Abstract
To study the regulation of lipid transport from the chloroplast envelope to the thylakoid, intact chloroplasts, isolated from fully expanded or still-expanding pea (Pisum sativum) leaves, were incubated with radiolabeled lipid precursors and thylakoid membranes subsequently were isolated. Incubation with UDP[3H]Gal labeled monogalactosyldiacylglycerol in both envelope membranes and digalactosyldiacylglycerol in the outer chloroplast envelope. Galactolipid synthesis increased with incubation temperature. Transport to the thylakoid was slow below 12°C, and exhibited a temperature dependency closely resembling that for the previously reported appearance and disappearance of vesicles in the stroma (D.J. Morré, G. Selldén, C. Sundqvist, A.S. Sandelius [1991] Plant Physiol 97: 1558–1564). In mature chloroplasts, monogalactosyldiacylglycerol transport to the thylakoid was up to three times higher than digalactosyldiacylglycerol transport, whereas the difference was markedly lower in developing chloroplasts. Incubation of chloroplasts with [14C]acyl-coenzyme A labeled phosphatidylcholine (PC) and free fatty acids in the inner envelope membrane and phosphatidylglycerol at the chloroplast surface. PC and phosphatidylglycerol were preferentially transported to the thylakoid. Analysis of lipid composition revealed that the thylakoid contained approximately 20% of the chloroplast PC. Our results demonstrate that lipids synthesized at the chloroplast surface as well as in the inner envelope membrane are transported to the thylakoid and that lipid sorting is involved in the process. Furthermore, the results also indicate that more than one pathway exists for galactolipid transfer from the chloroplast envelope to the thylakoid.
The chloroplast envelope is the site of synthesis of the major chloroplast membrane lipids, whereas the thylakoid membrane apparently lacks lipid-synthesizing activities (Dorne et al., 1990). The thylakoid lipid supply thus depends on lipid transport from the envelope to the thylakoid. In organello galactolipid transfer to the thylakoid has been demonstrated with isolated chloroplasts (Joyard et al., 1980; Bertrams et al., 1981; Rawyler et al., 1992, 1995) but the manner of transport has not been established. Structures apparently resembling fusions between the inner envelope membrane and the thylakoid have been observed in chloroplasts of expanding leaves (Carde et al., 1982; Morré et al., 1991b) and membrane vesicles have been observed in the stroma, close to the plastid envelope, both in embryonic leaf cells (Kaneko and Keegstra, 1996) and expanding leaves (Morré et al., 1991b). The number of vesicle-like structures in the stromal compartment of chloroplasts increased when the leaf discs were transferred from room temperature to 12°C. By analogy to the temperature dependence of vesicular trafficking between endoplasmic reticulum and the Golgi compartment in animal cells, it was proposed that the vesicles enriched in the stroma at low temperature represented transport vesicles that had blebbed off the envelope membrane, but which could not at the lowered temperature fuse with the thylakoid membrane (Morré et al., 1991b). Vesicular trafficking between endoplasmic reticulum and the Golgi compartment requires cytosolic proteins, hydrolysable nucleotides, and acyl-coenzyme A (CoA; Allan and Kallen, 1993). The findings that transfer of galactolipids from isolated envelope to isolated thylakoid membranes required stromal protein(s) and was further stimulated by ATP (Morré et al., 1991a), and that release of galactolipids from isolated envelope membranes depended on stromal protein(s) and was stimulated by hydrolysable nucleotides and acyl-CoA (Räntfors et al., 2000), thus support the suggestion of an intraplastidial vesicular lipid transfer.
Our objective with the present investigation was to determine whether the transfer of galactolipids from the chloroplast envelope to the thylakoid in organello showed temperature dependence resembling that of the previously reported appearance and disappearance of vesicles in the stroma compartment (Morré et al., 1991b). We also aimed to determine if and to what extent the phospholipids acylated in the envelope or the putative envelope-associated region of endoplasmic reticulum (Kjellberg et al., 2000) were transferred to the thylakoid membrane. Because ultrastructural studies suggested, age-different occurrence in vesicles or transient fusions (compare with above) and as in situ galactolipid synthesis was markedly more active in still-expanding than fully expanded pea (Pisum sativum) leaves (Hellgren 1996), we also wanted to assess whether the extent and regulation of lipid transport to the thylakoid depended on the stage of leaf development.
RESULTS
Fraction Purity of the Isolated Thylakoid Fractions
The fractional purity of thylakoid fractions were assayed in thylakoid fractions isolated from chloroplast batches corresponding in size to those later used for lipid transport assays. The average recovery of thylakoids from the intact chloroplasts routinely corresponded to 80% to 85%, based on chlorophyll (data not shown). The thylakoid fractions contained monogalactosyldiacylglycerol (MGDG), digalactosyldiacylglycerol (DGDG), sulfoquinovosyldiacylglycerol, phosphatidylglycerol (PG), phosphatidylcholine (PC), and phosphatidylinositol (PI), as determined by cochromatography with lipid standards. The qualitative lipid composition as determined by one-dimensional thin-layer chromatography (TLC) was also confirmed by two-dimensional TLC. The quantitative lipid composition of both chloroplasts and thylakoid fractions (Table I) were quite similar to previously published data on spinach (Spinacia oleracea; Block et al., 1983) and wheat thylakoids (Bahl et al., 1976). The percentage of PC, however, was somewhat lower in our thylakoid fractions and the PI content of the thylakoid fractions was too low to be reliably quantified (<0.5 mol %). The fatty acid compositions of MGDG and PC in intact chloroplasts and thylakoid fractions are shown in Table II. Whereas the fatty acid composition of MGDG was very similar between the intact chloroplast and thylakoid fractions, that of PC differed markedly between the two fractions. In thylakoid PC, 16:0 constituted twice as large a proportion than in the PC of intact chloroplasts and there was a slightly higher ratio of 18:3 to 18:2. We did not observe any significant differences in lipid or fatty acid compositions between fractions isolated from expanding leaves and fractions from whole seedlings (result not shown).
Table I.
The lipid composition of intact chloroplasts and thylakoid fractions
| Lipid | Amount in Intact Chloroplasts | Amount in Thylakoid Fractions |
|---|---|---|
| mol % | ||
| MGDG | 46.3 ± 4.0 | 50.7 ± 1.2 |
| DGDG | 32.1 ± 2.0 | 32.6 ± 3.8 |
| SQDGa | 7.4 ± 0.2 | 8.6 ± 0.2 |
| PG | 5.9 ± 0.4 | 5.4 ± 1.8 |
| PC | 7.3 ± 1.9 | 1.7 ± 0.1 |
| PI | 1.0 ± 0.1 | <0.5 |
Chloroplasts were isolated from 10-d-old pea seedlings and thylakoid fractions were obtained from chloroplast aliquots corresponding to 100 μg of chlorophyll. Lipids were extracted, separated by two-dimensional TLC, and methyl esters of the lipids were identified and quantified by gas chromatography. The data present mean values and the range of two independent chloroplast isolations made from separately cultivated pea.
SQDG, Sulfoquinovosyldiacylglycerol.
Table II.
The fatty acid composition of MGDG and PC in intact chloroplasts and thylakoid fractions
| Fatty Acid | Chloroplasts
|
Thylakoid
Fraction
|
||
|---|---|---|---|---|
| MGDG | PC | MGDG | PC | |
| mol % | ||||
| 16:0 | 1.1 ± 0.5 | 10.0 ± 1.1 | 1.5 ± 0.5 | 22.3 ± 1.9 |
| 18:0 | <0.5 | 1.6 ± 0.6 | <0.5 | <0.5 |
| 18:1 | 0.8 ± 0.1 | 2.9 ± 0.2 | 1.2 ± 0.7 | 6.2 ± 0.8 |
| 18:2 | 3.6 ± 1.6 | 48.4 ± 1.0 | 4.6 ± 1.1 | 26.6 ± 3.1 |
| 18:3 | 94.6 ± 2.2 | 37.5 ± 3.1 | 93.2 ± 2.8 | 44.9 ± 5.8 |
Chloroplasts were isolated from 10-d-old pea seedlings and thylakoid fractions were obtained from chloroplast aliquots corresponding to 100 μg of chlorophyll. Otherwise as in Table I.
Galactolipid synthesis from UDP-d-[6-3H]Gal was used as a marker for both inner and outer envelope membranes (Tietje and Heinz, 1998; Kjellberg et al., 2000). In thylakoids isolated from intact chloroplasts of fully expanded leaves or whole seedlings, the galactolipid synthesizing capacity corresponded to less than 2% of the capacity of the intact parent chloroplasts (not shown). In thylakoids obtained from expanding leaves, the corresponding activity was approximately twice as high (not shown).
To determine whether both envelope membranes co-isolated with the thylakoid, protein separations of intact chloroplasts and isolated thylakoids were immunoblotted with antibodies reactive to the inner envelope protein Tic110 (Fig. 1) and the outer envelope protein Toc75 (not shown), both central constituents of the envelope protein import machinery (Schleiff and Soll, 2000). Both Tic110 and Toc75 were detected at the expected Mrs in the separated proteins of intact chloroplasts isolated from both expanding leaves and whole seedlings. Neither Tic110 nor Toc75 were detectable in thylakoid fractions isolated from whole seedling chloroplasts. In thylakoids obtained from expanding leaf chloroplasts, the apparent amount of Tic110 was substantially lower than in the intact parent chloroplasts (Fig. 1), whereas Toc75 was barely detectable in this thylakoid fraction (not shown).
Figure 1.
Presence of Tic110 in intact chloroplasts and thylakoid fractions isolated from whole pea seedlings and still-expanding leaves. Intact chloroplasts (C) and thylakoid (T) fractions corresponding to 0.2 (subscript 1), 0.4 (2), and 1 (3) μg of chlorophyll isolated from expanding leaves and whole pea seedlings were separated by SDS-PAGE and immunoblotted with anti Tic110. Thylakoid fractions were isolated from batches of of chloroplasts corrsponding to 100 μg of chlorophyll.
We also assayed acyl-CoA thioesterase because the activity has been shown to be associated with the inner but not the outer envelope membrane (Andrews and Keegstra, 1983). However, as originally reported (Andrews and Keegstra, 1983), a substantial fraction of the activity of the intact parent chloroplasts, in our hands approximately 50%, was recovered in the thylakoid fraction, both with chloroplasts from expanding and fully expanded leaves (data not shown). Although this activity discriminates between the two envelope membranes, apparently it is not useful in discriminating between thylakoid and inner envelope fractions.
The original methodology report obtained thylakoid fractions of very high fractional purity from spinach chloroplasts corresponding to 1.5 mg of chlorophyll (Rawyler et al., 1992). It should be noted that when we used pea chloroplasts corresponding to more than 0.5 mg chlorophyll for the isolation of the thylakoid fraction (compare with above), regardless of tissue age used, the obtained thylakoid fraction always contained more than 20% of the MGDG synthase activity of the intact parent chloroplasts, and always contained both Tic110 and Toc75 (not shown).
Galactolipid Synthesis in Intact Chloroplasts and Transport to the Thylakoid
Galactolipid synthesis and transport to the thylakoid membrane was studied by incubating intact chloroplasts with UDP-d-[6-3H]Gal, followed by isolation of a thylakoid fraction. [3H]Gal was incorporated into MGDG and DGDG in a time-dependent fashion in both intact chloroplasts and thylakoid fractions (Fig. 2). Radiolabel incorporation into the chloroplast lipids increased linearly for at least 10 min. To obtain sufficient labeling of DGDG in the thylakoid fraction, 20 min was chosen as the standard incubation time.
Figure 2.
The time dependency of the incorporation of [3H]Gal into galactolipids of pea chloroplasts and their subsequent transfer to the thylakoid. Intact chloroplasts isolated from whole pea seedlings (corresponding to 100 μg of chlorophyll) were incubated with UDP-d-[6-3H]Gal at room temperature for the times indicated and a thylakoid fraction was subsequently isolated. White symbols denote chloroplast fraction and black symbols thylakoid fraction; squares, radiolabel recovered in MGDG; circles, radiolabel recovered in DGDG.
The transport of newly synthesized galactolipids to the thylakoid was studied with chloroplasts isolated from whole pea seedlings, from still-expanding pea leaves, or from fully expanded pea leaves (Table III). In the chloroplasts isolated from fully expanded leaves, 50% of the MGDG and 16% of the DGDG synthesized during the 20 min incubation were recovered in the thylakoid fraction, reflecting preferential transport of newly synthesized MGDG over newly synthesized DGDG. In the chloroplasts isolated from still-expanding leaves, 45% and 34% of the newly synthesized MGDG and DGDG, respectively, was recovered in the thylakoid fraction. Thus, in chloroplasts of still-expanding leaves, MGDG did not dominate galactolipid transport as in chloroplasts of fully expanded leaves. The transport of MGDG and DGDG to the thylakoid in chloroplasts isolated from whole seedlings was intermediate between the patterns observed for the chloroplasts isolated from still expanding and fully expanded leaves.
Table III.
The leaf age dependency of the transfer of newly synthesized galactolipids to the thylakoid
| Plant Material | Thylakoid
Label
|
Ratio of Radiolabel in MGDG/DGDG
|
||
|---|---|---|---|---|
| MGDG | DGDG | Chloroplast fraction | Thylakoid fraction | |
| % of chloroplast label | dpm dpm−1 | |||
| Fully expanded leaves | 50 ± 8 | 16 ± 2 | 4.8 ± 1.1 | 14.5 ± 2.6 |
| Expanding leaves | 45 ± 0 | 34 ± 3 | 3.1 ± 0.0 | 4.0 ± 0.3 |
| Whole seedlings | 38 ± 2 | 21 ± 3 | 4.4 ± 0.7 | 8.0 ± 0.4 |
Intact chloroplasts (corresponding to 70 μg of chlorophyll) were isolated from whole pea seedlings (10 d old), still expanding pea leaves (two lower most leaves of 7-d-old plants), or fully expanded pea leaves (two lower most leaves of 10-d-old plants) and incubated for 20 min at room temperature with UDP-d-[6-3H]-galactose. The radiolabel associated with MGDG and DGDG, respectively, were analyzed for the chloroplast and thylakoid fractions. Mean values ± the range of duplicate samples within one representative experiment are presented.
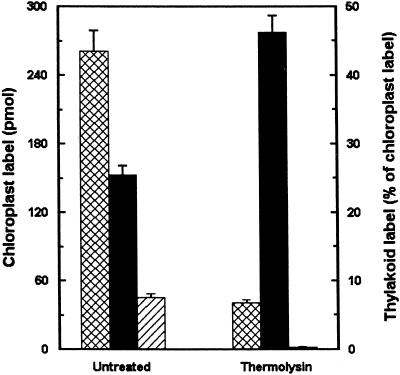
With chloroplasts isolated from whole pea seedlings, we investigated the effects of agents and conditions previously shown to affect galactolipid synthesis or trafficking to the thylakoid. Fluoride is considered a nonspecific phosphatase inhibitor (Telfer et al., 1983). Inclusion of 2 mm potassium fluoride (KF) inhibited MGDG synthesis by 20% to 50%, but did not affect DGDG synthesis (results not shown). The fractions transferred to the thylakoid increased for MGDG but decreased for DGDG (Fig. 3). To investigate whether the transfer of galactolipids from envelope to thylakoid depended on the presence of or activities of outer envelope-localized proteins, for example DGDG synthesis, intact chloroplasts from whole pea seedlings were incubated with the unspecific protease thermolysin prior to incubation with UDP-d-[6-3H]Gal. Thermolysin treatment almost completely abolished radiolabel incorporation into DGDG and drastically decreased radiolabel incorporation into MGDG, but the fraction of chloroplast radiolabel recovered in thylakoid MGDG increased markedly (Fig. 4).
Figure 3.
The effects of KF on the transfer of MGDG and DGDG to the thylakoid. Intact chloroplasts (100 μg of chlorophyll) isolated from whole pea seedlings were incubated with UDP-d-[6-3H]Gal for 20 min at room temperature with or without inclusion of 2 mm KF and thylakoid fractions were subsequently isolated. Black bars, Control; hatched bars, with inclusion of 2 mm KF. Mean values and the range from duplicate samples within one representative experiment are presented.
Figure 4.
The effects of thermolysin treatment of intact chloroplasts on the synthesis of galactolipids and on their subsequent transfer to the thylakoid. Thermolysin-treated intact chloroplasts (100 μg of chlorophyll), isolated from whole pea seedlings, were incubated with UDP-d-[6-3H]Gal for 20 min at room temperature and thylakoid fractions were subsequently isolated. Cross-hatched bars, Radiolabel recovered in chloroplast MGDG; black bars, radiolabel recovered in thylakoid MGDG; hatched bars, radiolabel recovered in chloroplast DGDG. Mean values and the range from duplicate samples within one representative experiment are presented.
Both galactolipid transport from isolated envelope to isolated thylakoid (Morré et al., 1991b) as well as galactolipid release from isolated chloroplast envelope (Räntfors et al., 2000) were stimulated by soluble proteins (stroma) and ATP. The galactolipid release was stimulated also by GTP but markedly inhibited by GTPγS. However, neither an ATP-generating system containing ATP (ATP/ATP-generating system, 50 μm ATP, 300 μm UTP, 2.0 mm creatine phosphate, and 1 unit mL−1 creatine phosphokinase; Balch et al., 1984) nor 200 μm GTPγS had any apparent effects on the fraction of MGDG or DGDG transported to the thylakoid (results not shown). Soluble leaf proteins (100 μg protein) had no apparent effects on the fraction of galactolipids transferred to the thylakoid but caused a 60% decrease in both MGDG and DGDG synthesis. The decrease could be partially restored by co-incubation with an ATP-generating system (results not shown), reflecting that the fraction of soluble proteins contained nucleosidase activities.
Temperature Dependence of Galactolipid Transport to the Thylakoid
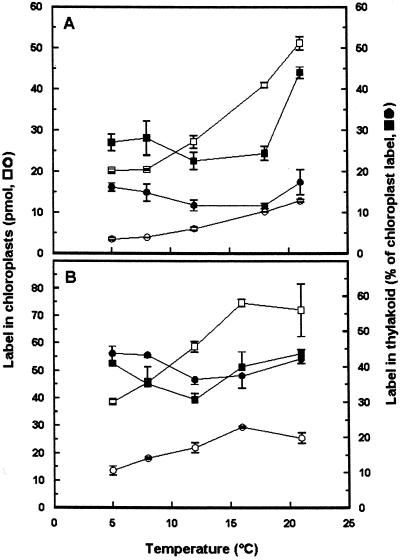
With chloroplasts isolated from fully expanded (Fig. 5A) or whole pea seedlings (results not shown), the synthesis of MGDG and DGDG increased with temperature up to the highest temperature investigated, 21°C. The fraction of radiolabeled MGDG recovered in the thylakoid fraction was significantly lower at 4°C than at 21°C, and was even lower at 12°C (Fig. 5A). The fraction of radiolabeled DGDG recovered in the thylakoid fraction exhibited a similar temperature dependency, except that the proportion transported at 21°C was not larger than the proportion transported at 4°C (Fig. 5A).
Figure 5.
The temperature dependency of galactolipid synthesis and transport to the thylakoid in intact chloroplasts isolated from fully expanded (A) or expanding (B) leaves. Intact chloroplasts (100 μg of chlorophyll) were isolated from the fully expanded two lowermost leaves of 10- or 7-d-old pea and incubated for 20 min with UDP-d-[6-3H]Gal at the temperatures indicated, followed by isolation of a thylakoid fraction. White symbols denote radiolabel in chloroplast fractions and black symbols radiolabel in thylakoid fractions; squares, radiolabel recovered in MGDG; circles, radiolabel recovered in DGDG. Mean values and the range from duplicate samples within one representative experiment are presented.
The temperature response of synthesis and transport of the galactolipids in chloroplasts isolated from still-expanding pea leaves was similar to that of the chloroplasts from fully expanded leaves in that synthesis increased with temperature, in this case up to 16°C, whereas transport was less efficient at 12°C (Fig. 5B). The rate of galactolipid synthesis was markedly higher in the chloroplasts from the still-expanding pea leaves than in those from the fully expanded leaves.
Phospholipid Acylation in Intact Chloroplasts and Transport to the Thylakoid
Isolated intact pea chloroplasts recently were reported to exhibit acyl specificity for acyl-CoA-dependent acyl group incorporation into PC and PG (Kjellberg et al., 2000). To determine whether the newly acylated phospholipids were transferred to the thylakoid membrane, intact pea chloroplasts were incubated with [14C]18:1-CoA or [14C]16:0-CoA and the acyl incorporation into phospholipids was assayed in the intact chloroplasts and the subsequently isolated thylakoid fractions (Table IV). In intact chloroplasts isolated from both whole 10-d-old pea seedlings (the bulk of the leaf mass corresponding to fully expanded leaves) or expanding pea leaves, [14C]18:1-CoA and [14C]16:0-CoA-labeled PC, PG, and FFA, as previously reported (Kjellberg et al., 2000). The fraction of [14C]acyl-CoA-labeled phospholipids transported to the thylakoid was roughly the same with both [14C]acyl-CoA substrates in both chloroplast ages. Between 7% and 11% of the [14C]acyl-CoA-labeled PC and PG and approximately 3% of the [14C]acyl-CoA-derived FFA were transferred to the thylakoid. In chloroplasts isolated from fully expanded leaves, [14C]acyl-CoA-labeled PC and FFA were to a similar extent transported to the thylakoid, but no PG label was detected in the thylakoid fractions (not shown), indicating that PG transfer only occurred in expanding leaves, also present as a minor tissue portion of the whole seedlings. The ratio of [14C]FFA/[14C]PC differed between leaf ages and acyl-CoA substrates, but in all instances, the ratio was substantially lower in the thylakoid fractions than in the parent chloroplasts, demonstrating lipid sorting prior to transport.
Table IV.
Labeling of chloroplast lipids by incubation with [14C]acyl-CoAs and the portion of the respective lipids transported to the thylakoid
| Plant Material | Substrate | Thylakoid
Label
|
Ratio of Radiolabel in FFA/PC
|
|||
|---|---|---|---|---|---|---|
| PC | PG | FFA | Chloroplast fraction | Thylakoid fraction | ||
| % of chloroplast label | dpm dpm−1 | |||||
| Expanding leaves | 16:0-CoA | 8.1 ± 0.1 | 7.0 ± 0.1 | 2.6 ± 0.3 | 6.3 ± 1.0 | 2.0 ± 0.1 |
| 18:1-CoA | 10.8 ± 0.7 | 9.5 ± 0.6 | 2.9 ± 0.7 | 3.5 ± 0.2 | 0.9 ± 0.1 | |
| Whole seedlings | 16:0-CoA | 9.7 ± 1.4 | 10.7 ± 1.9 | 2.5 ± 0.3 | 10.1 ± 1.5 | 2.6 ± 2.3 |
| 18:1-CoA | 11.2 ± 2.1 | 8.1 ± 1.1 | 3.4 ± 0.3 | 3.0 ± 0.2 | 0.9 ± 0.2 | |
Intact chloroplasts were isolated from still expanding pea leaves (two lower most leaves of 7-d-old plants) or whole 10-d-old seedlings and incubated (corresponding to 70 μg chlorophyll) for 30 min at room temperature with [14C]16:0-CoA or [14C]18:1-CoA. Thylakoids were subsequently isolated and the radiolabel associated with PC, PG, and free fatty acids (FFA), respectively, were analyzed for the chloroplast and thylakoid fractions. Mean values ± the range of duplicate samples within one representative experiment are presented.
Positional Distribution of Label on Thylakoid Lipids Acylated by Acyl-CoA
Radiolabel derived from [14C]16:0-CoA was recovered mainly in the sn-1 position of PG, whereas radiolabel derived from [14C]18:1-CoA was associated mainly with the sn-2 position of PC (Fig. 6). Both sn-1-[14C]16:0-PG and sn-2-[14C]18:1-PC were transported to the thylakoid and with both lipids, transport slightly favored molecules radiolabeled in the sn-2 position (Fig. 6). In the remaining two cases, [14C]16:0-CoA-derived labeling of PC and [14C]18:1-CoA-derived labeling of PG, the two acyl groups distributed close to even between the two positions (results not shown). However, in these cases labeling was too low to allow conclusions regarding transfer to the thylakoid.
Figure 6.
Positional distribution of radiolabeled acyl groups in phospholipids in intact chloroplasts and in the thylakoid. Intact chloroplasts were incubated with [14C]acyl-CoAs and a thylakoid fraction subsequently was isolated. Radiolabeled PC and PG of the chloroplast and thylakoid fractions were treated with phospholipase A2 and the distribution of radiolabel between the lysophospholipid and FFA determined. Hatched bars, The fraction of radioactivity associated with the sn-1 position; black bars, the fraction of radioactivity associated with the sn-2 position. Mean values and the range from duplicate samples within one representative experiment are presented.
DISCUSSION
The first issue to be met in undertaking an in organello study of lipid transfer from the envelope to the thylakoid membrane is the isolation of highly purified thylakoids from intact chloroplasts. The qualitative lipid composition, membrane recovery, and very low galactolipid synthesis capacity in the thylakoid fractions obtained from chloroplasts isolated from whole pea seedlings or fully expanded pea leaves were very similar to the corresponding values reported for thylakoid fractions isolated from spinach chloroplasts in the original methodology report (Rawyler et al., 1992). Because the pea thylakoids also lacked detectable amounts of Tic110 or Toc75, it is probably safe to assume that, as with spinach, the method yields thylakoid fractions of very high fraction purity also from mature pea chloroplasts, provided the amount of chloroplasts used in the thylakoid isolation step is markedly reduced.
The thylakoid fractions obtained from chloroplasts isolated from still-expanding pea leaves, however, exhibited about twice as high MGDG synthesis activity as that of thylakoid fractions obtained from whole seedling chloroplasts. The presence of Tic110 but barely detectable Toc75 suggest that the higher MGDG synthesis activity probably could be attributed to contamination with inner, but not outer, envelope fragments. It should be noted that both in chloroplasts isolated from still-expanding and fully expanded leaves, respectively, similarly small portions (about 3%) of the [14C]FFA formed in the inner envelope from [14C]acyl-CoA was recovered in the thylakoid fraction (compare with below). This result suggests that the higher MGDG-synthesizing activity in thylakoids obtained from chloroplasts of expanding leaves may not be ascribed to contamination by bulk inner envelope. Apparent fusions between the inner envelope and the thylakoid have been observed in ultrastructural studies of expanding leaves (Carde et al., 1982; Morré et al., 1991b) and galactolipid synthesis has been reported to occur in etioplast prothylakoids (Sandelius and Selstam, 1984) and cyanobacterial thylakoids (Omata and Murata, 1986).
In organello transfer of galactolipids from the envelope membrane to the thylakoid did not appear to depend on cytosolic conditions, such as exogenous ATP or cytosolic proteins, although the apparent fluoride sensitivity of the process suggests the involvment of phosphatases. Because phosphorylation of proteins (Bovet et al., 1997; Kjellberg, 2000) and lipids (Siegenthaler et al., 1997; Kjellberg, 2000; Müller et al., 2000) has been reported to occur in the chloroplast envelope, it can be hypothesized that phosphorylation of lipids and/or proteins is in some way involoved in the lipid transfer process. The release of galactolipids from isolated chloroplast envelope has been shown to be stimulated by hydrolysable nucleotides and inhibited by GTPγS (Räntfors et al., 2000). The fact that presence of exogenous GTPγS did not affect the in organello transfer indicates that the GTP requirement resides in or faces the stromal compartment and is not reachable from the outside of the chloroplast. Further evidence that cytosolic conditions do not influence galactolipid transport to the thylakoid comes from the incubations of the chloroplasts with thermolysin. MGDG transport to the thylakoid occurred independently of whether DGDG synthase and other outer envelope proteins exposed at the chloroplast surface had been damaged. The results suggest an intraplastidial galactolipid transport mechanism largely independent of extraplastidial conditions. In chloroplasts isolated from still-expanding leaves, a substantially larger proportion of newly synthesized DGDG was transferred to the thylakoid, demonstrating that lipid specificity of the transport process changes with plastid and/or leaf developmental stage. The preferential transport of MGDG over DGDG probably reflects that the former is the more dominating thylakoid constituent (compare with Table I).
In pea, synthesis of MGDG has been reported to occur either solely in the outer envelope (Cline and Keegstra, 1983) or in both envelope membranes (Tietje and Heinz, 1998; Kjellberg et al., 2000). In the present study, thermolysin abolished a portion but not all MGDG synthesis in the intact chloroplasts, demonstrating that also in the present case, the synthesis apparently occurred in both envelope membranes. Because the fraction of MGDG transferred to the thylakoid increased in thermolysin-treated chloroplasts, the results also reflect a possible preference for the transfer of inner envelope-synthesized MGDG to the thylakoid.
In intact chloroplasts isolated from fully expanded leaves, the transport of galactolipids responded to temperature with a clear decrease in the fraction of MGDG transported to the thylakoid between 10°C and 18°C, whereas the temperature effect on DGDG transfer was somewhat smaller. In chloroplasts isolated from still-expanding leaves, the temperature dependence of galactolipid transport resembled that of the expanded leaves. The results suggest the existence of two mechanisms of lipid transfer. At lower temperatures, a pathway with a low capacity functions, whereas at higher temperatures, this pathway is partially or completely replaced by a pathway that requires higher temperatures to work efficiently. The low-capacity pathway is able to keep up with the low rate of synthesis at low temperature, but as synthesis increase with temperature, the low-capacity pathway is unable to keep pace with synthesis, reflected as an apparent drop in the fraction of radiolabel transferred to the thylakoid. The temperature dependency of the transport of galactolipids from the envelope to the thylakoid closely mimics the temperature dependency reported for a proposed vesicle trafficking in the chloroplast stroma of tobacco (Nicotiana tabacum) and pea leaves (Morré et al., 1991b).
In this ultrastructural study, stroma vesicles were present at all temperatures investigates (4°C–25°C), but were markedly more prevalent at temperatures around 12°C. The findings were interpreted as a temperature-dependent vesicle formation from the inner envelope membrane, apparently occurring already at 4°C, whereas fusion with the thylakoid was blocked at temperatures up to 12°C (Morré et al., 1991b). Based on these results, the present findings suggest that the high-capacity pathway for intraplastidial galactolipid transport is mediated by vesicles. Membrane vesicle trafficking is dependent on the appropriate ability of membranes to fuse, which is related to the physical properties of the membrane lipids (Williams, 1998). It was recently reported that an Arabidopsis mutant deficient in chloroplast trienoic fatty acids was unable to regenerate thylakoids at low temperature (Routaboul et al., 2000). We suggest that one interpretation of this result is that the deficient capacity in the mutant to adjust the lipid desaturation level in the envelope and/or thylakoid lipids resulted in blocked vesicular lipid transfer to the thylakoid at the lowered temperature.
Although most reports on thylakoid lipid composition include PC, it is often assumed that the thylakoid PC represents envelope contamination. The results presented herein suggest that PC is a natural constituent of thylakoid membranes. From the quantitative analysis of the lipid composition (Table I) and assuming that the thylakoid contains at least 80% of the chloroplast lipids, it can be calculated that approximately 20% of the total chloroplast PC is associated with the thylakoid. This value is substantially higher than the calculated degree of contamination, approximately 2% to 3% of the envelope, as judged by MGDG synthase and presence of FFA from acyl-CoA thioesterase activity. The PC associated with the thylakoid had a markedly different fatty acid composition than the total chloroplast PC. We recently showed that 18:1-CoA-dependent acyl incorporation into PC in isolated pea chloroplasts occurred predominantly in the inner envelope membrane (Kjellberg et al., 2000). The present finding, that this freshly acylated PC is transferred to the thylakoid membrane at levels substantially higher than the calculated envelope contamination, also supports that PC is a natural constituent of the thylakoid membrane. Taken together, our results clearly show that the PC detected in the thylakoid is not due to contamination by bulk envelope. In light of the data presented by Dorne et al. (1990) that thylakoids isolated from spinach chloroplasts treated with phospholipase C are devoid of PC, it may be suggested that the PC associated with the thylakoid is rapidly metabolized. The transport may serve to deliver acyl groups for the repair of thylakoid lipids rather than PC itself. Another explanation would be species-specific differences between the lipid compositions of spinach and pea thylakoids.
Phospholipid acylation in pea chloroplasts isolated from still-expanding leaves also included a 16:0-CoA-dependent acyl group incorporation into PG that preferentially labeled the sn-1 position of the glycerol moiety (Kjellberg et al., 2000). Because the acylation pattern resembled the acylation pattern characteristic for phospholipid synthesis in the endoplasmic reticulum, PG acylation was sensitive to thermolysin, and marker activities for endoplasmic reticulum co-isolated with the developing chloroplasts, it was suggested that the activity could reside in a discrete chloroplast-associated region of the endoplasmic reticulum (Kjellberg et al., 2000). In the present study, the newly acylated PG was transported to the thylakoid to the same extent as PC. Thus, as with DGDG, PG synthesized at (or close to) the chloroplast surface can be transported to the thylakoid. Pea thylakoids contain a minor amount of PG with 16:0 at the sn-1 position, about 5% of the total PG (Dorne and Heinz, 1989). Our results suggest that during chloroplast development, the 16:0-CoA-dependent acyl group incorporation into PG in a putative chloroplast-associated region of the endoplasmic reticulum may be the origin of this minor thylakoid PG species.
To conclude, our results support previous suggestions that galactolipid transport from the envelope to the thylakoid occurs at least partly as vesicles. Our results also suggest the presence of an additional temperature-insensitive mechanism for galactolipid transfer to the thylakoid. The transport of galactolipids to the thylakoid appears to occur largely independent of extraplastidial factors. Furthermore, PC and PG are also transported to the thylakoid and our results reflect that lipid sorting occurs prior to transfer of galactolipids and phospholipids.
MATERIALS AND METHODS
Materials
Garden pea (Pisum sativum L. cv Kelvedon Wonder; Svalöv-Weibull, Sweden) was cultivated as previously described (Kjellberg et al., 2000). Lipid references and fine chemicals were from Sigma (St. Louis), inorganic salts, organic solvents, and TLC plates were from Merck (Darmstadt, Germany) and radiochemicals, Percoll, and Hyperfilm MP were from Amersham Pharmacia Biotech (Uppsala).
Isolation of Intact Chloroplasts
If not otherwise indicated, the entire above-ground tissue of 10-d-old pea seedlings were harvested and used for chloroplast isolation and the material will be referred to as whole pea seedlings. For experiments concerning age effects, the two oldest (lower most) leaves of 7- or 10-d-old pea seedlings were harvested and used for chloroplast isolation. These plant materials will be referred to as still-expanding and fully expanded leaves, respectively. Batches of 100 to 200 g of leaves were used for isolation of intact chloroplasts as described (Räntfors et al., 2000), except that the Percoll gradient was made of 4.0 mL 75% (v/v) and 10.0 mL 35% (v/v) Percoll. Chloroplast intactness was approximately 90%, as determined by the ferricyanide method (Lilley et al., 1975). The temperature was maintained at 4°C during the isolation procedure.
In Organello Labeling of Galactolipids
Intact chloroplasts, unless otherwise stated corresponding to 100 μg chlorophyll, were incubated with 0.47 μm of UDP-d-[6-3H]Gal (174 GBq mmol−1) in a total volume of 100 μL of 0.33 m Suc, 30 mm HEPES [4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid]/KOH (pH 7.0), 10 mm KCl, 2.5 mm MgCl2, and 0.75% (v/v) ethanol for 20 min at room temperature with shaking. Incubations were terminated by adding 2 mL of ice-cold 0.33 m Suc, 10 mm HEPES/KOH (pH 7.8), and 5 mm UDP. UDP was included by product inhibition to prevent any further incorporation of radiolabel into galactolipids. The chloroplasts were pelleted by centrifugation for 10 min at 1,500gmax, and resuspended in 500 μL of 0.33 m Suc, 10 mm HEPES/KOH (pH 7.8), and 5 mm UDP. A 100-μL aliquot of the suspended chloroplasts was removed for lipid extraction and the remainder was used for thylakoid isolation.
In Organello Labeling of Phospholipids
Acyl group incorporation into chloroplast phospholipids was assayed as previously described (Kjellberg et al., 2000), with minor modifications. Intact chloroplasts (100 μg chlorophyll unless otherwise stated), were incubated with 27 μm of [1-14C]16:0-CoA (2.04 GBq mmol−1) or [1-14C]18:1-CoA (2.07 GBq mmol−1) in a total volume of 100 μL of 0.33 m Suc, 30 mm HEPES/KOH (pH 7.0), 10 mm KCl, and 2.5 mm MgCl2 for 30 min at room temperature with shaking. Incubations were terminated by adding 2 mL of chilled 0.33 m Suc and 10 mm HEPES/KOH (pH 7.8). The chloroplasts were pelleted by centrifugation for 10 min at 1,500gmax, and resuspended in 500 μL 0.33 m Suc and 10 mm HEPES/KOH (pH 7.8). An aliquot, 100 μL, of the suspended chloroplasts was removed for lipid extraction and the remainder was used for thylakoid isolation.
Isolation of Thylakoid Membranes
Purified thylakoid membranes were isolated essentially as described (Rawyler et al., 1992). In brief, 400 μL of intact suspended chloroplasts (corresponding to approximately 80–100 μg chlorophyll) were hypotonically lysed by addition of 20 mL of 10 mm HEPES/KOH (pH 7.8) and 2 mm EDTA. After 2 min on ice, the osmolarity was restored by addition of 4 mL of 2.0 m Suc. The lysate was centrifuged at 16,500gmax for 5 min, the pellet was resuspended in 18 mL of 0.33 m Suc and 10 mm HEPES/KOH (pH 7.8) in a centrifuge tube, and 8 mL of 5% (v/v) Percoll in the same medium was injected below the dilute membrane suspension in the centrifuge tube. The thylakoids were pelleted through the Percoll cushion by centrifugation at 20,000gmax for 15 min. The pelleted thylakoid membranes were suspended in 30 mL of 0.33 m Suc and 10 mm HEPES/KOH (pH 7.8), pelleted at 16,500gmax, and resuspended in a small volume of the same medium. The temperature was maintained at 4°C during the isolation procedure.
Isolation of Soluble Proteins
Pea seedlings (50 g) were harvested and homogenized in 50 mL of 30 mm HEPES/KOH (pH 7.0), 10 mm KCl, and 2.5 mm MgCl2 supplemented with 1.5 mm of DTT, 9 mm ascorbate, and one complete protease inhibitor cocktail tablet (Boehringer Mannheim, Mannheim, Germany). The homogenate was filtered through Miracloth (Calbiochem-Novabiochem Co., Darmstadt, Germany) and centrifuged at 9,000gmax for 10 min and the supernatant was recentrifuged at 100,000gmax for 60 min. The resulting supernatant was centrifuged again at 200,000gmax for 30 min and the final supernatant was concentrated 10 times with a Centricon 10-kD cutoff filter (Amicon, Beverly, MA). The concentrated soluble protein fraction was frozen in liquid nitrogen and stored at −80°C.
Lipid Analysis
Lipids were extracted as described (Sommarin and Sandelius, 1988), dried under nitrogen, and dissolved in a small volume of chloroform. An aliquot of the lipid extract was transferred to a liquid scintillation vial and after addition of 1.0 mL methanol and 9 mL of Beckman Ready Safe, the total radioactivity was determined by liquid scintillation counting (Packard Tri-Carb 2100TR). Lipids were separated by TLC using the solvent system chloroform:methanol:acetic acid:aqeous 0.6 m ammonium chloride (80:20:10:3, v/v). Lipids were visualized by exposing the TLC plates to iodine vapor and identified by cochromatography with authentic lipid standards. The distribution of radiolabel between the lipids was determined by scanning the TLC plates one dimensionally in a Bioscan System 2000 Imaging Scanner, using NSCAN software for data evaluation or by liquid scintillation counting (as above) of lipid-containing silica areas scraped off the TLC plates.
Two-dimensional TLC plates were developed in the first dimension by chloroform:methanol:25% (w/v) aqueous ammonia (40:22:3, v/v) and in the second by chloroform:methanol:acetone:acetic acid:water (10:2:4:2:1, v/v). To visualize the lipids, the plates were sprayed with 0.1% (w/v) 2,7-dichlorofluorescein in ethanol and viewed under UV illumination. Lipids were identified by cochromatography with authentic lipid standards. Lipid spots were scraped off of the TLC plates and fatty acid methyl esthers were produced in the presence of the silica gel by base catalyzed methylation in 0.5 m sodium methoxide in the presence of a known amount of diheptadecanoyl-PC as internal standard (Christie, 1976). Fatty acid methyl esthers were separated and quantified by gas chromatography as described (Norberg and Liljenberg, 1991).
Other Assays
Chloroplasts and thylakoid fractions, separated by SDS-PAGE (Laemmli, 1970) on 10% (w/v) acrylamide gels, were electroblotted over night to Hybond (Amarsham Pharmacia Biotech, Uppsala) polyvinylidene difluoride membranes. The membranes were probed with rabbit anti Tic110 or anti Toc75 at 1:5,000 dilutions followed by peroxidase-labeled goat anti rabbit antibody at 1:4,000 dilution. The peroxidase activity was detected by enhanced chemiluminscence using the enhanced chemiluminescence immunodetection kit (Amersham Pharmacia Biotech AB).
Lipase digestion of phospholipids (Mongrand et al., 1997) and thermolysin treatment of intact chloroplasts (Cline et al., 1984; Lübeck et al., 1996) were assayed according to previously described modifications (Kjellberg et al., 2000). Assays of acyl-CoA thioesterase activity (Andrews and Keegstra, 1983), total chlorophyll (Arnon, 1949), and protein (Smith et al., 1985; bovine serum albumin as standard) were according to the published protocols.
Experimental Design
For each experimental setup, the data presented are representative of two to five separate experiments, using chloroplasts isolated from independently cultivated plant material. Error bars correspond to error range in duplicate samples within a representative experiment. The exception is Figure 2, which shows single values from one out of the three independent experiments performed. The trends in labeling over time were fully reproducible between these experiments, although absolute values differed.
ACKNOWLEDGMENTS
The authors wish to sincerely thank Dr. Lisa Heins and Prof. Jürgen Soll (Kiel University, Germany) for the generous gift of the antibodies against Tic110 and Toc75.
Footnotes
This work was supported by the Swedish Natural Science Research Council.
LITERATURE CITED
- Allan D, Kallen KJ. Transport of lipids to the plasma membrane in animal cells. Prog Lipid Res. 1993;32:195–219. doi: 10.1016/0163-7827(93)90015-o. [DOI] [PubMed] [Google Scholar]
- Andrews J, Keegstra K. Acyl-CoA synthetase is located in the outer membrane and acyl-CoA thioesterase in the inner membrane of pea chloroplast envelopes. Plant Physiol. 1983;72:735–740. doi: 10.1104/pp.72.3.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnon DI. Copper enzymes in isolated chloroplasts: polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949;24:1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahl J, Francke B, Monéger Lipid composition of envelopes, prolamellar bodies and other plastid membranes in etioloated, green and greening wheat leaves. Planta. 1976;129:193–201. doi: 10.1007/BF00398257. [DOI] [PubMed] [Google Scholar]
- Balch WE, Dunphy WG, Braell WA, Rothman JE. Reconstitution of the transport of protein between successive compartments of Golgi measured by the coupled incorporation of N-acetyl glucosamine. Cell. 1984;39:405–416. doi: 10.1016/0092-8674(84)90019-9. [DOI] [PubMed] [Google Scholar]
- Bertrams M, Wrage K, Heinz E. Lipid labeling in intact chloroplasts from exogenous nucelotide precursors. Z Naturforsch. 1981;36:62–70. [Google Scholar]
- Block MA, Dorne AJ, Joyard J, Douce R. Preparation and characterization of membrane fractions enriched in outer and inner envelope membranes from spinach chloroplasts: II. Biochemical characterization. J Biol Chem. 1983;258:13281–13286. [PubMed] [Google Scholar]
- Bovet L, Leplattenier B, Siegenthaler PA. In vitro and in organello phosphorylation of envelope proteins and phosphoglucomutase in spinach chloroplasts. Plant Sci. 1997;128:169–180. [Google Scholar]
- Carde JP, Joyard J, Douce R. Electron microscopic studies of envelope membranes from spinach chloroplasts. Biol Cell. 1982;44:315–324. [Google Scholar]
- Christie WW. Lipid Analysis. Oxford: Pergamon Press Ltd.; 1976. [Google Scholar]
- Cline K, Keegstra K. Galactosyltransferases involved in galactolipid biosynthesis are located in the outer membrane of pea chloroplast envelopes. Plant Physiol. 1983;78:366–372. doi: 10.1104/pp.71.2.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cline K, Werner-Washburne M, Andrews J, Keegstra K. Thermolysin is a suitable protease for probing the surface of intact pea chloroplasts. Plant Physiol. 1984;75:675–678. doi: 10.1104/pp.75.3.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorne AJ, Heinz E. Position and pairing of fatty acids in phosphatidylglycerol from pea leaf chloroplasts and mitochondria. Plant Sci. 1989;60:39–46. [Google Scholar]
- Dorne AJ, Joyard J, Douce R. Do thylakoids really contain phospahtidylcholine? Proc Natl Acad Sci USA. 1990;87:71–74. doi: 10.1073/pnas.87.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellgren LI. Plant membrane lipids: on the impact of ozone on lipid composition, lipid metabolism and the dynamical properties of plasma membranes. PhD Thesis. Sweden: Göteborg University; 1996. [Google Scholar]
- Joyard J, Douce R, Siebertz HP, Heinz E. Distribution of radioactive lipids between envelopes and thylakoids from chloroplasts labeled in vivo. Eur J Biochem. 1980;108:171–176. doi: 10.1111/j.1432-1033.1980.tb04709.x. [DOI] [PubMed] [Google Scholar]
- Kaneko Y, Keegstra K. Plastid biogenesis in embryonic pea leaf cells during early germination. Protoplasma. 1996;195:59–67. [Google Scholar]
- Kjellberg JM. Chloroplast biogenesis: the lipid supply. PhD Thesis. Sweden: Göteborg University; 2000. [Google Scholar]
- Kjellberg JM, Trimborn M, Andersson M, Sandelius AS. Acyl-CoA dependent acylation of phospholipids in the chloroplast envelope. Biochim Biophys Acta. 2000;1485:100–110. doi: 10.1016/s1388-1981(00)00040-8. [DOI] [PubMed] [Google Scholar]
- Laemmli U. Cleavage of structural proteins during the assembly of the head of the bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lilley R McC, Fitzgerald MP, Rienits KG, Walker DA. Criteria of intactness and the photosynthetic activity of spinach chloroplast preparations. New Phytol. 1975;75:1–10. [Google Scholar]
- Lübeck J, Soll J, Akita M, Nielsen E, Keegstra K. Topology of IEP110, a component of the chloroplastic import machinery present in the inner envelope membrane. EMBO J. 1996;16:4230–4238. [PMC free article] [PubMed] [Google Scholar]
- Mongrand S, Bessoule JJ, Cassagne C. A re-examination in vivo of the phosphatidylcholine-galactolipid metabolic relationship during plant lipid biosynthesis. Biochem J. 1997;327:853–858. doi: 10.1042/bj3270853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morré DJ, Morré JT, Morré SR, Sundqvist C, Sandelius AS. Chloroplast biogenesis: cell-free transfer of monogalactosylglycerides to thylakoids. Biochim Biophys Acta. 1991a;1070:437–445. doi: 10.1016/0005-2736(91)90084-l. [DOI] [PubMed] [Google Scholar]
- Morré DJ, Selldén G, Sundqvist C, Sandelius AS. Stromal low temperature compartment derived from the inner membrane of the chloroplast envelope. Plant Physiol. 1991b;97:1558–156. doi: 10.1104/pp.97.4.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller MO, Meylan-Bettex, Eckstein F, Martinoia E, Siegenthaler PA, Bovet L. Lipid phosporylation in chloroplast envelopes: evidence for galactolipid CTP-dependent kinase activities. J Biol Chem. 2000;275:19475–19481. doi: 10.1074/jbc.M002575200. [DOI] [PubMed] [Google Scholar]
- Norberg P, Liljenberg C. Lipids of plasma membranes prepared from oat root cells: effects of induced water-deficit tolerance. Plant Physiol. 1991;96:1136–1141. doi: 10.1104/pp.96.4.1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omata T, Murata N. Glucolipid synthesis activities in cytoplasmic and thylakoid membranes from the cyanobacterium Anacystis nidulans. Plant Cell Physiol. 1986;27:485–490. [Google Scholar]
- Räntfors M, Evertsson I, Kjellberg JM, Sandelius AS. Intraplastidial lipid trafficking: regulation of galactolipid release from isolated chloroplast envelope. Physiol Plant. 2000;110:262–270. [Google Scholar]
- Rawyler A, Meylan M, Siegenthaler PA. Galactolipid export from envelope to thylakoid in intact chloroplasts: I. Characterization and involvement in thylakoid lipid asymmetry. Biochim Biophys Acta. 1992;1104:331–341. doi: 10.1016/0005-2736(92)90048-q. [DOI] [PubMed] [Google Scholar]
- Rawyler A, Meylan-Bettex M, Siegenthaler PA. (Galacto) lipid export from envelope to thylakoid membrane in intact chloroplasts: II A general process with a key role for the envelope in the establishment of lipid asymmetry in thylakoid membranes. Biochim Biophys Acta. 1995;1233:123–133. doi: 10.1016/0005-2736(94)00248-n. [DOI] [PubMed] [Google Scholar]
- Routaboul JM, Fischer SF, Browse J. Trienoic fatty acids are required to maintain chloroplast function at low temperatures. Plant Physiol. 2000;124:1697–1705. doi: 10.1104/pp.124.4.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandelius AS, Selstam E. Localization of galactolipid biosynthesis in etioplasts isolated from dark-grown wheat (Triticum aestivum L.) Plant Physiol. 1984;76:1041–1046. doi: 10.1104/pp.76.4.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schleiff E, Soll J. Travelling of proteins through membranes: translocation into chloroplasts. Planta. 2000;211:449–456. doi: 10.1007/s004250000357. [DOI] [PubMed] [Google Scholar]
- Siegenthaler PA, Müller MO, Bovet L. Evidence for lipid kinase activities in spinach chloroplast envelope membranes. FEBS Lett. 1997;416:57–60. doi: 10.1016/s0014-5793(97)01168-x. [DOI] [PubMed] [Google Scholar]
- Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, Fujimoto EK, Goeke NM, Olson BJ, Klenk DC. Measurement of protein using bichinchoninic acid. Anal Biochem. 1985;150:76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Sommarin M, Sandelius AS. Phosphatidylinositol and phosphatidylinositol-phosphate kinases in plant plasma membranes. Biochim Biophys Acta. 1988;958:268–278. [Google Scholar]
- Telfer A, Allen JF, Barber J, Bennett J. Thylakoid protein phosphorylation during state 1-state 2 transitions in osmotically shocked chloroplasts. Biochim Biophys Acta. 1983;722:176–181. [Google Scholar]
- Tietje C, Heinz E. Uridine-diphospho-sulfoquinovose:diacylglycerol sulfoquinovosyltransferase activity is concentrated in the inner membrane of chloroplast envelopes. Planta. 1998;206:72–78. [Google Scholar]
- Williams EE. Membrane lipids: what membrane physical properties are conserved during physiochemically induced membrane restructuring? Am Zool. 1998;38:280–290. [Google Scholar]