.1 mm CaCl2, and 10 mm MES [-(N-morpholino) ethanesulfonic acid]/KOH, pH 5.5, standard recording medium for cell files) to remove external dye. After sedimentation at room temperature, cell files were embedded in a thin layer of low-melting-point agarose (1.3% [w/v] agarose VII, in buffer B, applied at about 40°C) on a prewarmed welled slide.
Freshly prepared longitudinal sections through the pulvinal region were rinsed and incubated for 60 min in 10 μm SNARF-1AM (1% [v/v] dimethyl sulfoxide, 22°C). Following dye loading they were washed three times with buffer C (0.1 mm CaSO4, 0.2 mm K2SO4, 0.1 mm NaCl, 0.5 mm MgCl2, and 10 mm MES/Tris, pH 5.5, standard recording medium for tissue sections) to remove excess dye. The slices were then embedded in a thin agarose layer (1.3% [w/v] agarose VII, in buffer C, applied at about 40°C) on a prewarmed welled slide.
For gravity experiments, welled slides were sealed with a coverslip using melted valap (vaseline:lanolin:paraffin, 1:1:1, w/w), whereas for perfusion experiments, the slide well (approximate volume 0.3 mL) was left open on two sides and had a small reservoir on each side. This allowed rapid media exchange using filter paper.
Unless otherwise stated, chemicals were obtained from Sigma Chemical Co. (St. Louis) and fluorescent dyes were obtained from Molecular Probes Inc. (Eugene, OR).
Confocal Imaging and Ratiometric pH Measurements
Experiments were conducted with a confocal microscope system (Leica SP, Leica). The ratiometric pH indicator SNARF-1 AM was excited with an argon laser at 514 nm, and fluorescence emission windows were set to 550 to 600 nm (peak of acidic form) and 620 to 670 nm (peak of basic form). These wavelengths avoided autofluorescence from the amyloplasts present within the bundle sheath cells. Concurrent differential interference contrast images were also recorded. Images were acquired with a 20× numerical aperture 0.6 dry objective at 5- to 10-s intervals for up to 40 min. Ratiometric image analysis was performed with Metafluor 4.01 software (Universal Imaging) with cytoplasmic regions marked and thresholds set at 3 and 253 (8-bit image). Ratios are given as emission intensity at 620 to 670 nm divided by emission intensity at 550 to 600 nm. In vitro calibrations were carried out with 50 μm SNARF-1 dextran (10 kD) in 100 mm KCl, 1 mm ATP, 1 mm MgCl2, and 10 mm HEPES [4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid]/Tris adjusted to pH 6.0, 6.5, 7.0, 7.5, and 8.0 (Bibikova et al., 1998).
For gravity experiments, the confocal unit was attached to an “upright” microscope (Leica model DMRX-A) that was mounted sideways in a cradle to give a horizontal light path. Specimens were mounted on a rotating stage and kept in an upright position for at least 30 min before the start of an experiment. Specimens were gravistimulated by rotating the stage, and imaged with centerable lenses adjusted to give a constant field of view as the stage rotated. Images were recorded before and after gravistimulation, but images taken during and directly after rotation were often blurred and could not be used for ratio analysis, resulting in gaps in the recording of between 25 and 180 s.
Perfusion experiments were performed in the horizontal position on an inverted microscope (Leica model DM-IRB) to facilitate solution changes.
Data Analysis
Emission ratios were routinely measured at the sides and base of the turned cells. Throughout the manuscript, the terms “side” and “base” of cells refers to their position after rotation. Average ratio values were compiled from three to nine independent data sets (if not indicated otherwise) and are presented as average values ± se (n = no. of cells or regions measured). The rate of change in the ratio after gravistimulation was determined using linear regression and a least-square fit. Data sets obtained for different cell regions/types were compared statistically using Student's t test for independent samples.
Supplementary Material
Footnotes
This work was supported by the National Aeronautics and Space Administration (NASA Specialized Center of Research and Training grant no. NAGW–4984).
The online version of this article contains Web-only data. The supplemental material is available at www.plantphysiol.org.
LITERATURE CITED
- Alexandre J, Lassalles JP, Kado RT. Opening of Ca2+ channels in isolated red beet vacuole membrane by inositol 1,4,5-trisphosphate. Nature. 1990;343:567–570. [Google Scholar]
- Allen GJ, Muir SR, Sanders D. Release of Ca2+ from individual plant vacuoles by both InsP3 and cyclic ADP-ribose. Science. 1995;268:735–735. doi: 10.1126/science.7732384. [DOI] [PubMed] [Google Scholar]
- Allen NS, Bennett MN, Cox, Shipley A, Ehrhardt DW, Long SR. Effects of nod factors on alfalfa root hair Ca++ and H+ currents and on cytoskeletal behavior. In: Daniels M, editor. Advances in Molecular Genetics. Vol. 3. Dordrecht, The Netherlands: Kluwer Academics; 1994. pp. 107–113. [Google Scholar]
- Baluška F, Hasenstein KH. Root cytoskeleton: its role in perception of and response to gravity. Planta. 1997;203:S69–S78. doi: 10.1007/pl00008117. [DOI] [PubMed] [Google Scholar]
- Beffagna N, Romani G, Gatti L. Changes in chloride fluxes and cytosolic pH induced by abscisic acid in Elodea densa leaves. Bot Acta. 1994;108:74–79. [Google Scholar]
- Behrens HM, Gradmann D, Sievers A. Membrane potential responses following gravistimulation in roots of Lepidium sativum L. Planta. 1985;163:463–472. doi: 10.1007/BF00392703. [DOI] [PubMed] [Google Scholar]
- Bibikova TN, Jacob T, Dahse I, Gilroy S. Localized changes in apoplastic and cytoplasmic pH are associated with root hair development in Arabidospis thaliana. Development. 1998;125:2925–2934. doi: 10.1242/dev.125.15.2925. [DOI] [PubMed] [Google Scholar]
- Björkman T, Leopold AC. An electric current associated with gravity sensing in maize roots. Plant Physiol. 1987;84:841–846. doi: 10.1104/pp.84.3.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blancaflor EB, Fasano JM, Gilroy S. Mapping the functional roles of cap cells in the response of Arabidopsis primary roots to gravity. Plant Physiol. 1998;116:213–222. doi: 10.1104/pp.116.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R, Rosen E, Masson PH. Update on development: gravitropism in higher plants. Plant Physiol. 1999;120:343–350. doi: 10.1104/pp.120.2.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collings DA, Winter H, Wyatt SE, Allen NS. Growth dynamics and cytoskeleton organization during stem maturation and gravity-induced stem bending in Zea mays L. Planta. 1998;207:246–258. doi: 10.1007/s004250050480. [DOI] [PubMed] [Google Scholar]
- Collings DA, Zsuppan G, Allen NS, Blancaflor EB. Demonstration of prominent actin filaments in the root columella. Planta. 2001;212:392–403. doi: 10.1007/s004250000406. [DOI] [PubMed] [Google Scholar]
- Dayanandan P, Franklin CI, Kaufman PB. Linkage between gravity perception and response in the grass leaf-sheath pulvinus. Physiologist. 1982;25:s101–s102. [Google Scholar]
- Estelle M. Plant tropisms: the ins and outs of auxin. Curr Biol. 1996;6:1589–1591. doi: 10.1016/s0960-9822(02)70780-x. [DOI] [PubMed] [Google Scholar]
- Evans ML. Gravitropism: interaction of sensitivity modulation and effector redistribution. Plant Physiol. 1991;95:1–5. doi: 10.1104/pp.95.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasano JM, Swanson SJ, Blancaflor EB, Dowd PE, Kao T, Gilroy S. Changes in root cap pH required for the gravity response of the Arabidopsis root. Plant Cell. 2001;13:907–921. doi: 10.1105/tpc.13.4.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felle H. Proton transport and pH control in Sinapis alba root hairs: a study carried out with double-barrelled pH microelectrodes. J Exp Bot. 1987;38:340–354. [Google Scholar]
- Felle H. Auxin causes oscillations of cytosolic free calcium and pH in Zea mays coleoptiles. Planta. 1988a;174:495–499. doi: 10.1007/BF00634478. [DOI] [PubMed] [Google Scholar]
- Felle H. Short-term pH regulation in plants. Physiol Plant. 1988b;74:583–591. [Google Scholar]
- Felle H. pH as a second messenger in plants. In: Boss WF, Morré DJ, editors. Second Messengers in Plant Growth Development. Alan R. New York: Liss; 1989. pp. 145–166. [Google Scholar]
- Felle H, Bertl A. Light-induced cytoplasmic pH changes and their interrelation to the activity of the electrogenic proton pump in Riccia fluitans. Biochim Biophys Acta. 1986;848:176–182. [Google Scholar]
- Felle H, Johannes E. The regulation of proton/amino acid symport in Riccia fluitans L. by cytosolic pH and proton pump activity. J Exp Bot. 1991;41:587–592. [Google Scholar]
- Felle HH, Kondorosi E, Kondorosi A, Schultze M. Rapid alkalinization in alfalfa root hairs in response to rhizobial lipochitooligosaccharide signals. Plant J. 1996;10:295–301. [Google Scholar]
- Firn RD, Wagstaff C, Digby J. The use of mutants to probe models of gravitropism. J Exp Bot. 2000;51:1323–1340. [PubMed] [Google Scholar]
- Frachisse J-M, Johannes E, Felle H. The use of weak acids as physiological tools: a study of the effects of fatty acids on intracellular pH and electrical plasmalemma properties of Riccia fluitans rhizoid cells. Biochim Biophys Acta. 1988;938:199–210. [Google Scholar]
- Fricker MD, Tester M, Gilroy S. Fluorescence and luminescence techniques to probe ion activities in living plant cells. In: Mason WT, editor. Fluorescent and Luminescent Probes for Biological Activity. London: Academic Press; 1993. pp. 360–377. [Google Scholar]
- Gehring CA, Irving HR, Parish RW. Effects of auxin and abscisic acid on cytosolic calcium and pH in plant cells. Proc Natl Acad Sci USA. 1990a;87:9645–9649. doi: 10.1073/pnas.87.24.9645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehring CA, Irving HR, Parish RW. Gibberellic acid induces cytoplasmic acidification in maize coleoptiles. Planta. 1994;194:532–540. [Google Scholar]
- Gehring CA, Williams DA, Cody SH, Parish RW. Phototropism and geotropism in maize coleoptiles are spatially correlated with increases in cytosolic calcium. Nature. 1990b;345:528–530. doi: 10.1038/345528a0. [DOI] [PubMed] [Google Scholar]
- Gibbon BC, Staiger C. Profilin. In: Staiger CJ, Baluška F, Volkmann D, Barlow PW, editors. Actin: A Dynamic Framework for Multiple Plant Cell Functions. Dordrecht, The Netherlands: Kluwer Academic Press; 2000. pp. 45–65. [Google Scholar]
- Grabov A, Blatt MR. Parallel control of the inward-rectifier K+ channel by cytosolic free Ca2+ and pH in Vicia guard cells. Planta. 1997;201:84–95. [Google Scholar]
- Guern J, Felle H, Mathieu Y, Kurkdjian A. Regulation of intracellular pH in plant cells. Int Rev Plant Physiol. 1991;127:111–173. [Google Scholar]
- Guern J, Mathieu Y, Pean M, Pasquier C, Beloeil J-C, Lallemand J-Y. Cytoplasmic pH regulation in Acer pseudoplatanus cells: I. A 31P NMR desciption of acid loading effects. Plant Physiol. 1986;82:840–845. doi: 10.1104/pp.82.3.840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guern J, Mathieu Y, Thomine S, Jouanneau JP, Beloeil JC. Plant cells counteract cytoplasmic pH changes but likely use these pH changes as secondary messages in signal perception. Curr Top Plant Biochem Physiol. 1992;11:249–269. [Google Scholar]
- Gungabissoon RA, Jiang C-J, Drøbak BK, Maciver SK, Hussey PJ. Interaction of maize actin-depolimerising factor with actin and phosphoinositides and its inhibition of plant phospholipase C. Plant J. 1998;16:689–696. [Google Scholar]
- Johannes E, Crofts A, Sanders D. Control of Cl− efflux in Chara corallina by cytosolic pH, free Ca2+, and phosphorylation indicates a role of plasma membrane anion channels in cytosolic pH regulation. Plant Physiol. 1998;118:173–181. doi: 10.1104/pp.118.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman PB, Brock TG, Song I, Rho YR, Ghoshseh NS. How cereal grass shoots perceive and respond to gravity. Am J Bot. 1987;74:1446–1457. [PubMed] [Google Scholar]
- Kaufman PB, Wu L, Brock TG, Kim D. Hormones and the orientation of growth. In: Davies PJ, editor. Plant Hormones. Dordrecht, The Netherlands: Kluwer Academic; 1995. pp. 547–571. [Google Scholar]
- Kiss JZ. Mechanisms of the early phases of plant gravitropism. Crit Rev Plant Sci. 2000;19:551–573. doi: 10.1080/07352680091139295. [DOI] [PubMed] [Google Scholar]
- Kovar DR, Staiger CJ. Actin depolymerizing factor. In: Staiger CJ, Baluška F, Volkmann D, Barlow PW, editors. Actin: A Dynamic Framework for Multiple Plant Cell Functions. Dordrecht, The Netherlands: Kluwer Academic Press; 2000. pp. 67–85. [Google Scholar]
- Kurkdjian A, Guern J. Intracellular pH: measurement and importance in cell activity. Annu Rev Plant Physiol Plant Mol Biol. 1989;40:271–303. [Google Scholar]
- Lacombe B, Pilot G, Gaymard F, Sentenac H, Thibaud JB. pH control of the plant outwardly rectifying potassium channel SKOR. FEBS Lett. 2000;466:351–354. doi: 10.1016/s0014-5793(00)01093-0. [DOI] [PubMed] [Google Scholar]
- Legué V, Blancalflor E, Wymer C, Perbal G, Fantin D, Gilroy S. Cytoplasmic free Ca2+ in arabidopsis roots changes in response to touch but not gravity. Plant Physiol. 1997;114:789–800. doi: 10.1104/pp.114.3.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masson PH. Root gravitropism. Bioessays. 1995;17:119–127. doi: 10.1002/bies.950170207. [DOI] [PubMed] [Google Scholar]
- Mathieu Y, Lapous D, Thomine S, Lauriére C, Guern J. Cytoplasmic acidification as an early phosphorylation dependent response of tobacco cells to elicitors. Planta. 1996;199:416–424. [Google Scholar]
- Miesenböck G, de Angelis DA, Rothman JE. Visualizing secretion and synaptic transmission with pH-sensitive green fluorescent proteins. Nature. 1998;394:192–195. doi: 10.1038/28190. [DOI] [PubMed] [Google Scholar]
- Muday GK. Interactions between the actin cytoskeleton and an auxin transport protein. In: Staiger CJ, Baluška F, Volkmann D, Barlow PW, editors. Actin: A Dynamic Framework for Multiple Plant Cell Functions. Dordrecht, The Netherlands: Kluwer Academic Press; 2000. pp. 541–571. [Google Scholar]
- Okazaki Y, Tazawa M, Iwassaki N. Light-induced changes in cytoplasmic pH in leaf cells of Egeria densa: measurements with pH-sensitive microelectrodes. Plant Cell Physiol. 1994;35:943–950. [Google Scholar]
- Paterson KM, Nimmo HG. Effects of pH on the induction of phosphoenolpyruvate carboxylase kinase in Kalanchoë fedtschenkoi. Plant Sci. 2000;154:135–141. doi: 10.1016/s0168-9452(99)00249-6. [DOI] [PubMed] [Google Scholar]
- Perera IY, Heilmann I, Boss WF. Transient and sustained increases in inositol 1,4,5-trisphosphate precede the differential growth response in gravistimulated maize pulvini. Proc Natl Acad Sci USA. 1999;96:5838–5843. doi: 10.1073/pnas.96.10.5838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera IY, Heilmann I, Chang SC, Boss WF, Kaufman PB (2001) A role for inositol 1,4,5-trisphosphate in gravitropic signaling and the retention of cold-perceived gravistimulation of oat (Avena sativa) shoot pulvini. Plant Physiol (in press) [DOI] [PMC free article] [PubMed]
- Portillo F. Regulation of plasma membrane H+-ATPase in fungi and plants. Biochim Biophys Acta. 2000;1469:31–42. doi: 10.1016/s0304-4157(99)00011-8. [DOI] [PubMed] [Google Scholar]
- Putnam R. Intracellular pH regulation. In: Sperelakis N, editor. Cell Physiology Source Book. San Diego: Academic Press; 1998. pp. 293–305. [Google Scholar]
- Quader H, Fast H. Influence of cytosolic pH changes on the organization of the endoplasmatic reticulum in epidermal cells of onion bulb scales: acidification by loading with weak organic acids. Protoplasma. 1990;157:216–224. [Google Scholar]
- Roos W. Ion mapping in plant cells: methods and applications in signal transduction research. Planta. 2000;210:347–370. doi: 10.1007/PL00008144. [DOI] [PubMed] [Google Scholar]
- Roos W, Evers S, Hieke M, Tschope M, Schumann B. Shifts of intracellular pH distribution as a part of the signal mechanism leading to the elicitation of benzophenanthridine alkaloids: phytoalexin biosynthesis in cultured cells of Eschscholtzia californica. Plant Physiol. 1998;118:349–364. doi: 10.1104/pp.118.2.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sack FD. Plastids and gravitropic sensing. Planta. 1997;203:S63–S68. doi: 10.1007/pl00008116. [DOI] [PubMed] [Google Scholar]
- Sack FD, Leopold AC. Cytoplasmic streaming affects gravity-induced amyloplast sedimentation in maize coleoptiles. Planta. 1985;164:52–62. [PubMed] [Google Scholar]
- Schulz-Lessdorf B, Lohse G, Hedrich H. GCAC1 recognizes the pH gradient across the plasma membrane: a pH-sensitive and ATP-dependent anion channel links guard cell membrane potential to acid and energy metabolism. Plant J. 1996;10:993–1004. [Google Scholar]
- Scott AC, Allen NS. Changes in cytosolic pH within Arabidopsis root columella cells play a key role in the early signaling pathway for root gravitropism. Plant Physiol. 1999;121:1291–1298. doi: 10.1104/pp.121.4.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedbrook JC, Chen RJ, Masson PH. ARG1 (altered response to gravity) encodes a DNAJ-like protein that potentially interacts with the cytoskeleton. Proc Natl Acad Sci USA. 1999;96:1140–1145. doi: 10.1073/pnas.96.3.1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sievers A, Busch MB. An inhibitor of the Ca2+-ATPase in the sarcoplasmic and endoplasmic reticula inhibits transduction of the gravity stimulus in cress roots. Planta. 1992;188:619–622. doi: 10.1007/BF00197057. [DOI] [PubMed] [Google Scholar]
- Sievers A, Sondag C, Trabacz K, Heijnowicz Z. Gravity-induced changes in intracellular potentials in statocytes of cress roots. Planta. 1995;197:392–398. doi: 10.1007/BF00202662. [DOI] [PubMed] [Google Scholar]
- Smith FA, Raven JA. Intracellular pH and its regulation. Annu Rev Plant Physiol. 1979;30:289–311. [Google Scholar]
- Tasaka M, Kato T, Fukaki H. The endodermis and shoot gravitropism. Trends Plant Sci. 1999;4:103–107. doi: 10.1016/s1360-1385(99)01376-x. [DOI] [PubMed] [Google Scholar]
- Vercesi AB, Moreno SNJ, Docampo R. Ca2+/H+ exchange in acidic vacuoles of Trypanosoma brucei. Biochem J. 1994;304:227–233. doi: 10.1042/bj3040227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DA, Cody SH, Dubbin PN. Introducing and calibrating fluorescent probes in cells and organelles. In: Mason WT, editor. Fluorescent and Luminescent Probes for Biological Activity. London: Academic Press; 1993. pp. 312–334. [Google Scholar]
- Winter H, Huber JL, Huber CS. Membrane association of sucrose synthase: changes during the graviresponse and possible control by protein phosphorylation. FEBS Lett. 1997;420:151–155. doi: 10.1016/s0014-5793(97)01506-8. [DOI] [PubMed] [Google Scholar]
- Zhou FS, Andersen CH, Burhenne K, Fischer PH, Collinge DB, Thordal-Christensen H. Proton extrusion is an essential signalling component in the HR of epidermal single cells in the barley-powdery mildew interaction. Plant J. 2000;23:245–254. doi: 10.1046/j.1365-313x.2000.00777.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.