Abstract
During eukaryotic gene expression, alternative splicing of messenger RNA precursors is critical in increasing protein diversity and regulatory complexity. Multiple transcript isoforms could be produced by alternative splicing from a single gene; they could eventually be translated into protein isoforms with deleted, added, or altered domains or produce transcripts containing premature termination codons that could be targeted by nonsense-mediated mRNA decay. Alternative splicing can generate proteins with similar, different, or even opposite functions. Increasingly strong evidence indicates that abnormal RNA splicing is a prevalent and crucial occurrence in cellular differentiation, tissue advancement, and the development and progression of cancer. Aberrant alternative splicing could affect cancer cell activities such as growth, apoptosis, invasiveness, drug resistance, angiogenesis, and metabolism. This systematic review provides a comprehensive overview of the impact of abnormal RNA alternative splicing on the development and progression of hepatocellular carcinoma.
Keywords: Aberrant splicing, Hepatocellular carcinoma, Cis-element, SR proteins, hnRNPs
Introduction
Hepatocellular carcinoma (HCC) is the fourth leading cause of cancer-related deaths and the sixth most prevalent cancer worldwide (Bray et al. 2018; Kim and Viatour 2020). The intricate etiology and mechanistic aspects underlying the process of carcinogenesis in HCC remain subjects of ongoing investigation. Despite notable advancements in early diagnostic approaches, the prognosis for individuals affected by HCC remains unfavorable due to the absence of efficacious therapeutic interventions. HCC typically occurs as a consequence of chronic inflammation arising from fibrotic processes. Liver cirrhosis, primarily stemming from chronic infections caused by hepatitis B or C viruses, alcohol misuse, exposure to chemical toxins, non-alcoholic cirrhosis, or metabolic dysregulation, represents the principal predisposing risk factor for HCC development (Marengo et al. 2016; Aravalli et al. 2013; Huang et al. 2023). The molecular pathogenesis of HCC remains inadequately elucidated. In addition to the role of transcriptional regulation, accumulating evidence suggests a correlation between liver cancer and aberrant RNA splicing (Liu et al. 2014; Berasain et al. 2014; Webster 2017; Climente-Gonzalez et al. 2017; Singh and Eyras 2017; Jimenez et al. 2018).
In humans, RNA splicing is one of the basic processes that regulate gene expression in eukaryotes. Normal physiological processes lead to alternative splicing in more than 95% of human genes, resulting in the encoding of splice variants (Wang et al. 2008; Jiang and Chen 2021; Choi et al. 2022). Alternative splicing offers the capacity to generate extensive diversity at the level of RNA and protein, thereby expanding the repertoire of functional molecules from a seemingly constrained number of genes within the genome. The generation of proteomic diversity in humans is attributed to the essential role played by alternative splicing. According to the most recent research, humans have 63,494 genes and 233,615 transcripts in total, with 19,969 genes (86,245 transcripts) expected to encode protein (Nurk et al. 2022). Splicing in humans is complex in producing more transcripts, and finally more proteins that are critical in chemical reactions for life. For example, the muscle protein coding gene TTN has 364 exons, and except for the first exon, which is non-coding, the rest are coding exons. TTN can theoretically produce more than one million splice variants through splicing, which is important for muscle development (Savarese et al. 2018).
Alternative splicing also has effects on mRNA stability, localization, or translation. Furthermore, the protein isoforms arising from alternative splicing events can exhibit distinct or even antagonistic functions, thereby exerting regulatory control over diverse biological processes in humans. Researchers believe that most alternative isoforms are not just splicing noise arising from the stochasticity of splicing (Wang et al. 2014). Alternative isoforms can be translated into proteins. In a study by Kim et al., employing high-resolution mass spectrometry (MS), isoform-specific peptides were successfully identified for a total of 2,861 protein isoforms originating from 2,450 genes (Kim et al. 2014). As MS technology develops, an increasing number of isoform-specific peptides could be identified in the future.
Alternative splicing regulates liver development
Tissue development in humans is a highly intricate process that involves not only transcriptional regulation but also the coordinated activity of alternative splicing networks. These networks exert a critical regulatory influence on various aspects of tissue development, including the formation and maturation of important organs such as the brain, heart, skeletal muscle, pancreas, germ line, and immune system (Baralle and Giudice 2017). Alternative splicing plays a pivotal role in various aspects of liver biology, encompassing liver development, maturation, homeostasis, and metabolism. Notably, alternative splicing specific to certain tissues is widespread in the human body. Research has shown that the brain, pancreas, liver, and peripheral nervous system exhibit the most unique types of alternative splicing. Analysis of expressed sequence tag (EST) data revealed that 3′ splice sites and other 5′ splice sites are used approximately 50–100% more frequently than in other human tissues (Yeo et al. 2004).
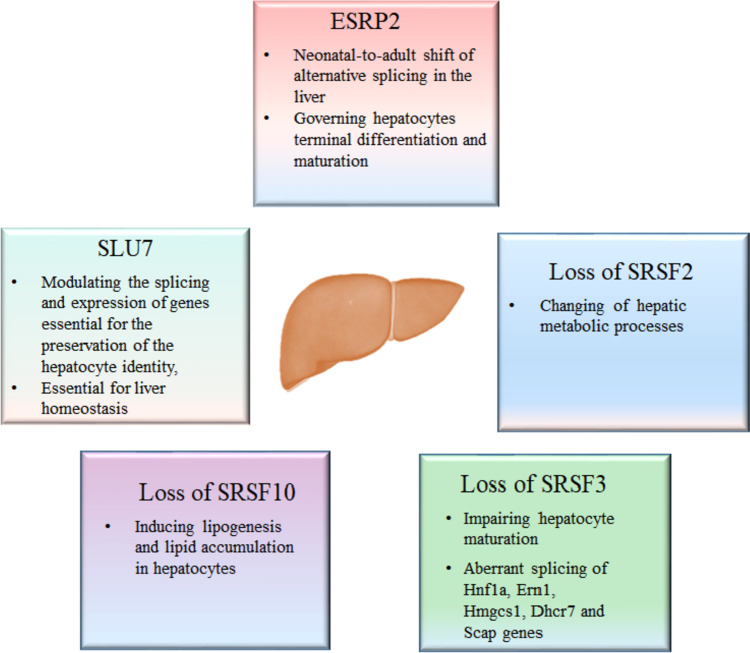
The liver undergoes a dynamic transition from a hematopoietic tissue during embryonic development to a vital metabolic organ in adulthood. This developmental switch is regulated by transcriptional machinery and simultaneously by a relevant repertoire of alternative splicing events. Bhate et al. conducted an in-depth investigation using high-resolution transcriptome analysis based on RNA-seq and bioinformatics techniques. They focused on poly(A)-enriched RNA samples derived from four distinct mouse liver developmental time points: embryonic day 18, postnatal day 14, postnatal day 28, and adult stages (Bhate et al. 2015). Through their comprehensive analysis, they identified 4,882 genes exhibiting differential expression between the embryonic day 18 and adult stages. Importantly, they also found 529 different alternative splicing events (percent spliced in (PSI) values changed > 20%) in 487 unique genes. These alternative splicing changes exhibited a high degree of conservation between mouse and human liver tissues, both during fetal development and at the adult stage. In addition, research has also revealed a primary controller of splicing, known as epithelial splicing regulatory protein 2 (ESRP2). This RNA-binding protein is among the limited number of such proteins that experience an increase in their levels during the development of liver cells. ESRP2 plays a critical role in the neonatal-to-adult shift of alternative splicing in the liver, governing hepatocyte terminal differentiation and maturation (Bangru et al. 2018).
Serine/arginine-rich splicing factor 1 (SRSF1) is a member of the serine/arginine-rich (SR-rich) protein family. Many studies have demonstrated that SRSF1 regulates alternative splicing within extensive gene networks and various biological pathways (Paz et al. 2021). The hepatocyte-specific depletion of SRSF1 demonstrated no discernible phenotype in mice, as they maintained normal health and typical lifespan and exhibited unaltered liver development and cellular functions, in contrast, the targeted deletion of SRSF2 specifically in hepatocytes led to significant perturbations in hepatic metabolic processes. This disruption resulted in the induction of endoplasmic reticulum (ER) stress, liver injury, and, ultimately, premature mortality within one month after birth (Cheng et al. 2016). Serine/arginine-rich splicing factor 2 (SRSF2) also belongs to the serine/arginine-rich (SR) protein family, and functional importantly by in pre-mRNA splicing regulation (Li and Wang 2021). The absence of SRSF2 had a profound impact on liver homeostasis, emphasizing its crucial role as a splicing regulator in this tissue. Notably, SRSF2 exerts its regulatory influence by modulating the splicing patterns of a broad range of genes implicated in autophagy and the regulation of cellular responses to stress (Cheng et al. 2016; He et al. 2015).
SRSF3, an additional member of the SR protein family, emerges as a pivotal transcriptional regulator of hepatic gene expression during the early postnatal phase. Its indispensability becomes evident, as SRSF3 plays a crucial role in liver maturation and metabolic function (Kumar et al. 2019). The selective elimination of SRSF3 in hepatocytes resulted in impaired hepatocyte maturation accompanied by perturbations in glucose, lipid, and cholesterol homeostasis. Notably, these mice displayed diminished glycogen storage, fasting hypoglycemia, heightened insulin sensitivity, and reduced cholesterol synthesis. The mismatched splicing of essential regulators responsible for glucose and lipid metabolism, such as the Hnf1a, Ern1, Hmgcs1, Dhcr7, and Scap genes, occurs due to the absence of SRSF3. The precise splicing regulation orchestrated by SRSF3 underscores its indispensable role in orchestrating liver development and ensuring the maintenance of metabolic homeostasis and normal physiology (Sen et al. 2013).
The splicing factor SRSF10 has been observed to exhibit downregulation in the liver and muscle tissues of obese individuals, as well as in mice subjected to a high-fat diet (Wu et al. 2021). Knockdown and overexpression experiments of SRSF10 have revealed its involvement in the induction of lipogenesis and the subsequent accumulation of lipids in hepatocytes. Notably, SRSF10 exerts its regulatory influence through splicing modulation of LPIN1, a pivotal regulator of lipid metabolism. The intricate splicing regulation mediated by SRSF10 holds the potential to modulate metabolic pathways that are intricately linked to obesity and associated metabolic phenotypes. This underscores the significance of SRSF10 in the regulation of metabolic processes critical to obesity and its related manifestations (Pihlajamaki et al. 2011).
The targeted depletion of the pre-mRNA splicing factor SLU7 in human and mouse liver cells elicited substantial alterations in pre-mRNA splicing patterns, underscoring the significant regulation by SLU7 in this process (Urtasun et al. 2016). Furthermore, SLU7 was found to exert modulatory effects on the splicing events and expression levels of genes crucial for the maintenance of hepatocyte identity, including the splicing factor SRSF3 and the hepatic differentiation regulator Hnf4α. The indispensable role of SLU7 in liver homeostasis was also established, further highlighting its critical involvement in maintaining the normal physiological equilibrium of the liver (Elizalde et al. 2014) (Fig. 1).
Fig. 1.
Splicing factors regulate liver development
Alternative splicing and HCC
Most alternative splicing events are cellular or tissue-specifically regulated. Alternative splicing can be modulated according to cell and tissue type, developmental stage, gender, or in response to external inner or environmental factors (Wang et al. 2008; Baralle and Giudice 2017; Keren et al. 2010; Witten and Ule 2011). Numerous human diseases result from mutations in the cis-acting sequence elements found in precursor mRNA (pre-mRNA), which play a crucial role in alternative splicing. These mutations can occur in various regions, including the 5′ and 3′ splice sites, as well as exonic and intronic splicing enhancer or silencer sequences. This highlights the significant biological importance of alternative splicing. Additionally, mutations in trans-acting splicing factors or other components of the spliceosome can also contribute to disease pathogenesis. Alternative splicing in cancer is influenced by various regulatory features, including trans-acting RNA-binding proteins and cis-acting regulatory elements. Numerous studies have demonstrated that trans-acting splicing factors can modulate splicing outcomes by interacting with cis-regulatory elements (Luo et al. 2017; Bruun et al. 2016; Thibault et al. 2021). Thus, alterations in the levels or mutations of RNA-binding proteins would lead to mis-regulation of alternative splicing. Notably, alternative splicing is implicated in numerous pathological conditions, including cancer (Wang and Burge 2008; Barash et al. 2010; Yu et al. 2020). Alternative splicing plays a pivotal role as a regulatory mechanism in cancer, exerting significant influence over diverse cellular processes, including cell proliferation, signal transduction pathways, apoptosis, metabolism, angiogenesis, invasion, drug resistance, and metastasis (Choi et al. 2022, 2023; Sveen et al. 2016).
In recent studies, a notable observation has been the frequent identification of aberrantly spliced variants in HCC, implying the potential involvement of these variants in the survival and progression of HCC cancer cells. It seems likely that these abnormal splicing events will benefit the diagnosis and treatment of HCC (Lin et al. 2018; Li et al. 2019; Wu et al. 2019a, b; Zhang et al. 2019; Luk et al. 2021). Furthermore, 404 somatic mutations in HCC underwent a detailed investigation of the genomic and epigenomic characteristics by Yu-Jia Zhao and associates. They pinpointed several notable mutational patterns, including RNF213, SF3B1, SPEN, NOVA1, and EEF1A1, and they attempted to identify the regulatory network in charge of these bodily changes. They aimed to learn more about the processes that control clinically significant alternative splicing occurrences (Zhao et al. 2021). The production of alternative isoforms through alternative splicing has the potential to facilitate the development of HCC by generating oncogenic proteins that remain persistently active or by interfering with the action of tumor suppressor proteins. In the present review, we aim to summarize and discuss the aberrant alternative splicing events that have been observed in HCC.
Cis-element mutations in HCC
After sequencing and analyzing the whole genomes of 27 HCCs, Fujimoto et al. found 52 splice-site mutations from all 27 HCC genomes that could alter proteins, indicating that these point mutations in the splicing site can modulate the splicing of genes in HCC (Fujimoto et al. 2012). Tian et al. used genotype data from the Cancer Genome Atlas and corresponding alternative splicing values and identified 119,209 splicing quantitative trait loci and 4407 genetic variants that affect splicing from 369 samples (Tian et al. 2019).
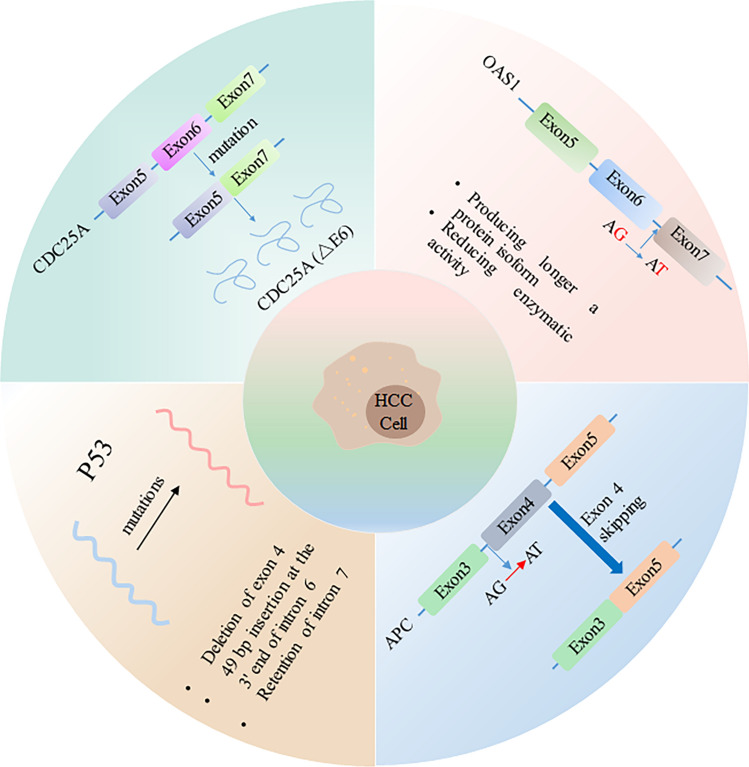
The p53 gene, recognized for its pivotal role in preserving genomic stability and suppressing tumor formation, serves as a prominent tumor suppressor (Hernández Borrero and El-Deiry 2021). A pancancer view revealed that P53 was one of the most mutated genes across cancer types. Alternative splicing is one of the factors that led to the identification of several p53 isoforms. Ubiquitination and degradation of wild-type p53 can be inhibited by its alternative splice isoform which translate into N-terminal truncation P53 protein (Ghosh et al. 2004). By genomic DNA sequencing, Lai et al. found that point mutations of the P53 gene at the 5′ or 3′ splice site led to the retention of intron 7 or 49 bp insertion at the 3′ end of intron 6 in 2 samples or deletion of exon 4. Notably, the patients harboring these splicing site mutations exhibited a younger age at diagnosis and presented with notably large tumors. These findings collectively suggest a potential implication of these specific p53 mutants in the development of liver cancers (Lai et al. 1993).
The adenomatous polyposis coli (APC) gene is responsible for inhibiting the formation of tumors and plays a crucial role in regulating beta-catenin levels. Additionally, APC interacts with E-cadherin, which is involved in maintaining cell adhesion, and acts as a tumor suppressor (Yamazaki et al. 2021). Kurahashi et al. reported that in hepatoblastoma, one allele was lost, and a point mutation at the splice acceptor site from splice site AG to AT mutation of the intron 3-exon 4 junction was detected, which caused skipping of exon 4. Exon 4 skipping of APC transcripts causes frameshifts and results in inactivation of the APC gene, which might play a causative role in tumorigenesis (Kurahashi et al. 1995).
The 2′,5′-oligoadenylate synthetase (2′5′AS) enzyme, which is encoded by OAS1, plays a crucial role in the innate immune system’s defense against viral infections (Choi et al. 2015). A SNP at the 3′ splice site of exon 7 mutates the splice site from AG to AT and results in the creation of a new 3′ splicing site located only one base downstream of the splice-site polymorphism. Usage of the new 3′ splicing site causes a frameshift and produces a longer protein isoform with reduced enzymatic activity. Virus-induced HCC occurs worldwide, and chronic hepatitis B virus (HBV), hepatitis delta virus (HDV), and hepatitis C virus (HCV) infection are the most significant etiological risk factors for HCC. Common mechanisms by which HBV, HCV, and HDV drive hepatocarcinogenesis include persistent liver inflammation with impaired antiviral immune responses, immune and viral protein-mediated oxidative stress, and dysregulation of cellular signaling pathways by viral proteins (D'Souza et al. 2020). Mutations at splicing sites can significantly impact a host’s susceptibility to viral infection, their disease progression, and even their response to IFN therapy when dealing with either hepatitis C or hepatitis B infections. Furthermore, such mutations have been found to be strongly associated with a higher degree of liver fibrosis, ultimately leading to cirrhosis and liver cancer (Lu et al. 2012; Awady et al. 2011).
CDC25A is a member of the Cdc25 phosphatase family. CDC25A activates cyclin-dependent kinase-2 (Cdk2) through the dephosphorylation of threonine and tyrosine residues, playing a vital role in the S phase and the transition from G2 to mitotic processes. This elevated expression of CDC25A has been found to be associated with a negative prognosis (Yuan et al. 2017). The deletion mutation of two ubiquitination (Lys150, Lys169) sites in exon 6 of CDC25A causes the CDC25A (△E6) subtype that skipped exons to remain stable in the nucleus. The stable subtype CDC25A (△E6) from AS has a stronger cell cycle impact on tumorigenesis of HCC (Liu et al. 2022) (Fig. 2).
Fig. 2.
Mechanisms of cis-element mutations regulate HCC
HCC-related mutations in core spliceosomal components
Recently, a recurring mutation has been identified in chronic lymphocytic leukemia and HCC. Specifically, there is a mutation at the third base of U1 snRNA where A is replaced by C. The presence of this mutation has the potential to interfere with the proper formation of base pairs between U1 snRNA and the 5′ splicing site, which is an essential process for maintaining accuracy in splicing (Yang et al. 2013). The A > G mutation could alter the splicing of 533 introns in 303 genes in HCC, and some of the genes are cancer driver genes. Furthermore, this mutation has been detected in the initial phases of HCC and is correlated with higher age at diagnosis, but it does not affect survival outcomes. This mutation was also found to be associated with heavy alcohol use in patients but not with infection with hepatitis B or C virus (Shuai et al. 2019). Li and colleagues observed over 40,000 possible driver variants, as well as approximately 80,000 splicing targets that may have become deregulated across 33 different types of cancer (Li et al. 2017).
SF3B is a crucial multiprotein complex that plays a significant role in the removal of introns from premessenger RNA during splicing. The SF3b complex comprises seven proteins with sizes varying from 10 to 155 kDa after purification. SF3b1-7 is the designation for these proteins. The complex’s biggest component is SF3b1, whereas SF3b2 lacks any discernible structural features. Each of the three BP domains that make up SF3b3 has seven lobes. SF3b4 features a PR domain at its C-terminus and two RRMs at its N-terminus. The remaining three components of the SF3b complex are small proteins (Sun 2020). As a U2 small nuclear ribonucleoprotein (snRNP) component, SF3B is involved in the recognition of the premessenger RNA branch site. The SF3B complex is an essential component of the spliceosome for mature mRNA processing (Tang et al. 2016).
SF3B1 is highly expressed in HCC according to immunohistochemistry analysis. As an important splicing factor, SF3B1 is a nuclear protein, but in HCC, SF3B1 is also secreted into extracellular fluid as a component of exosomes. The presence of anti-SF3B1 autoantibodies in the serum has been identified as a diagnostic biomarker for HCC (Hwang et al. 2018a). Recently, SF3B1 was also found to be frequently mutated in advanced HCC by whole-exome sequencing (Nault et al. 2019). In recent studies, scientists have discovered that when SF3B1 is silenced, the ability of various liver cancer cell lines to form tumors is reduced. They speculate that this effect may be due to altered how certain tumor-associated genetic variations, such as KLF6-SV1, are expressed (López-Cánovas et al. 2021).
Subunit 3 of the splicing Factor 3b protein complex is encoded by SF3B3. This complex is a part of the U2 snRNP complex, and its main function is to recognize the 3' splicing site during the splicing process (Sun 2020). According to Qiao and colleagues, the factor RALY has the potential to promote tumor growth. Furthermore, they discovered that the subunit SF3B3 of splicing Factor 3b can collaborate with RALY to regulate the splicing switch of transfer correlation 1 (MTA1) from MTA-S to MTA1-L. Normally, the MTA1-S isoform exhibits an inhibitory effect on cell proliferation by suppressing the transcription of cholesterol-synthesizing genes. The inhibitory effect of MTA1-S on cholesterol synthesis genes is weakened in HCC due to the reduced levels of MTA1-S. This weakening occurs through the synergistic regulation of the splicing switch of MTA1 by RALY and SF3B3. Furthermore, low levels of MTA1-S promote the proliferation of HCC cells at the same time (Qiao et al. 2022). Further studies have shown that the interaction of SF3B3 with long non-coding RNA (lncRNA) might contribute to the progression of HCC. It has been found that LINC01348 and SF3B3 may interact to regulate the alternative splicing of the EZH2 pre-mRNA, which in turn affects the metastasis of HCC cells (Lin et al. 2021).
SF3B4, another crucial component of the SF3B complex, has been found to exhibit significant upregulation in HCC. In HCC cells, SF3B4 knockdown has been shown to cause arrest in the G1 phase of the cell cycle. This effect is achieved by increasing the expression of p27Kip1 and decreasing the levels of cyclins and CDKs. In the initial phase of HCC, upregulation of SF3B4 resulted in modifications in the alternative splicing pattern of the Krüppel-like Factor 4 (KLF4) tumor suppressor gene, leading to the generation of nonfunctional exon-skipped transcripts with elevated expression levels. The inactivation of KLF4 contributes to the malignant transformation and growth of hepatocytes by transcriptionally suppressing p27Kip1 and simultaneously activating Slug genes. Splicing factor SF3B4 which belongs to the SF3B protein complex regulates alternative splicing in HCC and could function as a driver in early hepatocarcinogenesis (Shen and Nam 2018). In HCC, it was reported that the copy number of SF3B4 was increased and associated with intrahepatic metastasis and poor HCC prognosis (Iguchi et al. 2016). As an important potential oncogene, SF3B4 could be negatively regulated by miRNA-133b in HCC (Liu et al. 2018).
Mutations affecting CTNNB1 and TP53 genes have been implicated in alterations in protein phosphorylation, particularly in relation to actin filament organization and lipid metabolism (Zhou et al. 2014; Ng et al. 2022; Chen and Wang 2021). Ng et al. analyzed the differential expression of mutant and wild-type HCC. They identified 3067 genes that exhibited differential expression in the CTNNB1 mutant group, as well as 23 differentially expressed proteins specifically associated with the CTNNB1 mutation. They found that PPIE showed a difference at the protein level and was not associated with differential transcription. PPIE is a spliceosome component that regulates the splicing of the long-chain non-coding RNA FAST, which in turn regulates the β-catenin and Wnt signaling pathways. Therefore, CTNNB1 and TP53 mutations affect the protein expression of the associated splice components in HCC (Ng et al. 2022). Researchers have discovered that that PRPF8, a crucial protein involved in the catalytic activity of spliceosomes, likes connecting with exons in freshly generated mRNA molecules, many of which are connected to genes that produce proteins. Additionally, they discovered that HepG2 cells with decreased PRPF8 activity may exhibit less aggressive HCC due to altered splicing of critical cancer-related genes (López-Cánovas et al. 2023). Further research on these cells proved this. Another vital element of the spliceosome is Snrpb (Small Nuclear Ribonucleoprotein Polypeptide B and B1). According to a study by Yu-Ting Zhan et al., higher levels of SNRPB expression encourage HCC cell growth that is malignant and stem cell-like. On the other hand, the result is the reverse when SNRPB levels are decreased in HCC cells (Zhan et al. 2020).
Splicing regulators (SR proteins and hnRNPs) regulate aberrant splicing in HCC
SR proteins regulate aberrant splicing in HCC
The process of alternative splicing is under strict control by a trans-acting splicing factor that attaches to a splicing regulatory motif for HCC splicing (Wang et al. 2019a; Marasco and Kornblihtt 2022). SR proteins and hnRNPs are two significant families of splicing regulators that play vital roles in the splicing process. The RNA recognition motif (RRM) domain in SR proteins, usually in one or two copies, is responsible for binding to RNA with specificity. Additionally, these proteins contain an arginine/serine-rich (RS) domain that plays a crucial role in interacting with other proteins (Long and Caceres 2009; Jeong 2017). HnRNPs are another family of RNA-binding proteins that contain both RNA-binding domains and other auxiliary domains (Martinez-Contreras et al. 2007). SR proteins and hnRNPs have been found to play different roles in HCC.
SRSF1
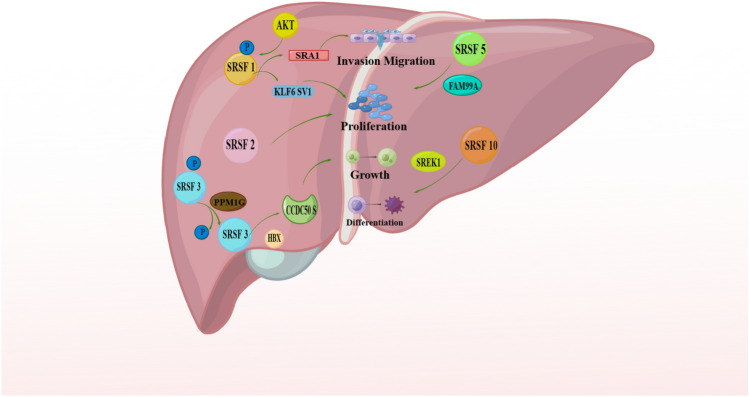
The tumor suppressor gene Krüppel-like Factor 6 (KLF6), a gene known to suppress tumor growth, produces a variant called KLF6 splice variant 1 (SV1) via alternative splicing, which occurs when a natural hidden splice site within exon 2 is utilized. KLF6 splice variant 1 is a growth-promoting variant that antagonizes full-length KLF6. Knockdown of SRSF1 was found to increase KLF6 SV1 mRNA expression in human HCC cell lines. SRSF1 is involved in regulating KLF6 alternative splicing. Activated AKT1 can phosphorylate SRSF1, thereby promoting the generation of KLF6 SV1 and subsequently enhancing cellular proliferation. Consequently, SRSF1 serves as a promoter of tumorigenesis in HCC. These findings highlight the intricate interplay between alternative splicing, signaling pathways (such as AKT1), and the tumorigenic potential of HCC, underscoring the significance of KLF6 and KLF6 SV1 in the pathogenesis of HCC (Narla et al. 2005; Yea et al. 2008; He et al. 2017).
Furthermore, it has been demonstrated that SRSF1 plays an equally crucial role in preserving the integrity of the genome. When targeted SRSF1 is depleted, hepatocytes experience an increase in lipid accumulation, resulting in cell death, inflammation, and fibrosis. These observations strongly suggest that abnormalities in SRSF1 are of great significance in HCC (Arif et al. 2023). A recent study by Sijia Lei et al. has demonstrated that SRA1, a steroid receptor RNA activator 1, functions as a unique coactivator that impacts the migration of cancer cells. They observed that SRSF1 controls the splicing of SRA1 by binding to specific regions within its exon 3. This splicing process yields two isoforms of SRA1, which are essential for facilitating cell invasion and migration (Lei et al. 2021).
SRSF2
SRSF2 increases the proliferation and tumorigenic potential of HCC cells by regulating cancer-related splicing events (Luo et al. 2017). In HCC patient samples, Luo and colleagues observed that the expression of SRSF2 is often increased. High SRSF2 expression is associated with poor prognosis and lower survival time in HCC patients. SRSF2 and its regulated isoforms have been shown to stimulate cancer cell proliferation both in vitro and in mouse models.
Recently, SRSF2 was shown to be involved in the process of HCC and is related to hepatic progenitor cells (HPCs). The deletion of SRSF2 was found to trigger the regeneration and activation of HPC-mediated oncofetal genes, thereby promoting the development and progression of HCC in mice (Zhang et al. 2020). These findings underscore the significance of SRSF2 in HCC pathogenesis, emphasizing its role in driving alternative splicing events, promoting the generation of protumorigenic isoforms, and influencing the behavior of HPCs in liver cancer.
SRSF3
SRSF3 was found to decrease expression or mislocalize in human HCC. According to prior research, a mutation in SRSF3 has been identified in 6.7% of HCC specimens from the RIKEN database. Sen et al. found that hepatocyte-specific deletion of SRSF3 causes liver fibrosis and eventually develops into metastatic HCC with aging (Sen et al. 2013).
Viruses are one of the major risk factors for the occurrence and development of HCC, accounting for 80% of cases. These viruses, such as HBV, can cause HCC by integrating their DNA into the body, causing epigenetic changes, immune dysfunction, and mutations in viral genes (Shen et al. 2023; Zhang et al. 2022). In addition, HBV-encoded X protein (HBx)-mediated cytosolic SRSF3 promotes upregulation of CCDC50S, a truncated splice variant of CCDC50, and promotes tumor growth and metastasis in HCC through Ras/forkhead box protein O4 (Foxo4) signaling (Wang et al. 2019b). The expression of insulin receptor subtype A and insulin-like growth factor 2 is increased due to the lack of SRSF3 in the livers of SRSF3-HKO mice, leading to abnormal activation of mitogenic signaling. Furthermore, acute loss of SRSF3 results in aberrant splicing of genes involved in epithelial-mesenchymal transition (EMT), such as leukemia inhibitory factor receptor, erythrocyte protein band 4.1-like 5, myosin 1B, catenin (cadherin-associated protein), delta 1, G-protein-coupled receptor kinase-interactor 2, and Slk, suggesting that SRSF3 directly regulates the EMT process in HCC. Moreover, the loss of SRSF3 activates β-catenin signaling and increases c-MYC expression (Sen et al. 2015).
Deepak Kumar et al. found that SRSF3 protein in HCC was reduced sixfold compared with normal liver tissue, but SRSF3 mRNA was increased. From published TCGA data, HCC patients with high SRSF3 mRNA expression exhibit poorer survival rates, as is that of patients with known SRSF3-dependent splicing event alterations. In conclusion, the results suggest that reduced SRSF3 splice activity may be a major driver of liver cancer (Kumar et al. 2022).
SRSF3 itself could be aberrantly spliced in HCC. Another isoform includes exon 4 of SRSF3, which contains an in-frame premature stop codon that promotes degradation of SRSF3, induced by knockdown of the repressed splicing regulator SLU7 in liver cancer (Elizalde et al. 2014). SLU7 is critical for the correct selection of the 3′ splice site of SRSF3, and it was significantly downregulated in human cirrhotic liver and HCC. RNA-CLIP assays showed that SLU7 binds SRSF3 mRNA and regulates SRSF3 splicing (Jimenez et al. 2019).
There is evidence that dephosphorylation at the protein level of SRSF3 is significant as well in HCC. SRSF3 can interact with and be dephosphorylated by the protein phosphatase PPM1G. The ability of SRSF3 to regulate alternative splicing is subsequently compromised due to its release from RNA (Chen et al. 2021).
SRSF5
Through the integration of multiple RNA-Seq datasets, it was discovered that SRSF5 was markedly downregulated in HCC samples in comparison with adjacent normal samples (Zhang et al. 2016). Other SR proteins are also important in other types of cancers. Typical “housekeeping” genes are deregulated in HCV-induced HCC, but SRSF4 is constantly expressed in HCV-induced HCC and controls human liver tissues (Waxman and Wurmbach 2007). In a study conducted by Mo et al., SRSF5 was identified as a potential binding partner of FAM99A through RNA pull-down and mass spectrometry assays. In patients diagnosed with HCC, FAM99A serves as an autonomous indicator of prognosis for their survival as a whole. The upregulation of FAM99A was found to inhibit the proliferation, colony formation, migration, and invasion capacity of HCC cells in vitro. Furthermore, subcutaneous xenograft tumor models revealed that the overexpression of FAM99A significantly suppressed tumor growth in HCC cells in vivo (Mo et al. 2022).
SRSF10
SRSF10, also known as SRp38 or FUSIP1, belongs to the SR protein family that plays a crucial role in RNA splicing through phosphorylation and interaction with small nuclear ribonucleoprotein particles. Recent studies have indicated the involvement of SRSF10 in various cellular processes, including the DNA damage response, HIV replication, cellular differentiation, glucose production, and the development of HCC in humans (Liu et al. 2022; Shin et al. 2004; Shin and Manley 2002). Chang et al. conducted a study to investigate the prognostic significance of SREK1 (SREK1L) and SRSF10 in HCC tumor tissue (HCC-T). The outcomes indicated that SREK1L had a connection with unfavorable prognosis by stimulating the development of tumors in both in vitro and in vivo HCC cells. On the other hand, SRSF10 was identified as a carcinogenic driver and exhibited a significant correlation with HCC recurrence. Further analysis revealed that SRSF10 played a crucial role in sustaining the inclusion of exon 10 in SREK1. SREK1 can promote hepatocarcinogenesis by forming SRSF10/SREK1 splicing signaling; thus, it may be regarded as the carcinogenic downstream effect of SRSF10 (Chang et al. 2022) (Fig. 3).
Fig. 3.
The roles of SR proteins in HCC
Since numerous kinases, including SRPK, regulate the subcellular localization of SR proteins, direct contact with or phosphorylation of SRSF2 by SRPK3 is crucial for its subnuclear distribution. In addition, SRPK1 forms an SRPK1-CLK1 complex and undergoes a series of steps that ultimately lead to the mobilization of SRSF1 from nuclear speckles to the nucleoplasm (Long et al. 2019). Numb, a protein involved in cell fate determination, undergoes alternative splicing, resulting in two isoforms: one containing a long proline-rich region (PRRL) and the other with a short proline-rich region (PRRS). In HCC, the expression of the Numb PRRL isoform is elevated and correlated with early recurrence and decreased overall survival following surgical intervention. Functionally, the PRRL isoform generally promotes cellular processes such as proliferation, migration, invasion, and colony formation, whereas the PRRS isoform exhibits suppressive effects on these activities in HCC cell lines. Numb PRR isoforms are regulated in a coordinated manner by the kinase serine/arginine protein-specific kinase 2 (SRPK2) and the splicing factor RNA-binding Fox domain 2 (RBFOX2). Knockdown of RBFOX2 leads to accumulation of PRRL, whereas knockdown of SRPK2 leads to accumulation of PRRS (Lu et al. 2015).
HnRNPs regulate aberrant splicing in HCC
In addition to SR proteins, hnRNPs are another family of well-studied splicing regulators. There are 20 hnRNP proteins that show greater structural diversity than SR proteins. Four unique RBDs, including the RNA recognition motif (RRM), the quasi-RRM, a glycine-rich domain constituting an RGG box (RGG), and a KH domain, were identified in hnRNP proteins (Geuens et al. 2016). Moreover, hnRNPs also have other auxiliary domains, which can mediate interactions between proteins or modulate protein localization (Geuens et al. 2016). Both SR proteins and hnRNPs play diverse roles in splicing regulation, promoting or inhibiting splice-site recognition and modulating the usage of alternative exons or splice sites (Jeong 2017).
The expression of splicing regulators is critical in alternative splicing regulation, and different research groups have reported increased mRNA and protein levels of hnRNPA1, hnRNPA2/B1, and hnRNP H in HCC tissue specimens compared with normal liver tissues (Chettouh et al. 2013). HnRNPA2/B1 was found to be distributed in the nucleus in hepatitis and well-differentiated HCC, but in poorly differentiated tumors, hnRNPA2/B1 was distributed mainly in the cytoplasm (Cui et al. 2010). HnRNPA1 is often highly expressed in tumors and can promote HCC cell invasion by regulating CD44 splicing. CD44 is a cell surface glycoprotein and assumes pivotal functions in cell motility, proliferation, and survival.
HnRNPA1
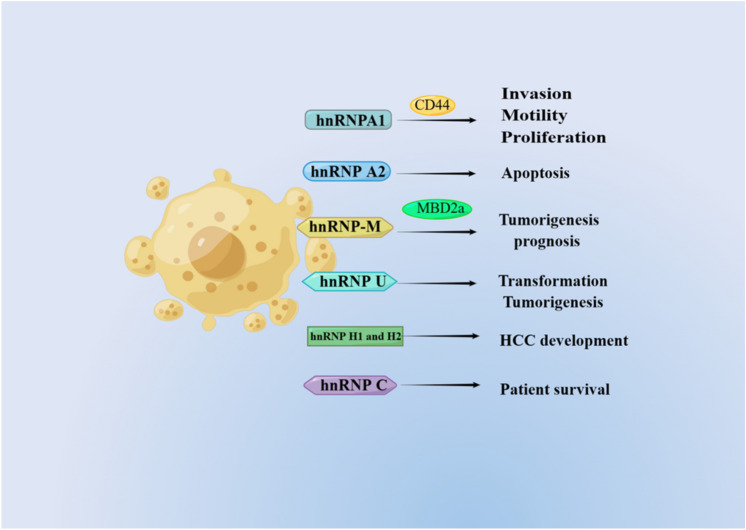
In HCC patients, hnRNP A1 promotes splicing of the CD44 v6 isoform. The CD44 v6 splice variant represents an alternative isoform of the CD44 glycoprotein found on the cell membrane, acting as a receptor for hyaluronic acid and contributing to the development and progression of various tumors, including HCC. High hnRNP A1 expression is associated with shorter overall survival and higher rates of tumor recurrence (Ke et al. 2019; Endo and Terada 2000). HnRNP A1 alone or in combination with CD44v6 can be used as an independent prognostic indicator for survival and recurrence time and could have potential as a therapeutic target (Endo and Terada 2000). Altered glucose metabolism is common in cancers. HnRNP A1 can be deacetylated by SIRT1 and SIRT6, leading to the inhibition of glycolysis, blocking the alternative splicing of PKM mRNA to PKM2, and suppressing the proliferation and tumorigenesis of HCC cells (Yang et al. 2019).
HnRNP A2
hnRNP A2/B1 encodes hnRNP A2 and its splicing variant hnRNP B1, which contains a specific 36-nucleotide mini-exon and produces an additional 12 amino acids near the N-terminus. Shilo et al. found that overexpression of hnRNP A1 or hnRNP A2, but not hnRNP B1, induced tumorigenesis. Knockdown of hnRNP A1 or hnRNP A2 inhibits human HCC cell line transformation and tumor growth in humans. More importantly, Shilo et al. found that overexpression of hnRNP A2 regulates A-Raf alternative splicing by reducing the production of a short dominant-negative isoform of A-Raf, which contains the Ras binding domain and lacks the kinase domain. In this way, hnRNP A2 overexpression causes constitutive activation of the Ras-MAPK-ERK pathway. Additionally, HCC cells with hnRNP A1/A2 overexpression are resistant to apoptosis induced by MEK inhibition, suggesting that hnRNP A2 upregulation might play an important role in drug resistance (Shilo et al. 2014).
HnRNP-M
HnRNP-M is an abundant nuclear protein that binds to pre-mRNA and is a component of the spliceosome complex. HnRNP-M affects the selection of both 5′ and 3′ alternative splice sites (Llères et al. 2010). Zhu et al. conducted a study in which they identified an oncofetal splicing factor known as heterogeneous nuclear ribonucleoprotein-M (hnRNP-M). It was discovered that this factor is stimulated in liver tissues of fetuses, inactive in liver tissues of adults, and markedly increased in liver tissues with HCC. Moreover, high hnRNP-M expression was determined to be a strong indicator of poor prognosis in HCC patients. MBD2 undergoes alternative splicing, resulting in a long isoform of MBD2a and a spliced short isoform of MBD2c. HnRNP-M promotes the splicing of MBD2 and leads to increased MBD2a expression and decreased MBD2c expression (Zhu et al. 2022) (Fig. 4). These findings significantly expand the understanding of different functions of MBD2 splicing variants in tumorigenesis and metastasis and elucidate the intrinsic function of hnRNP-M in regulating the HCC environment through an AS-dependent mechanism.
Fig. 4.
The roles of hnRNP proteins in HCC
Other hnRNP proteins
Another hnRNP family protein, hnRNP U, interacts directly with DIS3-like 3′-5′ exoribonuclease 2 (DIS3L2) and promotes the splicing of Rac1b, ultimately stimulating cellular transformation and tumorigenesis in HCC (Xing et al. 2019). HnRNP H1 and H2 are unregulated by c-Myc in HCC. HnRNP H1 and H2 regulate the mutually exclusive splicing of exons 3C and 3A in the KHK gene, leading to the high expression of the KHK-A isoform in HCC. Protein produced by KHK-A is a kinase that mediates the phosphorylation and activation of PRPS1 and is essential for HCC development (Li et al. 2016; Jiang et al. 2021). High hnRNP C expression was also found to be associated with reduced patient survival in HCC by Kaplan‒Meier analysis (Tremblay et al. 2016).
Other splicing regulatory proteins in HCC
PCBP1 is a versatile RNA-binding protein that controls alternative splicing, translation, and RNA stability in numerous cancer-related genes (Wang et al. 2019c). Researchers found that PCBP1 significantly affects the alternative splicing of genes essential for pathways connected to cancer by knocking down PCBP1 and using RNA-seq. Furthermore, PCBP1 has been recognized as a tumor suppressor gene in HCC and can impede the invasion of HCC cells (Huang et al. 2021). On the other hand, the development and treatment of HCC are closely associated with hypoxia and the resulting oxidative stress. The detail mechanism mediated by hypoxia in HCC is still under investigation (Shen et al. 2021), but some studies have shown that hypoxia can lead to a decrease in the level of the related splicing factor SRSF6, thereby weakening its binding to the 3′ splicing site (de Oliveira Freitas Machado et al. 2023). Scientists have discovered that nuclear scaffold protein p54nrb/NONO plays a crucial role in regulating hypoxic transcription signals, ultimately contributing to the advancement of liver cancer. NONO interacts with and stabilizes HIF-1 and HIF-2 complexes, thus activating the transcription of genes induced by hypoxia. Moreover, NONO also binds to the pre-mRNA and subsequent mRNA of these genes, promoting their splicing and maintaining mRNA stability (Shen et al. 2021).
Liver cancer risk factors and alternative splicing
HCC depends on several significant risk factors, including infections from the hepatitis B virus, chronic inflammation, and excessive alcohol consumption (Llovet et al. 2021). When HBV-related transcripts are transported out of the nucleus, they face obstacles in RNA processing. These difficulties include RNA degradation and unnecessary splicing, which ultimately impact the formation of protein complexes involved in viral transcription and post-transcriptional stages of the virus’s life cycle. Prior research has uncovered that TARDBP suppresses the splicing of genomic RNA, aiding the virus in exporting its non-standard RNA from the nucleus without unnecessary splicing (Makokha et al. 2019). Additionally, there have been reports on studies regarding alternative splicing in liver cancer induced by chronic inflammation. HCC is often associated with hypoxia in areas within the tumor. This hypoxic microenvironment mainly promotes tumor invasiveness and treatment resistance by activating the hypoxia-inducible factor (HIF) regulatory pathway. Inflammation is also one of the critical factors that shape the HCC microenvironment. Some studies have found that due to hypoxia-inducible factor 1α (HIF-1α), stability is increased, and tumor-associated macrophages (the main pro-inflammatory cells within tumors) secrete more interleukin 1β (IL-1β) under moderate hypoxic conditions, suggesting that liver potential benefits of anti-inflammatory strategies during cell carcinoma treatment (Zhang et al. 2018). Jeongeun Hyun and colleagues discovered that chronic inflammation hinders the expression of the splicing factor ESRP2, leading to an increase in YAP/TAZ activity downstream and ultimately facilitating the development of liver cancer in cases of chronic injury (Hyun et al. 2021). Furthermore, consuming large amounts of alcohol can also result in diseases such as alcoholic liver disease, cirrhosis, and HCC. Notably, a crucial enzyme called serine, arginine-rich protein kinase 2 (SRPK2), has been identified to regulate selective splicing, a process linked to fat production in individuals with alcoholic liver disease (Li et al. 2023). In conclusion, the process of alternative splicing, along with its associated risk factors, significantly contributes to the development of HCC.
Drug targets against aberrant AS in HCC
As previously stated, AS holds great significance in all facets of HCC, making it crucial to focus on the developing of drugs that target AS.
Metformin, taken orally to lower blood sugar, has been observed to have anti-cancer effects in various types of cancer, such as HCC. Research has indicated that metformin can decrease the expression of LGR4 through alternative splicing, lowering cell growth rates, movement, and infiltration. Analysis of the GEO database by the researchers revealed no variations in the LGR4 gene studied in HCC cell lines when treated with metformin, and 3′ UTR was shortened compared to the control group. However, the three isoforms of LGR displayed variations (Zhuo et al. 2022). Apart from metformin, melatonin is another drug used to treat HCC. Melatonin, a hormone produced by the pineal gland, has been found to possess anti-cancer properties. According to a study conducted by Tong-Hong Wang et al., melatonin hormones can potentially suppress the expression of RAF1 and the activation of oncogenic pathways downstream of RAF1. The patient’s quality of life is eventually improved due to this inhibition, which stops the development and spread of HCC cells (Wang et al. 2018). However, problems with drug resistance frequently occur throughout HCC therapy, which greatly impedes the entire therapeutic process. Therefore, it is imperative to develop new drugs to address this problem continuously.
Despite significant advancements in treating of HCC, such as immunotherapy, the prognosis remains unsatisfactory. Increasing evidence indicates that the tumor immune microenvironment (TME) plays a vital role in the progression of HCC and significantly affects the effectiveness of treatment (Sas et al. 2022). The TME encompasses various elements, including surrounding blood vessels, immune cells, fibroblasts, signaling molecules, and extracellular matrix. Acquired drug resistance is one of the primary reasons for the poor prognosis in HCC patients. One of the mechanisms contributing to resistance involves the hypoxia-inducible factor (HIF). Combining sorafenib with HIF-targeted inhibitors like ICI-118,551, melatonin, or genistein, which are potential synthetic natural compounds, may help overcome resistance to sorafenib (Méndez-Blanco et al. 2018). Furthermore, the phase III IMbrave150 research, which administered a combination of atezolizumab and bevacizumab, monoclonal antibodies that target PD-L1 and VEGF, respectively, proved successful (Kudo 2020). Hence, in the complex microenvironment of HCC, combination therapy holds more advantages than individual drugs, and personalized treatment plans are required to ensure maximum clinical benefits for patients.
Continuous reports have highlighted the progress in identifying potential drugs to target AS. Wai Kit Ma et al. researched the development of drugs to control alternative splicing of precursor RNA. According to their research, ASO medicines can convert PKM2 linked to cancer into PKM1 isoforms, inhibiting the proliferation of HCC cells. These results have also been confirmed in mouse models, illustrating the promising potential of ASO in HCC splicing therapy (Ma et al. 2022). Natasha T. Snider et al. identified NT5E-2, a new variant involved in pre-RNA splicing, which is expressed at a low level in typical human tissues but is increased in HCC. This variant, NT5E-2, codes for a shorter subtype of CD73. The researchers determined that by regulating CD73, NT5E-2 could have therapeutic implications in cancer treatment (Snider et al. 2014). Furthermore, it has been discovered by certain scientists that the androgen receptor (AR) may hold important implications in the treatment of HCC. The research conducted by Anees M Dauki et al. revealed through RNA-seq analysis that the presence of C-terminal truncated AR-SV played a critical role in promoting the advancement of HCC. Consequently, developing of treatment methods that specifically target AR-SV holds immense significance (Dauki et al. 2020).
Some potential drug targets have been identified through research on drug development that focuses on the core spliceosome and splicing factors. The study conducted by Biao Zhang et al. discovered that the splicing factor hnRNPU, when accompanied by the overexpression of c-Myc, can up-regulate its expression. This process promotes the proliferation and self-renewal of HCC. Therefore, targeting the positive feedback loop between hnRNPU and c-Myc could be a novel approach to treating HCC (Zhang et al. 2021). Another splicing factor, SRSF3, has also been investigated. Studies have demonstrated that inhibiting the degradation of SRSF3 has the potential to prevent progressive liver disease and could be valuable in the treatment of HCC (Kumar et al. 2019). More research is being done on possibly creating medications that target the spliceosome. In a study by Juan L Lopez-Cnovas et al., they discovered that the decreased expression of PRPF8, a component of the splicing body, was associated with a decrease in the invasiveness of HCC. This decrease also led to changes in the expression and splicing patterns of key cancer-related genes, making PRPF8 a potential target for drug therapy (López-Cánovas et al. 2023). Additionally, researchers found that the upregulation of SNRPB, another core component of the spliceosome, contributed to HCC cell proliferation. As a result, further research on SNRPB is expected to uncover new possibilities for drug treatment (Zhan et al. 2020).
Conclusions
The proper regulation of gene expression is important in human development, tissue homeostasis, and human disease. RNA splicing is a critical step in human pre-mRNA processing, so it could play a causal role in disease pathogenesis. Malfunctioning regulatory splicing factors, along with mutations in vital elements of the splicing machinery, splice sites, and cis-acting splicing sequences, can cause incorrect RNA splicing in a variety of ailments, including cancer. Furthermore, epigenetic modifications play a role in determining different patterns of pre-mRNA splicing. This is because the speed of RNA polymerase II (Pol II) elongation can have an impact on splice-site selection. Chromatin modifications, chromatin structure, and nucleosome occupancy are factors that can potentially affect Pol II elongation. Transcription and splicing must be coordinated with each other to promote acute splice site choice and to ultimately bring about correct alternative splicing (Alpert et al. 2017).
Alternative splicing is ubiquitous in humans. Over the last ten years, the swift progress of next-generation sequencing technology has provided a powerful means of exploring the transcriptome. This has led to an increasing identification of anomalous splicing occurrences in various cancers linked to tumorigenesis, such as HCC (Lin et al. 2018; Li et al. 2019). In the near future, the utilization of single-cell transcriptome RNA sequencing (MacParland et al. 2018; Hwang et al. 2018b; Shalek et al. 2013) along with third-generation single-molecule sequencing will enable researchers to gain deeper insights into the impact of mis-splicing on HCC and other diseases (Ardui et al. 2018). Recently, HCC cells from patients were subjected to third-generation single-molecule real-time (SMRT) sequencing. Three hundred and sixty-two new alternative spliced isoforms were identified, and many of them, such as ARHGEF2, DEK, ADRM1, CD44v8-10, and SRSF3, were tumor specific (Chen et al. 2019a). However, SMRT sequencing is limited by high mRNA quality. As the technology is developing, more aberrantly spliced variants could be identified.
The prognosis and treatment of HCC remain unsatisfying because of limited reliable early diagnostic and effective therapeutic targets. Tumors are highly heterogeneous, and diverse proteins produced by alternative splicing could be one of the strategies useful in tumor classification (Tremblay et al. 2016; Omenn et al. 2010). Alternative splicing studies will not only provide a deeper understanding of HCC occurrence but also suggest potential molecular therapeutic approaches and could benefit HCC biomarker research in the future (Chen et al. 2019b; Zhu et al. 2019).
Once the critical aberrant disease-associated alternative splicing events are confirmed, they could be developed as biomarkers for prognosis. More importantly, splicing modulatory strategies might be helpful for disease treatment. Antisense oligonucleotides have exhibited potential in treating disorders such as spinal muscular atrophy (SMA) during clinical trials. One of the ways they can be useful is by correcting specific mis-splicing occurrences. The drug called Spinraza was approved by the FDA in 2016 in the USA. Additionally, several bacterial fermentation products, plant products, and other small molecules that could modulate the function of the spliceosome have antitumoral properties (Bonnal et al. 2012; Spitali and Aartsma-Rus 2012). Antisense oligonucleotides targeting pre-mRNA or small molecule drugs which could disrupt spliceosome assembly has the potential to be developed as a novel therapeutic approach, which could improve drug efficacy and reduce side effects (Wang and Lee 2018; Lee and Abdel-Wahab 2016).
Acknowledgements
We thank Figdraw (www.figdraw.com) for expert assistance in the pattern drawing. The authors thanks Faustina Halm-Lai (University of Cape Coast) for proofreading the manuscript.
Author contributions
WS: designed the theme and direction of the manuscript. MS, YW, YZ, WL, XW, TK, PL, SW, and WS: drafted the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (82071770); Research Level Improvement Project of Anhui Medical University (2021xkjT001); Anhui Provincial Natural Science Foundation (2008085QH371); Scientific Research of BSKY in Anhui Medical University (XJ201601); and Research and Practical Innovation Projects of AHMU (YJS20230039).
Declarations
Conflict of interest
The authors declare no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Alpert T, Herzel L, Neugebauer KM (2017) Perfect timing: splicing and transcription rates in living cells. Wiley Interdiscip Rev RNA. 10.1002/wrna.1401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aravalli RN, Cressman EN, Steer CJ (2013) Cellular and molecular mechanisms of hepatocellular carcinoma: an update. Arch Toxicol 87:227–247. 10.1007/s00204-012-0931-2 [DOI] [PubMed] [Google Scholar]
- Ardui S, Ameur A, Vermeesch JR, Hestand MS (2018) Single molecule real-time (SMRT) sequencing comes of age: applications and utilities for medical diagnostics. Nucleic Acids Res 46:2159–2168. 10.1093/nar/gky066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arif W, Mathur B, Saikali MF, Chembazhi UV, Toohill K, Song YJ, Hao Q, Karimi S, Blue SM, Yee BA et al (2023) Splicing factor SRSF1 deficiency in the liver triggers NASH-like pathology and cell death. Nat Commun 14:551. 10.1038/s41467-023-35932-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangru S, Arif W, Seimetz J, Bhate A, Chen J, Rashan EH, Carstens RP, Anakk S, Kalsotra A (2018) Alternative splicing rewires Hippo signaling pathway in hepatocytes to promote liver regeneration. Nat Struct Mol Biol 25:928–939. 10.1038/s41594-018-0129-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baralle FE, Giudice J (2017) Alternative splicing as a regulator of development and tissue identity. Nat Rev Mol Cell Biol 18:437–451. 10.1038/nrm.2017.27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barash Y, Calarco JA, Gao W, Pan Q, Wang X, Shai O, Blencowe BJ, Frey BJ (2010) Deciphering the splicing code. Nature 465:53–59. 10.1038/nature09000 [DOI] [PubMed] [Google Scholar]
- Berasain C, Elizalde M, Urtasun R, Castillo J, Garcia-Irigoyen O, Uriarte I, Latasa MU, Prieto J, Avila MA (2014) Alterations in the expression and activity of pre-mRNA splicing factors in hepatocarcinogenesis. Hepat Oncol 1:241–252. 10.2217/hep.13.17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhate A, Parker DJ, Bebee TW, Ahn J, Arif W, Rashan EH, Chorghade S, Chau A, Lee JH, Anakk S et al (2015) ESRP2 controls an adult splicing programme in hepatocytes to support postnatal liver maturation. Nat Commun 6:8768. 10.1038/ncomms9768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnal S, Vigevani L, Valcarcel J (2012) The spliceosome as a target of novel antitumour drugs. Nat Rev Drug Discov 11:847–859. 10.1038/nrd3823 [DOI] [PubMed] [Google Scholar]
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68:394–424. 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- Bruun GH, Doktor TK, Borch-Jensen J, Masuda A, Krainer AR, Ohno K, Andresen BS (2016) Global identification of hnRNP A1 binding sites for SSO-based splicing modulation. BMC Biol 14:54. 10.1186/s12915-016-0279-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C, Rajasekaran M, Qiao Y, Dong H, Wang Y, Xia H, Deivasigamani A, Wu M, Sekar K, Gao H et al (2022) The aberrant upregulation of exon 10-inclusive SREK1 through SRSF10 acts as an oncogenic driver in human hepatocellular carcinoma. Nat Commun 13:1363. 10.1038/s41467-022-29016-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen LL, Wang WJ (2021) p53 regulates lipid metabolism in cancer. Int J Biol Macromol 192:45–54. 10.1016/j.ijbiomac.2021.09.188 [DOI] [PubMed] [Google Scholar]
- Chen H, Gao F, He M, Ding XF, Wong AM, Sze SC, Yu AC, Sun T, Chan AW, Wang X et al (2019a) Long-read RNA sequencing identifies alternative splice variants in hepatocellular carcinoma and tumor-specific isoforms. Hepatology 70:1011–1025. 10.1002/hep.30500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen QF, Li W, Wu P, Shen L, Huang ZL (2019b) Alternative splicing events are prognostic in hepatocellular carcinoma. Aging 11:4720–4735. 10.18632/aging.102085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D, Zhao Z, Chen L, Li Q, Zou J, Liu S (2021) PPM1G promotes the progression of hepatocellular carcinoma via phosphorylation regulation of alternative splicing protein SRSF3. Cell Death Dis 12:722. 10.1038/s41419-021-04013-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y, Luo C, Wu W, Xie Z, Fu X, Feng Y (2016) Liver-specific deletion of SRSF2 caused acute liver failure and early death in mice. Mol Cell Biol 36:1628–1638. 10.1128/MCB.01071-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chettouh H, Fartoux L, Aoudjehane L, Wendum D, Claperon A, Chretien Y, Rey C, Scatton O, Soubrane O, Conti F et al (2013) Mitogenic insulin receptor-A is overexpressed in human hepatocellular carcinoma due to EGFR-mediated dysregulation of RNA splicing factors. Can Res 73:3974–3986. 10.1158/0008-5472.CAN-12-3824 [DOI] [PubMed] [Google Scholar]
- Choi UY, Kang JS, Hwang YS, Kim YJ (2015) Oligoadenylate synthase-like (OASL) proteins: dual functions and associations with diseases. Exp Mol Med 47:e144. 10.1038/emm.2014.110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi S, Lee HS, Cho N, Kim I, Cheon S, Park C, Kim E-M, Kim W, Kim KK (2022) RBFOX2-regulated TEAD1 alternative splicing plays a pivotal role in Hippo-YAP signaling. Nucleic Acids Res 50:8658–8673. 10.1093/nar/gkac509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi S, Cho N, Kim KK (2023) The implications of alternative pre-mRNA splicing in cell signal transduction. Exp Mol Med 55:755–766. 10.1038/s12276-023-00981-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Climente-Gonzalez H, Porta-Pardo E, Godzik A, Eyras E (2017) The functional impact of alternative splicing in cancer. Cell Rep 20:2215–2226. 10.1016/j.celrep.2017.08.012 [DOI] [PubMed] [Google Scholar]
- Cui H, Wu F, Sun Y, Fan G, Wang Q (2010) Up-regulation and subcellular localization of hnRNP A2/B1 in the development of hepatocellular carcinoma. BMC Cancer 10:356. 10.1186/1471-2407-10-356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dauki AM, Blachly JS, Kautto EA, Ezzat S, Abdel-Rahman MH, Coss CC (2020) Transcriptionally active androgen receptor splice variants promote hepatocellular carcinoma progression. Cancer Res 80:561–575. 10.1158/0008-5472.Can-19-1117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Oliveira Freitas machado C, Schafranek M, Brüggemann M, Hernández Cañás MC, Keller M, Di Liddo A, Brezski A, Blümel N, Arnold B, Bremm A et al (2023) Poison cassette exon splicing of SRSF6 regulates nuclear speckle dispersal and the response to hypoxia. Nucleic Acids Res 51:870–890. 10.1093/nar/gkac1225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Souza S, Lau KC, Coffin CS, Patel TR (2020) Molecular mechanisms of viral hepatitis induced hepatocellular carcinoma. World J Gastroenterol 26:5759–5783. 10.3748/wjg.v26.i38.5759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Awady MK, Anany MA, Esmat G, Zayed N, Tabll AA, Helmy A, El Zayady AR, Abdalla MS, Sharada HM, El Raziky M et al (2011) Single nucleotide polymorphism at exon 7 splice acceptor site of OAS1 gene determines response of hepatitis C virus patients to interferon therapy. J Gastroenterol Hepatol 26:843–850. 10.1111/j.1440-1746.2010.06605.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elizalde M, Urtasun R, Azkona M, Latasa MU, Goñi S, García-Irigoyen O, Uriarte I, Segura V, Collantes M, Di Scala M et al (2014) Splicing regulator SLU7 is essential for maintaining liver homeostasis. J Clin Invest 124:2909–2920. 10.1172/jci74382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo K, Terada T (2000) Protein expression of CD44 (standard and variant isoforms) in hepatocellular carcinoma: relationships with tumor grade, clinicopathologic parameters, p53 expression, and patient survival. J Hepatol 32:78–84 [DOI] [PubMed] [Google Scholar]
- Fujimoto A, Totoki Y, Abe T, Boroevich KA, Hosoda F, Nguyen HH, Aoki M, Hosono N, Kubo M, Miya F et al (2012) Whole-genome sequencing of liver cancers identifies etiological influences on mutation patterns and recurrent mutations in chromatin regulators. Nat Genet 44:760–764. 10.1038/ng.2291 [DOI] [PubMed] [Google Scholar]
- Geuens T, Bouhy D, Timmerman V (2016) The hnRNP family: insights into their role in health and disease. Hum Genet 135:851–867. 10.1007/s00439-016-1683-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh A, Stewart D, Matlashewski G (2004) Regulation of human p53 activity and cell localization by alternative splicing. Mol Cell Biol 24:7987–7997. 10.1128/MCB.24.18.7987-7997.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He R, Peng J, Yuan P, Xu F, Wei W (2015) Divergent roles of BECN1 in LC3 lipidation and autophagosomal function. Autophagy 11:740–747. 10.1080/15548627.2015.1034404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He A-D, Xie W, Song W, Ma Y-Y, Liu G, Liang M-L, Da X-W, Yao G-Q, Zhang B-X, Gao C-J et al (2017) Platelet releasates promote the proliferation of hepatocellular carcinoma cells by suppressing the expression of KLF6. Sci Rep. 10.1038/s41598-017-02801-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández Borrero LJ, El-Deiry WS (2021) Tumor suppressor p53: Biology, signaling pathways, and therapeutic targeting. Biochim Biophys Acta Rev Cancer 1876:188556. 10.1016/j.bbcan.2021.188556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S, Luo K, Jiang L, Zhang XD, Lv YH, Li RF (2021) PCBP1 regulates the transcription and alternative splicing of metastasis-related genes and pathways in hepatocellular carcinoma. Sci Rep 11:23356. 10.1038/s41598-021-02642-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang DQ, Terrault NA, Tacke F, Gluud LL, Arrese M, Bugianesi E, Loomba R (2023) Global epidemiology of cirrhosis—aetiology, trends and predictions. Nat Rev Gastroenterol Hepatol 20:388–398. 10.1038/s41575-023-00759-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang HM, Heo CK, Lee HJ, Kwak SS, Lim WH, Yoo JS, Yu DY, Lim KJ, Kim JY, Cho EW (2018a) Identification of anti-SF3B1 autoantibody as a diagnostic marker in patients with hepatocellular carcinoma. J Transl Med 16:177. 10.1186/s12967-018-1546-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang B, Lee JH, Bang D (2018b) Single-cell RNA sequencing technologies and bioinformatics pipelines. Exp Mol Med 50:96. 10.1038/s12276-018-0071-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyun J, Al Abo M, Dutta RK, Oh SH, Xiang K, Zhou X, Maeso-Díaz R, Caffrey R, Sanyal AJ, Freedman JA et al (2021) Dysregulation of the ESRP2-NF2-YAP/TAZ axis promotes hepatobiliary carcinogenesis in non-alcoholic fatty liver disease. J Hepatol 75:623–633. 10.1016/j.jhep.2021.04.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iguchi T, Komatsu H, Masuda T, Nambara S, Kidogami S, Ogawa Y, Hu Q, Saito T, Hirata H, Sakimura S et al (2016) Increased copy number of the gene encoding SF3B4 indicates poor prognosis in hepatocellular carcinoma. Anticancer Res 36:2139–2144 [PubMed] [Google Scholar]
- Jeong S (2017) SR proteins: binders, regulators, and connectors of RNA. Mol Cells 40:1–9. 10.14348/molcells.2017.2319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang W, Chen L (2021) Alternative splicing: Human disease and quantitative analysis from high-throughput sequencing. Comput Struct Biotechnol J 19:183–195. 10.1016/j.csbj.2020.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H, Lin Q, Ma L, Luo S, Jiang X, Fang J, Lu Z (2021) Fructose and fructose kinase in cancer and other pathologies. J Genet Genom 48:531–539. 10.1016/j.jgg.2021.06.006 [DOI] [PubMed] [Google Scholar]
- Jimenez M, Arechederra M, Avila MA, Berasain C (2018) Splicing alterations contributing to cancer hallmarks in the liver: central role of dedifferentiation and genome instability. Transl Gastroenterol Hepatol 3:84. 10.21037/tgh.2018.10.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez M, Urtasun R, Elizalde M, Azkona M, Latasa MU, Uriarte I, Arechederra M, Alignani D, Barcena-Varela M, Alvarez-Sola G et al (2019) Splicing events in the control of genome integrity: role of SLU7 and truncated SRSF3 proteins. Nucleic Acids Res 47:3450–3466. 10.1093/nar/gkz014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke RS, Zhang K, Lv LZ, Dong YP, Pan F, Yang F, Cai QC, Jiang Y (2019) Prognostic value and oncogene function of heterogeneous nuclear ribonucleoprotein A1 overexpression in HBV-related hepatocellular carcinoma. Int J Biol Macromol 129:140–151. 10.1016/j.ijbiomac.2019.02.012 [DOI] [PubMed] [Google Scholar]
- Keren H, Lev-Maor G, Ast G (2010) Alternative splicing and evolution: diversification, exon definition and function. Nat Rev Genet 11:345–355. 10.1038/nrg2776 [DOI] [PubMed] [Google Scholar]
- Kim E, Viatour P (2020) Hepatocellular carcinoma: old friends and new tricks. Exp Mol Med 52:1898–1907. 10.1038/s12276-020-00527-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MS, Pinto SM, Getnet D, Nirujogi RS, Manda SS, Chaerkady R, Madugundu AK, Kelkar DS, Isserlin R, Jain S et al (2014) A draft map of the human proteome. Nature 509:575–581. 10.1038/nature13302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudo M (2020) Scientific rationale for combined immunotherapy with PD-1/PD-L1 antibodies and VEGF inhibitors in advanced hepatocellular carcinoma. Cancers (basel). 10.3390/cancers12051089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar D, Das M, Sauceda C, Ellies LG, Kuo K, Parwal P, Kaur M, Jih L, Bandyopadhyay GK, Burton D et al (2019) Degradation of splicing factor SRSF3 contributes to progressive liver disease. J Clin Invest 129:4477–4491. 10.1172/jci127374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar D, Das M, Oberg A, Sahoo D, Wu P, Sauceda C, Jih L, Ellies LG, Langiewicz MT, Sen S et al (2022) Hepatocyte Deletion of IGF2 prevents DNA damage and tumor formation in hepatocellular carcinoma. Adv Sci (weinh) 9:e2105120. 10.1002/advs.202105120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurahashi H, Takami K, Oue T, Kusafuka T, Okada A, Tawa A, Okada S, Nishisho I (1995) Biallelic inactivation of the APC gene in hepatoblastoma. Can Res 55:5007–5011 [PubMed] [Google Scholar]
- Lai MY, Chang HC, Li HP, Ku CK, Chen PJ, Sheu JC, Huang GT, Lee PH, Chen DS (1993) Splicing mutations of the p53 gene in human hepatocellular carcinoma. Can Res 53:1653–1656 [PubMed] [Google Scholar]
- Lee SC, Abdel-Wahab O (2016) Therapeutic targeting of splicing in cancer. Nat Med 22:976–986. 10.1038/nm.4165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei S, Zhang B, Huang L, Zheng Z, Xie S, Shen L, Breitzig M, Czachor A, Liu H, Luo H et al (2021) SRSF1 promotes the inclusion of exon 3 of SRA1 and the invasion of hepatocellular carcinoma cells by interacting with exon 3 of SRA1pre-mRNA. Cell Death Discov 7:117. 10.1038/s41420-021-00498-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li K, Wang Z (2021) Splicing factor SRSF2-centric gene regulation. Int J Biol Sci 17:1708–1715. 10.7150/ijbs.58888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Qian X, Peng LX, Jiang Y, Hawke DH, Zheng Y, Xia Y, Lee JH, Cote G, Wang H et al (2016) A splicing switch from ketohexokinase-C to ketohexokinase-A drives hepatocellular carcinoma formation. Nat Cell Biol 18:561–571. 10.1038/ncb3338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Sahni N, Pancsa R, McGrail DJ, Xu J, Hua X, Coulombe-Huntington J, Ryan M, Tychhon B, Sudhakar D et al (2017) Revealing the determinants of widespread alternative splicing perturbation in cancer. Cell Rep 21:798–812. 10.1016/j.celrep.2017.09.071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Hu Z, Zhao Y, Huang S, He X (2019) Transcriptome-wide analysis reveals the landscape of aberrant alternative splicing events in liver cancer. Hepatology 69:359–375. 10.1002/hep.30158 [DOI] [PubMed] [Google Scholar]
- Li G, Chen H, Shen F, Smithson SB, Shealy GL, Ping Q, Liang Z, Han J, Adams AC, Li Y et al (2023) Targeting hepatic serine-arginine protein kinase 2 ameliorates alcohol-associated liver disease by alternative splicing control of lipogenesis. Hepatology. 10.1097/hep.0000000000000433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin KT, Ma WK, Scharner J, Liu YR, Krainer AR (2018) A human-specific switch of alternatively spliced AFMID isoforms contributes to TP53 mutations and tumor recurrence in hepatocellular carcinoma. Genome Res. 10.1101/gr.227181.117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin YH, Wu MH, Liu YC, Lyu PC, Yeh CT, Lin KH (2021) LINC01348 suppresses hepatocellular carcinoma metastasis through inhibition of SF3B3-mediated EZH2 pre-mRNA splicing. Oncogene 40:4675–4685. 10.1038/s41388-021-01905-3 [DOI] [PubMed] [Google Scholar]
- Liu L, Xie S, Zhang C, Zhu F (2014) Aberrant regulation of alternative pre-mRNA splicing in hepatocellular carcinoma. Crit Rev Eukaryot Gene Expr 24:133–149 [DOI] [PubMed] [Google Scholar]
- Liu ZY, Li W, Pang YN, Zhou ZX, Liu SP, Cheng K, Qin Q, Jia Y, Liu SR (2018) SF3B4 is regulated by microRNA-133b and promotes cell proliferation and metastasis in hepatocellular carcinoma. EBioMedicine 38:57–68. 10.1016/j.ebiom.2018.10.067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Zheng Y, Xiao M, Chen X, Zhu Y, Xu C, Wang F, Liu Z, Cao K (2022) SRSF10 stabilizes CDC25A by triggering exon 6 skipping to promote hepatocarcinogenesis. J Exp Clin Cancer Res 41:353. 10.1186/s13046-022-02558-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llères D, Denegri M, Biggiogera M, Ajuh P, Lamond AI (2010) Direct interaction between hnRNP-M and CDC5L/PLRG1 proteins affects alternative splice site choice. EMBO Rep 11:445–451. 10.1038/embor.2010.64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llovet JM, Kelley RK, Villanueva A, Singal AG, Pikarsky E, Roayaie S, Lencioni R, Koike K, Zucman-Rossi J, Finn RS (2021) Hepatocell carcinoma. Nat Rev Dis Primers 7:6. 10.1038/s41572-020-00240-3 [DOI] [PubMed] [Google Scholar]
- Long JC, Caceres JF (2009) The SR protein family of splicing factors: master regulators of gene expression. Biochem J 417:15–27. 10.1042/BJ20081501 [DOI] [PubMed] [Google Scholar]
- Long Y, Sou WH, Yung KWY, Liu H, Wan SWC, Li Q, Zeng C, Law COK, Chan GHC, Lau TCK et al (2019) Distinct mechanisms govern the phosphorylation of different SR protein splicing factors. J Biol Chem 294:1312–1327. 10.1074/jbc.RA118.003392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Cánovas JL, Del Rio-Moreno M, García-Fernandez H, Jiménez-Vacas JM, Moreno-Montilla MT, Sánchez-Frias ME, Amado V, Fernando LL, Fondevila MF, Ciria R et al (2021) Splicing factor SF3B1 is overexpressed and implicated in the aggressiveness and survival of hepatocellular carcinoma. Cancer Lett 496:72–83. 10.1016/j.canlet.2020.10.010 [DOI] [PubMed] [Google Scholar]
- López-Cánovas JL, Hermán-Sánchez N, Del Rio-Moreno M, Fuentes-Fayos AC, Lara-López A, Sánchez-Frias ME, Amado V, Ciria R, Briceño J, de la Mata M et al (2023) PRPF8 increases the aggressiveness of hepatocellular carcinoma by regulating FAK/AKT pathway via fibronectin 1 splicing. Exp Mol Med 55:132–142. 10.1038/s12276-022-00917-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu ZX, Jiang P, Xing Y (2012) Genetic variation of pre-mRNA alternative splicing in human populations. Wiley Interdiscip Rev RNA 3:581–592. 10.1002/wrna.120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Xu W, Ji J, Feng D, Sourbier C, Yang Y, Qu J, Zeng Z, Wang C, Chang X et al (2015) Alternative splicing of the cell fate determinant Numb in hepatocellular carcinoma. Hepatology 62:1122–1131. 10.1002/hep.27923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luk K-C, Gersch J, Harris BJ, Holzmayer V, Mbanya D, Sauleda S, Rodgers MA, Cloherty G (2021) More DNA and RNA of HBV SP1 splice variants are detected in genotypes B and C at low viral replication. Sci Rep. 10.1038/s41598-021-03304-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo C, Cheng Y, Liu Y, Chen L, Liu L, Wei N, Xie Z, Wu W, Feng Y (2017) SRSF2 regulates alternative splicing to drive hepatocellular carcinoma development. Cancer Res 77:1168–1178. 10.1158/0008-5472.Can-16-1919 [DOI] [PubMed] [Google Scholar]
- Ma WK, Voss DM, Scharner J, Costa ASH, Lin KT, Jeon HY, Wilkinson JE, Jackson M, Rigo F, Bennett CF et al (2022) ASO-Based PKM splice-switching therapy inhibits hepatocellular carcinoma growth. Cancer Res 82:900–915. 10.1158/0008-5472.Can-20-0948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacParland SA, Liu JC, Ma XZ, Innes BT, Bartczak AM, Gage BK, Manuel J, Khuu N, Echeverri J, Linares I et al (2018) Single cell RNA sequencing of human liver reveals distinct intrahepatic macrophage populations. Nat Commun 9:4383. 10.1038/s41467-018-06318-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makokha GN, Abe-Chayama H, Chowdhury S, Hayes CN, Tsuge M, Yoshima T, Ishida Y, Zhang Y, Uchida T, Tateno C et al (2019) Regulation of the hepatitis B virus replication and gene expression by the multi-functional protein TARDBP. Sci Rep 9:8462. 10.1038/s41598-019-44934-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marasco LE, Kornblihtt AR (2022) The physiology of alternative splicing. Nat Rev Mol Cell Biol 24:242–254. 10.1038/s41580-022-00545-z [DOI] [PubMed] [Google Scholar]
- Marengo A, Rosso C, Bugianesi E (2016) Liver cancer: connections with obesity, fatty liver, and cirrhosis. Annu Rev Med 67:103–117. 10.1146/annurev-med-090514-013832 [DOI] [PubMed] [Google Scholar]
- Martinez-Contreras R, Cloutier P, Shkreta L, Fisette JF, Revil T, Chabot B (2007) hnRNP proteins and splicing control. Adv Exp Med Biol 623:123–147. 10.1007/978-0-387-77374-2_8 [DOI] [PubMed] [Google Scholar]
- Méndez-Blanco C, Fondevila F, García-Palomo A, González-Gallego J, Mauriz JL (2018) Sorafenib resistance in hepatocarcinoma: role of hypoxia-inducible factors. Exp Mol Med 50:1–9. 10.1038/s12276-018-0159-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mo M, Ma X, Luo Y, Tan C, Liu B, Tang P, Liao Q, Liu S, Yu H, Huang D et al (2022) Liver-specific lncRNA FAM99A may be a tumor suppressor and promising prognostic biomarker in hepatocellular carcinoma. BMC Cancer 22:1098. 10.1186/s12885-022-10186-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narla G, Difeo A, Reeves HL, Schaid DJ, Hirshfeld J, Hod E, Katz A, Isaacs WB, Hebbring S, Komiya A et al (2005) A germline DNA polymorphism enhances alternative splicing of the KLF6 tumor suppressor gene and is associated with increased prostate cancer risk. Can Res 65:1213–1222. 10.1158/0008-5472.CAN-04-4249 [DOI] [PubMed] [Google Scholar]
- Nault JC, Martin Y, Caruso S, Hirsch TZ, Bayard Q, Calderaro J, Charpy C, Copie-Bergman C, Ziol M, Bioulac-Sage P et al (2019) Clinical impact of genomic diversity from early to advanced hepatocellular carcinoma. Hepatology. 10.1002/hep.30811 [DOI] [PubMed] [Google Scholar]
- Ng CKY, Dazert E, Boldanova T, Coto-Llerena M, Nuciforo S, Ercan C, Suslov A, Meier MA, Bock T, Schmidt A et al (2022) Integrative proteogenomic characterization of hepatocellular carcinoma across etiologies and stages. Nat Commun 13:2436. 10.1038/s41467-022-29960-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurk S, Koren S, Rhie A, Rautiainen M, Bzikadze AV, Mikheenko A, Vollger MR, Altemose N, Uralsky L, Gershman A et al (2022) The complete sequence of a human genome. Science 376:44–53. 10.1126/science.abj6987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omenn GS, Yocum AK, Menon R (2010) Alternative splice variants, a new class of protein cancer biomarker candidates: findings in pancreatic cancer and breast cancer with systems biology implications. Dis Markers 28:241–251. 10.3233/DMA-2010-0702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paz S, Ritchie A, Mauer C, Caputi M (2021) The RNA binding protein SRSF1 is a master switch of gene expression and regulation in the immune system. Cytokine Growth Factor Rev 57:19–26. 10.1016/j.cytogfr.2020.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pihlajamaki J, Lerin C, Itkonen P, Boes T, Floss T, Schroeder J, Dearie F, Crunkhorn S, Burak F, Jimenez-Chillaron JC et al (2011) Expression of the splicing factor gene SFRS10 is reduced in human obesity and contributes to enhanced lipogenesis. Cell Metab 14:208–218. 10.1016/j.cmet.2011.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao Y, Shi Q, Yuan X, Ding J, Li X, Shen M, Huang S, Chen Z, Wang L, Zhao Y et al (2022) RNA binding protein RALY activates the cholesterol synthesis pathway through an MTA1 splicing switch in hepatocellular carcinoma. Cancer Lett 538:215711. 10.1016/j.canlet.2022.215711 [DOI] [PubMed] [Google Scholar]
- Sas Z, Cendrowicz E, Weinhäuser I, Rygiel TP (2022) Tumor microenvironment of hepatocellular carcinoma: challenges and opportunities for new treatment options. Int J Mol Sci. 10.3390/ijms23073778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savarese M, Jonson PH, Huovinen S, Paulin L, Auvinen P, Udd B, Hackman P (2018) The complexity of titin splicing pattern in human adult skeletal muscles. Skeletal Muscle 8:11. 10.1186/s13395-018-0156-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen S, Jumaa H, Webster NJ (2013) Splicing factor SRSF3 is crucial for hepatocyte differentiation and metabolic function. Nat Commun 4:1336. 10.1038/ncomms2342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen S, Langiewicz M, Jumaa H, Webster NJ (2015) Deletion of serine/arginine-rich splicing factor 3 in hepatocytes predisposes to hepatocellular carcinoma in mice. Hepatology 61:171–183. 10.1002/hep.27380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalek AK, Satija R, Adiconis X, Gertner RS, Gaublomme JT, Raychowdhury R, Schwartz S, Yosef N, Malboeuf C, Lu D et al (2013) Single-cell transcriptomics reveals bimodality in expression and splicing in immune cells. Nature 498:236–240. 10.1038/nature12172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Q, Nam SW (2018) SF3B4 as an early-stage diagnostic marker and driver of hepatocellular carcinoma. BMB Rep 51:57–58. 10.5483/bmbrep.2018.51.2.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen M, Zhang R, Jia W, Zhu Z, Zhao X, Zhao L, Huang G, Liu J (2021) Nuclear scaffold protein p54(nrb)/NONO facilitates the hypoxia-enhanced progression of hepatocellular carcinoma. Oncogene 40:4167–4183. 10.1038/s41388-021-01848-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen C, Jiang X, Li M, Luo Y (2023) Hepatitis virus and hepatocellular carcinoma: recent advances. Cancers (basel). 10.3390/cancers15020533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shilo A, Ben Hur V, Denichenko P, Stein I, Pikarsky E, Rauch J, Kolch W, Zender L, Karni R (2014) Splicing factor hnRNP A2 activates the Ras-MAPK-ERK pathway by controlling A-Raf splicing in hepatocellular carcinoma development. RNA 20:505–515. 10.1261/rna.042259.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin C, Manley JL (2002) The SR protein SRp38 represses splicing in M phase cells. Cell 111:407–417. 10.1016/s0092-8674(02)01038-3 [DOI] [PubMed] [Google Scholar]
- Shin C, Feng Y, Manley JL (2004) Dephosphorylated SRp38 acts as a splicing repressor in response to heat shock. Nature 427:553–558. 10.1038/nature02288 [DOI] [PubMed] [Google Scholar]
- Shuai SM, Suzuki H, Diaz-Navarro A, Nadeu F, Kumar SA, Gutierrez-Fernandez A, Delgado J, Pinyol M, Lopez-Otin C, Puente XS et al (2019) The U1 spliceosomal RNA is recurrently mutated in multiple cancers. Nature 574:712–716. 10.1038/s41586-019-1651-z [DOI] [PubMed] [Google Scholar]
- Singh B, Eyras E (2017) The role of alternative splicing in cancer. Transcription 8:91–98. 10.1080/21541264.2016.1268245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snider NT, Altshuler PJ, Wan S, Welling TH, Cavalcoli J, Omary MB (2014) Alternative splicing of human NT5E in cirrhosis and hepatocellular carcinoma produces a negative regulator of ecto-5’-nucleotidase (CD73). Mol Biol Cell 25:4024–4033. 10.1091/mbc.E14-06-1167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitali P, Aartsma-Rus A (2012) Splice modulating therapies for human disease. Cell 148:1085–1088. 10.1016/j.cell.2012.02.014 [DOI] [PubMed] [Google Scholar]
- Sun C (2020) The SF3b complex: splicing and beyond. Cell Mol Life Sci 77:3583–3595. 10.1007/s00018-020-03493-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sveen A, Kilpinen S, Ruusulehto A, Lothe RA, Skotheim RI (2016) Aberrant RNA splicing in cancer; expression changes and driver mutations of splicing factor genes. Oncogene 35:2413–2427. 10.1038/onc.2015.318 [DOI] [PubMed] [Google Scholar]
- Tang Q, Rodriguez-Santiago S, Wang J, Pu J, Yuste A, Gupta V, Moldon A, Xu YZ, Query CC (2016) SF3B1/Hsh155 HEAT motif mutations affect interaction with the spliceosomal ATPase Prp5, resulting in altered branch site selectivity in pre-mRNA splicing. Genes Dev 30:2710–2723. 10.1101/gad.291872.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thibault PA, Ganesan A, Kalyaanamoorthy S, Clarke JWE, Salapa HE, Levin MC (2021) hnRNP A/B proteins: an encyclopedic assessment of their roles in homeostasis and disease. Biology (Basel). 10.3390/biology10080712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian J, Wang Z, Mei S, Yang N, Yang Y, Ke J, Zhu Y, Gong Y, Zou D, Peng X et al (2019) CancerSplicingQTL: a database for genome-wide identification of splicing QTLs in human cancer. Nucleic Acids Res 47:D909–D916. 10.1093/nar/gky954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay MP, Armero VES, Allaire A, Boudreault S, Martenon-Brodeur C, Durand M, Lapointe E, Thibault P, Tremblay-Letourneau M, Perreault JP et al (2016) Global profiling of alternative RNA splicing events provides insights into molecular differences between various types of hepatocellular carcinoma. BMC Genom 17:683. 10.1186/S12864-016-3029-Z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urtasun R, Elizalde M, Azkona M, Latasa MU, García-Irigoyen O, Uriarte I, Fernández-Barrena MG, Vicent S, Alonso MM, Muntané J et al (2016) Splicing regulator SLU7 preserves survival of hepatocellular carcinoma cells and other solid tumors via oncogenic miR-17-92 cluster expression. Oncogene 35:4719–4729. 10.1038/onc.2015.517 [DOI] [PubMed] [Google Scholar]
- Wang Z, Burge CB (2008) Splicing regulation: from a parts list of regulatory elements to an integrated splicing code. RNA 14:802–813. 10.1261/rna.876308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang BD, Lee NH (2018) Aberrant RNA splicing in cancer and drug resistance. Cancers. 10.3390/cancers10110458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ET, Sandberg R, Luo S, Khrebtukova I, Zhang L, Mayr C, Kingsmore SF, Schroth GP, Burge CB (2008) Alternative isoform regulation in human tissue transcriptomes. Nature 456:470–476. 10.1038/nature07509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Zhang P, Shu Y, Yuan F, Zhang Y, Zhou Y, Jiang M, Zhu Y, Hu L, Kong X et al (2014) Alternative splicing at GYNNGY 5’ splice sites: more noise, less regulation. Nucleic Acids Res 42:13969–13980. 10.1093/nar/gku1253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang TH, Hsueh C, Chen CC, Li WS, Yeh CT, Lian JH, Chang JL, Chen CY (2018) Melatonin inhibits the progression of hepatocellular carcinoma through MicroRNA Let7i-3p mediated RAF1 reduction. Int J Mol Sci. 10.3390/ijms19092687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Lekbaby B, Fares N, Augustin J, Attout T, Schnuriger A, Cassard AM, Panasyuk G, Perlemuter G, Bieche I et al (2019a) Alteration of splicing factors’ expression during liver disease progression: impact on hepatocellular carcinoma outcome. Hep Intl 13:454–467. 10.1007/s12072-019-09950-7 [DOI] [PubMed] [Google Scholar]
- Wang H, Zhang CZ, Lu SX, Zhang MF, Liu LL, Luo RZ, Yang X, Wang CH, Chen SL, He YF et al (2019b) A coiled-coil domain containing 50 splice variant is modulated by serine/arginine-rich splicing factor 3 and promotes hepatocellular carcinoma in mice by the ras signaling pathway. Hepatology 69:179–195. 10.1002/hep.30147 [DOI] [PubMed] [Google Scholar]
- Wang X, Guo J, Che X, Jia R (2019c) PCBP1 inhibits the expression of oncogenic STAT3 isoform by targeting alternative splicing of STAT3 exon 23. Int J Biol Sci 15:1177–1186. 10.7150/ijbs.33103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waxman S, Wurmbach E (2007) De-regulation of common housekeeping genes in hepatocellular carcinoma. BMC Genom 8:243. 10.1186/1471-2164-8-243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster NJG (2017) Alternative RNA splicing in the pathogenesis of liver disease. Front Endocrinol 8:133. 10.3389/fendo.2017.00133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witten JT, Ule J (2011) Understanding splicing regulation through RNA splicing maps. Trends Genet TIG 27:89–97. 10.1016/j.tig.2010.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu P, Zhou D, Wang Y, Lin W, Sun A, Wei H, Fang Y, Cong X, Jiang Y (2019a) Identification and validation of alternative splicing isoforms as novel biomarker candidates in hepatocellular carcinoma. Oncol Rep 41:1929–1937. 10.3892/or.2018.6947 [DOI] [PubMed] [Google Scholar]
- Wu HY, Peng ZG, He RQ, Luo B, Ma J, Hu XH, Dang YW, Chen G, Pan SL (2019b) Prognostic index of aberrant mRNA splicing profiling acts as a predictive indicator for hepatocellular carcinoma based on TCGA SpliceSeq data. Int J Oncol 55:425–438. 10.3892/ijo.2019.4834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu P, Zhang M, Webster NJG (2021) Alternative RNA splicing in fatty liver disease. Front Endocrinol (lausanne) 12:613213. 10.3389/fendo.2021.613213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing S, Li Z, Ma W, He X, Shen S, Wei H, Li ST, Shu Y, Sun L, Zhong X et al (2019) DIS3L2 promotes progression of hepatocellular carcinoma via hnRNP U-mediated alternative splicing. Can Res 79:4923–4936. 10.1158/0008-5472.CAN-19-0376 [DOI] [PubMed] [Google Scholar]
- Yamazaki D, Hashizume O, Taniguchi S, Funato Y, Miki H (2021) Role of adenomatous polyposis coli in proliferation and differentiation of colon epithelial cells in organoid culture. Sci Rep 11:3980. 10.1038/s41598-021-83590-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang F, Wang XY, Zhang ZM, Pu J, Fan YJ, Zhou JH, Query CC, Xu YZ (2013) Splicing proofreading at 5’ splice sites by ATPase Prp28p. Nucleic Acids Res 41:4660–4670. 10.1093/nar/gkt149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Zhu R, Zhao X, Liu L, Zhou Z, Zhao L, Liang B, Ma W, Zhao J, Liu J et al (2019) Sirtuin-mediated deacetylation of hnRNP A1 suppresses glycolysis and growth in hepatocellular carcinoma. Oncogene 38:4915–4931. 10.1038/s41388-019-0764-z [DOI] [PubMed] [Google Scholar]
- Yea S, Narla G, Zhao X, Garg R, Tal-Kremer S, Hod E, Villanueva A, Loke J, Tarocchi M, Akita K et al (2008) Ras promotes growth by alternative splicing-mediated inactivation of the KLF6 tumor suppressor in hepatocellular carcinoma. Gastroenterology 134:1521–1531. 10.1053/j.gastro.2008.02.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo G, Holste D, Kreiman G, Burge CB (2004) Variation in alternative splicing across human tissues. Genome Biol 5:R74. 10.1186/gb-2004-5-10-r74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu L, Kim J, Jiang L, Feng B, Ying Y, Ji KY, Tang Q, Chen W, Mai T, Dou W et al (2020) MTR4 drives liver tumorigenesis by promoting cancer metabolic switch through alternative splicing. Nat Commun 11:708. 10.1038/s41467-020-14437-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan P, Li J, Zhou F, Huang Q, Zhang J, Guo X, Lyu Z, Zhang H, Xing J (2017) NPAS2 promotes cell survival of hepatocellular carcinoma by transactivating CDC25A. Cell Death Dis 8:e2704. 10.1038/cddis.2017.131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan YT, Li L, Zeng TT, Zhou NN, Guan XY, Li Y (2020) SNRPB-mediated RNA splicing drives tumor cell proliferation and stemness in hepatocellular carcinoma. Aging (Albany NY) 13:537–554. 10.18632/aging.202164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Liu X, Zhang X, Chen R (2016) Identification of important long non-coding RNAs and highly recurrent aberrant alternative splicing events in hepatocellular carcinoma through integrative analysis of multiple RNA-Seq datasets. Mol Genet Genom : MGG 291:1035–1051. 10.1007/s00438-015-1163-y [DOI] [PubMed] [Google Scholar]
- Zhang J, Zhang Q, Lou Y, Fu Q, Chen Q, Wei T, Yang J, Tang J, Wang J, Chen Y et al (2018) Hypoxia-inducible factor-1α/interleukin-1β signaling enhances hepatoma epithelial-mesenchymal transition through macrophages in a hypoxic-inflammatory microenvironment. Hepatology 67:1872–1889. 10.1002/hep.29681 [DOI] [PubMed] [Google Scholar]
- Zhang D, Duan Y, Wang Z, Lin J (2019) Systematic profiling of a novel prognostic alternative splicing signature in hepatocellular carcinoma. Oncol Rep 42:2450–2472. 10.3892/or.2019.7342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Shen L, Yuan W, Liu Y, Guo R, Luo Y, Zhan Z, Xie Z, Wu G, Wu W et al (2020) Loss of SRSF2 triggers hepatic progenitor cell activation and tumor development in mice. Commun Biol 3:210. 10.1038/s42003-020-0893-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Wang HY, Zhao DX, Wang DX, Zeng Q, Xi JF, Nan X, He LJ, Zhou JN, Pei XT et al (2021) The splicing regulatory factor hnRNPU is a novel transcriptional target of c-Myc in hepatocellular carcinoma. FEBS Lett 595:68–84. 10.1002/1873-3468.13943 [DOI] [PubMed] [Google Scholar]
- Zhang YZ, Zeb A, Cheng LF (2022) Exploring the molecular mechanism of hepatitis virus inducing hepatocellular carcinoma by microarray data and immune infiltrates analysis. Front Immunol 13:1032819. 10.3389/fimmu.2022.1032819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao YJ, Wu LY, Pang JS, Liao W, Chen YJ, He Y, Yang H (2021) Integrated multi-omics analysis of the clinical relevance and potential regulatory mechanisms of splicing factors in hepatocellular carcinoma. Bioengineered 12:3978–3992. 10.1080/21655979.2021.1948949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou G, Wang J, Zhao M, Xie TX, Tanaka N, Sano D, Patel AA, Ward AM, Sandulache VC, Jasser SA et al (2014) Gain-of-function mutant p53 promotes cell growth and cancer cell metabolism via inhibition of AMPK activation. Mol Cell 54:960–974. 10.1016/j.molcel.2014.04.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu GQ, Zhou YJ, Qiu LX, Wang B, Yang Y, Liao WT, Luo YH, Shi YH, Zhou J, Fan J et al (2019) Prognostic alternative mRNA splicing signature in hepatocellular carcinoma: a study based on large-scale sequencing data. Carcinogenesis. 10.1093/carcin/bgz073 [DOI] [PubMed] [Google Scholar]
- Zhu GQ, Wang Y, Wang B, Liu WR, Dong SS, Chen EB, Cai JL, Wan JL, Du JX, Song LN et al (2022) Targeting HNRNPM inhibits cancer stemness and enhances antitumor immunity in wnt-activated hepatocellular carcinoma. Cell Mol Gastroenterol Hepatol 13:1413–1447. 10.1016/j.jcmgh.2022.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuo H, Miao S, Jin Z, Zhu D, Xu Z, Sun D, Ji J, Tan Z (2022) Metformin suppresses hepatocellular carcinoma through regulating alternative splicing of LGR4. J Oncol 2022:1774095. 10.1155/2022/1774095 [DOI] [PMC free article] [PubMed] [Google Scholar]