Abstract
Since the description of zebrafish (Danio rerio) in 1822, the identity of its closest living relative has been unclear. To address this problem, we sequenced the exomes of 10 species in genus Danio, using the closely related Devario aequipinnatus as outgroup, to infer relationships across the 25 chromosomes of the zebrafish genome. The majority of relationships within Danio were remarkably consistent across all chromosomes. Relationships of chromosome segments, however, depended systematically upon their genomic location within zebrafish chromosomes. Regions near chromosome centers identified Danio kyathit and/or Danio aesculapii as the closest relative of zebrafish, while segments near chromosome ends supported only D. aesculapii as the zebrafish sister species. Genome-wide comparisons of derived character states revealed that danio relationships are inconsistent with a simple bifurcating species history but support an ancient hybrid origin of the D. rerio lineage by homoploid hybrid speciation. We also found evidence of more recent gene flow limited to the high recombination ends of chromosomes and several megabases of chromosome 20 with a history distinct from the rest of the genome. Additional insights gained from incorporating genome structure into a phylogenomic study demonstrate the utility of such an approach for future studies in other taxa. The multiple genomic histories of species in the genus Danio have important implications for comparative studies in these morphologically varied and beautiful species and for our understanding of the hybrid evolutionary history of zebrafish.
Keywords: zebrafish, danios, phylogenomics, genome structure, hybrid species, introgression
Graphical abstract
Graphical Abstract.
Introduction
Despite the status of Danio rerio as a major model organism, its recent evolutionary history remains unclear. Different phylogenetic studies of the genus Danio pointed to several different species as the sister group to zebrafish (Meyer et al. 1993; Fang 2003; Quigley et al. 2004; Mayden et al. 2007, 2008; Fang et al. 2009; Tang et al. 2010; McCluskey and Postlethwait 2015). This confusion was due in part to limited phylogenetic signal in the few genes examined and the ongoing discovery of danio biodiversity (Fang 1998; Kullander and Fang 2009a). The first study of genus Danio using a phylogenomic approach (McCluskey and Postlethwait 2015) proposed that the discordant results of past studies could be explained if different regions within a danio species had different genomic histories.
Determining relationships among closely related species relies on genetic variants in the form of shared derived characters (SDCs). These variants ultimately arise from ancestral alleles (denoted “A”) being converted to derived alleles (denoted “B”) via de novo mutation in an ancestral population. A single population history can contain several different genomic histories (Fig. 1, a–c). Discordant histories can arise from various processes that can be discerned by the relative frequencies of derived characters shared by different taxa across the genome. When species arise rapidly from an ancestral population, alleles segregating in the ancestral population can be inherited in a pattern that does not necessarily reflect the history of population bifurcation (Holder et al. 2001) in a process known as incomplete lineage sorting of alleles (ILS, Fig. 1d). Under ILS, the most frequent pattern of SDCs will be those shared by sister species, as new alleles are generated in an ancestral population shared by only 2 species (dashed lines in Fig. 1d). Alleles inherited by ILS will also occur, but will be balanced between sister taxa, with each sister species sharing roughly the same number of alleles with more distantly related species (Fig. 1d).
Fig. 1.
Demographic events and distribution of SDCs in related lineages. Ancestral alleles are denoted “A” and derived alleles “B,” with alleles segregating in multiple individuals in populations over time. Derived alleles present in the ancestral population of the 3 species are shown as colored lines. Alleles arising in ancestral populations after the split from the outgroup and shared by only 2 species (dashed lines) are shown with shading. a) Mutations can occur and reflect the history of the population tree. b and c) Alternative ways of inheriting ancestrally segregating polymorphisms by ILS of alleles. d) Expectations with ILS as the only source of multiple genomic histories (compared to panels b) and c)). e) Expectations with introgression from 1 species. Introgression can also occur from 2 different species, not shown. f) Expectations with HHS. HHS is a special type of HAS.
Alleles can also be exchanged between diverged populations via introgression (Fig. 1e). Introgression can be inferred from an excess of 1 pattern of alleles that can be inherited by ILS. The regions of the genome affected by introgression can be detected by an excess of derived character states relative to other regions in the genome (Durand et al. 2011; Eaton and Ree 2013; Supple et al. 2013; Martin et al. 2015; Li et al. 2016). Regions of high recombination are expected to preferentially retain alleles passed by introgression because they can be more easily separated from linked maladaptive alleles than alleles in low-recombination regions. The most extreme example of discordant genomic histories occurs in instances of hybridization-associated speciation (HAS), such as when a new species forms following a hybrid swarm, repeated gene flow following initial divergence, or via homoploid hybrid speciation (HHS) (Fig. 1f). Different forms of HAS can be difficult or impossible to distinguish using only genomic data (Schumer et al. 2014), but species originating from any type of HAS are more closely related to each parent species than to any other species and should harbor roughly equal SDCs inherited from each parent species. In contrast to introgression, signals of HAS will occur genome wide rather than mainly in regions with high rates of recombination.
Phylogenomic analyses of several taxa have used patterns of SDCs across the genome to infer population histories from local genome histories. Such groups include systems with ecology and mating systems predisposing them to gene flow, such as oaks, broomrape, cats, mosquitoes, and butterflies (Eaton and Ree 2013; Hipp et al. 2014; Fontaine et al. 2015; Li et al. 2016; Rosser et al. 2024); groups that radiated rapidly such as cichlid fish, swordtails, tomatoes, and birds (Meyer et al. 2015; Prum et al. 2015; Pease et al. 2016; Schumer et al. 2018; Du et al. 2024); and key model organisms including drosophila (Drosophila 12 Genomes Consortium et al. 2007; Garrigan et al. 2012) and primates (Scally et al. 2012; Ting and Sterner 2013; Prufer et al. 2014; Sankararaman et al. 2014; Gordon et al. 2016).
Several aspects of genome structure have been shown to correlate with ILS and/or introgression including chromosomal location, gene density, and recombination rate (Mallet 2005; Turner et al. 2005; Ellegren et al. 2012; Scally et al. 2012; Burri et al. 2015; Fontaine et al. 2015). These effects can result in distinct patterns across the genome, which are not apparent without incorporating genomic structure into the analysis.
As more research groups use the genus Danio as a model for evolution (Mahalwar et al. 2014; McMenamin et al. 2014; Patterson et al. 2014; McCluskey and Postlethwait 2015; Singh and Nusslein-Volhard 2015; Irion and Nusslein-Volhard 2019; Podobnik et al. 2020; Huang et al. 2021; McCluskey, Liang, et al. 2021a; McCluskey, Uji, et al. 2021b; Toomey et al. 2022; Podobnik et al. 2023), it becomes increasingly important to understand relationships within the genus to properly interpret the trajectory of evolutionary change. Here, we demonstrate how genome structure mediated admixture between the closest relatives of zebrafish. Our findings show that the D. rerio lineage arose from genome-wide admixture between 2 separate lineages, compatible with HHS.
Materials and methods
Library preparation and exome alignment creation
DNAs were collected from the following species: zebra danio (D. rerio, AB strain), orange-finned danio (Danio kyathit), panther danio (Danio aesculapii), spotted danio (Danio nigrofasciatus), pearl danio (Danio albolineatus), Kerr's danio (Danio kerri), glowlight danio (Danio choprae), celestial pearl danio (Danio margaritatus), emerald dwarf danio (Danio erythromicron), Meghalaya danio (Danio meghalayensis), and giant danio (Devario aequipinnatus). Fish were sourced from local hobby aquarium suppliers with the exception of D. rerio, which were AB strain and sourced from the Zebrafish International Research Center (Eugene, OR). The University of Oregon Animal Care and Use Committee approved all protocols associated with this work. We extracted and purified genomic DNA using a Blood and Tissue Kit (Qiagen) and constructed libraries and performed exome enrichment with the All Exon Zebrafish oligonucleotide bait panel (Agilent) according to the manufacturer's instructions. Exome-enriched libraries were quantified using a Qubit fluorometer and sequenced on the Illumina HiSeq 2500 (single-end 100 bp reads) and the Illumina NextSeq (single-end 75 bp reads). We quality-filtered Illumina reads with Trimmomatic (Bolger et al. 2014) using a per-base quality score minimum set to 20 and minimum read length of 30 nucleotides.
To generate orthologous sequence alignments for phylogenetic inference, we first aligned genomic reads to coding sequence for APPRIS primary gene models (Rodriguez et al. 2013) from the zebrafish genome GRCz10 v82 (Kersey et al. 2016). We used GSNAP release 2015-07-23 (Wu and Nacu 2010) with the following parameters to account for mapping genomic reads to a transcript reference and for the sequence divergence between species: -k 12 –expand-offsets = 1 –max-mismatches = 0.2 –npaths = 1. Consensus sequences for each species were generated with samtools v 1.1 (Danecek et al. 2021) using the mpileup command with parameters -B -C 0 -Q 0 -q 0 -m 1 -e 0 -F 0 -h 0 -o 0 followed by the “bcftools call” command with the -c flag. These parameters ensured that reads with any evidence of indels were flagged and excluded, while allowing soft clipping of the genomic reads to the reference transcripts. Consensus sequences from 11,635 genes each having at least 500 unambiguously aligned nucleotide positions with at least 10× coverage across each species were concatenated to form the 15.45 Mb unpartitioned, genome-wide data set. These aligned positions were split according to chromosome and position to create the 250 jackknife alignments.
To identify variants in danio species across the danio genome, we aligned Illumina reads for all 11 species to the zebrafish genome (GRCz10 v 82). To handle the amount of sequence divergence between the zebrafish reference genome and sequences from other species, we used bbmap (Bushnell 2014), a global alignment algorithm with permissive parameters, but required a single best alignment to the zebrafish genome. The BBMap parameters were ambiguous = best minidentity = 0.70 maxindel = 100 idtag = t k = 12. To extract variants from coding sequence across the genome, we used samtools mpileup with the following parameters: –no-BAQ –adjust-MQ 0 –min-BQ 13 –min-MQ 0 –min-ireads 1 –ext-prob 20 –gap-frac 0.002 –tandem-qual 100 –VCF –uncompressed –output-tags DP,DPR,DV,DP4,INFO/DPR,SP. We then filtered variants using the “vcf-annotate” function from vcftools v0.1.12a to include only sites with at least 5× coverage and exclude sites with 3 or more SNPs within 10 base pairs. The resulting variant calls were kept for subsequent analysis outlined below.
Comparisons to existing sequences
Reconstructed sequences for rho and rag1 from each species included in this study were compared to all available Danio (taxid:7954) and Devario (taxid:439832) sequences using BLASTN with default parameters on the NCBI BLAST site. The top BLAST hit to the appropriate species is included in Supplementary Table 2. In one instance (D. meghalayensis rag1), no sequences were available, so a match from the closely related Danio dangila was included. When multiple matches were present, the match with the highest percent identity was selected.
Derived characters shared by D. rerio and either D. kyathit or D. aesculapii were compared to D. rerio RAD-seq data from 2 studies (McCluskey and Postlethwait 2015); (Suurväli et al. 2020). Sequences from 2 individuals each of from 6 populations were used: AB (SRR2912296 and SRR2912297); TU (SRR2912299 and SRR2912300); WIK (SRR2912301 and SRR2912302); UT (SRR9696604 and SRR9696603); KHA (SRR9696610 and SRR9696617); and CHT (SRR9696613 and SRR9696612). RAD-seq data were aligned to the zebrafish reference genome using bwa mem with default parameters, converted to variant call format using bcftools mpileup (with “-Oz” specified to generate.vcf.gz output), and genotyped using bcftools call with the multiallelic-caller option. These genotypes were then compared to the danio exome data to find overlapping positions.
Maximum likelihood phylogenetic analyses and hybridization detection
To infer phylogenies for different jackknife windows, we partitioned data from each chromosome into 10 bins each representing 10% of the total aligned sequence for a chromosome. For the unpartitioned genomic alignment and each of the 250 jackknife partitions, we inferred maximum likelihood phylogenies and approximate likelihood ratio tests under a GTR + I+Γ model in RAxML v 8.2.3 (Stamatakis 2006) on the University of Oregon's super computer (http://aciss-computing.uoregon.edu). We determined topology frequencies and visualized phylogenies using DensiTree (Bouckaert 2010). We performed concordance analyses in BUCKy v 1.4.2 (Larget et al. 2010) with default settings.
To test our proposed model of a hybrid origin of D. rerio between the D. aesculapii and D. kyathit lineages, we used HyDe for hybrid detection (Blischak et al. 2018). The sequence alignments analyzed were the same as those used for the 250 jackknife trees, partitioned according to chromosome position. Taxa were input such that D. nigrofasciatus was the outgroup and the value of gamma returned by HyDe corresponded to the proportion of the D. rerio genome with ancestry coming from the D. kyathit lineage.
Genomic structure analyses
To investigate relationships along the genome at a finer scale, we extracted genotypes from .vcf files for the genomic alignments outlined previously. We recoded genotypes (A, C, G, or T) at biallelic sites to splits (A, ancestral, or B, derived) for subsequent analysis and visualization. To avoid confounding factors of regions of exceptionally low diversity, split frequencies were calculated for variable positions only. Due to variation in gene density and the discontiguous nature of our exome data across the genome, we standardized analyses to include the same number of splits rather than analyzing relative to windows of equal numbers of nucleotides in the nuclear genome. This approach ameliorated the effects of stochastic sampling that can cause false positives in certain types of genome scans (Martin et al. 2015). Chromoplots and line plots were plotted in R using the plot function of the ggplot2 package.
D-statistics were calculated as previously described (Durand et al. 2011) using windows of 200 ABBA/BABA sites and plotted for every site based on the calculated value from the surrounding 200 ABBA/BABA sites. Ancestral alleles were determined based on sequences for all available basal danios. Any sites with polymorphism between basal species were removed to avoid the possible influence of introgression with the outgroup. For the analysis of all 6 pairwise splits, sliding windows of 500 splits, jumping by 10 sites, were used. Regions enriched for particular splits were identified in R using a binomial distribution with an expected frequency of one sixth (there are 6 possible species pairs in the rerio species group) and the number of trials set to the window size of 500. RAD-site locations were from McCluskey and Postlethwait (2015). Gene locations were downloaded from Ensembl BioMart for protein-coding genes on the 25 chromosomes from GRCz10 v 82.
Results
Exome enrichment and data validation
To investigate the evolutionary history of the genus Danio, we used RNA baits complementary to annotated D. rerio nuclear mRNA genes to isolate and sequence exomes from 10 danio species varying in body size, pigment pattern, barbel number, and geographic distribution, as well as 1 species from the closely related Devario genus (Supplementary Table 1). We applied stringent filtering to ensure that only high quality sites were used for phylogenetic inference (see Materials and methods). Our main data set comprised 15.44 Mb of aligned nucleotides from 11,682 genes (1.1% of the nuclear genome sampled from nearly half of all protein-coding genes with annotated primary transcripts), which is over 100 times more sequence than the most recent Danio phylogenomic study based on reduced representation sequencing (McCluskey and Postlethwait 2015). This data set has notable limitations, however. Capture baits lacked mitochondrial genes, so these data can only address the history of the nuclear genome. Furthermore, filtering for codons with alignable sequence across all species means our data set will miss sequences that had identity too low to effectively bind to the RNA baits, a possibility for some rapidly evolving genes given the evolutionary distances involved. Lastly, we have sequenced only 1 sample per species, and all individuals were obtained either from established laboratory strains (AB strain D. rerio) or from the pet trade. Thus, some of the variation we see in these fish has been shaped by their domestication and is only a point estimate of the considerable variation present in the wild (Suurväli et al. 2020).
To validate the accuracy of this exome-sequencing method in recapitulating known sequences for each species and to determine its utility for constructing gene trees, we compared sequences generated by our pipeline to reference sequences for rag1 and rho, 2 genes used in previous phylogenetic studies with sequences for danios and related species available in GenBank. The reconstituted rho and rag1 sequences from our exome data all had BLAST hits to the correct species (when present in GenBank) with greater than 98% identity (Supplementary Table 2).
Widespread genealogical incongruence
To investigate relationships among danio species, we inferred maximum likelihood phylogenies using several partitioning strategies to explicitly account for genome structure, inheritance patterns, and multiple genomic histories. As a first approximation of the history of these species, we assembled all codons that passed filtering into a single alignment and inferred a single phylogeny from genes sampled from the entire nuclear genome (Fig. 2a). We refer to this phylogeny as the Unpartitioned Genome Phylogeny.
Fig. 2.
Genealogical discordance and the effects of chromosome structure. a) The unpartitioned genome phylogeny inferred from the concatenated sequence for each species with concordance factors based on the phylogenies inferred for the 250 jackknife partitions. Dashed lines denote relationships not found in the unpartitioned genome topology, but supported by at least 10% of jackknife partitions. Splits supported by <10% of trees are not shown. A gray box demarcates the D. rerio species group. b) Concordance factors of pairwise splits grouping 2 species as sister taxa within the D. rerio species group for the 250 jackknife partitions, with major concordances shown on the left and minor concordances listed on the right. c) Five genealogical histories in the D. rerio species group explain relationships inferred for 93.2% of windows across the genome and a minority of other topologies explain the rest (6.8%, gray). The frequency of the most common jackknife topologies binned according to their position across chromosomes, indicated as a folded chromosome with the 2 telomeres at the right and the chromosome middle on the left. Each bin contains 50 partitions corresponding, for example, to alignments near the ends of each of the 25 Danio chromosomes. The inferred sister species of D. rerio depends on the position of the partition along the chromosome, with the D. rerio—D. kyathit relationship (re, ky; green, see panel c)) supported across the middle of most chromosomes, while the D. rerio—D. aesculapii relationship (ae, re; rust) supported near the telomeres of most chromosomes. Note also that few (ky, ni; purple) trees occurred at chromosome centers and many (ky, ni; purple) trees occupied partitions near the telomeres. d) The relationship of D. choprae relative to other members of the genus phylogeny varied according to chromosome position. The centers of chromosomes supported the placement of D. choprae inferred by the unpartitioned genome phylogeny (green), while chromosome ends showed more variation in the relationships supported.
The phylogeny inferred from the unpartitioned genome-wide nuclear data set strongly supported the monophyly of the D. rerio species group (sensu Fang 1998). This group is represented in this study by D. rerio, D. aesculapii, D. kyathit, and D. nigrofasciatus. Other members of this group not sampled in this study likely include Danio quagga (formerly referred to as D. aff. kyathit) and Danio tinwini (McCluskey and Postlethwait 2015). This genome-wide inferred phylogeny placed D. aesculapii as the sister species to D. rerio, with small internal branches placing D. kyathit and D. nigrofasciatus as diverging more basally (Fig. 2a). Outside of the D. rerio species group, relationships were consistent with previous studies. Two species—D. albolineatus and D. kerri—fell just outside the D. rerio species group, followed by a group of 3 species—D. choprae, D. erythromicron, and D. margaritatus—with the large-bodied D. meghalayensis diverging at the base of the genus (Fig. 2a).
To test how much of the danio genome supported the unpartitioned genomic topology, we used a jackknife subsampling approach to divide the nuclear genome into 250 windows (10 windows for each of the 25 chromosomes) based on chromosome position in the D. rerio reference genome. Each window had the same number of nucleotides of filtered, aligned sequence as the other nine windows on that chromosome with jackknife windows ranging in size from 37 kb on chromosome 22 to 84 kb on chromosome 7 due to chromosomes varying in sequence length.
The chromosome-level structure of the zebrafish genome is a good approximation of the genomes of other danios. Like most species in family Cyprinidae, zebrafish has 25 pairs of chromosomes ranging from metacentric to subtelocentric (Pijnacker and Ferwerda 1995; Daga et al. 1996; Amores and Postlethwait 1999; Gornung et al. 2000; Sola and Gornung 2001; Traut and Winking 2001; Phillips et al. 2006) with increased recombination rates near their ends (Singer et al. 2002; Bradley et al. 2011; Anderson et al. 2012; Howe et al. 2013; Wilson et al. 2014). Translocations between different chromosomes are rare in cyprinids as demonstrated by comparisons of gene order between zebrafish and distantly related Cyprinids, including carp, goldfish, and grass carp (Xu et al. 2014; Wang et al. 2015; Chen et al. 2019). Intrachromosomal rearrangements, however, have occurred in cyprinids (Avise and Gold 1977), with some between D. rerio strains (Freeman et al. 2007). Using our jackknife strategy, we could infer the histories of physically linked genomic windows as well as determine the effects of recombination rate throughout the genome.
Nearly all relationships found in the unpartitioned genomic phylogeny were supported by more than 90% of the 250 jackknife windows (Fig. 2a) demonstrating the sufficiency of the jackknife windows to robustly infer phylogenetic relationships. It is striking, therefore, that 3 relationships within the Danio genus showed markedly low support. The placement of D. choprae in the unpartitioned genomic topology was supported by 63% of jackknife windows, but a large minority, 27% of windows, placed D. choprae basal to the D. rerio species group plus the D. albolineatus + D. kerri group (Fig. 2a). Analysis of chromosome structure showed that the placement of D. choprae tended to match the unpartitioned genomic topology at chromosome centers with D. choprae as sister to D. erythromicron + D. margaritatus, but matched the alternative placement as sister to the D. rerio group + D. albolineatus + D. kerri group at chromosome ends (Fig. 2d). A similar anomaly was observed for the placement of D. nigrofasciatus. The unpartitioned genome topology placed D. nigrofasciatus basal to D. rerio, D. aesculapii, and D. kyathit. This placement was supported by 71% of jackknife trees with a considerable bias for the center of chromosomes, while an alternative topology placed D. nigrofasciatus as sister to D. kyathit with support from 28% of windows occurring almost exclusively at chromosome ends (Figs. 2, a and c, and 3a).
Fig. 3.
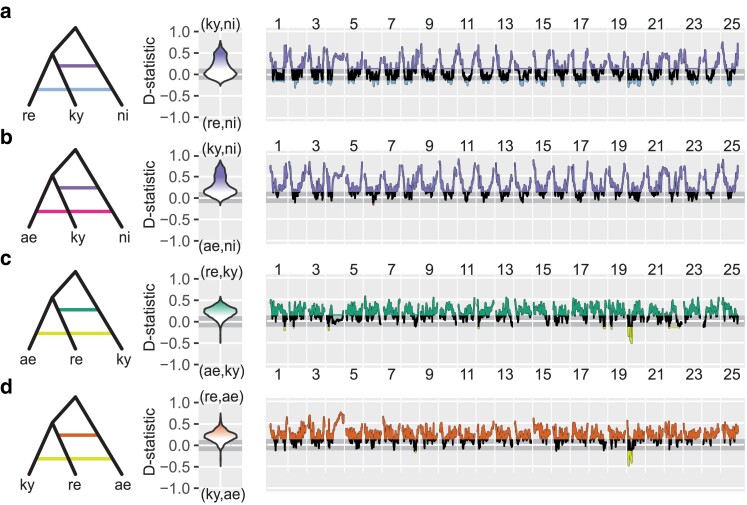
D-statistics show genomic regions affected by introgression. a) D-statistics testing for gene flow between D. nigrofasciatus and D. kyathit (purple) or between D. nigrofasciatus and D. rerio (blue). The assumed topology (left), distribution of D-statistic values across the genome (second from left), and D values across chromosomes for windows of 200 ABBA/BABA sites (right, note the spike near telomeres). The horizontal gray bar shows the 95% confidence intervals under the null expectation of equal ABBA/BABA frequencies. Color scheme as in Fig. 2. b) D-statistics testing for gene flow between D. nigrofasciatus and D. kyathit (purple) or between D. nigrofasciatus and D. aesculapii (pink). c) D-statistics testing for gene flow between D. kyathit and D. rerio (green) or between D. kyathit and D. aesculapii (yellow), demonstrating an anomaly on Chr20, but no consistent effect of chromosome position relative to telomeres. d) D-statistics testing for gene flow between D. aesculapii and D. rerio (rust) or between D. aesculapii and D. kyathit (yellow), again showing an anomaly on Chr20.
The placement of all species in the unpartitioned genomic topology was supported by the majority of the genome with one exception—the placement of D. rerio. A slight plurality of jackknife windows (42%) placed D. rerio as sister to D. kyathit, while 40% of jackknife windows recovered D. rerio as sister to D. aesculapii (Fig. 2b) in agreement with the unpartitioned genomic topology (Fig. 2a). The remaining trees placed D. rerio basal to 2 or more species in the D. rerio species group (Fig. 2, a and c). The finding that D. kyathit and D. aesculapii have nearly equal support to be the sister of D. rerio is consistent with the origin of D. rerio by HAS.
The effect of genome structure on the history of the D. rerio genome is not subtle. D. rerio was recovered as sister to D. kyathit in 72% of the 50 jackknife partitions from the centers of chromosomes (Fig. 2c left, green), but in 0% of the 50 partitions from chromosome ends. In contrast, at chromosome ends, D. rerio was placed as sister to D. aesculapii in 58% of partitions (Fig. 2c right, rust) or diverging more basally in the D. rerio species group (42% of partitions, Fig. 2c right, purple). Notably, the relationships within the D. rerio species group recovered by the unpartitioned genomic topology match only 22% of jackknife windows (Fig. 2c), demonstrating that the unpartitioned genomic topology is insufficient to fully explain the history of the D. rerio species group.
Genomic regions affected by gene flow and effects of recombination
To determine the sources of phylogenetic discordance within genus Danio, we focused on the most variable part of the phylogeny—the D. rerio species group—and patterns of pairwise splits, a subset of SDCs in which a derived character state is found in only 2 species. In a well-supported phylogeny with 3 species and an outgroup (Fig. 1), pairwise splits include BBAA splits shared by the most-closely related species, as well as ABBA and BABA splits (see Fig. 1). Several tests can use these “ABBA/BABA sites” to detect and quantify introgression and phylogenetic discordance (Martin et al. 2015; Pease and Hahn 2015; Blischak et al. 2018; Kubatko and Chifman 2019).
To determine if introgression could explain the 28% of the jackknife trees that recovered D. nigrofasciatus as sister to D. kyathit, we calculated Patterson's D-statistics genome wide (Table 1) and in windows of 200 ABBA/BABA sites across the genome (Fig. 3). Genome-wide D-statistics revealed a striking excess of characters supporting introgression between D. kyathit and D. nigrofasciatus using either D. rerio (D = 0.14) or D. aesculapii (D = 0.29) as the sister species to D. kyathit. Although ABBA/BABA patterns were roughly equal (D not significantly different from 0) over much of the genome, the ends of chromosomes harbored an excess of derived characters shared by D. kyathit and D. nigrofasciatus (Fig. 3, a and b, purple). This pattern of high introgression signal near the ends of chromosomes suggests introgression limited to the high recombination regions of the danio genome and explains why jackknife trees placing D. kyathit with D. nigrofasciatus were found almost exclusively at the ends of chromosomes (Fig. 2c).
Table 1.
Genome-wide D-statistics within the D. rerio species group.
| Assumed species tree (P1, P2), P3 | D-statistic | BBAA sites | ABBA sites | BABA sites |
|---|---|---|---|---|
| (D. rerio, D. kyathit), D. nigrofasciatus | 0.1405 | 63,343 | 48,670 | 36,676 |
| (D. aesculapii, D. kyathit), D. nigrofasciatus | 0.2978 | 46,262 | 49,991 | 27,046 |
| (D. aesculapii, D. rerio), D. kyathit | 0.2027 | 56,970 | 54,596 | 36,194 |
| (D. kyathit, D. rerio), D. aesculapii | 0.223 | 54,596 | 56,970 | 36,194 |
We similarly used D-statistics to test for evidence of admixture in D. rerio. Because 2 species—D. aesculapii and D. kyathit—had nearly equal support as the sister species of D. rerio (Fig. 2b), we calculated D-statistics for both possible relationships. Genome-wide D values were high for both comparisons (Table 1), providing strong support for an effect of admixture from both species on the history of the D. rerio genome. In stark contrast to the elevated D-statistics between D. kyathit and D. nigrofasciatus, which occurred almost exclusively at the ends of chromosomes (Fig. 3, a and b), D-statistics grouping D. rerio with D. kyathit and D. aesculapii were elevated across the majority of the genome (Fig. 3, c and d), supporting genome-wide admixture. These D-statistic tests, based on more than 80,000 ABBA/BABA sites per comparison, robustly reject ILS as the only source of variance in the D. rerio species group. Moreover, the genome-wide D values are greater than 0.14 in all comparisons, making them higher than in some other studies where introgression was either known a priori, or validated with follow up studies (Cui et al. 2013; Sankararaman et al. 2014; Li et al. 2016). While D-statistic tests rule out ILS as the only source of genealogical discordance in danios, these tests do not distinguish between introgression and HAS.
To supplement results from Patterson's D-Statistic tests and to compare all 4 species of the D. rerio group, we turned to a more generalized approach to investigate pairwise splits while also incorporating genomic position. Our data set contains over 200,000 pairwise splits that inform relationships within the D. rerio species group, allowing for a more fine-scale investigation than the jackknife trees of Fig. 2. If 4 species are all equally closely related, we expect to see each of the 6 pairwise splits occurring at equal frequency (16.7%). Regions of the genome having pairwise splits in considerable excess of this frequency support a closer relationship of the 2 species sharing the derived characters. Using a sliding window of 500 ancestry-informative SNPs, we find that each chromosome is a patchwork of genomic regions each enriched for a combination of pairwise splits (Fig. 4a; Supplementary Fig. 1).
Fig. 4.
Distinct genomic histories in the danio genome shown by patterns of SDCs. a) Proportions of the 6 pairwise splits (derived characters present in only 2 species) in the D. rerio species group in sliding windows of 500 pairwise splits across chromosome 2, which displays a representative pattern, and all chromosomes appear in Supplementary Fig. 2. The gray horizontal bar shows the 95% confidence interval under the null expectation of equal proportions of the 6 split patterns. The colored bar at the top shows which splits are enriched at each position along the chromosome. Color scheme as in Fig. 2. b) Distribution of pairwise split proportions from the entire genome (windows of 500 pairwise splits). c) Percentage of genomic windows showing enrichment for pairwise splits in at least 10 genomic windows. The bottom left graph shows the percent of the genome enriched for each combination of pairwise splits. d) Co-occurrence of pairwise split enrichment across all 25 zebrafish chromosomes. Chromosomes are shown unfolded and colored according to which splits are enriched at each position along the chromosome. Color scheme as in panel a). Genomic regions with no significant enrichment are dark gray. e) Effects of chromosome location on pairwise split proportions. Points are plotted according to relative chromosome location of a window along a folded chromosome (centers of chromosomes on the left and ends of chromosomes on the right) and the proportion of each pairwise split in that window. Splines are fitted to the data for each pairwise split across all 25 chromosomes in nonoverlapping windows of 500 pairwise splits. f) Proportion of D. rerio ancestry attributable to the D. aesculapii lineage (orange) or the D. kyathit lineage (green). Analysis was performed on the sequences used previously for jackknife trees binned according to chromosome position.
Genome-wide, all 6 pairwise splits appeared at appreciable frequency (Fig. 4b), suggesting a major role for ILS as danios speciated. Enrichment in genomic windows, however, was restricted almost exclusively to the 3 most common splits (re,ae; re,ky; ky,ni), which corresponded to relationships supported by both the jackknife trees (Fig. 2c) and the genome-wide D-statistics (Table 1).
The majority of genomic windows (65.2%) indicated that D. rerio is closely related to both D. aesculapii and D. kyathit (orange/green bars in Fig. 4c), suggesting that zebrafish arose from an ancient HAS event between ancestors of these 2 lineages. A further 10.4% of the genome indicated D. rerio was closely related to either D. aesculapii (6.4%) or D. kyathit (4.0%), but not the other species. A smaller proportion of windows (25.7%) indicated that D. rerio and D. aesculapii are closely related and that D. kyathit and D. nigrofasciatus are closely related (orange/purple bars in Fig. 4c). This set of relationships had been previously recovered with strong support (McCluskey and Postlethwait 2015). The separation of the danio genome into regions with 2 distinct histories is remarkably consistent when viewed across chromosomes, although small regions supporting other histories are apparent (Fig. 4d; Supplementary Fig. 2).
A small minority of genomic regions stood out in comparison to other chromosomes. One region spanning 15 Mb on the left half of chromosome 20 supported a history unique from the rest of the genome (Fig. 4d; Supplementary Fig. 1). This region was enriched for pairwise splits grouping D. rerio with D. nigrofasciatus (blue in figures) and D. aesculapii with D. kyathit (yellow in figures). Several other instances of discrete regions with distinct histories have been described when chromosomal inversions have a paraphyletic distribution relative to the species tree (Hohenlohe et al. 2012, 2010; Fontaine et al. 2015; Li et al. 2016; Pease et al. 2016). Furthermore, studies of cyprinid karyotypes suggest that pericentromeric inversions are common in cyprinids (Avise and Gold 1977). The hypothesis that the patterns on chromosome 20 correspond to one or more structural changes in the evolution of danios predicts that the gene order along the chromosome should differ between danio species.
The sex chromosome also showed a divergent pattern. SNPs grouping D. kyathit and D. nigrofasciatus were enriched at the ends of all 25 chromosomes (purple in Fig. 4d; Supplementary Fig. 2), but occurred at much lower frequency in the centers of all chromosomes except chromosome 4, which contains a major sex-determining gene in nature, with ZZ individuals always becoming male and ZW individuals usually developing as females, but with some sex reversal into neomales (Anderson et al. 2012; Wilson et al. 2014; Valdivieso et al. 2022). In contrast to natural strains of zebrafish, laboratory strains, including the AB strain used here and TU, have lost the Z chromosome and are chromosomally WW, relying on sex-reversed neomales to provide sperm, often leading to aberrant sex ratios (Lawrence et al. 2008; Wilson et al. 2014, 2024). The right arm of chromosome 4 is nonrecombining, repeat rich, and full of duplicated gene families that play roles in the maternal-to-zygotic transition, except for several megabases near the right telomere containing the sex locus (Anderson et al. 2012; Howe et al. 2013, 2016; Locati et al. 2017; Chang et al. 2022; Wilson and Postlethwait 2024). Despite spanning tens of megabases, because it does not recombine, the right arm of chromosome 4 is effectively a single locus near the end of the chromosome and thus is expected to match the history of chromosome ends, as the data showed (Fig. 4d).
Genome-wide, the 2 major histories of the D. rerio species group are partitioned according to chromosome location (Fig. 4, d and e). The first major history was apparent in the centers of chromosomes and supported a hybrid origin of D. rerio as evidenced by an excess of splits shared by D. rerio and D. kyathit and an excess of splits shared by D. rerio and D. aesculapii. Both splits occurred at nearly double the rate of splits exclusive to D. kyathit and D. aesculapii, demonstrating that the excess was not due to ILS. The second major history was apparent at the ends of chromosomes with D. rerio sister to D. aesculapii and D. kyathit sister to D. nigrofasciatus. Notably, as splits grouping D. kyathit and D. nigrofasciatus became more common toward the ends of chromosomes, splits grouping D. kyathit and D. rerio became less common. This anticorrelation suggests that introgression between D. kyathit and D. nigrofasciatus supplanted many derived characters exclusive to D. kyathit and D. rerio, thereby obscuring the relationships between the latter 2 species.
Several analyses herein suggested that D. rerio represents a hybrid species between the D. aesculapii and D. kyathit lineages. To test this hypothesis, we used the program HyDe (Blischak et al. 2018), which can detect evidence of hybridization and also estimate the fraction of ancestry attributable to each ancestral lineage. Using the 15.44 Mb of aligned coding sequence used previously for jackknife analyses (Fig. 2c), the hybrid detection results were clear and striking (Fig. 4f; Supplementary Table 3). The central 40% of the zebrafish genome strongly supported a HAS origin of D. rerio (P < 10e-10), with 50% ancestry estimates each for D. aesculapii and D. kyathit. The apparent contribution of the D. kyathit lineage, however, declined toward the ends of chromosomes with <1% ancestry estimated from D. kyathit. This finding suggests that alleles contributed by the D. kyathit lineage to the ends of chromosomes are no longer present in modern D. kyathit, possibly due to the high levels of introgression with D. nigrofasciatus seen at chromosome ends (Figs. 3, a and b, and 4e).
D. aesculapii alleles and D. kyathit alleles in laboratory and wild strains of D. rerio
If D. rerio is a hybrid species, many of the alleles inherited from the D. aesculapii or D. kyathit lineages in the founding population should be shared across distantly related populations of D. rerio. Alternatively, if the evidence of HAS is somehow due to the domestication of the laboratory AB strain of zebrafish used in this study, we would not expect the SDCs we identified to be shared across distantly related individuals. To distinguish these possibilities, we compared sites in our exome data with a derived state shared by D. rerio and D. aesculapii (orange in figures) or D. rerio and D. kyathit (green in figures) to sequences present in reduced-representation (RAD-seq) libraries from 3 laboratory D. rerio populations (McCluskey and Postlethwait 2015) and 3 wild D. rerio populations (Suurväli et al. 2020) spanning 3 countries. Of all the sites in the RAD-seq data sequenced across all individuals, 527 positions corresponded to derived characters also found in D. kyathit or D. aesculapii. These sites were widespread across the genome with little effect of chromosome structure (Supplementary Fig. 3). Most of these sites (89%) were fixed for the derived allele in all sampled D. rerio, consistent with fixation following HAS but before laboratory culture. The remaining 58 sites likely represent homoplastic mutations or standing genetic variation from the HAS event. Finding these SDCs distributed across diverse D. rerio populations further supports a hybrid origin of zebrafish.
Discussion
Admixture in the history of the Danio genus
This genome-wide analysis of exome sequences across 10 species of the genus Danio provides a novel understanding of the origins of the zebrafish D. rerio, a major biomedical model organism (Baldridge et al. 2021). Results showed that the genomes of zebrafish and its 3 closest allies harbor 2 histories that have remarkably consistent distributions across all chromosomes and a third history restricted to a small portion of a single chromosome arm. The genomic distributions of these disparate histories point to major effects of introgression and hybrid speciation within this group (Fig. 5).
Fig. 5.
The hybrid history of the zebrafish genome. a) A model for the recent population history of the D. rerio species group showing the hybrid origin of D. rerio from the D. aesculapii and D. kyathit lineages and the introgression of sequences between D. kyathit and D. nigrofasciatus retained mostly at chromosome ends. b) Graphic depicting, across a chromosome, the expected distribution of alleles originating in populations ancestral to only 2 species at the time of HAS. c) Approximate experimentally discovered distribution of alleles from populations shared by only 2 species in modern-day danios.
The first history we observed is that D. rerio is closely—and about equally—related to both D. aesculapii and D. kyathit across the majority of the genome, while the latter 2 species are more distantly related to each other. This history is supported by phylogenies inferred from jackknife windows (Fig. 2), D-statistics, (Fig. 3), and the correlated genomic distributions of derived character states (Fig. 4, c and e; Supplementary Fig. 1). All these results point to an ancient HAS origin of D. rerio from ancestors in the D. aesculapii and D. kyathit lineages (Fig. 5). The second history we observed occurred in the regions of high recombination at the ends of danio chromosomes (Figs. 2e and 5). These locations showed a striking excess of derived alleles shared by D. kyathit and D. nigrofasciatus, consistent with introgression limited to high recombination regions (Fig. 4, d and e; Supplementary Fig. 1). The third history is limited to a portion of chromosome 20, where D. rerio is not closely related to either D. aesculapii or D. kyathit, although the latter 2 species are closely related to each other in this region (Fig. 3d; Supplementary Fig. 3). A distinct history restricted to a single genomic region is consistent with a chromosomal inversion either inherited by ILS or passed by introgression as has been seen in other taxa (della Torre et al. 1997; Kulathinal et al. 2009; Fontaine et al. 2015; Barron et al. 2019; Edelman et al. 2019; Nelson et al. 2021; Soudi et al. 2023).
These distinct histories point to a hybrid origin of D. rerio, but is zebrafish a homoploid hybrid species? Opinions differ as to the criteria that define a homoploid hybrid species (Schumer et al. 2014; Nieto Feliner et al. 2017), but 3 proposed requirements include showing that the species is reproductively isolated from each parent species; there is genetic evidence of hybridization; and hybridization was responsible for the reproductive isolation (Schumer et al. 2014). Several studies have reported hybrid sterility in hybrids between D. rerio and other danios (Wong et al. 2011); (Podobnik et al. 2020); (Parichy and Johnson 2001), likely caused by aneuploid gametes (Endoh et al. 2020). The findings discussed in the current work provide ample evidence that the D. rerio genome shows evidence of hybridization between the D. aesculapii and D. kyathit lineages. Whether hybridization was responsible for the reproductive isolation of D. rerio remains uncertain and may be difficult to determine given one of the putative parental lineages shows evidence of subsequent gene flow. D. rerio may be a homoploid hybrid species, but further studies will be needed to understand better the origin of this model species.
Danios, and perhaps Cyprinids at large, offer a unique window into recombination variation and genomic histories because the group is remarkably diverse and clear patterns of recombination variation are present within each chromosome. The effects of recombination rate have been noted in several species across inversions, in sex chromosomes, and near recombination hot spots (Turner et al. 2005; Noor and Bennett 2009; Turner and Hahn 2010). Theory predicts that regions of lower recombination rates, such as the centers of danio chromosomes, will be resistant to gene flow (Felsenstein 1974; Hill and Robertson 2007). This distribution would occur especially in the case of pericentric inversions, which cytogenetic studies suggest are common among cyprinids (Avise and Gold 1977). Conversely, regions with high recombination rates, such as the ends of danio chromosomes, will be more susceptible to gene flow following the beginning of reproductive isolation and should better reflect the phylogeographic distribution of species. Interestingly, relationships at the ends of danio chromosomes more closely reflect present geographical distributions of danios than do relationships at the centers of chromosomes. This pattern holds for D. kyathit and D. nigrofasciatus, which both occur in the Irrawaddy hydrological basin; for D. rerio and D. aesculapii, which both occur in Bangladesh; and for D. choprae, which does not occur with D. erythromicron or D. margaritatus but does occur in the same drainage as several other danio species (McCluskey and Postlethwait 2015).
Much remains to be learned about the history of danios and the hybrid history of zebrafish. Inferences drawn here were based on ten Danio species, but the study of more danio species will help us better understand these relationships. Notably, 2 members of the D. rerio species group—the striped D. quagga and the spotted D. tinwini—may prove to be the best representatives of the lineages that experienced introgression in this group (Kullander et al. 2009; Kullander and Fang 2009b). Additionally, the inclusion of more individuals per species will provide better resolution of the timing and nature of gene flow as we saw with the comparison of our data to previous RAD-seq data from diverse zebrafish populations. Finally, the mitochondrial phylogeny for these species could not be inferred in this study because the exome enrichment baits targeted only nuclear genes. A better understanding of mitochondrial histories will provide insight into the history of these species.
With current taxon sampling and topologies of the best-supported trees, we cannot determine the direction of introgression in these species using conventional methods, which require comparison of 2 pairs of sister species (Durand et al. 2011; Martin et al. 2015; Pease et al. 2016) or estimates of historical effective population size (Hibbins and Hahn 2022). Future analyses incorporating more taxa, population level sampling across species, and whole mitochondrial and nuclear genome analyses may help to resolve better the complex history of members of the Danio genus.
Implications for future studies
The structured distribution of relationships across danio genomes has implications for the interpretation of past and future phylogenetic studies. First, when different genomic regions have different histories, the use of a small number of loci for phylogenetic inference will have considerable limitations. Previous phylogenetic studies involving the Danio genus (Mayden et al. 2007; Fang et al. 2009; Tang et al. 2010; McCluskey and Postlethwait 2015) arrived at different conclusions, likely in part because the loci used for inference were different in different studies and were sometimes sampled from chromosome regions that our data show to have different histories. The 2 nuclear markers most frequently used in these species are rhodopsin near the end of chromosome 8 and rag1 closer to the center of chromosome 25, regions we show tend to have different histories. Second, when large phylogenomic data sets are used without incorporating data on genome structure, systematic biases can lead to different inferred relationships than when genome structure is incorporated into analyses. For example, our previous phylogenomic study of Danio used short sequences flanking SbfI restriction sites sampled across the genome (McCluskey and Postlethwait 2015). Using a variety of data sets and phylogenetic inference methods, the best-supported topology for the D. rerio group in that study placed D. kyathit with D. nigrofasciatus and D. rerio with D. aesculapii, a topology supported by only 16.8% of jackknife windows in the current exome study with an extreme bias toward the ends of chromosomes. An analysis of the location of SbfI sites across the D. rerio genome showed that these restriction sites have an extreme bias for the ends of chromosomes (Supplementary Fig. 2), which we show here to be greatly enriched for SNPs supporting the topology recovered in the previous study. Interpretation of relationships in the context of genome structure will have important ramifications in future studies because drawing inferences under the supposition of different species trees can have considerable implications on inferred evolutionary origins of species-specific biology in the taxa examined (Thomas and Hahn 2015). Indeed, without accounting for genome structure, the history of the D. rerio species group inferred from the entire genome (the unpartitioned genomic topology) is supported by only 22.0% of genomic regions (Fig. 2c) and does not reflect the high levels of introgression between D. kyathit and D. nigrofasciatus or the hybrid origin of zebrafish.
Supplementary Material
Acknowledgments
This work benefited from access to the University of Oregon high performance computing cluster Talapas.
Contributor Information
Braedan M McCluskey, Minnesota Supercomputing Institute, University of Minnesota Twin Cities, Minneapolis, MN 55455, USA; Institute of Neuroscience, University of Oregon, Eugene, OR 97403, USA.
Peter Batzel, Institute of Neuroscience, University of Oregon, Eugene, OR 97403, USA.
John H Postlethwait, Institute of Neuroscience, University of Oregon, Eugene, OR 97403, USA.
Data availability
All exome sequence data described herein are available at the Short Read Archive under project number PRJNA1148758. Multiple sequence alignments, variant calls, and code used to analyze data and generate figures can be found at DOI 10.5281/zenodo.14201925. Additional data were reanalyzed from Suurväli et al. (2020).
Supplemental material available at G3 online.
Funding
We thank the support from the National Institutes of Health, United States of America (NIH), grants R01 OD011116 and R35 GM139635.
Literature cited
- Amores A, Postlethwait JH. 1999. Banded chromosomes and the zebrafish karyotype. Methods Cell Biol. 60:323–338. doi: 10.1016/S0091-679X(08)61908-1. [DOI] [PubMed] [Google Scholar]
- Anderson JL, Rodriguez Mari A, Braasch I, Amores A, Hohenlohe P, Batzel P, Postlethwait JH. 2012. Multiple sex-associated regions and a putative sex chromosome in zebrafish revealed by RAD mapping and population genomics. PLoS One. 7(7):e40701. doi: 10.1371/journal.pone.0040701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avise JC, Gold JR. 1977. Chromosomal divergence and speciation in two families of North American fishes. Evolution. 31(1):1–13. doi: 10.2307/2407539. [DOI] [PubMed] [Google Scholar]
- Baldridge D, Wangler MF, Bowman AN, Yamamoto S; Undiagnosed Diseases Network; Schedl T, Pak SC, Postlethwait JH, Shin J, Solnica-Krezel L, et al. 2021. Model organisms contribute to diagnosis and discovery in the undiagnosed diseases network: current state and a future vision. Orphanet J Rare Dis. 16(1):206. doi: 10.1186/s13023-021-01839-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barron MG, Paupy C, Rahola N, Akone-Ella O, Ngangue MF, Wilson-Bahun TA, Pombi M, Kengne P, Costantini C, Simard F, et al. 2019. A new species in the major malaria vector complex sheds light on reticulated species evolution. Sci Rep. 9(1):14753. doi: 10.1038/s41598-019-49065-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blischak PD, Chifman J, Wolfe AD, Kubatko LS. 2018. Hyde: a python package for genome-scale hybridization detection. Syst Biol. 67(5):821–829. doi: 10.1093/sysbio/syy023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolger AM, Lohse M, Usadel B. 2014. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 30(15):2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouckaert RR. 2010. DensiTree: making sense of sets of phylogenetic trees. Bioinformatics. 26(10):1372–1373. doi: 10.1093/bioinformatics/btq110. [DOI] [PubMed] [Google Scholar]
- Bradley KM, Breyer JP, Melville DB, Broman KW, Knapik EW, Smith JR. 2011. An SNP-based linkage map for zebrafish reveals sex determination loci. G3 (Bethesda). 1(1):3–9. doi: 10.1534/g3.111.000190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burri R, Nater A, Kawakami T, Mugal CF, Olason PI, Smeds L, Suh A, Dutoit L, Bureš S, Garamszegi LZ, et al. 2015. Linked selection and recombination rate variation drive the evolution of the genomic landscape of differentiation across the speciation continuum of Ficedula flycatchers. Genome Res. 25(11):1656–1665. doi: 10.1101/gr.196485.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushnell B. 2014. BBMap: a fast, accurate, splice-aware aligner. https://api.semanticscholar.org/CorpusID:114702182. Accessed 24 October 2017.
- Chang N-C, Rovira Q, Wells J, Feschotte C, Vaquerizas JM. 2022. Zebrafish transposable elements show extensive diversification in age, genomic distribution, and developmental expression. Genome Res. 32(7):1408–1423. doi: 10.1101/gr.275655.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Omori Y, Koren S, Shirokiya T, Kuroda T, Miyamoto A, Wada H, Fujiyama A, Toyoda A, Zhang S, et al. 2019. De novo assembly of the goldfish (Carassius auratus) genome and the evolution of genes after whole-genome duplication. Sci Adv. 5(6):eaav0547. doi: 10.1126/sciadv.aav0547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui R, Schumer M, Kruesi K, Walter R, Andolfatto P, Rosenthal GG. 2013. Phylogenomics reveals extensive reticulate evolution in Xiphophorus fishes. Evolution. 67(8):2166–2179. doi: 10.1111/evo.12099. [DOI] [PubMed] [Google Scholar]
- Daga RR, Thode G, Amores A. 1996. Chromosome complement, C-banding, Ag-NOR and replication banding in the zebrafish Danio rerio. Chromosome Res. 4(1):29–32. doi: 10.1007/BF02254941. [DOI] [PubMed] [Google Scholar]
- Danecek P, Bonfield JK, Liddle J, Marshall J, Ohan V, Pollard MO, Whitwham A, Keane T, McCarthy SA, Davies RM, et al. 2021. Twelve years of SAMtools and BCFtools. Gigascience. 10(2):giab008. doi: 10.1093/gigascience/giab008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- della Torre A, Merzagora L, Powell JR, Coluzzi M. 1997. Selective introgression of paracentric inversions between two sibling species of the Anopheles gambiae complex. Genetics. 146(1):239–244. doi: 10.1093/genetics/146.1.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drosophila 12 Genomes Consortium; Clark AG, Eisen MB, Smith DR, Bergman CM, Oliver B, Markow TA, Kaufman TC, Kellis M, Gelbart W, et al. 2007. Evolution of genes and genomes on the Drosophila phylogeny. Nature. 450(7167):203–218. doi: 10.1038/nature06341. [DOI] [PubMed] [Google Scholar]
- Du K, Ricci JMB, Lu Y, Garcia-Olazabal M, Walter RB, Warren WC, Dodge TO, Schumer M, Park H, Meyer A, et al. 2024. Phylogenomic analyses of all species of swordtail fishes (genus Xiphophorus) show that hybridization preceded speciation. Nat Commun. 15(1):6609. doi: 10.1038/s41467-024-50852-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durand EY, Patterson N, Reich D, Slatkin M. 2011. Testing for ancient admixture between closely related populations. Mol Biol Evol. 28(8):2239–2252. doi: 10.1093/molbev/msr048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton DAR, Ree RH. 2013. Inferring phylogeny and introgression using RADseq data: an example from flowering plants (Pedicularis: Orobanchaceae). Syst Biol. 62(5):689–706. doi: 10.1093/sysbio/syt032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelman NB, Frandsen PB, Miyagi M, Clavijo B, Davey J, Dikow RB, García-Accinelli G, Van Belleghem SM, Patterson N, Neafsey DE, et al. 2019. Genomic architecture and introgression shape a butterfly radiation. Science. 366(6465):594–599. doi: 10.1126/science.aaw2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellegren H, Smeds L, Burri R, Olason PI, Backstrom N, Kawakami T, Künstner A, Mäkinen H, Nadachowska-Brzyska K, Qvarnström A, et al. 2012. The genomic landscape of species divergence in Ficedula flycatchers. Nature. 491(7426):756–760. doi: 10.1038/nature11584. [DOI] [PubMed] [Google Scholar]
- Endoh M, Shima F, Havelka M, Asanuma R, Yamaha E, Fujimoto T, Arai K. 2020. Hybrid between Danio rerio female and Danio nigrofasciatus male produces aneuploid sperm with limited fertilization capacity. PLoS One. 15(5):e0233885. doi: 10.1371/journal.pone.0233885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang F. 1998. Danio kyathit, a new species of cyprinid fish from Myitkyina, northern Myanmar. Ichthyol Explor Freshw. 8(3):273–280. [Google Scholar]
- Fang F. 2003. Phylogenetic analysis of the Asian cyprinid genus Danio (Teleostei, Cyprinidae). Copeia. 2003(4):714–728. doi: 10.1643/IA03-131.1. [DOI] [Google Scholar]
- Fang F, Noren M, Liao TY, Kallersjo M, Kullander SO. 2009. Molecular phylogenetic interrelationships of the south Asian cyprinid genera Danio, Devario and Microrasbora (Teleostei, Cyprinidae, Danioninae). Zool Scr. 38(3):237–256. doi: 10.1111/j.1463-6409.2008.00373.x. [DOI] [Google Scholar]
- Felsenstein J. 1974. Evolutionary advantage of recombination. Genetics. 78(2):737–756. doi: 10.1093/genetics/78.2.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontaine MC, Pease JB, Steele A, Waterhouse RM, Neafsey DE, Sharakhov IV, Jiang X, Hall AB, Catteruccia F, Kakani E, et al. 2015. Mosquito genomics. Extensive introgression in a malaria vector species complex revealed by phylogenomics. Science. 347(6217):1258524. doi: 10.1126/science.1258524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman JL, Adeniyi A, Banerjee R, Dallaire S, Maguire SF, Chi J, Ng BL, Zepeda C, Scott CE, Humphray S, et al. 2007. Definition of the zebrafish genome using flow cytometry and cytogenetic mapping. BMC Genomics. 8(1):195. doi: 10.1186/1471-2164-8-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrigan D, Kingan SB, Geneva AJ, Andolfatto P, Clark AG, Thornton KR, Presgraves DC. 2012. Genome sequencing reveals complex speciation in the Drosophila simulans clade. Genome Res. 22(8):1499–1511. doi: 10.1101/gr.130922.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon D, Huddleston J, Chaisson MJP, Hill CM, Kronenberg ZN, Munson KM, Malig M, Raja A, Fiddes I, Hillier LW, et al. 2016. Long-read sequence assembly of the gorilla genome. Science. 352(6281):aae0344. doi: 10.1126/science.aae0344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gornung E, De Innocentiis S, Annesi F, Sola L. 2000. Zebrafish 5S rRNA genes map to the long arms of chromosome 3. Chromosome Res. 8(4):362. doi: 10.1023/A:1009252017097. [DOI] [PubMed] [Google Scholar]
- Hibbins MS, Hahn MW. 2022. Phylogenomic approaches to detecting and characterizing introgression. Genetics. 220(2):iyab173. doi: 10.1093/genetics/iyab173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill WG, Robertson A. 2007. The effect of linkage on limits to artificial selection. Genet Res. 89(5–6):311–336. doi: 10.1017/S001667230800949X. [DOI] [PubMed] [Google Scholar]
- Hipp AL, Eaton DAR, Cavender-Bares J, Fitzek E, Nipper R, Manos PS. 2014. A framework phylogeny of the American oak clade based on sequenced RAD data. PLoS One. 9(4):e93975. doi: 10.1371/journal.pone.0093975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohenlohe PA, Bassham S, Currey M, Cresko WA. 2012. Extensive linkage disequilibrium and parallel adaptive divergence across threespine stickleback genomes. Philos Trans R Soc Lond B Biol Sci. 367(1587):395–408. doi: 10.1098/rstb.2011.0245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohenlohe PA, Bassham S, Etter PD, Stiffler N, Johnson EA, Cresko WA. 2010. Population genomics of parallel adaptation in threespine stickleback using sequenced RAD tags. PLoS Genet. 6(2):e1000862. doi: 10.1371/journal.pgen.1000862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holder MT, Anderson JA, Holloway AK. 2001. Difficulties in detecting hybridization. Syst Biol. 50(6):978–982. doi: 10.1080/106351501753462911. [DOI] [PubMed] [Google Scholar]
- Howe K, Clark MD, Torroja CF, Torrance J, Berthelot C, Muffato M, Collins JE, Humphray S, McLaren K, Matthews L, et al. 2013. The zebrafish reference genome sequence and its relationship to the human genome. Nature. 496(7446):498–503. doi: 10.1038/nature12111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe K, Schiffer PH, Zielinski J, Wiehe T, Laird GK, Marioni JC, Soylemez O, Kondrashov F, Leptin M. 2016. Structure and evolutionary history of a large family of NLR proteins in the zebrafish. Open Biol. 6(4):160009. doi: 10.1098/rsob.160009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang D, Lewis VM, Foster TN, Toomey MB, Corbo JC, Parichy DM. 2021. Development and genetics of red coloration in the zebrafish relative Danio albolineatus. Elife. 10:e70253. doi: 10.7554/eLife.70253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irion U, Nusslein-Volhard C. 2019. The identification of genes involved in the evolution of color patterns in fish. Curr Opin Genet Dev. 57:31–38. doi: 10.1016/j.gde.2019.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kersey PJ, Allen JE, Armean I, Boddu S, Bolt BJ, Carvalho-Silva D, Christensen M, Davis P, Falin LJ, Grabmueller C, et al. 2016. Ensembl Genomes 2016: more genomes, more complexity. Nucleic Acids Res. 44(D1):D574–D580. doi: 10.1093/nar/gkv1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubatko LS, Chifman J. 2019. An invariants-based method for efficient identification of hybrid species from large-scale genomic data. BMC Evol Biol. 19(1):112. doi: 10.1186/s12862-019-1439-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulathinal RJ, Stevison LS, Noor MA. 2009. The genomics of speciation in Drosophila: diversity, divergence, and introgression estimated using low-coverage genome sequencing. PLoS Genet. 5(7):e1000550. doi: 10.1371/journal.pgen.1000550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kullander SO, Fang F. 2009a. Danio aesculapii, a new species of danio from south-western Myanmar (Teleostei: Cyprinidae). Zootaxa. 2164:41–48. doi: 10.11646/zootaxa.2164.1.4. [DOI] [Google Scholar]
- Kullander SO, Fang F. 2009b. Danio tinwini, a new species of spotted danio from northern Myanmar (Teleostei: Cyprinidae). Ichthyol Explor Freshw. 20(3):223–228. [Google Scholar]
- Kullander SO, Liao TY, Fang F. 2009. Danio quagga, a new species of striped danio from western Myanmar (Teleostei: Cyprinidae). Ichthyol Explor Freshw. 20(3):193–199. [Google Scholar]
- Larget BR, Kotha SK, Dewey CN, Ane C. 2010. BUCKy: gene tree/species tree reconciliation with Bayesian concordance analysis. Bioinformatics. 26(22):2910–2911. doi: 10.1093/bioinformatics/btq539. [DOI] [PubMed] [Google Scholar]
- Lawrence C, Ebersole JP, Kesseli RV. 2008. Rapid growth and out-crossing promote female development in zebrafish (Danio rerio). Environ Biol Fishes. 81(2):239–246. doi: 10.1007/s10641-007-9195-8. [DOI] [Google Scholar]
- Li G, Davis BW, Eizirik E, Murphy WJ. 2016. Phylogenomic evidence for ancient hybridization in the genomes of living cats (Felidae). Genome Res. 26(1):1–11. doi: 10.1101/gr.186668.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locati MD, Pagano JFB, Girard G, Ensink WA, van Olst M, van Leeuwen S, Nehrdich U, Spaink HP, Rauwerda H, Jonker MJ, et al. 2017. Expression of distinct maternal and somatic 5.8S, 18S, and 28S rRNA types during zebrafish development. RNA. 23(8):1188–1199. doi: 10.1261/rna.061515.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahalwar P, Walderich B, Singh AP, Nusslein-Volhard C. 2014. Local reorganization of xanthophores fine-tunes and colors the striped pattern of zebrafish. Science. 345(6202):1362–1364. doi: 10.1126/science.1254837. [DOI] [PubMed] [Google Scholar]
- Mallet J. 2005. Hybridization as an invasion of the genome. Trends Ecol Evol. 20(5):229–237. doi: 10.1016/j.tree.2005.02.010. [DOI] [PubMed] [Google Scholar]
- Martin SH, Davey JW, Jiggins CD. 2015. Evaluating the use of ABBA-BABA statistics to locate introgressed loci. Mol Biol Evol. 32(1):244–257. doi: 10.1093/molbev/msu269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayden RL, Tang KL, Conway KW, Freyhof J, Chamberlain S, Haskins M, Schneider L, Sudkamp M, Wood RM, Agnew M, et al. 2007. Phylogenetic relationships of Danio within the order cypriniformes: a framework for comparative and evolutionary studies of a model species. J Exp Zool B Mol Dev Evol. 308(5):642–654. doi: 10.1002/jez.b.21175. [DOI] [PubMed] [Google Scholar]
- Mayden RL, Tang KL, Wood RM, Chen W-J, Agnew MK, Conway KW, Yang L, Simons AM, Bart HL, Harris PM, et al. 2008. Inferring the Tree of Life of the order Cypriniformes, the earth's most diverse clade of freshwater fishes: implications of varied taxon and character sampling. J Syst Evol. 46(3):424–438. doi: 10.3724/SP.J.1002.2008.08062. [DOI] [Google Scholar]
- McCluskey BM, Liang Y, Lewis VM, Patterson LB, Parichy DM. 2021a. Pigment pattern morphospace of Danio fishes: evolutionary diversification and mutational effects. Biol Open. 10(9):bio058814. doi: 10.1242/bio.058814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCluskey BM, Postlethwait JH. 2015. Phylogeny of zebrafish, a “model species,” within Danio, a “model genus”. Mol Biol Evol. 32(3):635–652. doi: 10.1093/molbev/msu325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCluskey BM, Uji S, Mancusi JL, Postlethwait JH, Parichy DM. 2021b. A complex genetic architecture in zebrafish relatives Danio quagga and D. kyathit underlies development of stripes and spots. PLoS Genet. 17(4):e1009364. doi: 10.1371/journal.pgen.1009364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMenamin SK, Bain EJ, McCann AE, Patterson LB, Eom DS, Waller ZP, Hamill JC, Kuhlman JA, Eisen JS, Parichy DM. 2014. Thyroid hormone-dependent adult pigment cell lineage and pattern in zebrafish. Science. 345(6202):1358–1361. doi: 10.1126/science.1256251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer A, Biermann CH, Orti G. 1993. The phylogenetic position of the zebrafish (Danio rerio), a model system in developmental biology: an invitation to the comparative method. Proc Biol Sci. 252(1335):231–236. doi: 10.1098/rspb.1993.0070. [DOI] [PubMed] [Google Scholar]
- Meyer BS, Matschiner M, Salzburger W. 2015. A tribal level phylogeny of Lake Tanganyika cichlid fishes based on a genomic multi-marker approach. Mol Phylogenet Evol. 83:56–71. doi: 10.1016/j.ympev.2014.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson TC, Stathos AM, Vanderpool DD, Finseth FR, Yuan Y-W, Fishman L. 2021. Ancient and recent introgression shape the evolutionary history of pollinator adaptation and speciation in a model monkeyflower radiation (Mimulus section Erythranthe). PLoS Genet. 17(2):e1009095. doi: 10.1371/journal.pgen.1009095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieto Feliner G, Alvarez I, Fuertes-Aguilar J, Heuertz M, Marques I, Moharrek F, Piñeiro R, Riina R, Rosselló JA, Soltis PS, et al. 2017. Is homoploid hybrid speciation that rare? An empiricist's view. Heredity (Edinb). 118(6):513–516. doi: 10.1038/hdy.2017.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noor MAF, Bennett SM. 2009. Islands of speciation or mirages in the desert? Examining the role of restricted recombination in maintaining species. Heredity (Edinb). 103(6):439–444. doi: 10.1038/hdy.2009.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parichy DM, Johnson SL. 2001. Zebrafish hybrids suggest genetic mechanisms for pigment pattern diversification in Danio. Dev Genes Evol. 211(7):319–328. doi: 10.1007/s004270100155. [DOI] [PubMed] [Google Scholar]
- Patterson LB, Bain EJ, Parichy DM. 2014. Pigment cell interactions and differential xanthophore recruitment underlying zebrafish stripe reiteration and Danio pattern evolution. Nat Commun. 5(1):5299. doi: 10.1038/ncomms6299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pease JB, Haak DC, Hahn MW, Moyle LC. 2016. Phylogenomics reveals three sources of adaptive variation during a rapid radiation. PLoS Biol. 14(2):e1002379. doi: 10.1371/journal.pbio.1002379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pease JB, Hahn MW. 2015. Detection and polarization of introgression in a five-taxon phylogeny. Syst Biol. 64(4):651–662. doi: 10.1093/sysbio/syv023. [DOI] [PubMed] [Google Scholar]
- Phillips RB, Amores A, Morasch MR, Wilson C, Postlethwait JH. 2006. Assignment of zebrafish genetic linkage groups to chromosomes. Cytogenet Genome Res. 114(2):155–162. doi: 10.1159/000093332. [DOI] [PubMed] [Google Scholar]
- Pijnacker LP, Ferwerda MA. 1995. Zebrafish chromosome banding. Genome. 38(5):1052–1055. doi: 10.1139/g95-140. [DOI] [PubMed] [Google Scholar]
- Podobnik M, Frohnhofer HG, Dooley CM, Eskova A, Nusslein-Volhard C, Irion U. 2020. Evolution of the potassium channel gene Kcnj13 underlies colour pattern diversification in Danio fish. Nat Commun. 11(1):6230. doi: 10.1038/s41467-020-20021-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podobnik M, Singh AP, Fu Z, Dooley CM, Frohnhofer HG, Firlej M, Stednitz SJ, Elhabashy H, Weyand S, Weir JR, et al. 2023. Kcnj13 regulates pigment cell shapes in zebrafish and has diverged by cis-regulatory evolution between Danio species. Development. 150(16):dev201627. doi: 10.1242/dev.201627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prufer K, Racimo F, Patterson N, Jay F, Sankararaman S, Sawyer S, Heinze A, Renaud G, Sudmant PH, de Filippo C, et al. 2014. The complete genome sequence of a Neanderthal from the Altai Mountains. Nature. 505(7481):43–49. doi: 10.1038/nature12886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prum RO, Berv JS, Dornburg A, Field DJ, Townsend JP, Lemmon EM, Lemmon AR. 2015. A comprehensive phylogeny of birds (Aves) using targeted next-generation DNA sequencing. Nature. 526(7574):569–573. doi: 10.1038/nature15697. [DOI] [PubMed] [Google Scholar]
- Quigley IK, Turner JM, Nuckels RJ, Manuel JL, Budi EH, MacDonald EL, Parichy DM. 2004. Pigment pattern evolution by differential deployment of neural crest and post-embryonic melanophore lineages in Danio fishes. Development. 131(24):6053–6069. doi: 10.1242/dev.01526. [DOI] [PubMed] [Google Scholar]
- Rodriguez JM, Maietta P, Ezkurdia I, Pietrelli A, Wesselink J-J, Lopez G, Valencia A, Tress ML. 2013. APPRIS: annotation of principal and alternative splice isoforms. Nucleic Acids Res. 41(D1):D110–D117. doi: 10.1093/nar/gks1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosser N, Seixas F, Queste LM, Cama B, Mori-Pezo R, Kryvokhyzha D, Nelson M, Waite-Hudson R, Goringe M, Costa M, et al. 2024. Hybrid speciation driven by multilocus introgression of ecological traits. Nature. 628(8009):811–817. doi: 10.1038/s41586-024-07263-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sankararaman S, Mallick S, Dannemann M, Prufer K, Kelso J, Pääbo S, Patterson N, Reich D. 2014. The genomic landscape of Neanderthal ancestry in present-day humans. Nature. 507(7492):354–357. doi: 10.1038/nature12961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scally A, Dutheil JY, Hillier LW, Jordan GE, Goodhead I, Herrero J, Hobolth A, Lappalainen T, Mailund T, Marques-Bonet T, et al. 2012. Insights into hominid evolution from the gorilla genome sequence. Nature. 483(7388):169–175. doi: 10.1038/nature10842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumer M, Rosenthal GG, Andolfatto P. 2014. How common is homoploid hybrid speciation? Evolution. 68(6):1553–1560. doi: 10.1111/evo.12399. [DOI] [PubMed] [Google Scholar]
- Schumer M, Xu C, Powell DL, Durvasula A, Skov L, Holland C, Blazier JC, Sankararaman S, Andolfatto P, Rosenthal GG, et al. 2018. Natural selection interacts with recombination to shape the evolution of hybrid genomes. Science. 360(6389):656–660. doi: 10.1126/science.aar3684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer A, Perlman H, Yan Y, Walker C, Corley-Smith G, Brandhorst B, Postlethwait J. 2002. Sex-specific recombination rates in zebrafish (Danio rerio). Genetics. 160(2):649–657. doi: 10.1093/genetics/160.2.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh AP, Nusslein-Volhard C. 2015. Zebrafish stripes as a model for vertebrate colour pattern formation. Curr Biol. 25(2):R81–R92. doi: 10.1016/j.cub.2014.11.013. [DOI] [PubMed] [Google Scholar]
- Sola L, Gornung E. 2001. Classical and molecular cytogenetics of the zebrafish, Danio rerio (Cyprinidae, Cypriniformes): an overview. Genetica. 111(1–3):397–412. doi: 10.1023/A:1013776323077. [DOI] [PubMed] [Google Scholar]
- Soudi S, Jahani M, Todesco M, Owens GL, Bercovich N, Rieseberg LH, Yeaman S. 2023. Repeatability of adaptation in sunflowers reveals that genomic regions harbouring inversions also drive adaptation in species lacking an inversion. Elife. 12:RP88604. doi: 10.7554/eLife.88604.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatakis A. 2006. RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics. 22(21):2688–2690. doi: 10.1093/bioinformatics/btl446. [DOI] [PubMed] [Google Scholar]
- Supple MA, Hines HM, Dasmahapatra KK, Lewis JJ, Nielsen DM, Lavoie C, Ray DA, Salazar C, McMillan WO, Counterman BA. 2013. Genomic architecture of adaptive color pattern divergence and convergence in Heliconius butterflies. Genome Res. 23(8):1248–1257. doi: 10.1101/gr.150615.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suurväli J, Whiteley AR, Zheng Y, Gharbi K, Leptin M, Wiehe T. 2020. The laboratory domestication of zebrafish: from diverse populations to inbred substrains. Mol Biol Evol. 37(4):1056–1069. doi: 10.1093/molbev/msz289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang KL, Agnew MK, Hirt MV, Sado T, Schneider LM, Freyhof J, Sulaiman Z, Swartz E, Vidthayanon C, Miya M, et al. 2010. Systematics of the subfamily Danioninae (Teleostei: Cypriniformes: Cyprinidae). Mol Phylogenet Evol. 57(1):189–214. doi: 10.1016/j.ympev.2010.05.021. [DOI] [PubMed] [Google Scholar]
- Thomas GWC, Hahn MW. 2015. Determining the null model for detecting adaptive convergence from genomic data: a case study using echolocating mammals. Mol Biol Evol. 32(5):1232–1236. doi: 10.1093/molbev/msv013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ting N, Sterner KN. 2013. Primate molecular phylogenetics in a genomic era. Mol Phylogenet Evol. 66(2):565–568. doi: 10.1016/j.ympev.2012.08.021. [DOI] [PubMed] [Google Scholar]
- Toomey MB, Marques CI, Araujo PM, Huang D, Zhong S, Liu Y, Schreiner GD, Myers CA, Pereira P, Afonso S, et al. 2022. A mechanism for red coloration in vertebrates. Curr Biol. 32(19):4201–4214.e12. doi: 10.1016/j.cub.2022.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traut W, Winking H. 2001. Meiotic chromosomes and stages of sex chromosome evolution in fish: zebrafish, platyfish and guppy. Chromosome Res. 9(8):659–672. doi: 10.1023/A:1012956324417. [DOI] [PubMed] [Google Scholar]
- Turner TL, Hahn MW. 2010. Genomic islands of speciation or genomic islands and speciation? Mol Ecol. 19(5):848–850. doi: 10.1111/j.1365-294X.2010.04532.x. [DOI] [PubMed] [Google Scholar]
- Turner TL, Hahn MW, Nuzhdin SV. 2005. Genomic islands of speciation in Anopheles gambiae. PLoS Biol. 3(9):e285. doi: 10.1371/journal.pbio.0030285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdivieso A, Wilson CA, Amores A, da Silva Rodrigues M, Nobrega RH, Ribas L, Postlethwait JH, Piferrer F. 2022. Environmentally-induced sex reversal in fish with chromosomal vs. polygenic sex determination. Environ Res. 213:113549. doi: 10.1016/j.envres.2022.113549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Lu Y, Zhang Y, Ning Z, Li Y, Zhao Q, Lu H, Huang R, Xia X, Feng Q, et al. 2015. The draft genome of the grass carp (Ctenopharyngodon idellus) provides insights into its evolution and vegetarian adaptation. Nat Genet. 47(6):625–631. doi: 10.1038/ng.3280. [DOI] [PubMed] [Google Scholar]
- Wilson CA, Batzel P, Postlethwait JH. 2024. Direct male development in chromosomally ZZ zebrafish. Front Cell Dev Biol. 12:1362228. doi: 10.3389/fcell.2024.1362228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson CA, High SK, McCluskey BM, Amores A, Yan Y-L, Titus TA, Anderson JL, Batzel P, Carvan MJ III, Schartl M, et al. 2014. Wild sex in zebrafish: loss of the natural sex determinant in domesticated strains. Genetics. 198(3):1291–1308. doi: 10.1534/genetics.114.169284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson CA, Postlethwait JH. 2024. A maternal-to-zygotic-transition gene block on the zebrafish sex chromosome. G3 (Bethesda). 14(5):jkae050. doi: 10.1093/g3journal/jkae050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong T-T, Saito T, Crodian J, Collodi P. 2011. Zebrafish germline chimeras produced by transplantation of ovarian germ cells into sterile host larvae. Biol Reprod. 84(6):1190–1197. doi: 10.1095/biolreprod.110.088427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu TD, Nacu S. 2010. Fast and SNP-tolerant detection of complex variants and splicing in short reads. Bioinformatics. 26(7):873–881. doi: 10.1093/bioinformatics/btq057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu P, Zhang X, Wang X, Li J, Liu G, Kuang Y, Xu J, Zheng X, Ren L, Wang G, et al. 2014. Genome sequence and genetic diversity of the common carp, Cyprinus carpio. Nat Genet. 46(11):1212–1219. doi: 10.1038/ng.3098. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All exome sequence data described herein are available at the Short Read Archive under project number PRJNA1148758. Multiple sequence alignments, variant calls, and code used to analyze data and generate figures can be found at DOI 10.5281/zenodo.14201925. Additional data were reanalyzed from Suurväli et al. (2020).
Supplemental material available at G3 online.