Abstract
Background
The Hippo signaling pathway is an evolutionarily conserved signaling module that controls organ size in different species, and the disorder of the Hippo pathway can induce liver cancer in organisms, especially hepatocellular carcinoma (HCC). The exact mechanism that causes cancer is still unknown. Recent studies have shown that it is a classical kinase cascade that phosphorylates the Mst1/2-sav1 complex and activates the phosphorylation of the Lats1/2-mob1A/B complex for inactivating Yap and Taz. These kinases and scaffolds are regarded as primary regulators of the Hippo pathway, and help in activating a variety of carcinogenic processes. Among them, Yap/Taz is seen to be the main effector molecule, which is downstream of the Hippo pathway, and its abnormal activation is related to a variety of human cancers including liver cancer. Currently, since Yap/Taz plays a variety of roles in cancer promotion and tumor regeneration, the Hippo pathway has emerged as an attractive target in recent drug development research.
Methods
We collect and review relevant literature in web of Science and Pubmed.
Conclusion
This review highlights the important roles of Yap/Taz in activating Hippo pathway in liver cancer. The recent findings on the crosstalks between the Hippo and other cancer associated pathways and moleculars are also discussed.
Graphical abstract
In this review, we summarized and discussed recent breakthroughs in our understanding of how key components of the Hippo-YAP/TAZ pathway influence the hepatocellular carcinoma, including their effects on tumor occurrence and development, their roles in regulating metastasis, and their function in chemotherapy resistance. Further, the molecular mechanism and roles in regulating cross talk between Hippo-YAP/TAZ pathway and other cancer-associated pathways or oncogenes/cancer suppressor genes were summarized and discussed. More, many other inducers and inhibitors of this signaling cascade and available experimental therapies against the YAP/TAZ/TEAD axis were discussed. Targeting this pathway for cancer therapy may have great significance in the treatment of hepatocellular carcinoma. Graphical summary of the complex role of Hippo-YAP/TAZ signaling in hepatocellular carcinoma

Keywords: Hippo pathway, YAP/TAZ, Hepatocellular carcinoma, Signal transduction
Introduction
Hepatocellular carcinoma (HCC) is the most prevalent type of liver cancer, accounting for the sixth most common type of cancer among all cancers and the second major cause of mortality (Siegel et al. 2019, 2021; Sung et al. 2021). The latest study shows that the number of patients with HCC accounts for 85% of the patients with cirrhosis of the liver, and the survival rate of 5 years is only 18% after pancreatic cancer (Hao et al. 2021). With environmental factors, vaccination, and lifestyle changes, the prevalence and mortality of HCC in the world are increasing. The mortality rate of all other common cancers, such as breast cancer, lung cancer, and prostate cancer, has declined. Nevertheless, the death rate of HCC continues to increase by about 2–3% a year. However, most patients with HCC are diagnosed at late stages, when the beneficial treatments of hepatic resection, ablation, and liver transplantation cannot be applied (Kim and Viatour 2020; Wang and Wei 2020; Llovet et al. 2008). In general, HCC is not asymptomatic in the early stages, dramatically delaying its timely diagnosis. Future research must increase public understanding of HCC in order to facilitate early diagnosis and management (Grazie et al. 2017; Vogel et al. 2022; Bruix et al. 2017).
Although many people have tried to develop molecularly targeted drugs and immunotherapeutic methods, no effective drugs have been developed for the treatment of HCC. Furthermore, existing drugs are reported to cause a wide range of multi-system adverse reactions (Moukhadder et al. 2017). Therefore, there is a need to develop new treatment options for HCC patients. The Hippo signaling pathway was first identified in Drosophila melanogaster, and it has been intensively studied over the past 20 years. Transcriptional coregulatory factors YAP (Yes-associated proteins) and TAZ (transcriptional coactivators with PdZ-binding moths) play a crucial role in mediating various oncogenic processes. YAP/TAZ, as critical downstream effectors of the Hippo pathway, were overexpressed in human HCC and are vital for the initiation, progression, and recurrence (Smith et al. 2021; Zanconato et al. 2015).
Notably, YAP/TAZ incorporates inputs from various kinds of carcinogenic signaling pathways, such as KRAS, PI3K, EGFR, TGFβ, Wnt, GPCR, and TEAD. It can regulate genes working together to coordinate various carcinogenic processes, such as mechanical transduction, metabolic reprogramming, drug resistance, immunosuppression, and angiogenesis, many of which are considered cancer markers.
Cumulative evidence suggests that Hippo kinase, YAP/TAZ, and their partners are abnormally expressed in various human tumors, such as liver cancer (Zanconato et al. 2016a; Yimlamai et al. 2015; Noguchi et al. 2018). Moreover, the overexpression of YAP/TAZ can migrate to the nucleus and trigger TEAD-mediated gene expression associated with cell growth and proliferation (Fan et al. 2022; Luo et al. 2022; Pobbati and Hong 2020). Hippo kinase functions as a tumor suppressor in the healthy liver by suppressing hepatocyte proliferation and maintaining hepatocyte differentiation. The absence of Hippo kinase activity, on the other hand, can occur in mice with genetic deletions of Nf2, Mst1/2, Lats1/2, or Sav1, showed varying levels of YAP phosphorylation (a sign of high YAP activity), resulting in hepatomegaly and varieties of liver cancer, such as HCC, intrahepatic cholangiocacinoma (ICCA), and HCC/ICC mixed forms (Zanconato et al. 2016a; Nguyen and Yi 2019; Guo et al. 2022; Totaro et al. 2018). This type of evidence implies that YAP/TAZ activation is one of the critical effector factors of Hippo kinase activation and is crucial in the treatment of liver cancer.
The role of the Hippo signaling pathway in HCC
In general, the Hippo signaling pathway could affect various human cancers, especially HCC, in three aspects: rumor occurrence and development, tumor metastasis, and tumor drug resistance (Fig. 1). The discussions about the basic mechanisms are as follows.
Fig. 1.
Role of the Hippo signaling pathway in HCC: detailed description of the development, metastasis and drug resistance of the Hippo pathway in hepatocellular carcinoma
The hippo pathway in the onset and progression of HCC
The tumor genesis and progression is a multi-step process characterized by the transformation of healthy cells into malignant tumor cells and is driven by a variety of factors. The Hippo signaling pathway is mainly involved in the onset and progression of HCC by influencing cell proliferation, metabolic reprogramming, autophagy, and the immune microenvironment.
According to studies, two conditions must be met in order to facilitate the YAP/TAZ responses to function fully. These conditions are the promotion of nuclear accumulation of YAP/TAZ, for instance, by loss of Hippo signaling, and inhibition of ARID1A-SWI/SNF, which can occur either by genetic inactivation or as a result of increased cell mechanics (Chang et al. 2018). Therefore, inhibiting the molecular activity of the core kinase box of the Hippo signaling pathway or various factors leading to the increase of YAP/TAZ into the nucleus can promote tumor development. It has been shown that the transfer of YAP into the nucleus is critical for HCC cell proliferation in both HCC cells and mouse models, which is achieved through genetic and pharmacological interventions, and the results were consistent with iCCA (Ma and Huang 2021; Mranda et al. 2022). Recent studies suggested that liver cancer could be induced in mice hepatocytes by transgenic expression of activated Yes (Fan et al. 2022). Moreover, Yes phosphorylated the transcriptional coactivators YAP/TAZ, enhancing their nuclear accumulation and transcriptional activity in HCC cells and liver tumors (Guegan et al. 2022). These findings indicated that YAP and TAZ are effectors of Yes-dependent oncogenic transformation of hepatocytes.
Recent studies suggested that various kinds of proteins, which regulated phosphorylation and nuclear localization of YAP, were involved in the occurrence and development of HCC. For example, LATS-mediated phosphorylation of YAP at Ser127 suppresses its nuclear localization and transcriptional activity. However, YAP Nemo-like kinase (NLK) phosphorylates YAP at Ser128, blocks its interaction with 14-3-3, and enhances its nuclear localization (Tapon et al. 2002).
Src family kinase activation notably increased tumor load in mice by regulating phosphorylation and nuclear localization of YAP in human HCC (Guegan et al. 2022). HCC cell proliferation, infiltration, and migration can be suppressed via the mechanism of downregulating c-Src expression. Yes-associated protein transcriptional activity was enhanced by c-Src, which inhibited the translocation of YAP from the nucleus to the cytoplasm (Yang et al. 2021). In addition, peptide SMIM30 binds to the SRC/YES1 non-receptor tyrosine kinases to boost their membrane anchoring and phosphorylation, triggering the downstream MAPK signaling pathway and promoting the development of HCC. A novel class of noncoding RNA known as circular RNA (circRNA) has clear associations with the incidence and progression of numerous diseases, including tumors. Xiaopei Hao et al. suggested that CircPAK1 is expressed at a high level in tumor tissues and is linked to unfavorable outcomes in individuals with HCC. Moreover, by competitively binding 14-3-3 ζ with YAP, CircPAK1 performs its oncogenic role, boosting YAP nucleus localization and the downstream target gene amplification (Hao et al. 2022). Consistently, evidence highlighted that the input/output nucleus of YAP is closely regulated by 14-3-3-ζ, which is assembled with YAP to isolate it in the cytoplasm and prevent further amplification of its signal (Ren et al. 2010; Kanai et al. 2000).
A number of miRNAs have been established as well-known post-transcriptional regulators of the expression of Hippo-YAP/TAZ signaling components, and dysregulation of those miRNAs can result in liver cancer metastasis (Han 2019; Lee et al. 2021). Junjie Li et al. showed that miR-15b significantly increases the expression of LATS1, inhibits activation of the Hippo pathway, and promotes the proliferation of HCC cells (Li et al. 2021). Besides, a recent study reported that high miR-21-3p levels were linked to advanced tumor stages and are involved in promoting migration and invasion of HCC cells. Furthermore, miR-21-3p has shown significantly elevated YAP1 expression partly via downregulating expression of its direct target SMAD7 (Hong et al. 2021). Together, these results indicate that miRNAs are closely linked to Hippo-YAP/TAZ signaling in controlling HCC development.
For tumor onset and progression, metabolic reprogramming is necessary (Tapon et al. 2002; Jeong et al. 2018; Thompson 2020).
Previous findings highlight the role of the Hippo pathway in glycolysis reprogramming which is required for tumor cells to acquire the necessary energy and building blocks and to promote cancer development (Martinez-Reyes and Chandel 2021; Zhang et al. 2018). On the other hand, Liu et al. have reported that glycogen accumulation blocks signal transduction activity, subsequently activating YAP and contributing to liver enlargement and cancer development (Liu et al. 2021). Sox9 is a marker of liver progenitor cells, which is also a transcription factor regulated by YAP/TAZ in the liver (Yimlamai et al. 2014). According to research by Yuchen Liu et al., a transition from mature hepatocytes to hepatic progenitor cells and ultimately to bile duct lining cells occurs as a result of YAP activation in hepatocytes. Moreover, during YAP-induced hepatocarcinogenesis, Sox9 is essential for hepatocyte transformation to epithelial-like cells (Liu et al. 2022).
Meanwhile, Hippo is also crucial for preventing the occurrence and advancement of HCC. Hippo interacts with the AKT signaling pathway to prevent NAFLD (non-alcoholic fatty liver disease) and HCC progression. Conversely, it accelerates the formation of NAFLD and HCC once the interaction between the two pathways is impaired (Jeong et al. 2018; Jeong and Lim 2018; Foglia et al. 2022).
These studies are crucial for further evaluating the activation of YAP/TAZ activity in the Hippo pathway for the occurrence and development of HCC as well as for precise therapy.
The role of the Hippo-YAP/TAZ signaling pathway in HCC metastasis
Both high HCC prevalence and its high mortality are primarily due to tumor metastasis; however, the exact mechanism via which it occurs is still unclear. Recent studies have shown that Hippo pathway components, such as LAT1/2, MST1/2, YAP, and TAZ, are crucial in regulating HCC metastasis (Hu et al. 2018; Zhou et al. 2019; Ni et al. 2017). There are two possible mechanisms for the potential impact of the Hippo pathway on tumor metastasis.
Firstly, the Hippo pathway regulates epithelial–mesenchymal transformation (EMT) to promote migration and invasion (Zanconato et al. 2016a; Thompson 2020; Fu et al. 2022). EMT refers to the process of transforming the phenotype of epithelial cells to that of mesenchymal cells, which can lead to the reduction of intercellular adhesion and promote the metastasis of tumor cells. It is an essential feature of the enhanced migration of cancer cells (Fu et al. 2022). Huang et al. reported that the deletion of PDLIM1 protein in HCC would induce excessive F-actin formation, lead to LATS1 dephosphorylation and YAP activation, induce EMT of HCC cells, improve their invasion ability, and promote metastasis in vitro and in vivo (Zheng et al. 2019). On the other hand, the hypoxic environment-induced inactivation of YAP/TAZ significantly inhibited the EMT of tumor cells, thereby inhibiting the migration and metastasis of tumors in HCC (Weiler et al. 2020; Stoel et al. 2020). The inactivation of Hippo, which regulates the EGFR-RAS-RAF-MEK-ERK-mediated interaction of Hippo signaling, is greatly influenced by RAS signaling (Fu et al. 2022; Solberg et al. 2019). In PDAC mouse models, YAP is found to be a key promoter of mutated KRAS carcinogenic programs as it particularly induces the expression of secreting factors, including CTGF and CYR61, and also interacts with proto-oncogenes FOS for regulating the expression of genes linked to EMT including E-cadherin, SLUG, SNAIL, and Vimentin (Zhang et al. 2014a; Shao et al. 2014). In addition, small noncoding RNAs called the microRNAs (miRNAs) also promote the metastasis and EMT of hepatocellular carcinoma by direct inhibition of the sensors of Hippo-YAP/TAZ signaling (Zheng et al. 2019; Han et al. 2018a, b; Lu et al. 2021).
Secondly, the Hippo pathway can promote tumor metastasis by inhibiting anchorage-dependent cell death and apoptosis caused by loss of attachment between cells and ECM (extracellular matrix). Recently, previous research reported that DLC1 (deleted in liver cancer 1), a new transcriptional target of the YAP/TAZ–TEAD complex, is necessary for integrin-based focal adhesion disassembly, cell polarization, collective cell migration, and angiogenic sprouting. Moreover, they demonstrated that ectopic DLC1 expression in YAP-depleted endothelial cells restores their potential for migration and angiogenic sprouting (Stoel et al. 2020). Moreover, individuals with HCC have elevated levels of integrin-V (ITGAV), a novel TAZ-specific target gene, which is linked to a poorer clinical prognosis. Functionally, ITGAV disruption decreases nuclear YAP/TAZ protein levels and actin fiber production, indicating that ITGAV is involved in the assembly of actin stress fibers, cancer cell migration, and invasion (Weiler et al. 2020).
Based on these findings, the key elements of the Hippo-YAP/TAZ signaling pathway act as a switch for HCC metastasis and might be a potential target for anti-tumor metastasis therapy.
Development of drug resistance of Hippo-YAP/TAZ signaling pathway in HCC
A variety of therapies are available for HCC, with surgery, immunotherapy, chemotherapy, and targeted therapy as the four major anti-HCC therapies. However, it is known that the aforementioned therapeutic effects are impaired to varying degrees due to tumor resistance. Recent research revealed that the Hippo pathway was closely linked to a poor prognosis and cancer cells’ responses to chemotherapeutic treatments (Yuan et al. 2018; Kim et al. 2013; Xiao et al. 2015; Sohn et al. 2016). Therefore, it is crucial to find promising strategies for overcoming Hippo-induced chemotherapy resistance.
Both previous and recent studies have demonstrated that YAP/TAZ, as the major component of the Hippo pathway, was responsible for chemotherapeutic resistance in HCC, such as cisplatin (CDDP) and doxorubicin (Dox) (Hall et al. 2010; Huang et al. 2013; Li et al. 2019; Mao et al. 2014; Chen et al. 2018).
Specifically, Zhang et al. demonstrated that TAZ was associated with chemoresistance in HCC cells by directly attaching to the promoter of IL-8 and activating its transcription. Additionally, siRNAs targeting TAZ inhibition can restore CDDP and Dox sensitivity in chemoresistant HCC cells (Zhang et al. 2020). Moreover, Sun et al. found that sorafenib enhanced the accumulation and activation of nucleus YAP, thereby promoting the resistance of sorafenib by inhibiting apoptosis of HCC cells through the downstream mediator of YAP and survivin (Sun et al. 2021). Furthermore, Gao et al. highlighted that the expression of SLC7A11 was induced by YAP/TAZ for maintaining intracellular glutathione homeostasis in a TEAD-dependent manner, facilitating HCC cells to overcome ferroptosis caused by sorafinib (Gao et al. 2021). Our research consistently demonstrated that in HCC cells, upregulated YAP expression conferred resistance to cisplatin. Interestingly, this research also highlighted that the edible flavonoid myricetin considerably improved the effectiveness of cisplatin therapy in vivo via the mechanism of activating LATS1/2 and subsequently inducing phosphorylation and degradation of YAP (Li et al. 2019).
These outcomes indicate that regulating the Hippo pathway, particularly YAP/TZA activity, could be a promising approach to overcoming chemoresistance in HCC.
Multiple signal regulation mechanisms
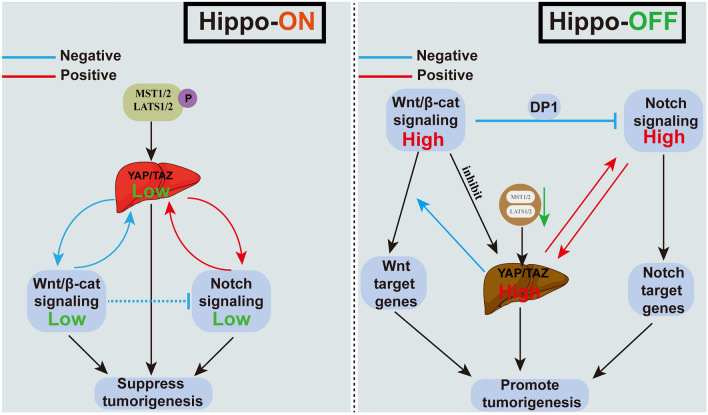
Apart from the established molecular cascades, lots of pathways that involve growth factor receptors or their cytoplasmic intermediates and pathways critical for cell differentiation were found to be frequently changing in HCC (Garcia-Lezana et al. 2021). Among these dysregulated pathways, about 5–10% of human HCCs have been reported to have genomic amplification of genomic sites involving YAP (Overholtzer et al. 2006). Moreover, about 60% of human HCCs are reported to increase the YAP and TAZ activity (Tao et al. 2014; Zhang et al. 2012; Wu et al. 2013). This suggests that the increase in YAP/TAZ level and activity may be closely associated with HCCs. On the other hand, the stability and activity of YAP and TAZ were reported to be regulated by multiple mechanisms (Zhang and Zhou 2019). A number of signaling pathways, including the Wnt/beta-catenin and Notch pathways, are found to interfere with Hippo signaling pathways for adjusting the YAP/TAZ activity in the development of HCC (Fig. 2).
Fig. 2.
Concatenation of other signaling pathways and the Hippo signaling pathway affects HCC: details of the interconnection of the Wnt/beta-catenin and Notch pathways with the Hippo pathway when the Hippo pathway is on or off
The concatenation of other signaling pathways and the Hippo signaling pathway affects HCC
The Hippo signaling pathway is the primary barrier that prevents liver tumors from developing. Increasing evidence suggested that the Hippo signaling pathway interacts with other signaling pathways, including Notch and Wnt/beta-catenin pathways, which can lead to hepatoma when abnormally activated.
Wnt/β-catenin signaling is a conserved molecular pathway, which is responsible for regulating cell fate during embryogenesis and hepatobiliary development, as well as liver homeostasis and repair in adults. Dysregulation of the Wnt/β-catenin pathway accelerates the progression of various kinds of liver diseases, such as HCC and iCCA. In recent years, a growing body of research suggested the cross talk between Hippo and Wnt/β-catenin pathway. According to recent reports, the Hippo signaling pathway, together with Wnt/beta-catenin pathways, establishes an interactive signaling network for maintaining the size of the liver and regulating the tumorigenesis of the HCC (Hanahan and Weinberg 2011; Yu et al. 2014; Miyajima et al. 2014). A previous study also found that liver carcinogenesis is suppressed by interactions between Hippo signaling and Wnt/-catenin signaling. Relieving the positive feedback loop between Hippo-YAP/TAZ and Notch signaling is an important link to inhibit tumor formation. Moreover, key events, Mst1 and Mst2, knockdown accelerated HCC progression and activated YAP/TAZ, Wnt/-catenin, and STAT3 signaling (Kim et al. 2017).
Notch signaling is another conserved pathway, which has been reported to be crucial to the occurrence and development of HCC via regulating cell invasion, growth, and apoptosis. Recent evidence has suggested that the cross talk between YAP and Notch signaling induced severe hepatomegaly and also accelerated HCC onset and progression (Kim et al. 2017; Lv et al. 2018; Wang et al. 2021a). Yang et al. (2023) reported that YAP gene expression might be transcriptionally induced in liver macrophages by Notch1 signaling. Reciprocally, YAP transcriptionally elevated the expression level of the Notch ligand Jagged1, which was necessary for Notch1-mediated macrophage polarization. In addition, other groups showed that the transcription of Notch2 and other Notch pathway genes was directly regulated by YAP/TEAD to relate to Notch signaling (Yimlamai et al. 2014).
Moreover, Hippo-YAP/TAZ plays a role upstream of the signaling of Notch, and its activity is essential for the dedifferentiation and clonal growth of hepatocyte ducts. The notch may act as an effector of the direct downstream effects of YAP, and YAP-driven HCC can benefit from the treatment of Notch inhibitors (Hu et al. 2022; Cigliano et al. 2022).
Interestingly, Wantae Kim et al. found that the Hippo, Wnt/β-catenin, and Notch pathways established an interacting network for maintaining the liver size and repressed liver tumorigenesis. They also showed that deficiency of the Mst1 and Mst2 accelerated the progression of HCC and triggered YAP/TAZ, STAT3, Wnt/β-catenin, and Notch signaling pathways. Notch signaling creates a positive feedback loop with YAP/TAZ, the Hippo signaling effector, for promoting severe hepatomegaly as well as the quick onset and progression of HCC. Moreover, suppressing the positive feedback loop between YAP/TAZ and Notch signaling by triggering Wnt/β-catenin signaling significantly inhibited the development of HCC (Kim et al. 2017; Wang et al. 2021a). These findings demonstrated the interaction of the three signaling pathways at the molecular level and may provide advanced perspectives for the development of novel HCC treatment approaches.
In conclusion, multiple signaling pathways and Hippo signaling pathways interfere with each other, which not only directly inhibits the occurrence and development of HCC but also prevents some liver diseases from developing into HCC. This makes it easier to comprehend the significance of the interacting signaling network instead of a single signaling pathway in suppressing the development of liver tumors (Li et al. 2019).
Genes and proteins regulating Hippo-YAP/TAZ pathway signaling in hepatocellular carcinoma
Recently, a growing body of research has shown that some common mutations in HCC can lead to the dysregulation of the Hippo pathway directly or indirectly and the activation of YAP and TAZ (Fig. 3).
Fig. 3.
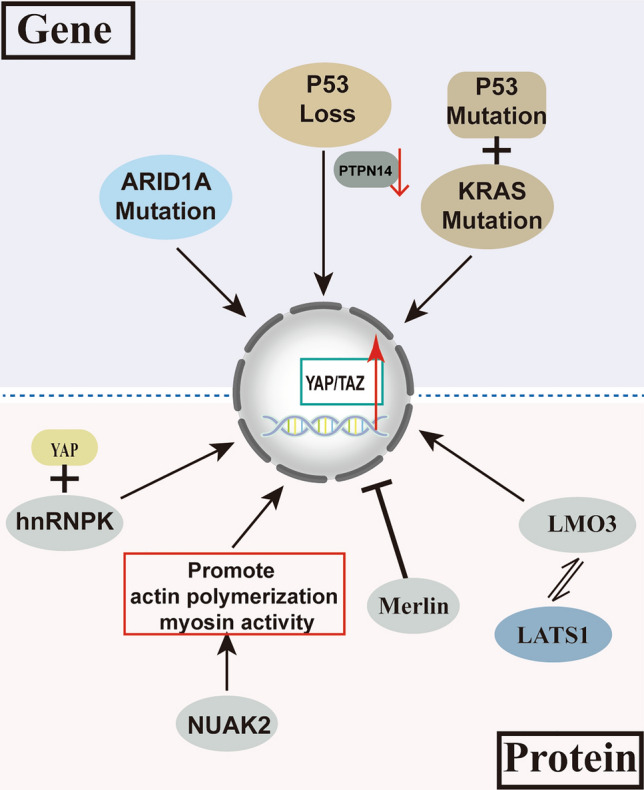
Genes and proteins regulating Hippo-YAP/TAZ pathway signaling in hepatocellular carcinoma
ARID1A (AT-Rich Interaction Domain 1A) is a part of the massive ATP-dependent chromatin remodeling complex SNF/SWI, which is necessary for the transcriptional activation of genes normally suppressed by chromatin. ARID1A mutation occurred in 10–15% of hepatocellular carcinoma patients and 20% of HCC-CCa patients (Xue et al. 2019; Fujimoto et al. 2012; Guichard et al. 2012). Recent research has highlighted that loss or mutation of ARID1A changes the level of YAP/TAZ or other regulators of the Hippo pathway and further regulates the activation of YAP/TAZ in HCC (Driskill and Pan 2021). Mechanistically, ARID1A was discovered to bind to YAP, inhibiting its transcriptional output. Furthermore, the knockdown of Arid1a significantly promoted HCC formation and CCa combinations in mice of an Nf2-null background (Chang et al. 2018).
P53 is one of the most critical proteins in cancer biology. Moreover, p53 mutations are prevalent in cancers, including HCC. A recent study revealed that p53 expression was considerably more frequent in YAP/TAZ-positive human HCC tissues, indicating a link between YAP/TAZ positivity and p53 (Park et al. 2021). Indeed, Nan Bai et al. showed that YAP overexpression in HCC cells caused a higher expression of p53. On the other hand, by attaching to the YAP promoter, p53 might have positive feedback on YAP expression (Bai et al. 2013). Furthermore, the deletion of p53 could affect the activity of YAP and TAZ by reducing PTPN14 expression (Chang et al. 2018). In addition, mutated p53 binding to KRAS, another frequently mutated gene in HCC, is sufficient to drive HCC formation as well as YAP activity, thereby maintaining tumor deterioration (Weiler et al. 2020; Hill et al. 2018). These results indicated that p53 is an important regulator of YAP/TAZ.
Heterogeneous nuclear RNA protein K (hnRNPK), directly binding to YAP, can promote YAP activation via TNFA-TNFR2 signaling and subsequently acts synergistically at the target loci in the whole genome. In addition, YAP, hnRNPK, and TNFR2 were all found to be upregulated in PLC and exhibited a positive correlation with each other (Meng et al. 2021).
Furthermore, the NUAK2, a NUAK family SNF1-like kinase 2, an important mediator of YAP, maximizes YAP activity via promoting actin polymerization as well as myosin activity to drive liver overgrowth and tumorigenesis (Yuan et al. 2018). Merlin, also known as the protein neurofibromin 2 (NF2), is a widely studied tumor suppressor that causes the activation of the Hippo pathway for inhibiting the YAP/TAZ activity. In addition, YAP/TAZ is sustainedly activated when NF2 function is lost, which accelerates hepatocyte proliferation and dedifferentiation and promotes the growth of mutant cells, enabling them to survive chronic carcinogenic stress (Hyun et al. 2021). Dysregulation of the ESRP2-NF2-YAP/TAZ axis stimulates hepatobiliary carcinogenesis in non-alcoholic fatty liver disease (NAFLD). In addition, Cheng et al. demonstrated that LMO3 inhibited Hippo signaling pathway and promoted HCC cell invasion and growth by interacting with LATS1 (Sohn et al. 2016).
Together, the discovery of such pieces of evidence suggested that the activated Hippo-YAP/TAZ signaling pathway is indeed the key to preventing the development of liver tumors.
Available experimental therapies against the YAP/TAZ-TEAD axis
In order to regulate cell growth and differentiation during development and the advancement of neoplastic disease, YAP and TAZ, paralog transcriptional regulators, can integrate mechanical, metabolic, and signaling inputs (Reggiani et al. 2021). A high similarity has been observed between YAP and TAZ proteins, which have several functional domains, such as the TEAD binding domain and the transcriptional activation domain. Comparing the most similar variants of human YAP and TAZ shows an overall similarity of about 53% (Wang et al. 2021b). An increasing body of evidence supports the distinct roles of YAP and TAZ in numerous contexts, from organ morphogenesis and tissue homeostasis to the onset and progression of cancer, despite the majority of literature suggesting that YAP and TAZ are functionally similar (Morin-Kensicki et al. 2006; Hossain et al. 2007; Liu et al. 2018).
Hiromitsu Hayashi et al. found that the TAZ expression in HCC was higher than that of YAP. Under normal conditions, knockout of TAZ could inhibit the growth of HCC cells and improve the tolerance of cells to pentafluorouracil. Notably, TAZ knockdown induced compensatory YAP expression. A transition to predominant YAP expression upon TAZ depletion gave rise to cancer stem cell-like features in HCC, such as chemoresistance and tumorigenicity. YAP knockdown inhibits the growth of HCC cells more prominently than TAZ knockdown. Combined YAP/TAZ knockdown can significantly reduce drug resistance and tumorigenicity (Hayashi et al. 2015).
YAP and TAZ, as Hippo pathway effectors, are the primary regulators of multiple cellular processes, but YAP may have a stronger influence than TAZ. These unique functions may depend on protein structural variations, expression differences, specific regulatory mechanisms, and the effect of different interacting partners (Kaan et al. 2017; Oudhoff et al. 2013). This might be pivotal for mechanistic and translational studies aimed at using YAP and TAZ as therapeutic targets and disease biomarkers against HCC.
YAP and TAZ confer various carcinogenic traits on cells, allowing them to maintain proliferation, suppress apoptosis, preserve stem cell characteristics, exhibit response to mechanical stimuli, involve metabolism, promote angiogenesis, suppress immune responses, and generate resistance to therapy (Pobbati and Hong 2020). In the absence of Hippo signaling, YAP and TAZ coactivators translocate to the nucleus, where they interact with members of the TEAD family to develop YAP/ TAZ-TEAD complexes that activate target genes involved in cell proliferation (Mo et al. 2014). Thus, the YAP/TAZ-TEAD transcription complex is a prospective target for cancer therapy, and inhibition of YAP/TAZ-TEAD is a promising and practical approach for innovative cancer therapies.
Some reports have highlighted that when the TEAD family of transcription factors and YAP/TAZ are unable to interact with each other, they will no longer be carcinogenic (Huh et al. 2019). Moreover, there are currently relatively few medications that can specifically target the Hippo-YAP/TAZ-TEAD cascade (Bae et al. 2017; Zanconato et al. 2016b). Pobbati et al. divided YAP/TAZ inhibitors into three groups. I: agents acted on upstream regulators and prevented YAP and TAZ from entering the nucleus; II: drugs directly acted on YAP/TAZ or TEAD, interfered with the formation of YAP/ TAZ-TEAD complex or directly inhibited TEAD; III: drugs targeted oncogenic proteins that are upregulated by YAP and TAZ transcription (Yong et al. 2021). Recently, many studies have demonstrated that YAP-TEAD blocking can inhibit YAP-induced tumor cell overgrowth (Liu-Chittenden et al. 2012; Brodowska et al. 2014). Representative inhibitors that block YAP-TEAD complex, such as Vitiprofen, CA3, super-TDU and Tankyrases inhibitors, play an important role in gastric cancer (Liu-Chittenden et al. 2012; Jiao et al. 2014; Giraud et al. 2020; Yao et al. 2019), colon cancer (Jiao et al. 2017), retinoblastoma (Brodowska et al. 2014), lung cancer (Hsu et al. 2016; Zhang et al. 2014b; Wang et al. 2016), and esophageal adenocarcinoma (Song et al. 2018).
A photosensitizer called verteporfin is clinically used to treat macular degeneration. It is the only small molecule capable of directly targeting the transcriptional activity mediated by YAP-TEAD. It has been discovered that verteporfin has shown effectiveness in inhibiting HCC cell growth both in vitro and in vivo (Liu-Chittenden et al. 2012). Using YAP RNAi- lipid nanoparticles (siYAP-LNP) is another way to inhibit YAP. Fitamant et al. demonstrated that siYAP-LNP could effectively induce HCC regression in liver-specific Mst1/Mst2 knockout mice (Fitamant et al. 2015). In addition, Tankyrases, as key signal transmitters in the Wnt pathway, can also inhibit HCC cell growth by targeting the YAP pathway. Jia et al. showed that administering Tankyrase inhibitors increased AMOTL1 and AMOTL2 expression in HCC cells, resulting in reduced YAP protein levels and transcriptional activity. Moreover, in HCC having a higher YAP activity, Tankyrase inhibitors can inhibit YAP to prevent activation of the Wnt pathway and further inhibit HCC cell growth (Jia et al. 2017). Additionally, researches have been demonstrated that a novel class of YAP inhibitors can be utilized to potentially and effectively treat HCC, such as calcimimetic agent cinacalcet and the immunomodulatory drug fingolimod (Zheng et al. 2021; Du et al. 2022).
Previous studies show that YAP may result in developing resistance against chemotherapeutic agents like sorafenib in HCC, as well as the fact that efficient YAP/TAZ inhibitors can act synergistically with chemotherapy. Zhou et al. demonstrated that YAP inhibitors (verteporfin) in combination with chemotherapy agents (5-fluorouracil, doxorubicin) could prove beneficial in overcoming chemical resistance in HCC cell lines and in vivo (Zhou et al. 2019). Chemical library screening led to the discovery of a small molecule called CA3, a new YAP1 inhibitor. Esophageal cancer has been researched for its anticancer efficacy (Song et al. 2018). Han et al. (2022) discovered that targeting YAP/TAZ with the new YAP1 inhibitor CA3 increased sensitivity to sorafenib, particularly in HCC with high YAP/TAZ expression levels. These works provide useful evidence to elucidate the function and utility of TEAD-YAP blockade in the treatment of hepatocellular carcinoma, but some of the limitations of TEAD-YAP inhibitors cannot be ignored (Fan et al. 2022). In a recent study by some scholars, it has been shown that TEAD-YAP blockade may result in slowing down tumor growth in vitro and in vivo in the nucleus accumbens (Yuan et al. 2020; Holden and Cunningham 2018; Guerrant et al. 2016), however, no significant apoptosis could be observed (Dasari et al. 2017; Zhang et al. 2015), which may occur through activation of certain pro-cell survival feedback signals. Sun et al. (Sun et al. 2022) found that VGLL3-mediated activation of the sox4/pik3c2b/AKT axis may be the limiting anti-tumor activity of TEAD-YAP inhibitors. And the toxicity problem of YAP inhibitors (Kakiuchi-Kiyota et al. 2019; Lu et al. 2018) is likely to be another challenge for YAP inhibitors. Although the bypass mechanism and toxicity issues will bring some troubles to the development of TEAD-YAP inhibition, YAP/TAZ-regulated genes can work together to orchestrate a variety of oncogenic processes, many of which are considered cancer hallmarks (Hanahan and Weinberg 2011); therefore, inhibition of YAP/TAZ-TEAD is still an attractive and viable option for novel cancer therapies (Pobbati et al. 2023; Gibault et al. 2018). Overall, these investigations imply that targeting Hippo-YAP/TAZ-TEAD axis to suppress YAP/TAZ activity serves as a potential treatment strategy for HCC (Fig. 4).
Fig. 4.
Multiple YAP inhibitors target the YAP/TAZ-TEAD axis to inhibit YAP activity in hepatocellular carcinoma
Conclusion
A large number of evidences show that Hippo-YAP/TAZ signaling pathway is one of the most common dysregulated pathways in human tumor. The YAP/TAZ activity can affect the occurrence, development, metastasis, invasion, and drug sensitivity of liver cancer. Various pathways and molecules may also affect the Hippo signaling pathway and further affect YAP/TAZ to regulate liver cancer. YAP/TAZ is a key oncogenic driver of liver carcinogenesis, and dysregulation of the Hippo pathway, which may lead to tumorigenesis and malignancy. Targeting this pathway for cancer therapy may have great significance in the treatment of liver cancer. Thus, understanding the mechanisms exhibited by the Hippo-YAP/TAZ pathway is critical to the development of novel targeted therapies.
Acknowledgements
We thank for the support of work in our lab by the Natural Science Foundation of Shandong Province (Grant no. ZR2020MH120 to Li Minjing, Grant no. ZR2020LZL020 to Yin Yancun) and the Introduction and Cultivation Project for Young Creative Talents of Higher Education of Shandong Province (to Yin Yancun and Li Minjing Li).
Author contributions
ML, YY, DL, YL, HS, and YZ conceived the review and wrote the manuscript. YZ and MC edited the references. HS and MC prepared the figures. All authors read and approved the final manuscript.
Data availability statement
Not applicable.
Declarations
Conflict of interest
The authors declare no competing interests.
Funding
Funding was supported by the Natural Science Foundation of Shandong Province (ZR2020LZL020, ZR2020MH120).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Hewen Shi, Ying Zou and Weiwei Zhong contributed equally to this work and share first authorship.
Contributor Information
Ying Liu, Email: 78075669@qq.com.
Minjing Li, Email: liminjing512@126.com.
References
- Bae JS, Kim SM, Lee H (2017) The Hippo signaling pathway provides novel anti-cancer drug targets. Oncotarget 8(9):16084–16098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai N, Zhang C, Liang N, Zhang Z, Chang A, Yin J et al (2013) Yes-associated protein (YAP) increases chemosensitivity of hepatocellular carcinoma cells by modulation of p53. Cancer Biol Ther 14(6):511–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodowska K, Al-Moujahed A, Marmalidou A, Meyer ZHM, Cichy J, Miller JW et al (2014) The clinically used photosensitizer Verteporfin (VP) inhibits YAP-TEAD and human retinoblastoma cell growth in vitro without light activation. Exp Eye Res 124:67–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruix J, Qin S, Merle P, Granito A, Huang YH, Bodoky G et al (2017) Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 389(10064):56–66 [DOI] [PubMed] [Google Scholar]
- Chang L, Azzolin L, Di Biagio D, Zanconato F, Battilana G, Lucon XR et al (2018) The SWI/SNF complex is a mechanoregulated inhibitor of YAP and TAZ. Nature 563(7730):265–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M, Wu L, Tu J, Zhao Z, Fan X, Mao J et al (2018) miR-590-5p suppresses hepatocellular carcinoma chemoresistance by targeting YAP1 expression. EBioMedicine 35:142–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cigliano A, Zhang S, Ribback S, Steinmann S, Sini M, Ament CE et al (2022) The Hippo pathway effector TAZ induces intrahepatic cholangiocarcinoma in mice and is ubiquitously activated in the human disease. J Exp Clin Cancer Res 41(1):192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasari VR, Mazack V, Feng W, Nash J, Carey DJ, Gogoi R (2017) Verteporfin exhibits YAP-independent anti-proliferative and cytotoxic effects in endometrial cancer cells. Oncotarget 8(17):28628–28640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driskill JH, Pan D (2021) The Hippo pathway in liver homeostasis and pathophysiology. Annu Rev Pathol Mech 16:299–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du J, Qian M, Yuan T, Zhang B, Chen X, An N et al (2022) Fingolimod exerts in vitro anticancer activity against hepatocellular carcinoma cell lines via YAP/TAZ suppression. Acta Pharm 72(3):427–436 [DOI] [PubMed] [Google Scholar]
- Fan S, Gao Y, Qu A, Jiang Y, Li H, Xie G et al (2022) YAP-TEAD mediates PPAR alpha-induced hepatomegaly and liver regeneration in mice. Hepatology 75(1):74–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan M, Lu W, Che J, Kwiatkowski NP, Gao Y, Seo HS et al (2022) Covalent disruptor of YAP-TEAD association suppresses defective Hippo signaling. Elife 11:e78810 [DOI] [PMC free article] [PubMed]
- Fitamant J, Kottakis F, Benhamouche S, Tian HS, Chuvin N, Parachoniak CA et al (2015) YAP inhibition restores hepatocyte differentiation in advanced HCC, leading to tumor regression. Cell Rep 10(10):1692–1707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foglia B, Sutti S, Cannito S, Rosso C, Maggiora M, Autelli R et al (2022) Hepatocyte-specific deletion of HIF2alpha prevents NASH-related liver carcinogenesis by decreasing cancer cell proliferation. Cell Mol Gastroenter 13(2):459–482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu M, Hu Y, Lan T, Guan KL, Luo T, Luo M (2022) The Hippo signalling pathway and its implications in human health and diseases. Signal Transduct Target 7(1):376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto A, Totoki Y, Abe T, Boroevich KA, Hosoda F, Nguyen HH et al (2012) Whole-genome sequencing of liver cancers identifies etiological influences on mutation patterns and recurrent mutations in chromatin regulators. Nat Genet 44(7):760–764 [DOI] [PubMed] [Google Scholar]
- Gao R, Kalathur R, Coto-Llerena M, Ercan C, Buechel D, Shuang S et al (2021) YAP/TAZ and ATF4 drive resistance to Sorafenib in hepatocellular carcinoma by preventing ferroptosis. EMBO Mol Med 13(12):e14351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Lezana T, Lopez-Canovas JL, Villanueva A (2021) Signaling pathways in hepatocellular carcinoma. Adv Cancer Res 149:63–101 [DOI] [PubMed] [Google Scholar]
- Gibault F, Sturbaut M, Bailly F, Melnyk P, Cotelle P (2018) Targeting transcriptional enhanced associate domains (TEADs). J Med Chem 61(12):5057–5072 [DOI] [PubMed] [Google Scholar]
- Giraud J, Molina-Castro S, Seeneevassen L, Sifre E, Izotte J, Tiffon C et al (2020) Verteporfin targeting YAP1/TAZ-TEAD transcriptional activity inhibits the tumorigenic properties of gastric cancer stem cells. Int J Cancer 146(8):2255–2267 [DOI] [PubMed] [Google Scholar]
- Guegan JP, Lapouge M, Voisin L, Saba-El-Leil MK, Tanguay PL, Levesque K et al (2022) Signaling by the tyrosine kinase Yes promotes liver cancer development. Sci Signal 15(717):j4743 [DOI] [PubMed] [Google Scholar]
- Guerrant W, Kota S, Troutman S, Mandati V, Fallahi M, Stemmer-Rachamimov A et al (2016) YAP mediates tumorigenesis in neurofibromatosis type 2 by promoting cell survival and proliferation through a COX-2-EGFR signaling axis. Cancer Res 76(12):3507–3519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guichard C, Amaddeo G, Imbeaud S, Ladeiro Y, Pelletier L, Maad IB et al (2012) Integrated analysis of somatic mutations and focal copy-number changes identifies key genes and pathways in hepatocellular carcinoma. Nat Genet 44(6):694–698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y, Luo J, Zou H, Liu C, Deng L, Li P (2022) Context-dependent transcriptional regulations of YAP/TAZ in cancer. Cancer Lett 527:164–173 [DOI] [PubMed] [Google Scholar]
- Hall CA, Wang R, Miao J, Oliva E, Shen X, Wheeler T et al (2010) Hippo pathway effector Yap is an ovarian cancer oncogene. Cancer Res 70(21):8517–8525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Y (2019) Analysis of the role of the Hippo pathway in cancer. J Transl Med 17(1):116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han LL, Yin XR, Zhang SQ (2018a) miR-103 promotes the metastasis and EMT of hepatocellular carcinoma by directly inhibiting LATS2. Int J Oncol 53(6):2433–2444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han LL, Yin XR, Zhang SQ (2018b) miR-650 promotes the metastasis and epithelial–mesenchymal transition of hepatocellular carcinoma by directly inhibiting LATS2 expression. Cell Physiol Biochem 51(3):1179–1192 [DOI] [PubMed] [Google Scholar]
- Han S, Lim JY, Cho K, Lee HW, Park JY, Ro SW et al (2022) Anti-cancer effects of YAP inhibitor (CA3) in combination with sorafenib against hepatocellular carcinoma (HCC) in patient-derived multicellular tumor spheroid models (MCTS). Cancers 14(11):2733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144(5):646–674 [DOI] [PubMed] [Google Scholar]
- Hao X, Sun G, Zhang Y, Kong X, Rong D, Song J et al (2021) Targeting immune cells in the tumor microenvironment of HCC: new opportunities and challenges. Front Cell Dev Biol 9:775462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao X, Zhang Y, Shi X, Liu H, Zheng Z, Han G et al (2022) CircPAK1 promotes the progression of hepatocellular carcinoma via modulation of YAP nucleus localization by interacting with 14-3-3zeta. J Exp Clin Cancer Res 41(1):281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi H, Higashi T, Yokoyama N, Kaida T, Sakamoto K, Fukushima Y et al (2015) An imbalance in TAZ and YAP expression in hepatocellular carcinoma confers cancer stem cell-like behaviors contributing to disease progression. Cancer Res 75(22):4985–4997 [DOI] [PubMed] [Google Scholar]
- Hill MA, Alexander WB, Guo B, Kato Y, Patra K, O’Dell MR et al (2018) Kras and Tp53 mutations cause cholangiocyte- and hepatocyte-derived cholangiocarcinoma. Cancer Res 78(16):4445–4451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holden JK, Cunningham CN (2018) Targeting the Hippo pathway and cancer through the TEAD family of transcription factors. Cancers 10(3):81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong Y, Ye M, Wang F, Fang J, Wang C, Luo J et al (2021) MiR-21-3p promotes hepatocellular carcinoma progression via SMAD7/YAP1 regulation. Front Oncol 11:642030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossain Z, Ali SM, Ko HL, Xu J, Ng CP, Guo K et al (2007) Glomerulocystic kidney disease in mice with a targeted inactivation of Wwtr1. Proc Natl Acad Sci USA 104(5):1631–1636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu PC, You B, Yang YL, Zhang WQ, Wang YC, Xu Z et al (2016) YAP promotes erlotinib resistance in human non-small cell lung cancer cells. Oncotarget 7(32):51922–51933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y, Yang C, Yang S, Cheng F, Rao J, Wang X (2018) miR-665 promotes hepatocellular carcinoma cell migration, invasion, and proliferation by decreasing Hippo signaling through targeting PTPRB. Cell Death Dis 9(10):954 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Hu S, Molina L, Tao J, Liu S, Hassan M, Singh S et al (2022) NOTCH-YAP1/TEAD-DNMT1 axis drives hepatocyte reprogramming into intrahepatic cholangiocarcinoma. Gastroenterology 163(2):449–465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang JM, Nagatomo I, Suzuki E, Mizuno T, Kumagai T, Berezov A et al (2013) YAP modifies cancer cell sensitivity to EGFR and survivin inhibitors and is negatively regulated by the non-receptor type protein tyrosine phosphatase 14. Oncogene 32(17):2220–2229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huh HD, Kim DH, Jeong HS, Park HW (2019) Regulation of TEAD transcription factors in cancer biology. Cells Basel 8(6):600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyun J, Al AM, Dutta RK, Oh SH, Xiang K, Zhou X et al (2021) Dysregulation of the ESRP2-NF2-YAP/TAZ axis promotes hepatobiliary carcinogenesis in non-alcoholic fatty liver disease. J Hepatol 75(3):623–633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong SH, Lim DS (2018) Insulin receptor substrate 2: a bridge between Hippo and AKT pathways. BMB Rep 51(5):209–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong SH, Kim HB, Kim MC, Lee JM, Lee JH, Kim JH et al (2018) Hippo-mediated suppression of IRS2/AKT signaling prevents hepatic steatosis and liver cancer. J Clin Investig 128(3):1010–1025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia J, Qiao Y, Pilo MG, Cigliano A, Liu X, Shao Z et al (2017) Tankyrase inhibitors suppress hepatocellular carcinoma cell growth via modulating the Hippo cascade. PLoS ONE 12(9):e184068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao S, Wang H, Shi Z, Dong A, Zhang W, Song X et al (2014) A peptide mimicking VGLL4 function acts as a YAP antagonist therapy against gastric cancer. Cancer Cell 25(2):166–180 [DOI] [PubMed] [Google Scholar]
- Jiao S, Li C, Hao Q, Miao H, Zhang L, Li L et al (2017) VGLL4 targets a TCF4-TEAD4 complex to coregulate Wnt and Hippo signalling in colorectal cancer. Nat Commun 8:14058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaan H, Chan SW, Tan S, Guo F, Lim CJ, Hong W et al (2017) Crystal structure of TAZ-TEAD complex reveals a distinct interaction mode from that of YAP-TEAD complex. Sci Rep UK 7(1):2035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakiuchi-Kiyota S, Schutten MM, Zhong Y, Crawford JJ, Dey A (2019) Safety considerations in the development of hippo pathway inhibitors in cancers. Front Cell Dev Biol 7:156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanai F, Marignani PA, Sarbassova D, Yagi R, Hall RA, Donowitz M et al (2000) TAZ: a novel transcriptional co-activator regulated by interactions with 14-3-3 and PDZ domain proteins. EMBO J 19(24):6778–6791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim E, Viatour P (2020) Hepatocellular carcinoma: old friends and new tricks. Exp Mol Med 52(12):1898–1907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim GJ, Kim H, Park YN (2013) Increased expression of Yes-associated protein 1 in hepatocellular carcinoma with stemness and combined hepatocellular-cholangiocarcinoma. PLoS ONE 8(9):e75449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim W, Khan SK, Gvozdenovic-Jeremic J, Kim Y, Dahlman J, Kim H et al (2017) Hippo signaling interactions with Wnt/beta-catenin and Notch signaling repress liver tumorigenesis. J Clin Investig 127(1):137–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Grazie M, Biagini MR, Tarocchi M, Polvani S, Galli A (2017) Chemotherapy for hepatocellular carcinoma: the present and the future. World J Hepatol 9(21):907–920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee NH, Kim SJ, Hyun J (2021) MicroRNAs regulating Hippo-YAP signaling in liver cancer. Biomedicines 9(4):347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Chen J, Yu X, Xu S, Li D, Zheng Q et al (2019) Myricetin suppresses the propagation of hepatocellular carcinoma via down-regulating expression of YAP. Cells Basel 8(4):358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Xue J, Ling M, Sun J, Xiao T, Dai X et al (2021) MicroRNA-15b in extracellular vesicles from arsenite-treated macrophages promotes the progression of hepatocellular carcinomas by blocking the LATS1-mediated Hippo pathway. Cancer Lett 497:137–153 [DOI] [PubMed] [Google Scholar]
- Liu H, Du S, Lei T, Wang H, He X, Tong R et al (2018) Multifaceted regulation and functions of YAP/TAZ in tumors (review). Oncol Rep 40(1):16–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Li J, Zhang W, Xiao C, Zhang S, Nian C et al (2021) Glycogen accumulation and phase separation drives liver tumor initiation. Cell 184(22):5559–5576 [DOI] [PubMed] [Google Scholar]
- Liu Y, Zhuo S, Zhou Y, Ma L, Sun Z, Wu X et al (2022) Yap-Sox9 signaling determines hepatocyte plasticity and lineage-specific hepatocarcinogenesis. J Hepatol 76(3):652–664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu-Chittenden Y, Huang B, Shim JS, Chen Q, Lee SJ, Anders RA et al (2012) Genetic and pharmacological disruption of the TEAD-YAP complex suppresses the oncogenic activity of YAP. Gene Dev 26(12):1300–1305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF et al (2008) Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 359(4):378–390 [DOI] [PubMed] [Google Scholar]
- Lu L, Finegold MJ, Johnson RL (2018) Hippo pathway coactivators Yap and Taz are required to coordinate mammalian liver regeneration. Exp Mol Med 50(1):E423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X, Yang C, Hu Y, Xu J, Shi C, Rao J et al (2021) Upregulation of miR-1254 promotes hepatocellular carcinoma cell proliferation, migration, and invasion via inactivation of the Hippo-YAP signaling pathway by decreasing PAX5. J Cancer 12(3):771–789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo M, Xu Y, Chen H, Wu Y, Pang A, Hu J et al (2022) Advances of targeting the YAP/TAZ-TEAD complex in the Hippo pathway for the treatment of cancers. Eur J Med Chem 244:114847 [DOI] [PubMed]
- Lv Y, Kim K, Sheng Y, Cho J, Qian Z, Zhao YY et al (2018) YAP controls endothelial activation and vascular inflammation through TRAF6. Circ Res 123(1):43–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J, Huang X (2021) Research progress in role of Hippo signaling pathway in diagnosis and treatment for hepatocellular carcinoma. Zhong Nan Da Xue Xue Bao Yi Xue Ban 46(6):637–643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao B, Hu F, Cheng J, Wang P, Xu M, Yuan F et al (2014) SIRT1 regulates YAP2-mediated cell proliferation and chemoresistance in hepatocellular carcinoma. Oncogene 33(11):1468–1474 [DOI] [PubMed] [Google Scholar]
- Martinez-Reyes I, Chandel NS (2021) Cancer metabolism: looking forward. Nat Rev Cancer 21(10):669–680 [DOI] [PubMed] [Google Scholar]
- Meng Y, Zhao Q, An L, Jiao S, Li R, Sang Y et al (2021) A TNFR2-hnRNPK axis promotes primary liver cancer development via activation of YAP signaling in hepatic progenitor cells. Cancer Res 81(11):3036–3050 [DOI] [PubMed] [Google Scholar]
- Miyajima A, Tanaka M, Itoh T (2014) Stem/progenitor cells in liver development, homeostasis, regeneration, and reprogramming. Cell Stem Cell 14(5):561–574 [DOI] [PubMed] [Google Scholar]
- Mo JS, Park HW, Guan KL (2014) The Hippo signaling pathway in stem cell biology and cancer. EMBO Rep 15(6):642–656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin-Kensicki EM, Boone BN, Howell M, Stonebraker JR, Teed J, Alb JG et al (2006) Defects in yolk sac vasculogenesis, chorioallantoic fusion, and embryonic axis elongation in mice with targeted disruption of Yap65. Mol Cell Biol 26(1):77–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moukhadder HM, Halawi R, Cappellini MD, Taher AT (2017) Hepatocellular carcinoma as an emerging morbidity in the thalassemia syndromes: a comprehensive review. Cancer Am Cancer Soc 123(5):751–758 [DOI] [PubMed] [Google Scholar]
- Mranda GM, Xiang ZP, Liu JJ, Wei T, Ding Y (2022) Advances in prognostic and therapeutic targets for hepatocellular carcinoma and intrahepatic cholangiocarcinoma: the Hippo signaling pathway. Front Oncol 12:937957 [DOI] [PMC free article] [PubMed]
- Nguyen C, Yi C (2019) YAP/TAZ signaling and resistance to cancer therapy. Trends Cancer 5(5):283–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni W, Zhang Y, Zhan Z, Ye F, Liang Y, Huang J et al (2017) A novel lncRNA uc.134 represses hepatocellular carcinoma progression by inhibiting CUL4A-mediated ubiquitination of LATS1. J Hematol Oncol 10(1):91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi S, Saito A, Nagase T (2018) YAP/TAZ signaling as a molecular link between fibrosis and cancer. Int J Mol Sci 19(11):3674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oudhoff MJ, Freeman SA, Couzens AL, Antignano F, Kuznetsova E, Min PH et al (2013) Control of the Hippo pathway by Set7-dependent methylation of Yap. Dev Cell 26(2):188–194 [DOI] [PubMed]
- Overholtzer M, Zhang J, Smolen GA, Muir B, Li W, Sgroi DC et al (2006) Transforming properties of YAP, a candidate oncogene on the chromosome 11q22 amplicon. Proc Natl Acad Sci USA 103(33):12405–12410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H, Lee Y, Lee K, Lee H, Yoo JE, Ahn S et al (2021) The clinicopathological significance of YAP/TAZ expression in hepatocellular carcinoma with relation to hypoxia and stemness. Pathol Oncol Res 27:604600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pobbati AV, Hong W (2020) A combat with the YAP/TAZ-TEAD oncoproteins for cancer therapy. Theranostics 10(8):3622–3635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pobbati AV, Kumar R, Rubin BP, Hong W (2023) Therapeutic targeting of TEAD transcription factors in cancer. Trends Biochem Sci 48(5):450–462 [DOI] [PubMed] [Google Scholar]
- Reggiani F, Gobbi G, Ciarrocchi A, Sancisi V (2021) YAP and TAZ are not identical twins. Trends Biochem Sci 46(2):154–168 [DOI] [PubMed] [Google Scholar]
- Ren F, Zhang L, Jiang J (2010) Hippo signaling regulates Yorkie nuclear localization and activity through 14-3-3 dependent and independent mechanisms. Dev Biol 337(2):303–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao DD, Xue W, Krall EB, Bhutkar A, Piccioni F, Wang X et al (2014) KRAS and YAP1 converge to regulate EMT and tumor survival. Cell 158(1):171–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel RL, Miller KD, Jemal A (2019) Cancer statistics, 2019. CA Cancer J Clin 69(1):7–34 [DOI] [PubMed] [Google Scholar]
- Siegel RL, Miller KD, Fuchs HE, Jemal A (2021) Cancer statistics, 2021. CA Cancer J Clin 71(1):7–33 [DOI] [PubMed] [Google Scholar]
- Smith JL, Rodriguez TC, Mou H, Kwan SY, Pratt H, Zhang XO et al (2021) YAP1 withdrawal in hepatoblastoma drives therapeutic differentiation of tumor cells to functional hepatocyte-like cells. Hepatology 73(3):1011–1027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohn BH, Shim JJ, Kim SB, Jang KY, Kim SM, Kim JH et al (2016) Inactivation of Hippo pathway is significantly associated with poor prognosis in hepatocellular carcinoma. Clin Cancer Res 22(5):1256–1264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solberg NT, Melheim M, Strand MF, Olsen PA, Krauss S (2019) MEK inhibition induces canonical WNT signaling through YAP in KRAS mutated HCT-15 cells, and a cancer preventive FOXO3/FOXM1 ratio in combination with TNKS inhibition. Cancers 11(2):164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song S, Xie M, Scott AW, Jin J, Ma L, Dong X et al (2018) A novel YAP1 inhibitor targets CSC-enriched radiation-resistant cells and exerts strong antitumor activity in esophageal adenocarcinoma. Mol Cancer Ther 17(2):443–454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun T, Mao W, Peng H, Wang Q, Jiao L (2021) YAP promotes sorafenib resistance in hepatocellular carcinoma by upregulating survivin. Cell Oncol 44(3):689–699 [DOI] [PubMed] [Google Scholar]
- Sun Y, Hu L, Tao Z, Jarugumilli GK, Erb H, Singh A et al (2022) Pharmacological blockade of TEAD-YAP reveals its therapeutic limitation in cancer cells. Nat Commun 13(1):6744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A et al (2021) Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71(3):209–249 [DOI] [PubMed] [Google Scholar]
- Tao J, Calvisi DF, Ranganathan S, Cigliano A, Zhou L, Singh S et al (2014) Activation of beta-catenin and Yap1 in human hepatoblastoma and induction of hepatocarcinogenesis in mice. Gastroenterology 147(3):690–701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapon N, Harvey KF, Bell DW, Wahrer DC, Schiripo TA, Haber D et al (2002) Salvador promotes both cell cycle exit and apoptosis in Drosophila and is mutated in human cancer cell lines. Cell 110(4):467–478 [DOI] [PubMed] [Google Scholar]
- Thompson BJ (2020) YAP/TAZ: drivers of tumor growth, metastasis, and resistance to therapy. BioEssays 42(5):e1900162 [DOI] [PubMed] [Google Scholar]
- Totaro A, Panciera T, Piccolo S (2018) YAP/TAZ upstream signals and downstream responses. Nat Cell Biol 20(8):888–899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Stoel M, Schimmel L, Nawaz K, van Stalborch AM, de Haan A, Klaus-Bergmann A et al (2020) DLC1 is a direct target of activated YAP/TAZ that drives collective migration and sprouting angiogenesis. J Cell Sci 133(3):jcs239947 [DOI] [PubMed]
- Vogel A, Meyer T, Sapisochin G, Salem R, Saborowski A (2022) Hepatocellular carcinoma. Lancet 400(10360):1345–1362 [DOI] [PubMed] [Google Scholar]
- Wang W, Wei C (2020) Advances in the early diagnosis of hepatocellular carcinoma. Genes Dis 7(3):308–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Lu B, Castillo J, Zhang Y, Yang Z, McAllister G et al (2016) Tankyrase inhibitor sensitizes lung cancer cells to endothelial growth factor receptor (EGFR) inhibition via stabilizing angiomotins and inhibiting YAP signaling. J Biol Chem 291(29):15256–15266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Song X, Liao H, Wang P, Zhang Y, Che L et al (2021a) Overexpression of mothers against decapentaplegic homolog 7 activates the Yes-associated protein/NOTCH cascade and promotes liver carcinogenesis in mice and humans. Hepatology 74(1):248–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Wang J, Zhang S, Jia J, Liu X, Zhang J et al (2021b) Distinct and overlapping roles of Hippo effectors YAP and TAZ during human and mouse hepatocarcinogenesis. Cell Mol Gastroenter 11(4):1095–1117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiler S, Lutz T, Bissinger M, Sticht C, Knaub M, Gretz N et al (2020) TAZ target gene ITGAV regulates invasion and feeds back positively on YAP and TAZ in liver cancer cells. Cancer Lett 473:164–175 [DOI] [PubMed] [Google Scholar]
- Wu H, Xiao Y, Zhang S, Ji S, Wei L, Fan F et al (2013) The Ets transcription factor GABP is a component of the hippo pathway essential for growth and antioxidant defense. Cell Rep 3(5):1663–1677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao H, Jiang N, Zhou B, Liu Q, Du C (2015) TAZ regulates cell proliferation and epithelial-mesenchymal transition of human hepatocellular carcinoma. Cancer Sci 106(2):151–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue R, Chen L, Zhang C, Fujita M, Li R, Yan SM et al (2019) Genomic and transcriptomic profiling of combined hepatocellular and intrahepatic cholangiocarcinoma reveals distinct molecular subtypes. Cancer Cell 35(6):932–947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Zhang X, Liu L, Yang X, Qian Q, Du B (2021) c-Src promotes the growth and tumorigenesis of hepatocellular carcinoma via the Hippo signaling pathway. Life Sci 264:118711 [DOI] [PubMed] [Google Scholar]
- Yang Y, Ni M, Zong R, Yu M, Sun Y, Li J et al (2023) Targeting Notch1-YAP circuit reprograms macrophage polarization and alleviates acute liver injury in mice. Cell Mol Gastroenterol 15(5):1085–1104 [DOI] [PMC free article] [PubMed]
- Yao Y, Wang Y, Li L, Xiang X, Li J, Chen J et al (2019) Down-regulation of interferon regulatory factor 2 binding protein 2 suppresses gastric cancer progression by negatively regulating connective tissue growth factor. J Cell Mol Med 23(12):8076–8089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yimlamai D, Christodoulou C, Galli GG, Yanger K, Pepe-Mooney B, Gurung B et al (2014) Hippo pathway activity influences liver cell fate. Cell 157(6):1324–1338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yimlamai D, Fowl BH, Camargo FD (2015) Emerging evidence on the role of the Hippo/YAP pathway in liver physiology and cancer. J Hepatol 63(6):1491–1501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yong J, Li Y, Lin S, Wang Z, Xu Y (2021) Inhibitors targeting YAP in gastric cancer: current status and future perspectives. Drug Des Devel Ther 15:2445–2456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H, Lee H, Herrmann A, Buettner R, Jove R (2014) Revisiting STAT3 signalling in cancer: new and unexpected biological functions. Nat Rev Cancer 14(11):736–746 [DOI] [PubMed] [Google Scholar]
- Yuan WC, Pepe-Mooney B, Galli GG, Dill MT, Huang HT, Hao M et al (2018) NUAK2 is a critical YAP target in liver cancer. Nat Commun 9(1):4834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Y, Park J, Feng A, Awasthi P, Wang Z, Chen Q et al (2020) YAP1/TAZ-TEAD transcriptional networks maintain skin homeostasis by regulating cell proliferation and limiting KLF4 activity. Nat Commun 11(1):1472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanconato F, Forcato M, Battilana G, Azzolin L, Quaranta E, Bodega B et al (2015) Genome-wide association between YAP/TAZ/TEAD and AP-1 at enhancers drives oncogenic growth. Nat Cell Biol 17(9):1218–1227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanconato F, Cordenonsi M, Piccolo S (2016a) YAP/TAZ at the roots of cancer. Cancer Cell 29(6):783–803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanconato F, Battilana G, Cordenonsi M, Piccolo S (2016b) YAP/TAZ as therapeutic targets in cancer. Curr Opin Pharmacol 29:26–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Zhou D (2019) Role of the transcriptional coactivators YAP/TAZ in liver cancer. Curr Opin Cell Biol 61:64–71 [DOI] [PubMed] [Google Scholar]
- Zhang T, Zhang J, You X, Liu Q, Du Y, Gao Y et al (2012) Hepatitis B virus X protein modulates oncogene Yes-associated protein by CREB to promote growth of hepatoma cells. Hepatology 56(6):2051–2059 [DOI] [PubMed] [Google Scholar]
- Zhang W, Nandakumar N, Shi Y, Manzano M, Smith A, Graham G et al (2014a) Downstream of mutant KRAS, the transcription regulator YAP is essential for neoplastic progression to pancreatic ductal adenocarcinoma. Sci Signal 7(324):a42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Gao Y, Li P, Shi Z, Guo T, Li F et al (2014b) VGLL4 functions as a new tumor suppressor in lung cancer by negatively regulating the YAP-TEAD transcriptional complex. Cell Res 24(3):331–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Ramakrishnan SK, Triner D, Centofanti B, Maitra D, Gyorffy B et al (2015) Tumor-selective proteotoxicity of verteporfin inhibits colon cancer progression independently of YAP1. Sci Signal 8(397):A98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Zhao H, Li Y, Xia D, Yang L, Ma Y et al (2018) The role of YAP/TAZ activity in cancer metabolic reprogramming. Mol Cancer 17(1):134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Yu QL, Meng L, Huang H, Liu H, Zhang N et al (2020) TAZ-regulated expression of IL-8 is involved in chemoresistance of hepatocellular carcinoma cells. Arch Biochem Biophys 693:108571 [DOI] [PubMed] [Google Scholar]
- Zheng X, Chen L, Zhou Y, Wang Q, Zheng Z, Xu B et al (2019) A novel protein encoded by a circular RNA circPPP1R12A promotes tumor pathogenesis and metastasis of colon cancer via Hippo-YAP signaling. Mol Cancer 18(1):47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng L, Du J, Ge F, Qian M, Yang B, He Q et al (2021) The calcimimetic agent cinacalcet inhibits hepatocellular carcinoma via YAP/TAZ suppression. Pharmazie 76(10):511–514 [DOI] [PubMed] [Google Scholar]
- Zhou Y, Wang Y, Zhou W, Chen T, Wu Q, Chutturghoon VK et al (2019) YAP promotes multi-drug resistance and inhibits autophagy-related cell death in hepatocellular carcinoma via the RAC1-ROS-mTOR pathway. Cancer Cell Int 19:179 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.