Abstract
Introduction
Colorectal cancer (CRC) is the third most prevalent cancer and the second significant cause of cancer-associated death worldwide. The microRNA-30 is a substantial member of the miRNA family and plays a vital role in expanding several cancers. This microRNA potentially targets interleukin 6 as an inflammatory cytokine in CRC.
Materials and methods
MSCs were isolated and identified from mice bone marrow and then transduced with lentiviruses containing miR-30C. Transfected MSCs were collected to evaluate IL-6 levels, CT-26 cells were also co-cultured with MSCs, and the effect of apoptosis and IL-6 on the supernatant was assessed.
Results
Our result showed the expression of IL-6 mRNA and the level of protein were decreased in the supernatant of miR-30-transduced MSC cells compared to the control group. In addition, the rate of apoptosis was assessed, and the obtained data revealed the induction of apoptosis in CT-26 cells when they are in the vicinity of miR-30c-transduced MSCs.
Discussion and conclusion
We demonstrated that downregulation of miR-30c was significantly correlated with CRC progression and survival. So, the present study elucidated the anticancer effects of miR-30c in CRC and presented a novel target for CRC therapy.
Keywords: Mesenchymal stem cells, Interleukin 6, MicroRNA-30, Colorectal cancer, Anticancer
Introduction
Colorectal cancer (CRC) is the third most prevalent cancer and the second significant cause of cancer-associated death worldwide (Siegel et al. 2017). Although numerous therapeutic approaches, including surgery, chemotherapy, and radiotherapy, have significantly enhanced, CRC remains a highly lethal cancer (Arnold et al. 2017). While there are various risk factors for CRC (including obesity, smoking, dietary habits, physical inactivity, and genetic and epigenetic factors), a deep comprehension of the molecular mechanisms that contribute to CRC and describing the factors that induce the expansion of the tumor will provide new helpful strategies for the CRC treatment (Mármol et al. 2017).
The tumour initiation and development are not only impressed by genetic changes in the tumor cell itself but possibly also by non‐tumor cells existing in the microenvironment. The tumor environment is formed of cancer cells and numerous cellular members, including endothelial cells, fibroblasts, mesenchymal stem cells (MSCs), and various immune system cells (Lazennec and Lam 2016; Mahjoor et al. 2021a; Moslemi et al. 2020, 2021; Sohrabi et al. 2021). MSCs are multipotent stromal cells that are recognized to exist in different parts like the bone marrow, fat, and dental pulp. Although some researchers propose that MSCs show tumor-suppressive function, other studies have indicated their tumor-promoting functions in various cancers (Lee and Hong 2017). It has been suggested that MSCs can home to tumor locations and cooperate in tumor development. MSCs generate several types of molecules, such as chemokines, cytokines, and growth factors, which operate in a paracrine manner on their specific receptors on the surface of cancer cells, thereby controlling tumor progression (Cuiffo and Karnoub 2012; Ridge et al. 2017).
Of these factors and cytokines, interleukin 6 (IL-6) indicates a vital role in the pathogenesis and development of numerous cancers. This inflammatory cytokine is involved in tumor survival, proliferation, angiogenesis, and metastasis by stimulating some signal transduction networks, including JAK/STAT3, RAS/ERK, and PI3K/AKT signaling networks (Jones and Jenkins 2018). A meaningfully high level of IL-6 has been noticed in serum samples of patients experiencing breast, pancreatic, esophageal, gastric, hepatic, pancreatic, non-small cell lung, renal, and colorectal cancer (Wolfe et al. 2016).
Numerous studies determine that microRNAs (miRNAs) have a significant role in human diseases, particularly cancers. miRNAs belong to a group of short, highly conserved non-coding RNAs identified to stop protein-coding gene expression via binding to the 3′ untranslated region (3′UTR) of downstream target genes (Shah et al. 2016). In the physical condition, miRNAs have central roles in various biological processes, including cell apoptosis, proliferation, and differentiation. In addition, several types of research have shown that dysregulation of miRNAs results in tumorigenesis, progression, and metastasis in numerous cancers, including CRC (Bracken et al. 2016).
The microRNA-30 (miR-30) family is a significant member of the miRNA family and plays a vital role in expanding several cancers. MicroRNA-30c has been informed as a tumor suppressor in different cancers, like lung, ovarian, pancreatic, and breast cancer (Mao et al. 2018; McCann et al. 2019). A recent study indicated that the miR-30c expression was significantly lessened in lung cancer cells and that knocking out miR-30c can meaningfully intensify the aggressive behavior of lung cancer (Spinelli et al. 2017). Also, another study examined high-flux miRNA chips and demonstrated that miR-30c is downregulated in osteosarcoma patients (Sun et al. 2018).
In this study, we provided several pieces of evidence representing that IL-6 is a target of miR-30c. We showed that miR-30c is downregulated in CRC and might function as a tumor suppressor and stop tumorigenesis by targeting IL-6.
Materials and methods
Strains, vectors, and culture media
The recombinant pCDH lenti-vector containing microRNA-30c was used in the current study. The heat-shock protocol was used for bacterial cell transformation (Derakhshani et al. 2019), and transformed clones were collected using an LB agar medium comprising 100 μg/mL ampicillin. The cultivated colonies with the specific primers were used for the colony PCR (Table 1). In addition, plasmids extraction was conducted for further analysis based on the manufacture of the Thermo Scientific GeneJET Plasmid Miniprep Kit (Thermo Scientific, USA).
Table 1.
The sequences of primers
| Name | Sequences |
|---|---|
| CMV-F primer | AAT GGG CGG TAG GCG TGT A |
| EF1-R primer | GGA CTG TGG GCG ATG TGC |
Extraction of bone marrow (BM)
Mouse BM was obtained from the femur of two mice. First, BM was transferred aseptically into the K2EDTA tube. A buffy coat was then isolated by centrifugation (450 g, for 15 min), suspended in 1.5 mL PBS, and used for culture. Next, the separated buffy coat was layered onto an equal volume of Ficoll and centrifuged (400g, for 20 min). Finally, the cells at the interface were separated and washed twice with PBS.
Mesenchymal stem cell culture
Mesenchymal stem cells were isolated from mouse bone marrow with a yield of approximately 5 × 106 cells. Also, human embryonic kidney 293 (HEK 293), U-251, and CT-26 cell lines were purchased from the cell bank of Pasture Institute (Tehran, Iran). All cells were grown in DMEM medium (Dulbecco's Modified Eagles Medium, Gibco, UK) enriched with ten percent fetal bovine serum (FBS; GIBCO, Carlsbad, CA) and containing two mM L-glutamine was maintained at 37 °C with 5% CO2 in a humidified chamber.
Characterization of human mesenchymal stem cells by differentiation
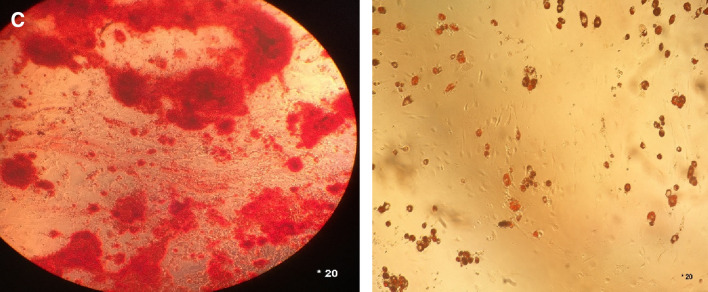
After incubation of isolated cells for characterization of the adherent cells, for adipogenesis, cells were cultured in an adipogenic medium (StemPro® Adipogenesis Differentiation Kit and also, for chondrogenesis, cells were maintained in a chondrogenic medium (StemPro® Chondrogenesis Differentiation Kit (Thermofisher), then Alizarin and S Red Oil (Sigma Aldrich) were used for osteoblastic and lipid droplet staining respectively.
Evolution of cluster of differentiation
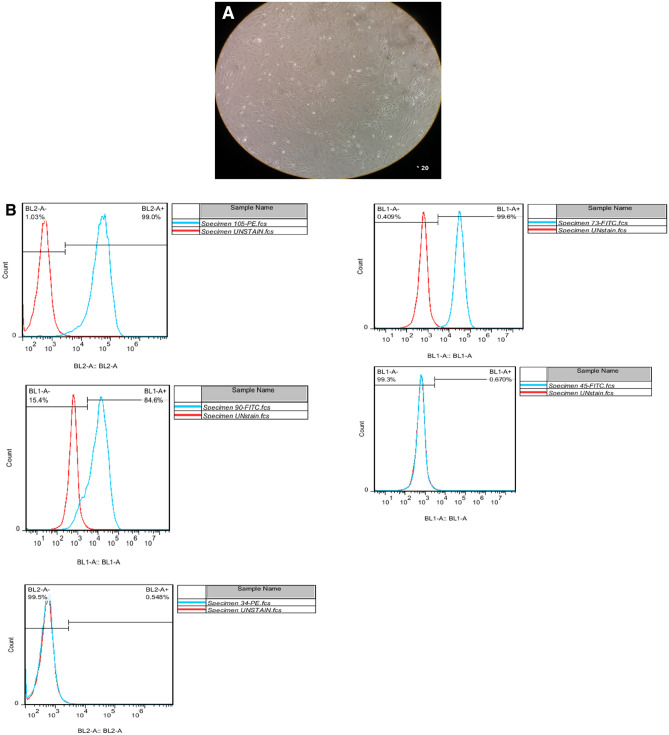
Flow cytometry was used for assessing the profile of MSCs, using the norm for MSC as defined by the International Society for Cellular Therapy (ISCT), and cells (P2-3) were collected, pelleted, and re-suspended in 3% rat serum in PBS, and counted. Each population containing 2*105 cells was used for flow cytometry. The cells were stained with directly PE (phycoerythrin)-conjugated antibodies against CD34, CD45, CD90, CD105, and CD73. In addition, a proper isotype-matched control antibody named mouse IgG1 K Iso control (Santa Cruz Biotechnology, Santa Cruz, CA, USA) was used in all evaluates.
Lentiviral packaging and virus collection
After transfection of HEK 293 T cells with psPAX2, pMD2.G, and pCDH vectors containing microRNA were packaged and produced. First, the HEK 293 T cells were trypsinized, and the cell density was adjusted to 1 × 106 cells/mL with a complete culture medium. After that, cells were seeded and cultured for 24 h before transfection. The cells converged 90% on the day of transfection. Then, the recombinant vector encoding the microRNA-30c and the three packaging plasmids, including pCDH, pMD2.G, and psPAX2, were extracted with a plasmid extraction kit (Thermo Scientific, USA). And co-transfected into HEK 293 T cells based on the Kit's instructions, and after passing eight hours of transfection, the medium of cells was replaced by a new one. After 24 h transfection, eGFP expression was assessed, and then after 48 h of transfection, to eradicate any cell debris, the culture medium was collected and centrifuged at 4000g, 4 °C for 10 min. The supernatant was purified through a Plus-20 centrifugal ultrafiltration device through a 0.45-μm filter and centrifuged at 4000g to achieve a high lentivirus titer. The lentivirus was used as the negative control and was developed similarly to the inhibitor vector. To assess the efficacy of recombinant lentivirus infecting target cells, U-251 cells were transduced with the recombinant lentivirus.
Apoptosis assay
Apoptosis was conducted using Annexin V/propidium iodide (PI) Kit. CT-26 cells were seeded at a density of 105 cells on six-well plates and tested in control and transfected groups. The transfected and control cells were removed with trypsin/EDTA and centrifuged for 8 min at 1500 g following 24 h. The cells were then performed according to the manufacturer's instructions with PE Annexin V Apoptosis Detection Kit with 7-AAD Sony Biotechnology, Inc, San Jose, CA, USA). Finally, the flow cytometry instrument was used to analyze the samples. FlowJo software (Tree Star, San Carlos, CA) was performed to evaluate the rate of apoptosis.
RNA extraction, cDNA synthesis, and qRT-PCR
The expression of microRNA-30c and interleukin 6 (IL-6) was performed by qRT-PCR and proper primer pairs (Table 2). RNA extraction was performed through TRIzol reagent (RiboEx). The qRT-PCR was carried out using Applied Biosystems Real-Time PCR (Mannheim, Germany) after cDNA synthesis with a kit (Bio Reality, Daejeon, South Korea).
Table 2.
The sequences of IL-6 and miR-30c primer pairs
| Name | Sequences |
|---|---|
| IL-6 forward | AGACAGCCACTCACCTCTTCAG |
| IL-6 reverse | TTCTGCCAGTGCCTCTTTGCTG |
| miR-30c forward | AACACGTGTGTAAACATCCTACA |
| miR-30c SL | GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACGCTGAG |
| Universal reverse | GTCGTATCCAGTGCAGGGT |
Cytokines measurement
The concentration of IL-6 in the medium of transduced and non-transduced MSCs as well as the co-cultured CT-26 cells with transduced and non-transduced MSCs was determined by commercial ELISA kits (Biosource International Inc, USA) following the manufacturer's instructions. All samples were checked in duplicate, and the average was used for analysis. The detection limit was > 0.01 IU/ml for IL-6.
Statistical methods
The statistical significance of variables was evaluated using T test analyses using GraphPad Prism 6 software (San Diego, CA, USA). P value < 0.05 was considered a significant result.
Results
Isolation and identification of mesenchymal stem cells
After 7–10 days and subsequent passage cells, isolated cells from BM grew within a week with a complete DMEM-F12 medium and reached a density of 80–70%. Finally, third passaged mesenchymal stem cells were used in the experiments. Isolated mesenchymal stem cells have a spindle-shaped, elongated, wavy appearance (Fig. 1A). Bone marrow mesenchymal stem cells were also examined by flow cytometry, and CD34, CD73, CD45, CD90, and CD105 markers were analyzed. Flow cytometry results are shown in Fig. 1B. To confirm the presence of bone marrow mesenchymal stem cells, their differentiability into fat cells and osteoblast cells was examined. Third passage mesenchymal stem cells then were cultured at the number of 125 cells in a 24-well plate, and the medium was changed twice a week. Differentiated fat cells after 12 days and differentiated osteoblast cells after 41 days were stained with oil-red-O and bone-stained (alizarin red). The staining results are shown in Fig. 1C.
Fig. 1.
A MSCs derived from human fat tissue. The spindle-shaped are obviously easy to see in different growth rate. B Detection of fat and osteoblast cells in MSCs derived from mice femur. To detect these cells, BL-2 is used as well as flow cytometry method. C Oil-red-O and alizarin red staining of MSCs to objectively show osteoblast and fat cells surrounding MSCs
Extraction and restriction digestion of pCDH plasmid
After culturing the bacteria-containing pCDH vector, the plasmid was extracted, and 4 μl of it was electrophoresed on the 1% agarose gel shown in Fig. 2A. The desired vector size is 8189 bp. To place the desired gene fragment, the pCDH plasmid was enzymatically digested and then cleaned up, and 4 μl of the plasmid and linear and cleaned plasmid were electrophoresed on 1% agarose gel (Fig. 2B).
Fig. 2.
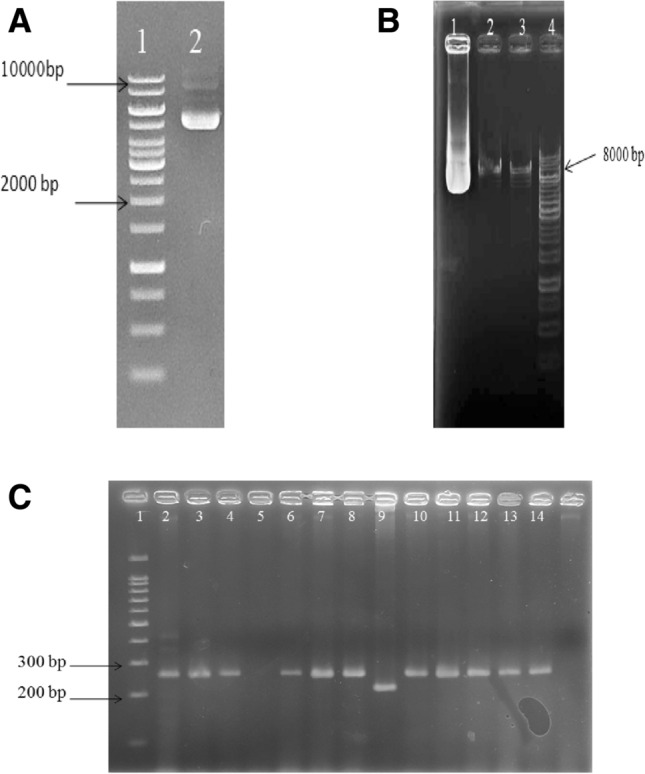
A Electrophoresed pCDH plasmid, well 1 the bioron1kb molecular weight marker and well 2 the pCDH plasmid. B Electrophoresing digested pCDH. Well1 undigested plasmid, well 2 digested plasmids, well 3 digested and cleaned up plasmids, well 4 molecular weight marker. C Colony PCR electrophoresis. Well 1 molecular weight marker, well 9 control or backbone plasmids
Colony PCR to detect the vectors containing miR-30c
To confirm the presence of the desired microRNA in the pCDH vector, colony PCR was applied with vector-specific primers for the formed colonies. As shown in Fig. 2C, the recombinant plasmids, which contain the fragments we are looking for, are much heavier than the control plasmids and stand in a higher position.
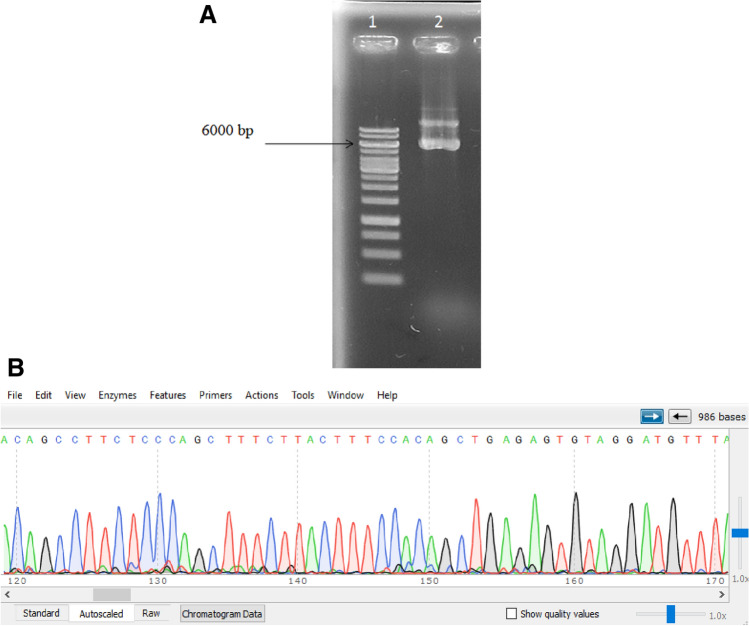
Extraction and sequencing of the recombinant plasmid
After the colony containing the desired gene was identified, it was cultured, and the plasmid was extracted (Fig. 3A). For cloning confirmations, a plasmid that was positive in the colony PCR stage for the presence of the gene fragment was sent for sequencing. The sequence obtained was then blasted on the Nucleotide-BLAST online tool, and the sequence was verified (Fig. 3B).
Fig. 3.
A The electrophoresis result of recombinant plasmid. Well 1 the molecular weight marker, well 2 the recombinant plasmid, which show the presence of the related plasmid and approve the efficacy of extraction. B The results of sequencing. It demonstrates that the sequenced plasmid is contained miR-30c sequence as the inner portion
Production of recombinant lentivirus
After total extraction of pAX and pMD2 plasmids and those containing the desired fragment, the plasmids were transfected into HEK293T cells using the calcium phosphate method. The results of transfection efficacy are shown in Fig. 4A. The sizes of pAX and pMD2 vectors are 12,273 bp and 5842 bp, respectively. After 24 h of the transfection, the efficacy of vector delivery was 70%, and after 48 h, it was approximately 90% (Fig. 4B and C).
Fig. 4.
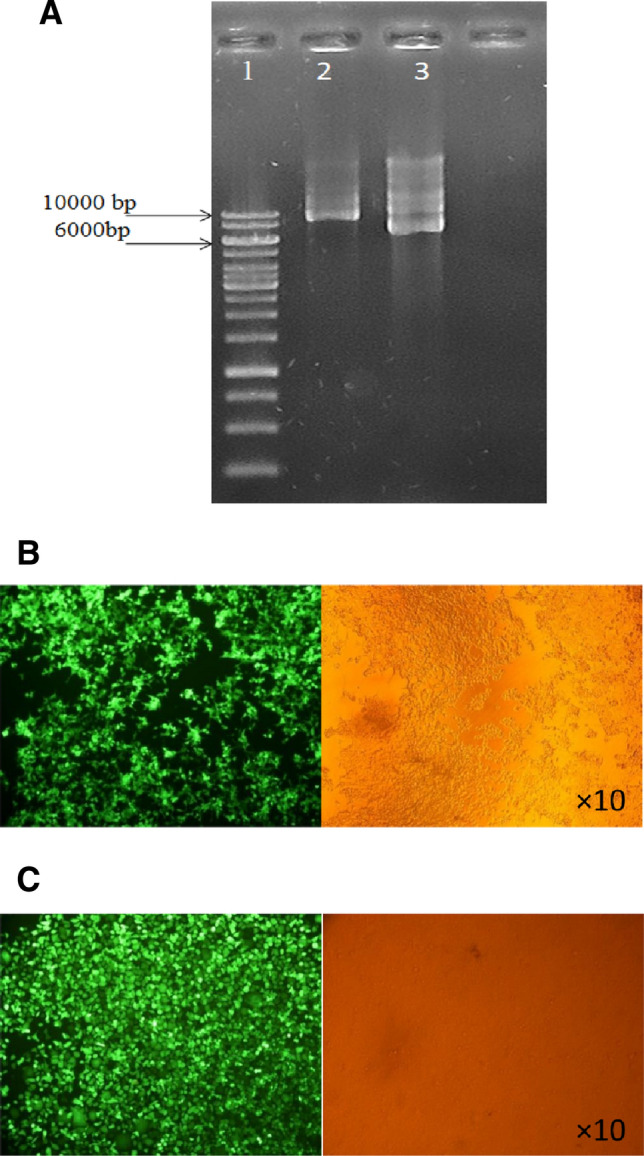
A Electrophoresis of extracted recombinant plasmid. Well 1 molecular weight marker, well 2 pAX plasmid with 10,073 bp in length, well 3 pMD plasmid with 5824 bp in length. B HEK293T cells 24 h after the transfection. The efficacy of transfection was evaluated about 70%. The transfected cells are shown with green color after fluorescent microscopy. C HEK293T cells 48 h after the transfection. The efficacy of transfection was evaluated about 90%
Titration of virus particles
After producing and concentrating on the virus, the titer of the generated virus was determined using flow cytometry. The GFP-positive cells in plate 1 were at a higher level (97%) and in plate 3 were at a lower level (40.4%) (Fig. 5A). Also, in Table 3, the amount of virus particles calculated for each cell plate, which is calculated according to flow cytometry results. After titration of the produced viruses, a certain number of viruses (MOI = 5) were transduced to U-251 cells to control their performance and efficiency. As it is clear in the figure, approximately 100% of U-251 cells are received desired vector (Fig. 5B).
Fig. 5.
A Assessment of GFP in three different plates containing virus particles as well as control group. Well 1 to 4 are control, plate 1, plate 2, and plate 3 respectively. B transduced U-251 cells imaged by fluorescent microscopy. 24 h after the transduction, the efficacy is 90% and after the 48 h of transduction of U-251 cells with recombinant lentivirus the efficacy is about 100%
Table 3.
The number of virus particles in each well
| Plate number | Virus (particle/ml) |
|---|---|
| 1 | 5,200,000 |
| 2 | 6,000,000 |
| 3 | 6,800,000 |
Transduction of MSC via the recombinant lentiviruses and co-culturing
After titration and determination of the relative number of viruses, when the confluency of MSC reached 30–40%, these cells were transduced with a certain amount of virus (MOI = 5). The results showed that 72 h after the transduction of CT-26 cells with recombinant lentivirus, the number of cells received this virus had a relatively increasing trend compared to 48 h (Fig. 6A). Moreover, the results demonstrated that the co-culture of transduced MSCs with colon cancer cells (CT-26) could affect the cancerous cells, by which the number of cancer cells was reduced (Fig. 6B).
Fig. 6.
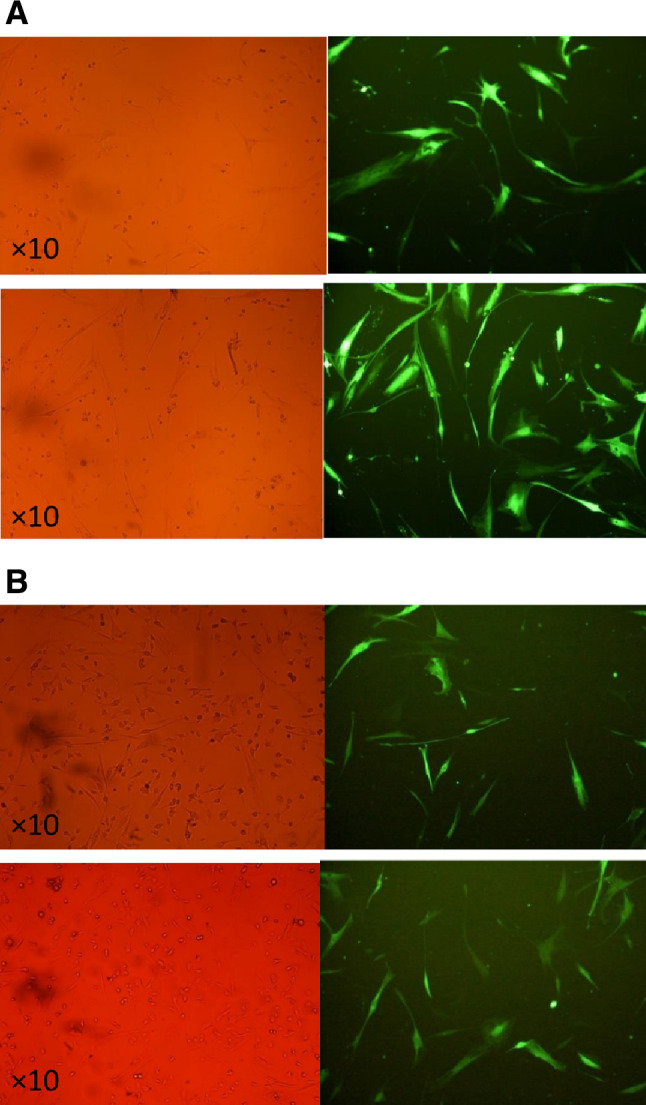
A Transduced MSCs with recombinant lentivirus carrying miR-30c 48 and 72 h after the transduction. B Co-cultured MSCs and CT-26 cells after the 48 and 72 h upon the co-culturing stained by GFP to find the cells expressed miR-30c
Evaluation of IL-6 and miR-30c expression
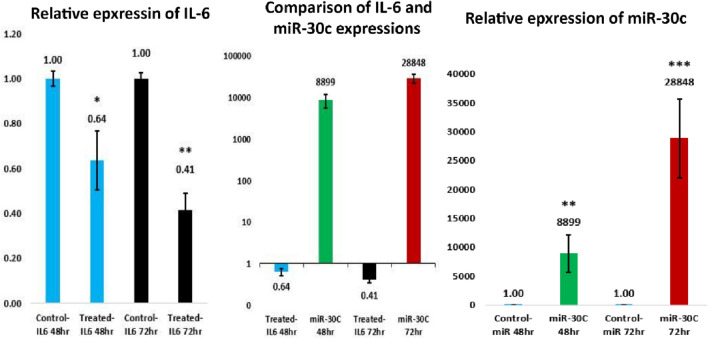
The result of qRT-PCR showed that after transduction of MSCs with the recombinant lentiviruses carrying the miR-30c gene and co-culturing of these cells with CT-26 cells, the expression level of miR-30c was significantly increased in CT-26 cells after 72 h about 28,848 times more than control cells. On the other hand, this transduction gave rise to the reduced level of IL-6 in these cells after 72 h, which suggested an associated expression between miR-30c and IL-6 (Fig. 7).
Fig. 7.
Altered expression of IL-6 and miR-30c in CT-26 cells in different period of time. The results show significant decreasing in IL-6 level. (*P < 0.05, **P < 0.01, ***P < 0.001)
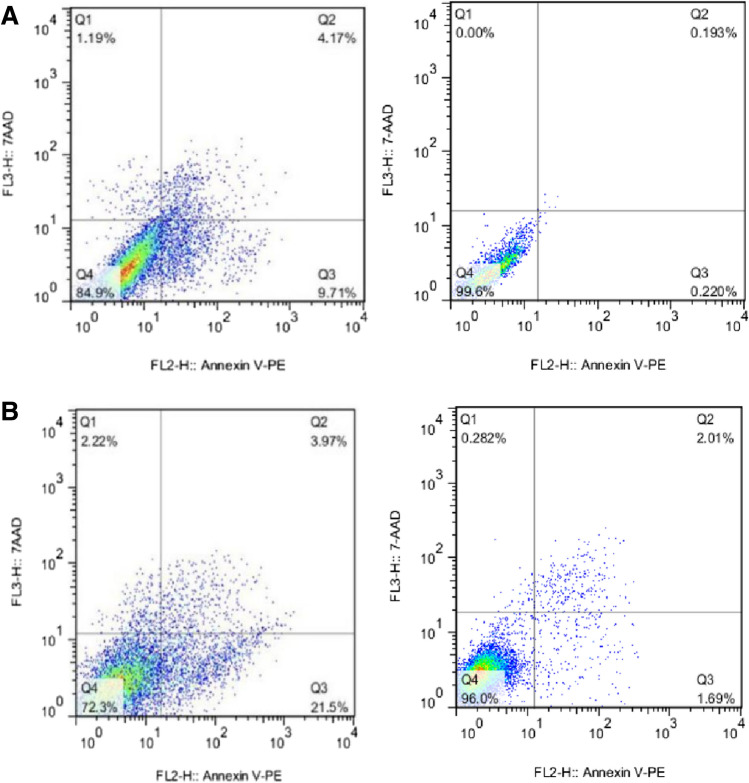
Effects of miR-30c-transduced MSCs and non-transduced-MSC on the apoptosis of CT-26 cells
Forty-eight and seventy-two h after the co-culturing of miR-30c-transduced MSCs and non-transduced-MSC with CT-26 cells, the apoptosis rate was assessed, and the obtained data revealed the induction of apoptosis in CT-26 cells when they are in the vicinity of miR-30c-transduced MSCs. The relative apoptosis rat at 48 h and 72 h was about 13.88 and 25.47%, respectively (Fig. 8).
Fig. 8.
A The effect of MSCs-transduced and non-transduced co-culturing on CT-26 cells 48 h after the co-culturing. B The effect of MSCs-transduced and non-transduced co-culturing on CT-26 cells 72 h after the co-culturing
Releasing of IL-6 from the transduced MSCs and non-transduced-MSC as well as CT-26 cells co-cultured with them
The supernatant of transduced and non-transduced MSCs with lentiviruses containing miR-30c was collected after 48 and 74 h to evaluate the change in the amount of IL-6 produced by these cells and was measured by the ELISA. There was a significant decrease in IL-6 secretion in the transduced MSCs supernatant compared to the control group. Moreover, to evaluate the change in the concentration of IL-6 produced by CT-26 cells co-cultured with transduced and non-transduced MSCs, after 28 and 74 h, the supernatant was collected from the culture medium. The results showed a significant reduction in the secretion of IL-6 in the supernatant of CT-26 cells cultured with both transduced and non-transduced MSCs (Fig. 9).
Fig. 9.
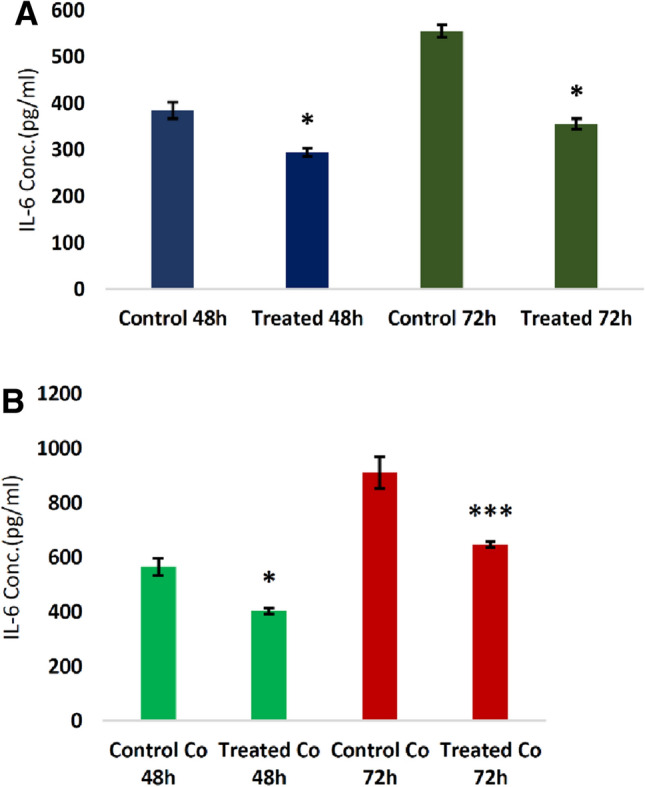
A Releasing of IL-6 in the medium of transduced and non-transduced MSCs after 48 and 72 h upon the transduction. The results showed a significant reduced level of IL-6. B Releasing of IL-6 in the medium of co-cultured CT-26 cells with transduced and non-transduced MSCs. The results showed a significant reduced level of IL-6. (*P < 0.05, ***P < 0.001)
Discussion
MSCs could vary in various forms of cells and are a heterogeneous population of self-renewable cells with multiple abilities (Ullah et al. 2015). They can move to inflammatory sites and exert inflammatory and immunomodulatory functions through the interaction with the immune system (Zhao et al. 2016). A better understanding of MSC biology and work on them can further be enhanced by a greater knowledge of the inherent gene regulation and the external environmental stimuli and instructions that control MSC fate decisions. MSC-CM plays a key role in controlling tumor initiation and development by its positive or negative effect on invasion, migration, or apoptosis resistance of tumor cells in collecting secreted cytokines and growth factors (Kyurkchiev et al. 2014). Among these secreted factors, IL-6 as a pro-inflammatory cytokine has been proven to contribute to metastatic cancers (Mi and Gong 2017), including HCC (Mano et al. 2013), gastric cancer (Wang et al. 2013), breast cancer (Hartman et al. 2013), and lung cancer (Li et al. 2014), and colorectal cancer (Zhang et al. 2018). IL-6 is a cytokine known by various names, reflecting its various cell types' actions. Several clinical series independently reported an association between IL-6 and patient prognosis in different cancer types (Lippitz and Harris 2016). Research has shown that IL-6 levels can be useful for diagnosis, recurrence, and recurrence (Unver and McAllister 2018). Komoda et al. found that IL-6 levels in malignant tissues in 32 patients diagnosed with CRC were substantially higher than normal colorectal tissues, concluding that IL-6 could play a role in tumor development (Komoda et al. 1998). Zeng et al. also reported that IL-6 can be a valuable prognostic predictor, as expression was significantly higher in CRC tissues relative to non-cancerous tissues (Zeng et al. 2017). In CRC, elevated IL-6 serum levels were also associated with lower disease outcome (Chung and Chang 2003).
MiRNAs can directly control several protein-coding genes, and bioinformatics estimates suggest that miRNAs control more than 30–60% of protein-coding genes in the human genome. Among several miRNAs, the microRNA family, miR-30, plays several roles in these main processes of cancer transformation, metastasis, and clinical outcomes. (Han et al. 2020). MiR-30c belongs to the miR-30 miRNA family. These family members share a similar seeding sequence near the 5′ end but differ in the sequences near the 3′ end. This variation in compensatory sequences between family members enables miRNAs from the same family to target different genes and pathways (Brennecke et al. 2005) microRNA-30c functions as a tumor suppressor or a tumor promoter in multiple human malignancies. Published articles suggest that loss of expression of miR-30c leads to numerous malignancies, including breast cancer, endometrial cancer, lung cancer, esophageal squamous cell carcinoma, and CRC (Ma et al. 2018; Zhang et al. 2015; Bockhorn et al. 2013a; Zhou et al. 2012; Xia et al. 2013). In line with these results, we established IL-6 as a direct or indirect miR-30c target and demonstrated that miR-30c upregulation in MSC could significantly reduce IL-6 expression. In this research, we revealed that transduced MSCs co-cultured with CT-26 cells could significantly decrease IL-6 expression as well as the reduced protein level of this pro-inflammatory factor in the medium that surrounds these cells. Our previous study highlighted that miR-30c could target IL-6 in glioblastoma multiform cells and decrease its expression in these cancerous cells (Mahjoor et al. 2021b). Zhou et al. documented that microRNA-30-3p inhibits inflammatory factor-induced cell injury by targeting TCF21. This research reveals that miR-30-5p suppresses NF-κB and MAPK-induced inflammatory responses by targeting TCF21 in the in vitro atherosclerosis model (Zhou et al. 2012). Also, microRNA-30c-5p was found to inhibit NLRP3 inflammasome-mediated endothelial cell pyroptosis through downregulation in atherosclerosis by Forkhead box O3 (FOXO3) (Li et al. 2018). Duecker et al. depicted that compared to controls, bronchiolitis patients showed considerably more airway inflammation in produced sputum, as evidenced by the expression level of IL-6. They also revealed that the expression of miR-30c was reduced in these patients, and this miRNA mostly functions in cytokine–cytokine receptor interaction (Duecker et al. 2022). Besides, it is reported that miRNA-30c, a prognostic marker for human breast cancer, plays a key role in chemoresistance by targeting the actin-binding protein twinfilin 1, which promotes epithelial-to-mesenchymal transition. Interleukin-11 (IL-11), a member of the IL-6 family, has been discovered as a secondary target of twinfilin 1 in the miRNA-30c signaling pathway. miRNA-30c expression in primary breast tumors correlates negatively with IL-11 expression, and low IL-11 corresponds with relapse-free survival in breast cancer patients (Bockhorn et al. 2013b).
In the present study, the results of Annexin V staining confirmed that miR-30c plays a substantial role in the stimulation of apoptosis in co-cultured cells. Flow cytometry was also used to define cell apoptosis following miR-30c expression. According to a current study, it is revealed that compared with cells transfected with the negative control, transfection with the miR-30c mimics leads to significantly increased rates of apoptosis. In our previous study, the effect of MSC-transduced miR-30c on the induction of apoptosis in glioblastoma multiform co-cultured with transduced MSCs was considered, and the results showed that the elevated expression of this miRNA could significantly increase the apoptosis rate in glioblastoma cells (Mahjoor et al. 2021b). Ying Wang et al. also suggested a decrease in cell viability and higher apoptotic rates of the transfection of miR‐30c mimics into prostate cancer cells. Transfection with the miR‐30c inhibitor, in contrast, led to lower apoptosis rates for prostate cancer cells relative to negative control groups (Wang et al. 2020). Moreover, Barez et al. add to the growing data that miR-30c-2-3p may be treated by inhibiting XBP1 transcription in ovarian cancer cells, triggering apoptosis via XBP1/CHOP/BIM mediators (Barez et al. 2021).
Conclusion
Our research provides new insight into the activity of the miR-30c/IL-6 axis in developing CRC. We demonstrated that upregulation of miR-30c in MSC could significantly affect the expression of IL-6 in CRC cells and trigger apoptosis in these cells. So, the present study elucidated the probable in vitro anticancer effects of miR-30c-upregulated MSC in CRC and shed new light on CRC treatment prospects.
Author contributions
MM: data collection; HA: data collection; MN: writing manuscript; AN: help to mn for writing manuscript; SK: corresponding author.
Funding
There is no funding sources.
Declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Arnold M, Sierra MS, Laversanne M, Soerjomataram I, Jemal A, Bray F (2017) Global patterns and trends in colorectal cancer incidence and mortality. Gut 66(4):683–691 [DOI] [PubMed] [Google Scholar]
- Barez SR, Attar AM, Aghaei M (2021) MicroRNA-30c-2-3p regulates ER stress and induces apoptosis in ovarian cancer cells underlying ER stress. EXCLI J 20:922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bockhorn J, Yee K, Chang Y-F, Prat A, Huo D, Nwachukwu C et al (2013a) MicroRNA-30c targets cytoskeleton genes involved in breast cancer cell invasion. Breast Cancer Res Treat 137(2):373–382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bockhorn J, Dalton R, Nwachukwu C, Huang S, Prat A, Yee K et al (2013b) MicroRNA-30c inhibits human breast tumour chemotherapy resistance by regulating TWF1 and IL-11. Nat Commun 4(1):1–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bracken CP, Scott HS, Goodall GJ (2016) A network-biology perspective of microRNA function and dysfunction in cancer. Nat Rev Genet 17(12):719 [DOI] [PubMed] [Google Scholar]
- Brennecke J, Stark A, Russell RB, Cohen SM (2005) Principles of microRNA–target recognition. PLoS Biol 3(3):e85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung YC, Chang YF (2003) Serum interleukin-6 levels reflect the disease status of colorectal cancer. J Surg Oncol 83(4):222–226 [DOI] [PubMed] [Google Scholar]
- Cuiffo BG, Karnoub AE (2012) Mesenchymal stem cells in tumor development: emerging roles and concepts. Cell Adh Migr 6(3):220–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derakhshani A, Kamyar KF, Barzegari Banadkoki S, Shirazi FH, Barati M, Fereidouni M et al (2019) Optimization of induction parameters, structure quality assessment by ATR-FTIR and in silico characterization of expressed recombinant polcalcin in three different strains of Escherichia coli. Int J Biol Macromol 138:97–105 [DOI] [PubMed] [Google Scholar]
- Duecker RP, De Mir MI, Jerkic SP, Kochems A, Gottwald G, Moreno-Galdó A et al (2022) Epigenetic regulation of inflammation by microRNAs in post-infectious bronchiolitis obliterans. Clin Transl Immunol 11(2):e1376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han W, Cui H, Liang J, Su X (2020) Role of MicroRNA-30c in cancer progression. J Cancer 11(9):2593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman ZC, Poage GM, Den Hollander P, Tsimelzon A, Hill J, Panupinthu N et al (2013) Growth of triple-negative breast cancer cells relies upon coordinate autocrine expression of the proinflammatory cytokines IL-6 and IL-8. Can Res 73(11):3470–3480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SA, Jenkins BJ (2018) Recent insights into targeting the IL-6 cytokine family in inflammatory diseases and cancer. Nat Rev Immunol 18(12):773–789 [DOI] [PubMed] [Google Scholar]
- Komoda H, Tanaka Y, Honda M, Matsuo Y, Hazama K, Takao T (1998) Interleukin-6 levels in colorectal cancer tissues. World J Surg 22(8):895–898 [DOI] [PubMed] [Google Scholar]
- Kyurkchiev D, Bochev I, Ivanova-Todorova E, Mourdjeva M, Oreshkova T, Belemezova K et al (2014) Secretion of immunoregulatory cytokines by mesenchymal stem cells. World J Stem Cells 6(5):552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazennec G, Lam PY (2016) Recent discoveries concerning the tumor-mesenchymal stem cell interactions. Biochimica Et Biophysica Acta (BBA) 1866(2):290–299 [DOI] [PubMed] [Google Scholar]
- Lee HY, Hong IS (2017) Double-edged sword of mesenchymal stem cells: cancer-promoting versus therapeutic potential. Cancer Sci 108(10):1939–1946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Han R, Xiao H, Lin C, Wang Y, Liu H et al (2014) Metformin sensitizes EGFR-TKI–resistant human lung cancer cells in vitro and in vivo through inhibition of IL-6 signaling and EMT reversal. Clin Cancer Res 20(10):2714–2726 [DOI] [PubMed] [Google Scholar]
- Li P, Zhong X, Li J, Liu H, Ma X, He R et al (2018) MicroRNA-30c-5p inhibits NLRP3 inflammasome-mediated endothelial cell pyroptosis through FOXO3 down-regulation in atherosclerosis. Biochem Biophys Res Commun 503(4):2833–2840 [DOI] [PubMed] [Google Scholar]
- Lippitz BE, Harris RA (2016) Cytokine patterns in cancer patients: a review of the correlation between interleukin 6 and prognosis. Oncoimmunology 5(5):e1093722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma T, Zhao Y, Lu Q, Lu Y, Liu Z, Xue T et al (2018) MicroRNA-30c functions as a tumor suppressor via targeting SNAI1 in esophageal squamous cell carcinoma. Biomed Pharmacother 98:680–686 [DOI] [PubMed] [Google Scholar]
- Mahjoor M, Afkhami H, Mollaei M, Nasr A, Shahriary S, Khorrami S (2021a) MicroRNA-30c delivered by bone marrow-mesenchymal stem cells induced apoptosis and diminished cell invasion in U-251 glioblastoma cell line. Life Sci 279:119643 [DOI] [PubMed] [Google Scholar]
- Mahjoor M, Afkhami H, Mollaei M, Nasr A, Shahriary S, Khorrami S (2021b) MicroRNA-30c delivered by bone marrow-mesenchymal stem cells induced apoptosis and diminished cell invasion in U-251 glioblastoma cell line. Life Sci 279:119643 [DOI] [PubMed] [Google Scholar]
- Mano Y, Aishima S, Fujita N, Tanaka Y, Kubo Y, Motomura T et al (2013) Tumor-associated macrophage promotes tumor progression via STAT3 signaling in hepatocellular carcinoma. Pathobiology 80(3):146–154 [DOI] [PubMed] [Google Scholar]
- Mao L, Liu S, Hu L, Jia L, Wang H, Guo M et al (2018) miR-30 family: a promising regulator in development and disease. BioMed Res Int 2018:1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mármol I, Sánchez-de-Diego C, Pradilla Dieste A, Cerrada E, Rodriguez Yoldi MJ (2017) Colorectal carcinoma: a general overview and future perspectives in colorectal cancer. Int J Mol Sci 18(1):197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCann JV, Xiao L, Kim DJ, Khan OF, Kowalski PS, Anderson DG et al (2019) Endothelial miR-30c suppresses tumor growth via inhibition of TGF-β–induced Serpine1. J Clin Invest 129(4):1654–1670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mi F, Gong L (2017) Secretion of interleukin-6 by bone marrow mesenchymal stem cells promotes metastasis in hepatocellular carcinoma. Biosci Rep. 10.1042/BSR20170181 [DOI] [PMC free article] [PubMed]
- Moslemi M, Sohrabi E, Azadi N, Zekri A, Afkhami H, Khaledi M et al (2020) Expression analysis of EEPD1 and MUS81 genes in breast Cancer. Bio J Sci Tech Res 29:22556–22564 [Google Scholar]
- Moslemi M, Moradi Y, Dehghanbanadaki H, Afkhami H, Khaledi M, Sedighimehr N et al (2021) The association between ATM variants and risk of breast cancer: a systematic review and meta-analysis. BMC Cancer 21(1):1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridge SM, Sullivan FJ, Glynn SA (2017) Mesenchymal stem cells: key players in cancer progression. Mol Cancer 16(1):31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah MY, Ferrajoli A, Sood AK, Lopez-Berestein G, Calin GA (2016) microRNA therapeutics in cancer—an emerging concept. EBioMedicine 12:34–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel RL, Miller KD, Fedewa SA, Ahnen DJ, Meester RG, Barzi A et al (2017) Colorectal cancer statistics, 2017. CA: A Cancer J Clinicians 67(3):177–193 [DOI] [PubMed] [Google Scholar]
- Sohrabi E, Moslemi M, Rezaie E, Nafissi N, Khaledi M, Afkhami H et al (2021) The tissue expression of MCT3, MCT8, and MCT9 genes in women with breast cancer. Genes Genomics 43(9):1065–1077 [DOI] [PubMed] [Google Scholar]
- Spinelli SV, Fernández RDV, Zoff L, Bongiovanni B, Díaz A, D’Attilio L et al (2017) miR-30c is specifically repressed in patients with active pulmonary tuberculosis. Tuberculosis 105:73–79 [DOI] [PubMed] [Google Scholar]
- Sun R, Muheremu A, Hu Y (2018) miRNA-30c can be used as a target in the diagnosis and treatment of osteosarcoma. Onco Targets Ther 11:9091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullah I, Subbarao RB, Rho GJ (2015) Human mesenchymalstem cells—current trends and future prospective 35(2):e00191 [DOI] [PMC free article] [PubMed]
- Unver N, McAllister F (2018) IL-6 family cytokines: key inflammatory mediators as biomarkers and potential therapeutic targets. Cytokine Growth Factor Rev 41:10–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Si X, Xu A, Meng X, Gao S, Qi Y et al (2013) Activation of STAT3 in human gastric cancer cells via interleukin (IL)-6-type cytokine signaling correlates with clinical implications. PLoS ONE 8(10):e75788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Pei X, Xu P, Tan Z, Zhu Z, Zhang G et al (2020) E2F7, regulated by miR-30c, inhibits apoptosis and promotes cell cycle of prostate cancer cells. Oncol Rep 44(3):849–862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe AR, Trenton NJ, Debeb BG, Larson R, Ruffell B, Chu K et al (2016) Mesenchymal stem cells and macrophages interact through IL-6 to promote inflammatory breast cancer in pre-clinical models. Oncotarget 7(50):82482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Y, Chen Q, Zhong Z, Xu C, Wu C, Liu B et al (2013) Down-regulation of miR-30c promotes the invasion of non-small cell lung cancer by targeting MTA1. Cell Physiol Biochem 32(2):476–485 [DOI] [PubMed] [Google Scholar]
- Zeng J, Tang Z-H, Liu S, Guo S-S (2017) Clinicopathological significance of overexpression of interleukin-6 in colorectal cancer. World J Gastroenterol 23(10):1780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Yu L, Qin D, Huang R, Jiang X, Zou C et al (2015) Role of microRNA-30c targeting ADAM19 in colorectal cancer. PLoS ONE 10(3):e0120698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Hu F, Li G, Li G, Yang X, Liu L et al (2018) Human colorectal cancer-derived mesenchymal stem cells promote colorectal cancer progression through IL-6/JAK2/STAT3 signaling. Cell Death Dis 9(2):1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Q, Ren H, Han Z (2016) Mesenchymal stem cells: Immunomodulatory capability and clinical potential in immune diseases. J Cell Immunother 2(1):3–20 [Google Scholar]
- Zhou H, Xu X, Xun Q, Yu D, Ling J, Guo F et al (2012) microRNA-30c negatively regulates endometrial cancer cells by targeting metastasis-associated gene-1. Oncol Rep 27(3):807–812 [DOI] [PubMed] [Google Scholar]
- Zhou Z, Chen Y, Zhang D, Wu S, Liu T, Cai G et al (2019) MicroRNA-30–3p suppresses inflammatory factor-induced endothelial cell injury by targeting TCF21. Mediators Inflammation 2019:1–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Mi F, Gong L (2017) Secretion of interleukin-6 by bone marrow mesenchymal stem cells promotes metastasis in hepatocellular carcinoma. Biosci Rep. 10.1042/BSR20170181 [DOI] [PMC free article] [PubMed]