Abstract
Background
Breast cancer (BC) is a prevalent and highly lethal malignancy affecting women worldwide. Immunotherapy has emerged as a promising therapeutic strategy for BC, offering potential improvements in patient survival. Neoadjuvant therapy (NAT) has also gained significant clinical traction. With the advancement of computer technology, Artificial Intelligence (AI) has been increasingly applied in pathology research, expanding and redefining the scope of the field. This narrative review aims to provide a comprehensive overview of the current literature on the application of computational pathology in BC, specifically focusing on diagnosis, immune microenvironment recognition, and the evaluation of immunotherapy and NAT response.
Methods
A thorough examination of relevant literature was conducted, focusing on studies investigating the role of computational pathology in BC diagnosis, immune microenvironment recognition, and immunotherapy and NAT assessment.
Results
The application of computational pathology has shown significant potential in BC management. AI-based techniques enable improved diagnosis and classification of BC subtypes, enhance the identification and characterization of the immune microenvironment, and facilitate the evaluation of immunotherapy and NAT response. However, challenges related to data quality, standardization, and algorithm development still need to be addressed.
Conclusion
The integration of computational pathology and AI has transformative implications for BC patient care. By leveraging AI-based technologies, clinicians can make more informed decisions in diagnosis, treatment planning, and therapeutic response assessment. Future research should focus on refining AI algorithms, addressing technical challenges, and conducting large-scale clinical validation studies to facilitate the translation of computational pathology into routine clinical practice for BC patients.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00432-023-05002-8.
Keywords: Computational pathology, Artificial intelligence, Breast cancer, Tumor immune microenvironment, Immunotherapy
Background
According to the latest national cancer statistics released by the National Cancer Center in February 2022, there were 306,000 new cases of breast cancer (BC) in China in 2016, ranking fifth among all malignant tumor. In 2020, the number of new BC cases in Chinese women surpassed that of lung cancer, making it the most common malignant tumor among women (Sung et al. 2021). The incidence and mortality of BC showed an increasing trend from 2000 to 2016 (Zheng et al. 2022). Currently, surgery with adjuvant radiotherapy, chemotherapy, endocrine therapy, and molecular targeted therapy is the main treatment for BC patients (Ben-Dror et al. 2022). However, the side effects of conventional treatments are often intolerable, and to a certain extent, it is difficult to significantly improve the survival prognosis of BC patients, especially those with advanced stages. As a novel treatment method, immunotherapy is expected to further improve the quality of life and prognosis of BC patients (Emens 2018; Keenan and Tolaney 2020).
Immunotherapy can enhance the immune response of the human body against tumor tissues and activate the patient's immune system to overcome tumor immunosuppression, ultimately leading to the destruction of tumor cells (Keenan and Tolaney 2020). Common clinical immunotherapies include immune-checkpoint inhibitors, chimeric antigen receptor T-cell immunotherapy, active specific immunotherapy, and adoptive cell transfer therapy (Nishino et al. 2017). The rise of immunotherapy benefits from the continuous development of studies on tumor microenvironment in recent years. Of particular significance is the tumor-immune microenvironment (TIME), which holds paramount importance in modulating the immune response through the interaction of numerous immune cells, such as T cells, B cells, NK cells, DC cells, and T-reg cells, as well as immunoregulatory molecules, with the tumor cells (Pansy et al. 2021; Yuan et al. 2021). The in-depth study of TIME provides a theoretical basis for the development of strategies that can improve the efficacy of immunotherapy. In addition, the combination of neoadjuvant therapy (NAT) and immunotherapy has shown promising synergistic effects in the treatment of BC (Ahn et al. 2022; Lau et al. 2022). These treatment modalities, such as chemotherapy, targeted therapy, and radiation therapy, can modulate the tumor microenvironment and potentially enhance the immune response against tumor cells. The interaction between NAT, immunotherapy, and the TIME is a key factor in optimizing treatment outcomes. Evaluating the efficacy of NAT can provide insights for the development of strategies to improve immunotherapy (Zhang et al. 2023).
To date, pathological evaluation of immunotherapy and NAT response in BC patients has relied on direct visual evaluation of slides by pathologists. With the continuous improvement of precision medicine, pathologists need to evaluate an increasing number of variables in their reports and to perform a series of pathological tests, such as immunohistochemical (IHC) staining and immunofluorescence. The accuracy of pathological diagnosis is extremely dependent on the working experience of pathologists, which is inevitably affected by subjective factors. Digital methods offer a possible solution to these problems.
In the past few decades, based on the development of computer technology, the application of artificial intelligence (AI) in pathology research is increasingly extensive, and the content of pathology research is constantly expanded and redefined, resulting in the emergence of computational pathology (Bera et al. 2019). Our study introduced the application of AI in computational pathology and over-reviewed the literature on computational pathology in immune microenvironment recognition and immunotherapy effect evaluation in BC. Finally, we proposed the future development trends of clinical decision-making for BC based on computational pathology.
Applications of AI in computational pathology
In the age of AI, pathology research is gradually showing a development trend to “omics”. Computational pathology is based on AI methods to analyze a large amount of data extracted from digital pathological images, including morphological features, biological features, texture features, edge gradient features, etc., and quantify these features to support the pathological diagnosis and prognosis assessment of diseases (Bera et al. 2019; Sobhani et al. 2021).
The development of AI models in computational pathology gradually shifted from expert systems to traditional machine learning (ML) and then to deep learning (DL) (Zhu et al. 2022). The expert system is a specific knowledge database established by experts and knowledge engineers by human–computer interaction, and it depends on the rules defined by experts. Feature-based approaches are conventional methods used in ML for data extraction. These methods involve separating the process of feature extraction from classifier construction. Feature extraction entails transforming input data into feature vectors, while the classifier employs these feature vectors to categorize the data into different classes. In this approach, feature extraction is manually performed, often necessitating the expertise of domain specialists to select and design suitable features. The resulting manually crafted features can then be utilized as input for common classifiers, such as support vector machines (VM), random forests (RF), and naive Bayes, for training and classification purposes (Erickson 2021). The limitations of these methods include the necessity of selecting important features from each image, which becomes increasingly cumbersome as the number of classes increases. Furthermore, defining each feature requires handling a significant number of parameters that must be adjusted by engineers. Additionally, a substantial amount of training data needs to be accumulated for each domain, which demands significant human and material resources for annotation (Currie et al. 2019). Despite the numerous limitations, traditional ML methods still hold an irreplaceable position due to their model interpretability, fast computation speed, ease of implementation, and low memory consumption.
DL is a subset of traditional ML. What sets it apart is its ability to directly extract information from raw data and automatically learn features, perform classification, and make predictions through end-to-end deep learning approaches (Sultan et al. 2020). In this approach, the input data are transformed through a series of hierarchical non-linear transformations into high-level abstract features. These features are then utilized by a final output layer for classification or regression tasks (Madabhushi and Lee 2016). Compared to ML, DL offers significant advantages in terms of reducing human resources and efforts required for feature analysis. As the volume of data increases and the depth of DL models grows, their performance continues to improve. However, DL models also introduce certain challenges that researchers need to consider. These include the lack of interpretability associated with the deep structure of the models, substantial memory requirements, and the high computational costs associated with training and inference. Researchers must carefully weigh these considerations when deciding to adopt DL methods.
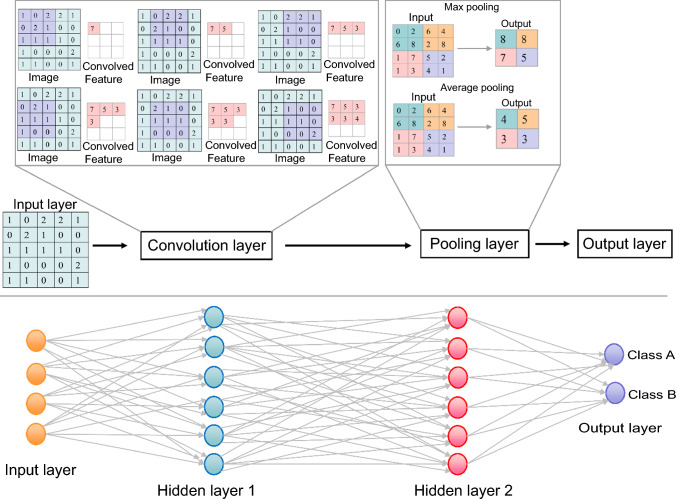
Deep neural networks (DNNs) have the capability to directly and effectively extract information from high-density and complex histopathological images (Jiang et al. 2020). DNNs are widely regarded to outperform the traditional algorithms (Deng et al. 2020), and with the increasing use of deep learning methods in the field of computer vision, the manual feature extraction process that relies on pathological diagnostic knowledge and expertise has been replaced by the iterative process of using deep learning architectures, which requires computer science skills (Niazi et al. 2019). Due to its intelligent nature, DNNs have achieved significant success and are widely applied in tasks, such as image classification, object detection, speech recognition, and other functionalities. Common types of DNNs include convolutional neural networks (CNNs), recurrent neural networks, deep belief networks, generative adversarial networks, and autoencoders (Jiang et al. 2020; Robertson et al. 2018). Among them, CNNs, including UNet, VGGNet, GoogleNet, ResNet, and DenseNet, are most widely used in computational pathology due to their advantages in image processing. A typical CNN includes three types of network layers: a convolution layer, a fully connected layer, and a pooling connected layer (Stamoulakatos et al. 2020). The convolution layer is used to extract images features from different layers, the pooling layer is used to extract the key information from the image features obtained from the convolution layer, and the fully connected layer is used to assemble the image feature information into a complete picture through the weight matrix and classify the results (Voulodimos et al. 2018) (Fig. 1).
Fig. 1.
Diagram of convolutional neural networks
Application of computational pathology in pathological diagnosis of BC
BC is the most common malignancy in women, and early diagnosis is crucial for improving the cure rate and prognosis. Currently, pathological diagnosis remains the gold standard for BC diagnosis. In the diagnostic process, distinguishing between benign and malignant lesions is a critical step. Spanhol et al. (2016) proposed an ML model for classifying benign and malignant lesions. They employed support vector machine (SVM) and RF classification algorithms to perform image classification on 7909 BC histopathology images from 82 patients collected in the BreakHis dataset. The model achieved an accuracy of 80–85% in distinguishing between benign and malignant tumors. In the recent publication, Srikantamurthy et al. (2023) developed a different model from Spanhol et al. They employed deep learning algorithm and built a hybrid model based on CNN and long short-term memory recurrent neural networks (LSTM-RNN) to enhance the robustness of the classifier. The study focused on classifying four benign and four BC subtypes within the BreakHis dataset. The proposed model achieved an accuracy of 99% in distinguishing between benign and malignant lesions and a subtype differentiation accuracy of 92.5%. Compared to ML models, the CNN-LSTM model based on deep learning significantly improved the accuracy of lesion classification without the need for feature extraction or dimensionality reduction. It also addressed the issues of overfitting or underfitting. The model has the potential for application in other diseases as well. However, it should be noted that the model lacks interpretability of the underlying data and comes with expensive computational costs. Similarly, using the BreakHis dataset, Alkhathlan and Saudagar (2022) developed three models for classifying benign and malignant lesions: an ML model based on the SVM algorithm, a DL model using CNNs, and a hybrid model that combined CNNs with transfer learning (TL) techniques. The results demonstrated that the SVM model achieved an accuracy of 92%, the CNNs’ model achieved an accuracy of 94%, and the TL-CNNs’ model achieved an accuracy of 97%.
In complex pathological diagnoses, simple classification of benign and malignant lesions may not meet the practical diagnostic needs. For example, distinguishing between BC and high-risk proliferative breast lesions is a common challenge for pathologists. And determining the invasiveness of lesions and assessing the distribution and structural characteristics of tumor cells are important indicators for assisting in the diagnosis. Mercan et al. (2019) annotated whole-slide images (WSIs) of BC with eight labels, including background, normal stroma, malignant epithelium, etc. They attempted to develop a CNN-based classification model and used a three-degree polynomial kernel to train and test a SVM classifier. The model extracted features related to tissue distribution and structure to assist in the diagnosis. The results showed that the model achieved accuracy rates close to those of pathologists in distinguishing between invasive cancer non-invasive cancer (accuracy rates of tissue distribution features, structure features, and pathologists were 0.94, 0.91, and 0.98, respectively). Furthermore, the model outperformed pathologists in distinguishing between atypia and ductal carcinoma in situ (accuracy rates of 0.83, 0.85, and 0.80 for tissue distribution features, structure features, and pathologists, respectively). However, the model had lower accuracy rates than pathologists in classifying atypia tissue or ductal carcinoma in situ versus benign tissue (accuracy rates of 0.70, 0.70, and 0.81 for tissue distribution features, structure features, and pathologists, respectively). These findings suggest that with the advancement of deep learning, improvements can be made in certain aspects of pathologists' diagnostic accuracy.
With the introduction of the concept of precision medicine, targeted therapy has been increasingly employed in clinical settings. The determination of molecular subtypes plays a pivotal role in predicting the prognosis of BC and guiding the selection of targeted therapies. Niyas et al. (2023) proposed a DL model for molecular subtyping of BC. The model utilized an enhanced LadderNet architecture, which consisted of two U-Net encoders connected to provide additional learnable pathways to the basic LadderNet architecture. Features were extracted from 600 IHC images of ER, PR, HER2, and Ki-67 markers obtained from 15 BC patients. The model achieved an accuracy of 93.3% in predicting the molecular subtypes of BC patients. Predicting tumor recurrence is also an important application of ML models in computational pathology. Lauritzen et al. (2023) utilized three classification models, namely vector magnitude, logistic regression (LR), and RF, to train an ML model on a dataset encompassing 5333 known recurrence patients and three times as many non-recurrent females. The model was capable of predicting post-diagnosis recurrence in BC patients, achieving an area under the curve (AUC) of 0.93 on the training set and 0.86 on the validation set. The study leveraged existing ML models and incorporated simple encoding to obtain a well-performing training model, thereby optimizing the clinical workflow of manual assessment of recurrence by clinicians.
Lymph-node metastasis and distant metastasis are key determining factors that influence the overall staging and prognosis of BC patients. Fan et al. (2023) developed a novel AI platform called MEAI, which can directly predict tumor metastasis on pathological slides of primary BC patients. The core architecture of this platform, called MECNN, utilizes CNN to extract features from WSI of BC tissue. ResNet50 is chosen as the backbone network for feature extraction in MECNN. The predictive performance of the model was validated on a test set comprising cases with distant metastasis and lymph-node metastasis, achieving an AUC of 0.934. This study establishes a predictive model for metastasis in BC tissue slides, providing a non-invasive method for assessing the likelihood of metastasis in BC patients. Up to 50% of initially lymph-node-positive BC patients experience lymph-node false negativity after receiving NAT (Hyder et al. 2021). The presence of lymph-node metastasis also influences the selection of NAT strategies for BC patients. Therefore, predicting the presence of lymph-node metastasis in BC patients who are about to undergo NAT is of significant importance for both patients and healthcare providers. Vrdoljak et al. (2023) trained three machine learning models (LR, RF, and XGBoost) in BC patients eligible for NAT to predict lymph-node metastasis. Among these models, the one trained with the RF algorithm exhibited the best predictive performance, with an AUC of 0.793. This model has the potential to provide more accurate disease staging and improve treatment selection by predicting lymph-node metastasis in BC patients.
Application of computational pathology in recognition of immune microenvironment and evaluation of immunotherapy response
With the 2018 Nobel Prize in Physiology and Medicine being awarded for discoveries related to harnessing the immune system to attack cancer, cancer immunotherapy has become a hot topic. Tumor-infiltrating lymphocytes (TILs) including T cells and NK cells are heterogeneous lymphocytes in the tumor stroma, playing an important role in recognizing, resisting, and attacking the tumor cells (Dushyanthen et al. 2015; Lin et al. 2020). Tumor patients with higher TILs in and around tumor tissues showed better immunotherapy response. Identification of the number and distribution of TILs can help clinicians to identify patients who benefit most from immunotherapy and to assess patients’ survival outcomes (Stanton and Disis 2016). Fassler et al. (2022) used CNN networks Resnet-34 and VGG16 to locate BC cells and to detect the spatial distribution of TILs in digital whole-slide images of BC stained with hematoxylin and eosin (HandE). After that, a tumor-TIL map was generated by combining the outputs of tumor and TILs detection. This study provides a strategy for assessing the abundance and distribution of peritumoral and intra-tumoral TILs as biomarkers to predict immunotherapy effectiveness and survival outcomes. Consistently, Lu et al. (2020) also constructed a U-net–based deep neural network to identify TILs map and quantify TILs’ spatial features on HandE-stained histopathologic images. The authors found that density and distribution of TIL clusters were important biomarkers of the BC patient outcomes. Hayashi et al. (2022) further classified TILs in different subtypes of BC using an automated DL-based quantitative pathology imaging system (Vectra Polaris) based on multiple immunohistochemistry and T-cell subpopulation markers, immune-modified markers, and TILs’ localization. The results showed that the number and distribution of TILs differed among different BC subtypes, which explains the different sensitivity to immunotherapy in different types of BC patients.
Triple negative breast cancer (TNBC) is a type of BC with negative expression of estrogen receptor, progesterone receptor, and human epidermal growth factor 2 (HER2), which has a high recurrence, metastasis rate, and poor clinical prognosis (Chen et al. 2017; Sharma 2016; Vagia et al. 2020). As a therapeutic approach following surgery, chemotherapy, endocrine therapy, and molecular targeted therapy, immunotherapy can effectively prolong the survival of TNBC patients, but not all patients are sensitive to immunotherapy. Identification of TIME characteristics in TNBC is helpful for assessing patient response to immunotherapy. Keren et al. (2018) used DL model-based automated image analysis pipelines to order the immune components of TNBC, and found significant differences in the quantity and constituent of immune cells in different patients, which may partly explain the individual differences in immunotherapy responses. On the basis of Keren et al., Patwa et al. (2021) presented a computational pipeline for the examination of TIME using a DL-based multiplexed ion beam imaging in TNBC, and demonstrated that functional proteins related to cell-to-cell interactions in TIME could predict recurrence and overall survival. As the subtype of BC with the worst prognosis, there is still a lack of clinically approved biomarkers for risk stratification and guided treatment of TNBC patients. The assessment of TIME by computational pathology may be a promising approach to fill these gaps.
Application of computational pathology in evaluation of neoadjuvant therapy
NAT is a preoperative systemic treatment to reduce the patient's pathological grade, including chemotherapy, endocrine therapy, targeted therapy, immunotherapy, and possibly even radiotherapy (Joo et al. 2021). In recent years, NAT has been increasingly used in the treatment of BC, especially for patients with advanced cancer or those who have been deprived of surgery due to the size of the tumors (Takada and Toi 2020). After receiving NAT, patients have access to surgery due to the reduction of the tumor size (Tufano et al. 2021), and the success rates of breast-conserving surgery and post-operative survival are improved (Harbeck 2022). The evaluation of tumor response to NAT is of great significance for determining further treatment options. Pathology complete response (pCR) and pathology part response were used to evaluate tumor response to NAT, and pCR predicted favorable prognosis (Thompson and Moulder-Thompson 2012; von Minckwitz et al. 2008). Previous study reported that TILs can be used as a predictor of pCR of NAT for BC patients (Asano et al. 2018). However, there may be subjective differences in the quantitative performance and reproducibility of TIL evaluations. Computational pathology is used to solve this problem. Abe et al. (2020) utilized an ML-based digital image analysis (DIA) model to quantitatively evaluate TILs in digital pathological images of HER2-positive BC and to investigate the correlation between pathological image features and pathological responses to NAT. The DIA process begins with the classification of epithelial and stromal elements using the RF algorithm, followed by the detection of TILs based on cell nuclear features and finally a combined analysis of the first two steps. The results showed that the AI-based methods were able to quantitatively assess TIL density, which appeared to be an independent predictor of neoadjuvant pCR for HER2-positive BC. Ali et al. (2017) reconfirmed that an increase in TILs’ density before and after NAT treatment was negatively correlated with pCR in BC patients based on a multicenter Phase III randomized controlled trial using an ML-based computational pathology analysis pipeline. TILs’ density in biopsies has been shown to be an independent predictor of pCR in BC. Multi-omics studies have found widespread application in the field of life sciences. Sammut et al. (2022) utilized clinical data, pathological WSI information, genomic data, and transcriptomic data from 168 BC patients to integrate multi-omics landscapes into a predictive model using ML methods. They predicted the pCR to NAT in an external validation cohort, achieving an area under the curve of 0.87. This study integrated diverse biological information from different levels and types, enhancing the accuracy and reliability of computational models and promoting personalized and precision medicine research in the field of oncology. It represents a promising direction for future computational pathology studies.
Perspectives of the application of computational pathology
To sum up, leveraging AI technology, computational pathology systematically integrates various pathological analysis tasks, such as qualitative and quantitative analysis of pathological features, evaluation of therapeutic effects, and prediction of prognosis of diseases, and has been widely used in many fields of modern medicine. The AI model developed for clinicopathological samples based on multiple tissue imaging techniques can not only compensate for the diagnostic risks brought by the subjective experience of pathologists, but also improve the accuracy of diagnosis in a shorter time, which has the potential to achieve an accurate description of the spatial organization of the complex tumor ecosystem. Despite significant advancements in ML techniques for visual image processing in recent years, there are still limitations. For example, SVM is primarily used for binary classification tasks and demonstrates strong generalization capabilities, making it well suited for high-dimensional datasets. However, when dealing with massive amounts of data, SVM increases the complexity of the data space, resulting in longer computational times. Additionally, SVM is sensitive to missing data and requires high data completeness. Conversely, LR is a relatively simple algorithm that is easy to implement. However, it is prone to underfitting and lacks high classification accuracy. RF algorithm, although effective in many cases, can encounter overfitting issues in certain regression problems and other challenges (Erickson 2021). Considering the limitations of traditional ML algorithms and the laborious task of feature extraction and data dimensionality reduction, DL algorithms have become the preferred technique for most AI-related problems due to their precision, strong adaptability, and deep data processing capabilities. However, the advantages of DL come with challenges. The large amount of data required for training, high computational demands, and complex parameter tuning make the iteration process of DL slower compared to the traditional ML.
Multi-omics research reveals the holistic view of biological systems and provides a more comprehensive understanding of complex diseases. With the aid of computational pathology, pathomorphology information can be synergized with radiomics, genomics, proteomics, and other fields, enabling the discovery of rules that are difficult to be summarized and explored through single-disciplinary approaches. This multi-omics integration fosters interdisciplinary collaboration, resulting in a more accurate and non-invasive diagnosis of diseases. Furthermore, the collaboration between data scientists and clinical pathologists may advance the application and refinement of AI in the field of precision medicine. While computational pathology methods have great potential in clinical practice, they still have several limitations. Future research should focus on refining AI algorithms, addressing technical challenges, and conducting large-scale clinical validation studies to facilitate the translation of computational pathology into routine clinical practice for BC patients.
Supplementary Information
Below is the link to the electronic supplementary material.
Author contributions
JL and XL wrote the original draft. K-LW prepared Fig. 1. GC revised the manuscript. D-DX managed the project and revised the manuscript.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability
The data used in the study are available from the corresponding author on reasonable request.
Declarations
Conflict of interest
The authors declared that they have no conflicts of interest.
Ethics approval and consent to participate
Ethics approval was not applicable in this review.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abe N, Matsumoto H, Takamatsu R, Tamaki K, Takigami N, Uehara K et al (2020) Quantitative digital image analysis of tumor-infiltrating lymphocytes in HER2-positive breast cancer. Virchows Arch 476(5):701–709. 10.1007/s00428-019-02730-6 [DOI] [PubMed] [Google Scholar]
- Ahn HK, Sim SH, Suh KJ, Kim MH, Jeong JH, Kim JY et al (2022) Response rate and safety of a neoadjuvant pertuzumab, atezolizumab, docetaxel, and trastuzumab regimen for patients with ERBB2-positive stage II/III breast cancer: the neo-PATH phase 2 nonrandomized clinical trial. JAMA Oncol 8(9):1271–1277. 10.1001/jamaoncol.2022.2310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali HR, Dariush A, Thomas J, Provenzano E, Dunn J, Hiller L et al (2017) Lymphocyte density determined by computational pathology validated as a predictor of response to neoadjuvant chemotherapy in breast cancer: secondary analysis of the ARTemis trial. Ann Oncol 28(8):1832–1835. 10.1093/annonc/mdx266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkhathlan L, Saudagar AKJ (2022) Predicting and classifying breast cancer using machine learning. J Comput Biol 29(6):497–514. 10.1089/cmb.2021.0236 [DOI] [PubMed] [Google Scholar]
- Asano Y, Kashiwagi S, Goto W, Takada K, Takahashi K, Hatano T et al (2018) Prediction of treatment response to neoadjuvant chemotherapy in breast cancer by subtype using tumor-infiltrating lymphocytes. Anticancer Res 38(4):2311–2321. 10.21873/anticanres.12476 [DOI] [PubMed] [Google Scholar]
- Ben-Dror J, Shalamov M, Sonnenblick A (2022) The history of early breast cancer treatment. Genes (basel). 10.3390/genes13060960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bera K, Schalper KA, Rimm DL, Velcheti V, Madabhushi A (2019) Artificial intelligence in digital pathology—new tools for diagnosis and precision oncology. Nat Rev Clin Oncol 16(11):703–715. 10.1038/s41571-019-0252-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Wu Q, Ding Y, Zhou W, Liu R, Chen H et al (2017) YD277 suppresses triple-negative breast cancer partially through activating the endoplasmic reticulum stress pathway. Theranostics 7(8):2339–2349. 10.7150/thno.17555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currie G, Hawk KE, Rohren E, Vial A, Klein R (2019) Machine learning and deep learning in medical imaging: intelligent imaging. J Med Imaging Radiat Sci 50(4):477–487. 10.1016/j.jmir.2019.09.005 [DOI] [PubMed] [Google Scholar]
- Deng S, Zhang X, Yan W, Chang EI, Fan Y, Lai M, Xu Y (2020) Deep learning in digital pathology image analysis: a survey. Front Med 14(4):470–487. 10.1007/s11684-020-0782-9 [DOI] [PubMed] [Google Scholar]
- Dushyanthen S, Beavis PA, Savas P, Teo ZL, Zhou C, Mansour M et al (2015) Relevance of tumor-infiltrating lymphocytes in breast cancer. BMC Med 13:202. 10.1186/s12916-015-0431-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emens LA (2018) Breast cancer immunotherapy: facts and hopes. Clin Cancer Res 24(3):511–520. 10.1158/1078-0432.CCR-16-3001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson BJ (2021) Basic artificial intelligence techniques: machine learning and deep learning. Radiol Clin North Am 59(6):933–940. 10.1016/j.rcl.2021.06.004 [DOI] [PubMed] [Google Scholar]
- Fan J, Zhang L, Lv T, Liu Y, Sun H, Miao K et al (2023) MEAI: an artificial intelligence platform for predicting distant and lymph node metastases directly from primary breast cancer. J Cancer Res Clin Oncol. 10.1007/s00432-023-04787-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fassler DJ, Torre-Healy LA, Gupta R, Hamilton AM, Kobayashi S, Van Alsten SC et al (2022) Spatial characterization of tumor-infiltrating lymphocytes and breast cancer progression. Cancers (basel). 10.3390/cancers14092148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harbeck N (2022) Neoadjuvant and adjuvant treatment of patients with HER2-positive early breast cancer. Breast 62(Suppl 1):S12–S16. 10.1016/j.breast.2022.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi K, Nogawa D, Kobayashi M, Asakawa A, Ohata Y, Kitagawa S et al (2022) Quantitative high-throughput analysis of tumor infiltrating lymphocytes in breast cancer. Front Oncol 12:901591. 10.3389/fonc.2022.901591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyder T, Bhattacharya S, Gade K, Nasrazadani A, Brufsky AM (2021) Approaching neoadjuvant therapy in the management of early-stage breast cancer. Breast Cancer (dove Med Press) 13:199–211. 10.2147/BCTT.S273058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y, Yang M, Wang S, Li X, Sun Y (2020) Emerging role of deep learning-based artificial intelligence in tumor pathology. Cancer Commun (lond) 40(4):154–166. 10.1002/cac2.12012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joo S, Ko ES, Kwon S, Jeon E, Jung H, Kim JY et al (2021) Multimodal deep learning models for the prediction of pathologic response to neoadjuvant chemotherapy in breast cancer. Sci Rep 11(1):18800. 10.1038/s41598-021-98408-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keenan TE, Tolaney SM (2020) Role of immunotherapy in triple-negative breast cancer. J Natl Compr Canc Netw 18(4):479–489. 10.6004/jnccn.2020.7554 [DOI] [PubMed] [Google Scholar]
- Keren L, Bosse M, Marquez D, Angoshtari R, Jain S, Varma S et al (2018) A structured tumor-immune microenvironment in triple negative breast cancer revealed by multiplexed ion beam imaging. Cell 174(6):1373-1387 e1319. 10.1016/j.cell.2018.08.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau KH, Tan AM, Shi Y (2022) New and emerging targeted therapies for advanced breast cancer. Int J Mol Sci. 10.3390/ijms23042288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauritzen AD, Berg T, Jensen MB, Lillholm M, Knoop A (2023) Identifying recurrent breast cancer patients in national health registries using machine learning. Acta Oncol. 10.1080/0284186X.2023.2201687 [DOI] [PubMed] [Google Scholar]
- Lin B, Du L, Li H, Zhu X, Cui L, Li X (2020) Tumor-infiltrating lymphocytes: warriors fight against tumors powerfully. Biomed Pharmacother 132:110873. 10.1016/j.biopha.2020.110873 [DOI] [PubMed] [Google Scholar]
- Lu Z, Xu S, Shao W, Wu Y, Zhang J, Han Z et al (2020) Deep-learning-based characterization of tumor-infiltrating lymphocytes in breast cancers from histopathology images and multiomics data. JCO Clin Cancer Inform 4:480–490. 10.1200/CCI.19.00126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madabhushi A, Lee G (2016) Image analysis and machine learning in digital pathology: challenges and opportunities. Med Image Anal 33:170–175. 10.1016/j.media.2016.06.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercan E, Mehta S, Bartlett J, Shapiro LG, Weaver DL, Elmore JG (2019) Assessment of machine learning of breast pathology structures for automated differentiation of breast cancer and high-risk proliferative lesions. JAMA Netw Open 2(8):e198777. 10.1001/jamanetworkopen.2019.8777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niazi MKK, Parwani AV, Gurcan MN (2019) Digital pathology and artificial intelligence. Lancet Oncol 20(5):e253–e261. 10.1016/S1470-2045(19)30154-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishino M, Ramaiya NH, Hatabu H, Hodi FS (2017) Monitoring immune-checkpoint blockade: response evaluation and biomarker development. Nat Rev Clin Oncol 14(11):655–668. 10.1038/nrclinonc.2017.88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niyas S, Bygari R, Naik R, Viswanath B, Ugwekar D, Mathew T et al (2023) Automated molecular subtyping of breast carcinoma using deep learning techniques. IEEE J Transl Eng Health Med 11:161–169. 10.1109/JTEHM.2023.3241613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pansy K, Uhl B, Krstic J, Szmyra M, Fechter K, Santiso A et al (2021) Immune regulatory processes of the tumor microenvironment under malignant conditions. Int J Mol Sci. 10.3390/ijms222413311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patwa A, Yamashita R, Long J, Risom T, Angelo M, Keren L, Rubin DL (2021) Multiplexed imaging analysis of the tumor-immune microenvironment reveals predictors of outcome in triple-negative breast cancer. Commun Biol 4(1):852. 10.1038/s42003-021-02361-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson S, Azizpour H, Smith K, Hartman J (2018) Digital image analysis in breast pathology-from image processing techniques to artificial intelligence. Transl Res 194:19–35. 10.1016/j.trsl.2017.10.010 [DOI] [PubMed] [Google Scholar]
- Sammut SJ, Crispin-Ortuzar M, Chin SF, Provenzano E, Bardwell HA, Ma W et al (2022) Multi-omic machine learning predictor of breast cancer therapy response. Nature 601(7894):623–629. 10.1038/s41586-021-04278-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma P (2016) Biology and management of patients with triple-negative breast cancer. Oncologist 21(9):1050–1062. 10.1634/theoncologist.2016-0067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobhani F, Robinson R, Hamidinekoo A, Roxanis I, Somaiah N, Yuan Y (2021) Artificial intelligence and digital pathology: opportunities and implications for immuno-oncology. Biochim Biophys Acta Rev Cancer 1875(2):188520. 10.1016/j.bbcan.2021.188520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanhol FA, Oliveira LS, Petitjean C, Heutte L (2016) A dataset for breast cancer histopathological image classification. IEEE Trans Biomed Eng 63(7):1455–1462. 10.1109/TBME.2015.2496264 [DOI] [PubMed] [Google Scholar]
- Srikantamurthy MM, Rallabandi VPS, Dudekula DB, Natarajan S, Park J (2023) Classification of benign and malignant subtypes of breast cancer histopathology imaging using hybrid CNN-LSTM based transfer learning. BMC Med Imaging 23(1):19. 10.1186/s12880-023-00964-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamoulakatos A, Cardona J, McCaig C, Murray D, Filius H, Atkinson R et al (2020) Automatic annotation of subsea pipelines using deep learning. Sensors (basel). 10.3390/s20030674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanton SE, Disis ML (2016) Clinical significance of tumor-infiltrating lymphocytes in breast cancer. J Immunother Cancer 4:59. 10.1186/s40425-016-0165-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sultan AS, Elgharib MA, Tavares T, Jessri M, Basile JR (2020) The use of artificial intelligence, machine learning and deep learning in oncologic histopathology. J Oral Pathol Med 49(9):849–856. 10.1111/jop.13042 [DOI] [PubMed] [Google Scholar]
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F (2021) Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71(3):209–249. 10.3322/caac.21660 [DOI] [PubMed] [Google Scholar]
- Takada M, Toi M (2020) Neoadjuvant treatment for HER2-positive breast cancer. Chin Clin Oncol 9(3):32. 10.21037/cco-20-123 [DOI] [PubMed] [Google Scholar]
- Thompson AM, Moulder-Thompson SL (2012) Neoadjuvant treatment of breast cancer. Ann Oncol 23(Suppl 10):x231-236. 10.1093/annonc/mds324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tufano AM, Teplinsky E, Landry CA (2021) Updates in neoadjuvant therapy for triple negative breast cancer. Clin Breast Cancer 21(1):1–9. 10.1016/j.clbc.2020.07.001 [DOI] [PubMed] [Google Scholar]
- Vagia E, Mahalingam D, Cristofanilli M (2020) The landscape of targeted therapies in TNBC. Cancers (basel). 10.3390/cancers12040916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Minckwitz G, Kummel S, Vogel P, Hanusch C, Eidtmann H, Hilfrich J et al (2008) Intensified neoadjuvant chemotherapy in early-responding breast cancer: phase III randomized GeparTrio study. J Natl Cancer Inst 100(8):552–562. 10.1093/jnci/djn089 [DOI] [PubMed] [Google Scholar]
- Voulodimos A, Doulamis N, Doulamis A, Protopapadakis E (2018) Deep learning for computer vision: a brief review. Comput Intell Neurosci 2018:7068349. 10.1155/2018/7068349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrdoljak J, Boban Z, Baric D, Segvic D, Kumric M, Avirovic M et al (2023) Applying explainable machine learning models for detection of breast cancer lymph node metastasis in patients eligible for neoadjuvant treatment. Cancers (basel). 10.3390/cancers15030634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan X, Wang J, Huang Y, Shangguan D, Zhang P (2021) Single-cell profiling to explore immunological heterogeneity of tumor microenvironment in breast cancer. Front Immunol 12:643692. 10.3389/fimmu.2021.643692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Zhang M, Tian Q, Yang J (2023) A novel model associated with tumor microenvironment on predicting prognosis and immunotherapy in triple negative breast cancer. Clin Exp Med. 10.1007/s10238-023-01090-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng R, Zhang S, Zeng H, Wang S, Sun K, Chen R et al (2022) Cancer incidence and mortality in China, 2016. J Natl Cancer Center 2(1):1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Liu M, Li X (2022) Progress on deep learning in digital pathology of breast cancer: a narrative review. Gland Surg 11(4):751–766. 10.21037/gs-22-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data used in the study are available from the corresponding author on reasonable request.