Abstract
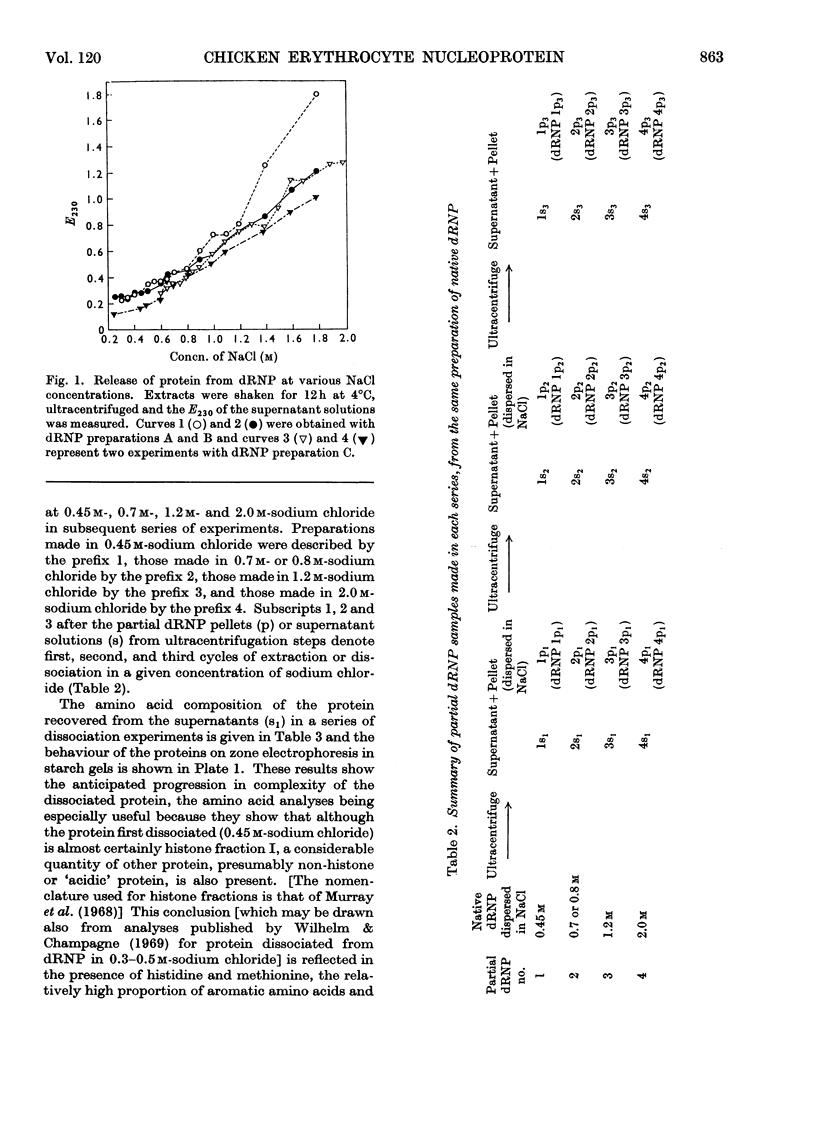
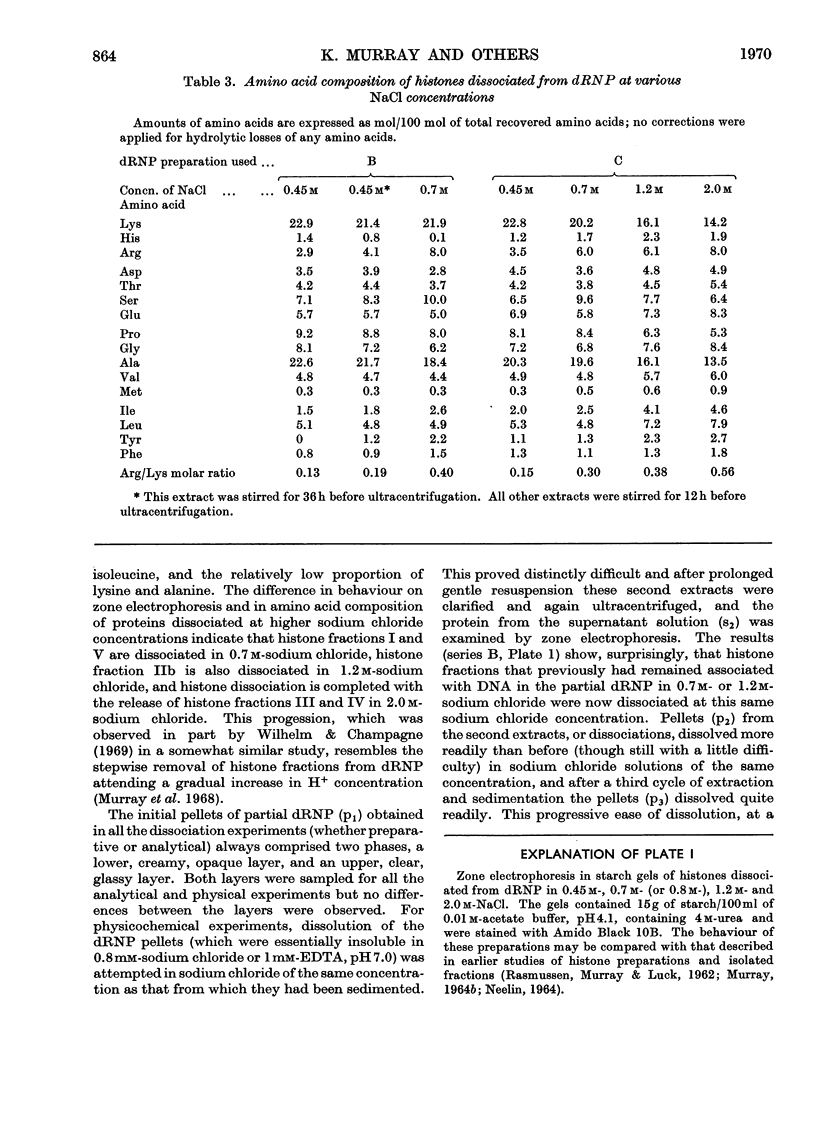
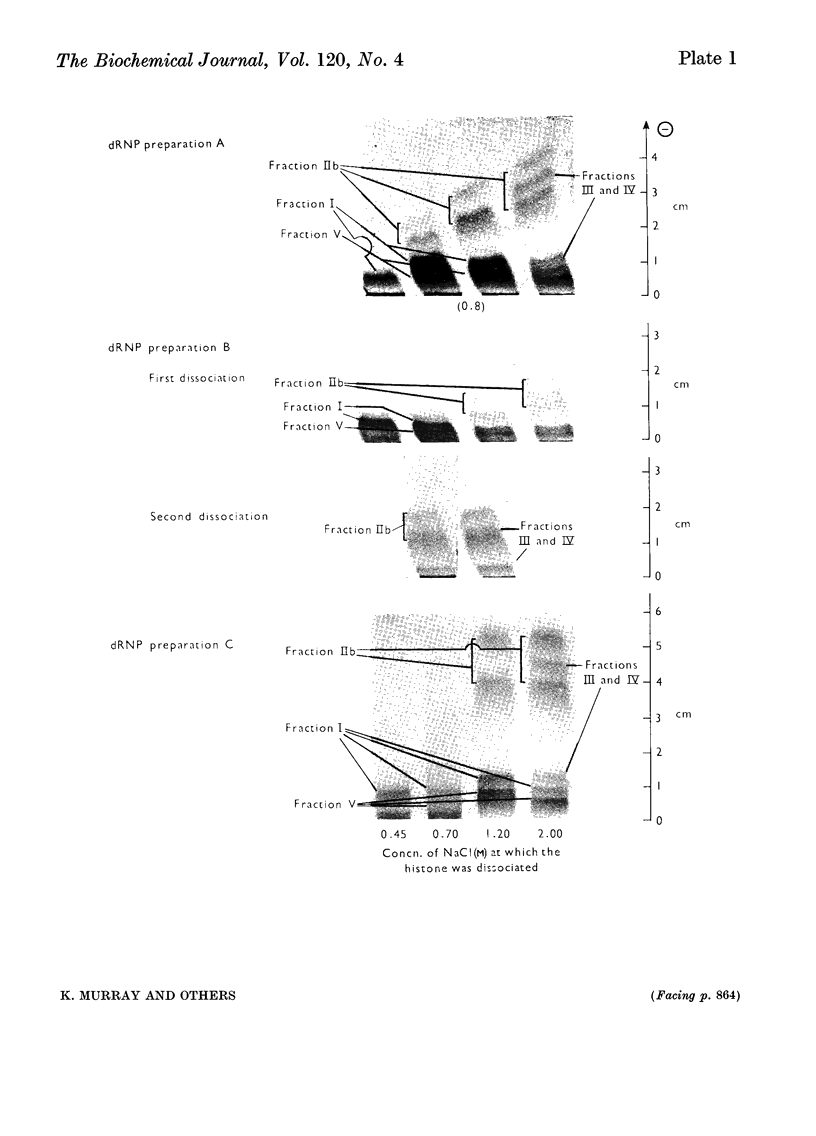
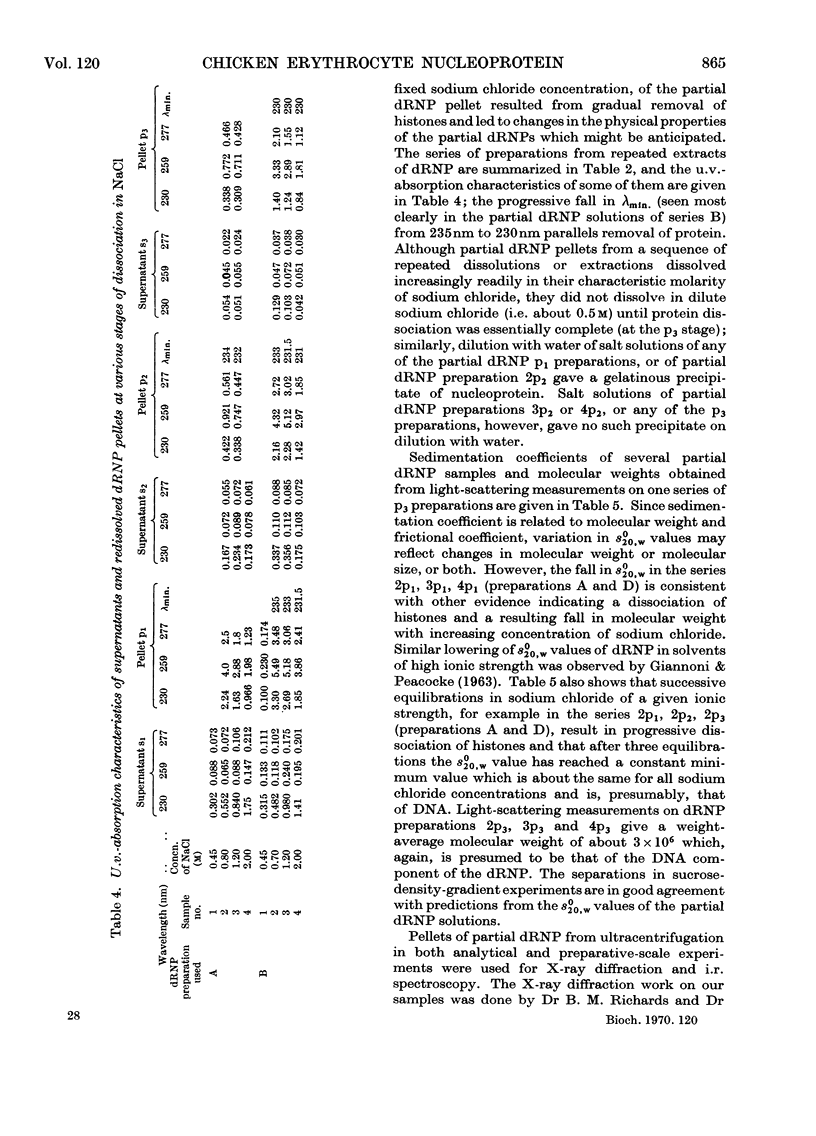
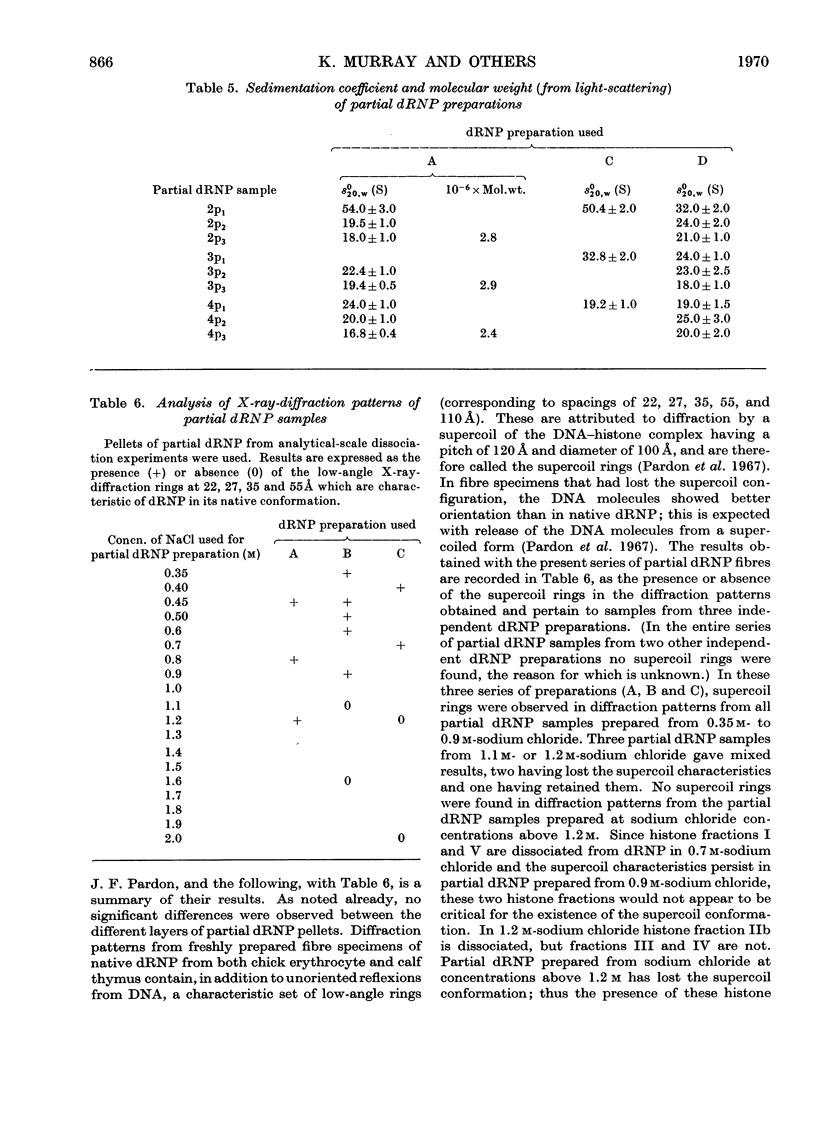
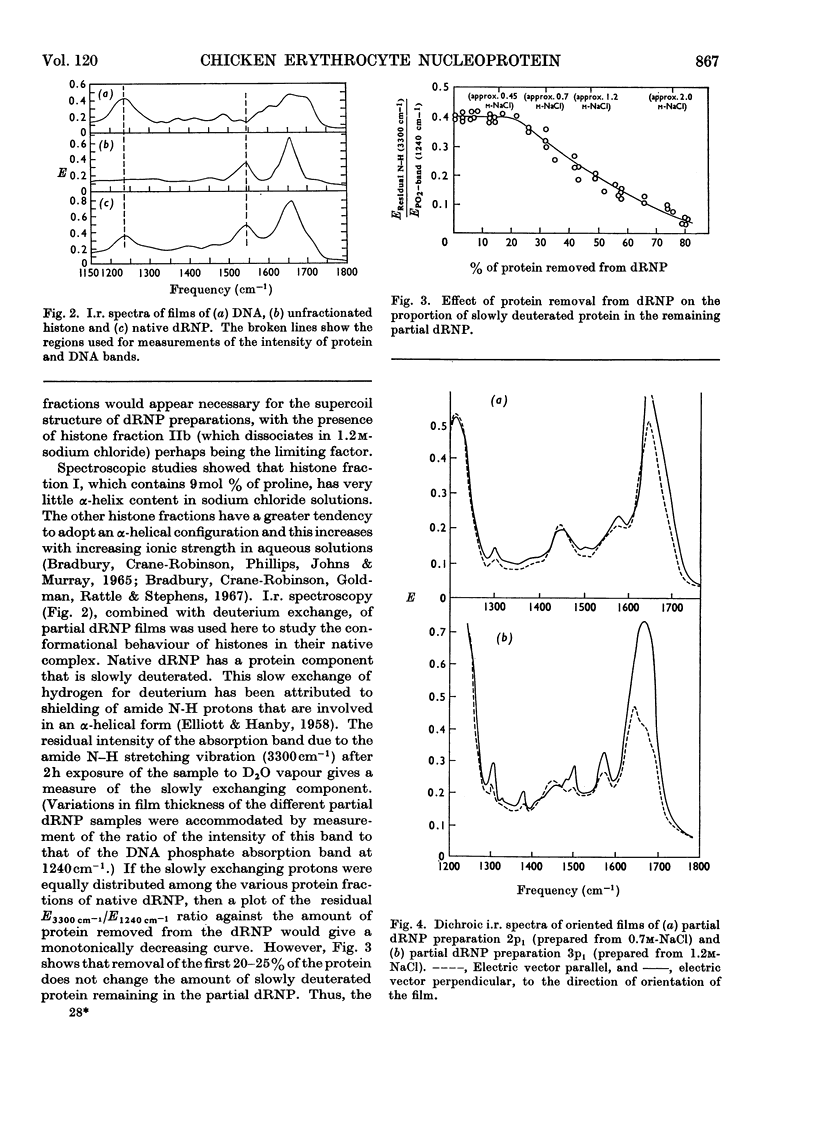
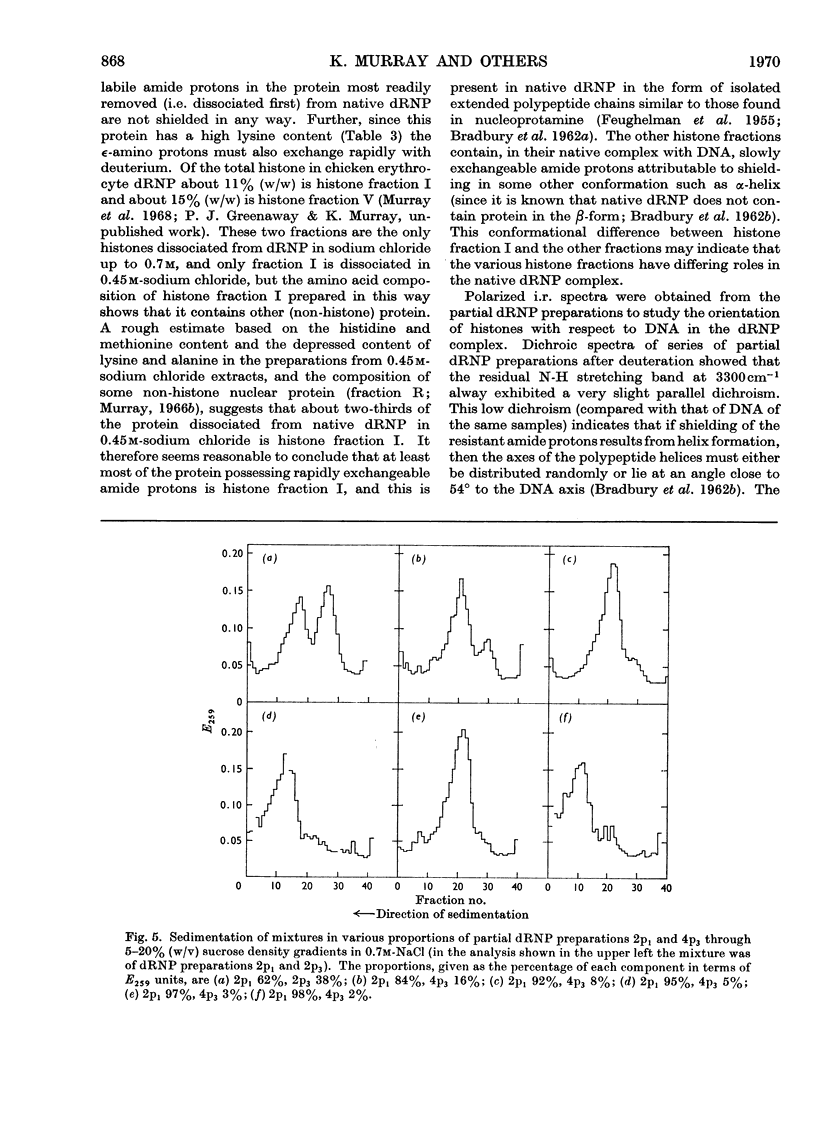
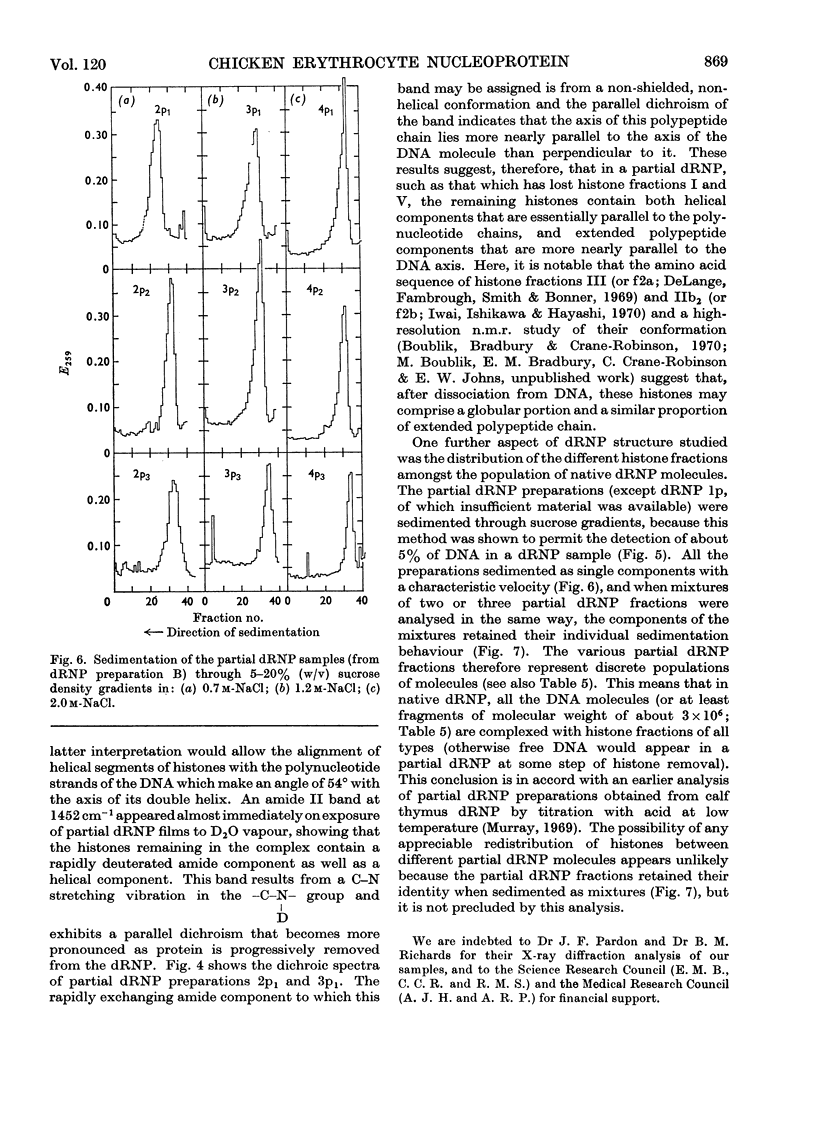
Histones were completely dissociated from their native complex with DNA in 2.0m-sodium chloride. Histone fractions IIb, V and I were dissociated in 1.2m-sodium chloride, fractions V and I in 0.7m-sodium chloride and fraction I in 0.45m-sodium chloride. Repeated extraction of partial dRNP (deoxyribonucleoprotein) preparations with sodium chloride of the same concentration as that from which they were prepared resulted in release of histones that previously had remained associated with the DNA of the complex. Gradual removal of histones from dRNP was paralleled by an improvement in solubility, a decrease in wavelength of the u.v.-absorption minimum, and a fall in sedimentation coefficient of the remaining partial dRNP. X-ray diffraction patterns of partial dRNP preparations showed that removal of histone fractions I and V from dRNP did not destroy the super-coil structure of the dRNP, but further removal of histones did. Infrared spectra of partial dRNP preparations showed that in native dRNP histone fraction I was present in the form of extended, isolated polypeptide chains, and that the other histone fractions probably contain a helical component that lies roughly parallel to the polynucleotide chains in the double helix and an extended polypeptide component that is more nearly parallel to the DNA helix axis. An analysis of the sedimentation of partial dRNP preparations on sucrose gradients showed that native dRNP consists of DNA molecules each complexed with histone fractions of all types.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bekhor I., Kung G. M., Bonner J. Sequence-specific interaction of DNA and chromosomal protein. J Mol Biol. 1969 Jan;39(2):351–364. doi: 10.1016/0022-2836(69)90322-2. [DOI] [PubMed] [Google Scholar]
- Bendit E. G. Infrared absorption spectrum of keratin. I. Spectra of alpha-, beta-, and supercontracted keratin. Biopolymers. 1966 Jun;4(5):539–559. doi: 10.1002/bip.1966.360040506. [DOI] [PubMed] [Google Scholar]
- Boublík M., Bradbury E. M., Crane-Robinson C. An investigation of the conformational changes in histones F1 and F2a1 by proton magnetic resonance spectroscopy. Eur J Biochem. 1970 Jul;14(3):486–497. doi: 10.1111/j.1432-1033.1970.tb00315.x. [DOI] [PubMed] [Google Scholar]
- CRAMPTON C. F., LIPSHITZ R., CHARGAFF E. Studies on nucleoproteins. II. Fractionation of deoxyribonucleic acids through fractional dissociation of their complexes with basic proteins. J Biol Chem. 1954 Nov;211(1):125–142. [PubMed] [Google Scholar]
- CRAMPTON C. F. Studies on the dissociation of histones from the nucleohistone of calf thymus. J Biol Chem. 1957 Jul;227(1):495–504. [PubMed] [Google Scholar]
- DeLange R. J., Fambrough D. M., Smith E. L., Bonner J. Calf and pea histone IV. II. The complete amino acid sequence of calf thymus histone IV; presence of epsilon-N-acetyllysine. J Biol Chem. 1969 Jan 25;244(2):319–334. [PubMed] [Google Scholar]
- ELLIOTT A., HANBY W. E. Deuterium exchange in polypeptides. Nature. 1958 Sep 6;182(4636):654–655. doi: 10.1038/182654b0. [DOI] [PubMed] [Google Scholar]
- FEUGHELMAN M., LANGRIDGE R., SEEDS W. E., STOKES A. R., WILSON H. R., HOOPER C. W., WILKINS M. H., BARCLAY R. K., HAMILTON L. D. Molecular structure of deoxyribose nucleic acid and nucleoprotein. Nature. 1955 May 14;175(4463):834–838. [PubMed] [Google Scholar]
- FRENSTER J. H., ALLFREY V. G., MIRSKY A. E. REPRESSED AND ACTIVE CHROMATIN ISOLATED FROM INTERPHASE LYMPHOCYTES. Proc Natl Acad Sci U S A. 1963 Dec;50:1026–1032. doi: 10.1073/pnas.50.6.1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIANNONI G., PEACOCKE A. R. Thymus deoxyribonucleoprotein. III. Sedimentation behaviour. Biochim Biophys Acta. 1963 Feb 26;68:157–166. doi: 10.1016/0006-3002(63)90131-8. [DOI] [PubMed] [Google Scholar]
- Georgiev G. P., Ananieva L. N., Kozlov J. V. Stepwise removal of protein from a deoxyribonucleoprotein complex and de-repression of the genome. J Mol Biol. 1966 Dec 28;22(2):365–371. doi: 10.1016/0022-2836(66)90140-9. [DOI] [PubMed] [Google Scholar]
- Gilmour R. S., Paul J. RNA transcribed from reconstituted nucleoprotein is similar to natural RNA. J Mol Biol. 1969 Feb 28;40(1):137–139. doi: 10.1016/0022-2836(69)90301-5. [DOI] [PubMed] [Google Scholar]
- HEARST J. E. The specific volume of various cationic forms of deoxyribonucleic acid. J Mol Biol. 1962 May;4:415–417. doi: 10.1016/s0022-2836(62)80024-2. [DOI] [PubMed] [Google Scholar]
- Hnilica L. S. The specificity of histones in chicken erythrocytes. Experientia. 1964 Jan 15;20(1):13–14. doi: 10.1007/BF02146014. [DOI] [PubMed] [Google Scholar]
- Iwai K., Ishikawa K., Hayashi H. Amino-acid sequence of slightly lysine-rich histone. Nature. 1970 Jun 13;226(5250):1056–1058. doi: 10.1038/2261056b0. [DOI] [PubMed] [Google Scholar]
- Leng M., Felsenfeld G. The preferential interactions of polylysine and polyarginine with specific base sequences in DNA. Proc Natl Acad Sci U S A. 1966 Oct;56(4):1325–1332. doi: 10.1073/pnas.56.4.1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray K., Vidali G., Neelin J. M. The stepwise removal of histones from chicken erythrocyte nucleoprotein. Biochem J. 1968 Mar;107(2):207–215. doi: 10.1042/bj1070207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlenbusch H. H., Olivera B. M., Tuan D., Davidson N. Selective dissociation of histones from calf thymus nucleoprotein. J Mol Biol. 1967 Apr 28;25(2):299–315. doi: 10.1016/0022-2836(67)90143-x. [DOI] [PubMed] [Google Scholar]
- Pardon J. F., Wilkins M. H., Richards B. M. Super-helical model for nucleohistone. Nature. 1967 Jul 29;215(5100):508–509. doi: 10.1038/215508a0. [DOI] [PubMed] [Google Scholar]
- SCHUMAKER V. N., SCHACHMAN H. K. Ultracentrifugal analysis of dilute solutions. Biochim Biophys Acta. 1957 Mar;23(3):628–639. doi: 10.1016/0006-3002(57)90386-4. [DOI] [PubMed] [Google Scholar]
- Skalka A., Fowler A. V., Hurwitz J. The effect of histones on the enzymatic synthesis of ribonucleic acid. J Biol Chem. 1966 Feb 10;241(3):588–596. [PubMed] [Google Scholar]
- Wilhelm X., Champagne M. Dissociation de la nucléoprotéine d'érythrocytes de poulets par les sels. Eur J Biochem. 1969 Aug;10(1):102–109. [PubMed] [Google Scholar]



