Abstract
Purpose
Radiotherapy is an integral treatment for non-small cell lung cancer (NSCLC); however, radiation-induced toxicities such as radiation pneumonitis (RP) present a considerable challenge. Herein, we aimed to evaluate the potential of salivary metabolomics as an independent risk factor for predicting RP.
Methods
This study included 62 consecutive patients with NSCLC who underwent thoracic radiotherapy at Tokyo Medical University between September 2016 and December 2018. The median age of the patients was 75 years (range: 41–89), comprising 47 (75.8%) males and 15 (24.2%) females. Patients with stage I NSCLC received 75 Gy in 30 fractions, whereas those with stage II and III NSCLC received 66 Gy in 33 fractions. Saliva samples were collected before treatment and at 2 weeks, 1 month, 3 months, and 1 year after initiating radiotherapy. Clinical RP was defined as grade 2 according to the Common Toxicity Criteria for Adverse Events. Salivary metabolomics were analyzed using capillary electrophoresis-mass spectrometry. Salivary metabolites were evaluated as potential predictors of RP.
Results
Clinical RP was observed in 11 patients (17.7%); no RP-related deaths were observed. Clinical RP developed at a median of 4 months (range: 2–6 months) after initiating radiotherapy. Three metabolites, butyrate, propionate, and hexanoate, collected before radiotherapy exhibited predictive ability for clinical RP. Multivariate logistic analysis indicated butyrate (P = 0.033) as a predictive factor, along with the previously known factor of lung volume irradiated with > 20 Gy (P = 0.045).
Conclusion
Salivary metabolite butyrate was an independent risk factor for clinical RP.
Keywords: Radiation pneumonitis, Saliva, Metabolomics, Prognosis
Introduction
Lung cancer is the leading and second leading cause of cancer-related deaths among males and females, respectively (National Cancer Center Japan 2023). Non-small cell lung cancer (NSCLC) reportedly accounts for 84% of all lung cancers (Utada et al. 2019). Radiotherapy is integral for treating NSCLC, either alone or in combination with surgery or chemotherapy. High-dose radiotherapy using advanced techniques has shown promising results in patients with early-stage cancer, improving survival rates (Tandberg et al. 2018). However, chemoradiotherapy remains the standard of care in advanced stages with poor clinical outcomes (Spigel et al. 2022).
Radiotherapy is a less invasive alternative to surgery; however, radiation-induced toxicities such as radiation pneumonitis (RP) can be life-threatening (Palma et al. 2013). Although several RP-related factors have been identified, accurate prediction remains an ongoing challenge. Molecular biomarkers for the early prediction of RP have been explored (Yang et al. 2021). The metabolic profiles and quantified patterns of small organic molecules in the blood can demonstrate abnormal changes due to radiation toxicity (Vicente et al. 2020). Mass-based imaging techniques have revealed that lipid patterns reflect lung inflammation and fibrosis levels (Carter et al. 2017). Furthermore, radiation was found to affect salivary metabolomic profiles associated with jaw osteonecrosis and oral mucositis (Yatsuoka et al. 2019, 2021). Metabolites in these low- or non-invasive biofluids have shown potential in detecting RP; however, few studies have explored the potential applications of these molecules.
In the present study, we investigated the potential of salivary metabolites and their combinations as predictors of RP. The predictive ability of salivary metabolites was evaluated using known risk factors.
Methods
The Ethics Committee of Tokyo Medical University Hospital granted permission to conduct a cohort study to investigate RP using salivary metabolomics to treat lung cancer (Approval Number: SH4092). The inclusion criteria were as follows: patients aged ≥ 20 years; patients diagnosed with NSCLC based on histology or diagnosed by a cancer board conference if the histological diagnosis was uncertain; patients eligible for curative radiotherapy; and patients with no history of thoracic radiotherapy. The exclusion criteria were as follows: patients with active interstitial pneumonia, severe liver cirrhosis, diabetes mellitus, or cardiovascular disease, and those with malignancies other than lung cancer. Written informed consent was obtained from all participants of this study.
For treating stage I NSCLC, the clinical target volume (CTV) was defined as the tumor volume of NSCLC, and the planning target volume (PTV) was determined to cover the CTV with adequate margins to include respiratory motion. In addition, 95% of the prescribed dose of 75 Gy in 30 fractions was determined to cover 95% of the PTV. The PTV was irradiated with noncoplanar intensity-modulated radiation therapy using solid compensated filters (Decimal Co. Ltd., Florida, USA) (Itonaga et al. 2019).
To treat stage II and III NSCLC, CTV1 was defined as the primary tumor and associated lymph nodes, and CTV2 was defined as the primary tumor and gross tumor of associated lymph nodes. PTV1 was designed to cover CTV1 with sufficient margins to allow for respiratory motion, whereas PTV2 was designed to cover CTV2 with sufficient margins to avoid excessive radiation to the spinal cord. Using three-dimensional conformal radiotherapy (3DCRT), PTV1 received 40 Gy in 20 fractions, and PTV2 received an additional 26 Gy in 13 fractions. A total dose of 66 Gy in 33 fractions was administered to the primary tumor concurrently with cisplatin and vindesine chemotherapy. Radiation treatment was planned using a radiation treatment planning system (Xi, ver. 4.6, Elekta AB, Stockholm, Sweden).
Patients were followed up every 3 months for 2 years after treatment initiation and every 6 months thereafter. Follow-up included consultations with radiation oncologists and surgeons, blood tests, chest CT scans, and collection of saliva samples. Clinical RP was defined as grade 2 or higher RP according to the National Cancer Institute (NCI) Common Terminology Criteria for Adverse Events (CTCAE) ver. 5.0. We evaluated the potential patient-related risk factors, including age, sex, smoking, presence or absence of interstitial pneumonia, and tumor location (upper or lower lobe). In addition, treatment-related risk factors were examined, including a combination of chemotherapy, mean lung dose (MLD), and a lung-irradiated volume greater than 20 Gy (V20) or 5 Gy (V5) for all patients. For determining the cutoff value of MLD, V20, and V5, we used the median values specific to our dataset. A higher value of MLD, V20, and V5 corresponds to a group with values equal to or greater than the median, whereas a lower value indicates a group with values smaller than the median (Chun et al. 2017).
For each patient, saliva samples were collected at five different time points: pretreatment, 2 weeks, 1 month, 3 months, and 1 year after initiating radiotherapy (Fig. 1). For sample collection, patients were instructed to abstain from all food except water after the last dinner. All saliva samples were collected between 9:00 and 12:00 to minimize diurnal variation. Stimulation of the oral cavity, such as brushing and exercise, was also restricted at least 1 h before sampling. The participants were required to rinse their mouths with water immediately before sample collection. Approximately 400 μL of unstimulated saliva was collected from each patient in a 50 mL polypropylene tube. Then, saliva samples were immediately stored at −80 °C in a deep freezer until metabolomics analyses. Capillary electrophoresis time-of-flight mass spectrometry (CE-TOFMS) was used to profile the hydrophilic metabolites (Sugimoto et al. 2010, 2020). Additionally, we performed liquid chromatography-triple quadrupole MS (LC-QQQMS) to profile the polyamines (Nose et al. 2023; Tomita et al. 2018). The raw data were processed to eliminate noise, subtract the background, and align multiple data points to generate a data matrix (samples × metabolites) (Sugimoto et al. 2012). Metabolite identification was conducted by matching migration or retention times and m/z values with those of standard compounds to calculate the relative peak areas. The peak areas of each metabolite were divided by peak areas of the internal standards to eliminate fluctuations in MS sensitivity. The absolute concentrations were calculated by comparing the relative peak areas of the saliva samples and standard compound mixtures measured in identical batches (Sugimoto et al. 2020).
Fig. 1.

Study design. Saliva samples were collected at five-time points. RP was diagnosed 2–6 months after initiation of radiotherapy. RP radiation pneumonitis
Frequently detected metabolites (390% of the samples) were subjected to statistical analyses. The quantified metabolite concentrations were visualized using a heat map. Subsequently, we calculated the fold-change (FC) in the average concentration of each metabolite in patients with RP (RP group) and those without RP (non-RP group). Metabolites showing FC > 1.5 or FC < 0.5 were used. Each metabolite was assigned a Z-score for color assignment. Metabolites were then subjected to cluster analysis using Pearson’s correlation coefficient to identify patterns of metabolic responses associated with RP. The Mann–Whitney U test was used to evaluate differences between the RP and non-RP groups. Univariate analysis was performed for all variables, and multivariate analysis was performed for variables that were significant in the univariate analysis. Statistical significance was set at P < 0.05. Statistical analyses were performed using the MetaboAnalyst (ver. 5) (Pang et al. 2021), GraphPad Prism (ver. 9.2.0, GraphPad Software Inc., San Diego, CA, USA), and Stata (ver. 16.0, StataCorp, TX, USA).
Results
In total, 62 patients were enrolled in the current study, with a median age of 75 (range: 41–89) years. Of these, 47 (75.8%) were male, and 15 (24.2%) were female. Table 1 summarizes the patient characteristics.
Table 1.
Patient characteristics
| Characteristic | Number of patients |
|---|---|
| Age (years) | |
| Median | 75 |
| Range | 41–89 |
| Sex | |
| Men | 47 |
| Women | 15 |
| Smoking | |
| + | 54 |
| − | 8 |
| NSCLC stage | |
| I | 20 |
| II | 6 |
| III | 36 |
| Interstitial pneumonia | |
| + | 13 |
| − | 49 |
| Radiotherapy | |
| IMRT | 16 |
| 3DCRT | 46 |
| Total dose | |
| Median | 66 |
| Range | 50–75 |
| V20 | |
| ≥ 30 | 11 |
| < 30 | 51 |
| Chemotherapy | |
| + | 26 |
| − | 36 |
3DCRT three-dimensional conformal radiotherapy; IMRT intensity-modulated radiotherapy; V20 a lung-irradiated volume greater than 20 Gy
The median radiation dose was 66 (range: 50–75) Gy. Thirteen patients died in September 2019: 7 due to primary disease, three from pneumonia, and three from other diseases, and no RP-related deaths were noted. Overall, 11 (17.7%) patients presented with clinical RP, diagnosed at a median of 4 (range: 2–6) months after initiation of radiotherapy. These patients were primarily treated with corticosteroids, with all symptoms satisfactorily resolved. No patient developed progressive RP.
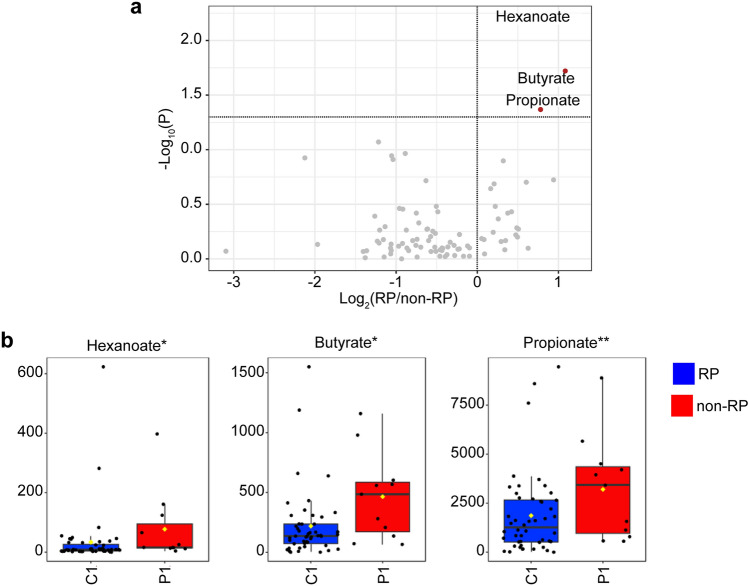
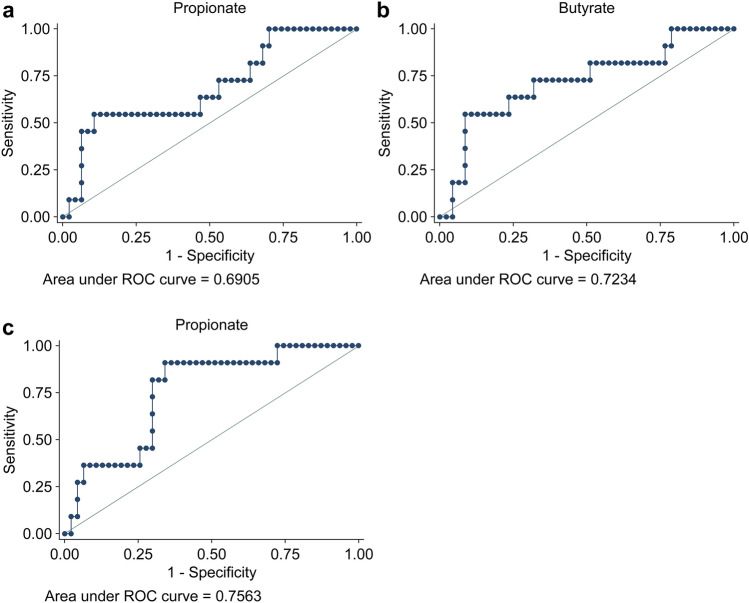
In the current study, CE-TOFMS and LC-QQQMS were performed to profile hydrophilic and polyamine metabolites using 522 and 25 standard compounds, respectively. At least 321 metabolites were identified in each sample, 90 of which were frequently detected. Volcano plots were used to visualize differences in salivary metabolite concentrations between the RP and non-RP groups in the pretreatment data (Fig. 2). The RP group exhibited significantly higher levels of three metabolites, hexanoate, butyrate, and propionate, than the non-RP group (Mann–Whitney U test). Metabolite concentrations at all time points were visualized using a heatmap (Fig. 3). Data included several amino acids, such as arginine (Arg), methionine (Met), glycine (Gly), alanine (Ala), leucine (Leu), and isoleucine (Ile). Lactate, an end product of glycolysis, and citrate, the first intermediate metabolite of the tricarboxylic acid cycle, were also included. Creatinine and creatine levels were measured. The metabolites shown in Fig. 2 were aligned adjacent to each other, indicating similar time-course patterns for these three metabolites. Figure 4 presents the time courses for hexanoate, butyrate, and propionate. Notably, significant differences between the groups were observed at pretreatment, which became smaller, except for hexanoate data at 3 months. The receiver operating characteristic (ROC) curves of pretreatment levels of three metabolites, i.e., propionate, butyrate, and hexanoate, demonstrated a relationship with RP (Fig. 5).
Fig. 2.
Volcano plots (a) and box plots (b) to visualize the pretreatment metabolite data. a The X-axis and Y-axis indicate log2 of the fold-change of (RP group/non-RP) and −log10 of the P value (Mann–Whitney test), respectively. b Box plots of non-RP (left) and RP (right) groups. RP radiation pneumonitis
Fig. 3.
Heatmap showing the averaged metabolite concentration of the non-RP and RP groups. Of the 90 quantified metabolites, 28 metabolomes showing FC > 1.5 or < 0.5 between the two groups at pretreatment were used. Each metabolite concentration was normalized by the median value, log-transformed, and auto-scaled (Z-score). Pearson correlation was used for clustering. The metabolites showing P < 0.05 (Mann–Whitney) at pretreatment are indicated in red color. RP radiation pneumonitis; FC fold-change; Pre pretreatment; w week; m month; yr year; Arg arginine; GABA gamma-aminobutyric acid; Ser serine; Gly-Leu glycyl-l-leucine; 2AB 2-aminobutyric acid; Met methionine; Ala alpha-lipoic acid; Ile isoleucine
Fig. 4.
The time-course of metabolites showing significant differences between the groups at pretreatment (P < 0.05; Mann–Whitney test). The dot and error bars indicate the average and standard deviations. *P < 0.05 and **P < 0.01. RP radiation pneumonitis; Pre pretreatment; w week; m month; y year
Fig. 5.
The receiver operating characteristic (ROC) curves of pretreatment salivary levels of propionate (a), butyrate (b), and hexanoate (c) indicated that these metabolites were predictors of radiation pneumonitis, with areas under ROC curves of 0.6905, 0.7234, and 0.7563, respectively
Based on univariate analysis, age, clinical stage, combination with chemotherapy, MLD, V5, V20, and butyrate levels were identified as potential risk factors. However, sex, smoking status, tumor location, interstitial pneumonia, and hexanoate or propionate metabolites were not identified as potential risk factors. Given that the combination of chemotherapy, NSCLC stage, MLD, V5, and V20 confounded with each other, we omitted stage, MLD, and V5 as independent risk factors in the multivariate analysis. Multivariate logistic analysis revealed that the butyrate metabolome and V20 were prognostic factors for RP (Table 2).
Table 2.
Predictive factors for radiation pneumonitis using univariate and multivariate analyses
| Univariate analysis | Multivariate analysis | |||||||
|---|---|---|---|---|---|---|---|---|
| Odds ratio | P value | 95% confidence interval | Odds ratio | P value | 95% confidence interval | |||
| Age (high/low) | 0.183 | 0.041 | 0.036 | 0.930 | 0.791 | 0.861 | 0.0573 | 10.92 |
| Sex (male/female) | 0.292 | 0.080 | 0.074 | 1.156 | ||||
| Smoking ( ±) | 0.600 | 0.568 | 0.104 | 3.463 | 0.517 | 0.603 | 0.0431 | 6.21 |
| Tumor location (lower/upper) | 0.991 | 0.998 | 2.230 | 4.278 | 0.762 | 0.8 | 0.0931 | 6.24 |
| Interstitial pneumonia ( ±) | 0.292 | 0.262 | 2.234 | 2.509 | 1.072 | 0.971 | 0.0259 | 44.28 |
| NSCLC stage (II–III/I) | 8.219 | 0.050 | 0.998 | 67.670 | ||||
| Chemotherapy ( ±) | 20.000 | 0.006 | 2.361 | 169.392 | 8.95 | 0.166 | 0.4014 | 199.65 |
| V20 (high/low) | 14.286 | 0.014 | 1.698 | 120.283 | 14.2 | 0.045 | 1.06 | 190.3 |
| V5 (high/low) | 5.932 | 0.032 | 1.163 | 30.254 | ||||
| MLD (high/low) | 14.286 | 0.014 | 1.698 | 120.203 | ||||
| Butyrate ( ±) | 5.200 | 0.020 | 1.295 | 20.890 | 9.514 | 0.033 | 1.1997 | 75.45 |
| Hexanoate ( ±) | 2.476 | 0.213 | 0.595 | 10.300 | ||||
| Propionate ( ±) | 2.400 | 0.197 | 0.635 | 9.074 | ||||
MLD mean lung dose; V20, V5 a lung-irradiated volume greater than 20 or 5 Gy
Discussion
RP is a well-known toxicity associated with radiotherapy and is characterized by inflammation of lung tissue due to radiation exposure (Hanania et al. 2019). Prompt and effective treatment of RP can minimize its impact on patients undergoing radiation therapy.
Several studies have explored risk factors associated with RP. A history of respiratory comorbidities, right-lung location, MLD, and V20 have been identified as risk factors for stereotactic body radiotherapy at an early stage (Liu et al. 2021). In cases of locally advanced disease, a meta-analysis of individual patient data has revealed that age, combined chemotherapy with carboplatin and paclitaxel, and V20 were risk factors for RP (Palma et al. 2013). Machine learning and multivariate analyses were employed to analyze a cohort of patients with stage II–III disease and identify risk factors associated with RP, including maximum esophageal dose, V20, MLD, and smoking (Luna et al. 2019). A review article identified several risk factors, including lung dose, V20, dose per fraction, and combined chemotherapy or immunotherapy as treatment-related factors; tumor location, histology, staging, and tumor volume as tumor-related factors; smoking; comorbid conditions, such as chronic obstructive lung disease and interstitial lung disease; sex; age; and genetic phenotype as patient-related factors (Arroyo-Hernández et al. 2021). In the present study, we identified age, sex, smoking, interstitial pneumonia, tumor location, tumor stage, combined chemotherapy, MLD, V5, and V20 as possible factors associated with RP. Several of these factors may have complex relationships or influence each other differently.
RP is a multifactorial process involving several mechanisms that remain poorly clarified. During the classical phase of RP, cellular injury leads to the release of cytokines that cause acute RP. Sporadic RP mimics hypersensitivity pneumonitis (Hanania et al. 2019), suggesting that a complex interplay of cellular and molecular pathways may contribute to the development of RP. The blood protein and metabolite profiles were found to be associated with radiation-related toxicities and could predict potential toxicities prior to radiotherapy initiation (Jelonek et al. 2017). The current study focused on analyzing saliva biofluids, which can be collected non-invasively, repeatedly, and safely. Salivary metabolomic profiles have been frequently reported as biomarkers for cancer detection (Asai et al. 2018) or for monitoring responses to therapeutic management (Yatsuoka et al. 2019, 2021). Accordingly, we used this technique to predict the occurrence of RP by analyzing salivary samples collected at five time points, including pretreatment.
CE-TOFMS- and LC-QQQMS-based analyses identified three pretreatment salivary metabolites, namely butyrate, propionate, and hexanoate, as potential risk factors for predicting the occurrence of RP. Butyrate is a short-chain fatty acid produced by bacterial fermentation in the colon. This metabolite has been detected in human breath as a volatile compound (Dallinga et al. 2010); therefore, human saliva may also contain this metabolite because of its hydrophilicity. Propionate, an organic acid produced by the colonic microbiome, has been detected at relatively high concentrations in human saliva (Takeda et al. 2009). Hexanoate, or caproic acid, is a medium-chain fatty acid produced by β-oxidation and fatty acid synthesis. Hexanoate has been previously detected in human saliva (Dallmann et al. 2012; Silwood et al. 2002). Although no previous report has assessed the relationship between these metabolites and RP, the detection of these three metabolites in the salivary samples was reasonable.
The observed clinical and metabolomic data were integrated and analyzed to predict RP. Univariate regression analyses revealed that age, tumor stage, combined chemotherapy, V5, V20, and salivary butyrate metabolism were significantly associated with RP. However, because stage and combination chemotherapy were confounded with MLD, V5, and V20, we excluded tumor stage, MLD, and V5 from the multivariate analyses. According to multivariate logistic regression analysis, the salivary butyrate metabolome and V20 were significant risk factors for RP. Notably, these risk factors were distinct from other general or treatment factors, indicating their unique contribution to the risk of RP. In this study, our aim was to identify potential salivary factors associated with RP. In particular, butyrate, a short-chain fatty acid, plays a role in differentiating regulatory T cells responsible for immune tolerance in peripheral tissues. Further study is required to evaluate the detailed mechanism of the relationship with RP (Arpaia et al 2013).
The limitations of the current study include the small number of patients, selection bias, and the inability to draw definitive conclusions or analyze confounding factors and generalize the findings to different populations. We plan to further explore our findings using a larger sample size to predict tumor control rates and collect more data to validate and extend our findings.
Conclusions
The study results suggest that butyrate, the identified salivary metabolite, could be an independent risk factor among other known risk factors. With the implementation of additional patient studies, the risk of RP could be detected non-invasively using pretreatment saliva sampling.
Acknowledgements
We thank M. Kaneko, S. Ota, and A. Enomoto for their help with the sample measurements. This study was supported by JSPS KAKENHI (grant numbers JP19K08106, JP16K10404, and JP22H00595).
Author contributions
All authors contributed to the conception and design of the study. Material preparation and data collection were performed by SS. Analysis was performed by MS and KT. The first draft of the manuscript was written by SS and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
This study was supported by JSPS KAKENHI (grant numbers JP19K08106, JP16K10404, and JP22H00595).
Data availability
Research data are not available at this time. The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethics approval
The Ethics Committee of Tokyo Medical University Hospital granted permission to conduct a cohort study to investigate radiation pneumonitis using salivary metabolomics to treat lung cancer (SH4092).
Informed consent
Written informed consent was obtained from all participants of this study.
Consent to publish
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Arpaia N, Campbell C, Fan X et al (2013) Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature 504:451–455. 10.1038/nature12726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arroyo-Hernández M, Maldonado F, Lozano-Ruiz F, Muñoz-Montaño W, Nuñez-Baez M, Arrieta O (2021) Radiation-induced lung injury: current evidence. BMC Pulm Med 21:9. 10.1186/s12890-020-01376-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asai Y, Itoi T, Sugimoto M et al (2018) Elevated polyamines in saliva of pancreatic cancer. Cancers (Basel) 10:43. 10.3390/cancers10020043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter CL, Jones JW, Farese AM, MacVittie TJ, Kane MA (2017) Lipidomic dysregulation within the lung parenchyma following whole-thorax lung irradiation: markers of injury, inflammation and fibrosis detected by MALDI-MSI. Sci Rep 7:10343. 10.1038/s41598-017-10396-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun SG, Hu C, Choy H et al (2017) Impact of intensity-modulated radiation therapy technique for locally advanced non-small-cell lung cancer: a secondary analysis of the NRG oncology RTOG 0617 randomized clinical trial. J Clin Oncol 35:56–62. 10.1200/JCO.2016.69.1378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallinga JW, Robroeks CM, van Berkel JJ et al (2010) Volatile organic compounds in exhaled breath as a diagnostic tool for asthma in children. Clin Exp Allergy 40:68–76. 10.1111/j.1365-2222.2009.03343.x [DOI] [PubMed] [Google Scholar]
- Dallmann R, Viola AU, Tarokh L, Cajochen C, Brown SA (2012) The human circadian metabolome. Proc Natl Acad Sci U S A 109:2625–2629. 10.1073/pnas.1114410109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanania AN, Mainwaring W, Ghebre YT, Hanania NA, Ludwig M (2019) Radiation-induced lung injury: assessment and management. Chest 156:150–162. 10.1016/j.chest.2019.03.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itonaga T, Mikami R, Nakayama H et al (2019) Phase II study of compensator-based non-coplanar intensity-modulated radiotherapy for stage I non-small-cell lung cancer. J Radiat Res 60:387–393. 10.1093/jrr/rrz009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelonek K, Pietrowska M, Widlak P (2017) Systemic effects of ionizing radiation at the proteome and metabolome levels in the blood of cancer patients treated with radiotherapy: the influence of inflammation and radiation toxicity. Int J Radiat Biol 93:683–696. 10.1080/09553002.2017.1304590 [DOI] [PubMed] [Google Scholar]
- Liu Y, Wang W, Shiue K et al (2021) Risk factors for symptomatic radiation pneumonitis after stereotactic body radiation therapy (SBRT) in patients with non-small cell lung cancer. Radiother Oncol 156:231–238. 10.1016/j.radonc.2020.10.015 [DOI] [PubMed] [Google Scholar]
- Luna JM, Chao HH, Diffenderfer ES et al (2019) Predicting radiation pneumonitis in locally advanced stage II–III non-small cell lung cancer using machine learning. Radiother Oncol 133:106–112. 10.1016/j.radonc.2019.01.003 [DOI] [PubMed] [Google Scholar]
- National Cancer Center Japan (2023) Cancer statistics in Japan 2023. https://ganjoho.jp/public/qa_links/report/statistics/2023_en.html, Accessed Aug 14, 2023
- Nose D, Sugimoto M, Muta T, Miura SI (2023) Salivary polyamines help detect high-risk patients with pancreatic cancer: a prospective validation study. Int J Mol Sci 24:2998. 10.3390/ijms24032998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palma DA, Senan S, Tsujino K et al (2013) Predicting radiation pneumonitis after chemoradiation therapy for lung cancer: an international individual patient data meta-analysis. Int J Radiat Oncol Biol Phys 85:444–450. 10.1016/j.ijrobp.2012.04.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang Z, Chong J, Zhou G et al (2021) MetaboAnalyst 5.0: narrowing the gap between raw spectra and functional insights. Nucleic Acids Res 49:W388–W396. 10.1093/nar/gkab382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silwood CJ, Lynch E, Claxson AW, Grootveld MC (2002) 1H and (13)C NMR spectroscopic analysis of human saliva. J Dent Res 81:422–427. 10.1177/154405910208100613 [DOI] [PubMed] [Google Scholar]
- Spigel DR, Faivre-Finn C, Gray JE et al (2022) Five-year survival outcomes from the PACIFIC trial: durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. J Clin Oncol 40:1301–1311. 10.1200/JCO.21.01308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimoto M, Wong DT, Hirayama A, Soga T, Tomita M (2010) Capillary electrophoresis mass spectrometry-based saliva metabolomics identified oral, breast and pancreatic cancer-specific profiles. Metabolomics 6:78–95. 10.1007/s11306-009-0178-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimoto M, Kawakami M, Robert M, Soga T, Tomita M (2012) Bioinformatics tools for mass spectroscopy-based metabolomic data processing and analysis. Curr Bioinform 7:96–108. 10.2174/157489312799304431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimoto M, Ota S, Kaneko M, Enomoto A, Soga T (2020) Quantification of salivary charged metabolites using capillary electrophoresis time-of-flight-mass spectrometry. Bio Protoc 10:e3797. 10.21769/BioProtoc.3797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda I, Stretch C, Barnaby P et al (2009) Understanding the human salivary metabolome. NMR Biomed 22:577–584. 10.1002/nbm.1369 [DOI] [PubMed] [Google Scholar]
- Tandberg DJ, Tong BC, Ackerson BG, Kelsey CR (2018) Surgery versus stereotactic body radiation therapy for stage I non-small cell lung cancer: a comprehensive review. Cancer 124:667–678. 10.1002/cncr.31196 [DOI] [PubMed] [Google Scholar]
- Tomita A, Mori M, Hiwatari K et al (2018) Effect of storage conditions on salivary polyamines quantified via liquid chromatography-mass spectrometry. Sci Rep 8:12075. 10.1038/s41598-018-30482-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utada M, Yonehara S, Ozasa K (2019) Historical changes in histological diagnosis of lung cancer. J Epidemiol 29:238–240. 10.2188/jea.JE20180037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicente E, Vujaskovic Z, Jackson IL (2020) A systematic review of metabolomic and lipidomic candidates for biomarkers in radiation injury. Metabolites 10:259. 10.3390/metabo10060259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S, Yao Y, Dong Y et al (2021) Prediction of radiation pneumonitis using genome-scale flux analysis of RNA-seq derived from peripheral blood. Front Med (lausanne) 8:715961. 10.3389/fmed.2021.715961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yatsuoka W, Ueno T, Miyano K et al (2019) Metabolomic profiling reveals salivary hypotaurine as a potential early detection marker for medication-related osteonecrosis of the jaw. PLoS ONE 14:e0220712. 10.1371/journal.pone.0220712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yatsuoka W, Ueno T, Miyano K et al (2021) Time-course of salivary metabolomic profiles during radiation therapy for head and neck cancer. J Clin Med 10:2631. 10.3390/jcm10122631 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Research data are not available at this time. The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.