Abstract
Glucosinolates are a large group of plant secondary metabolites found mainly in the order Capparales, which includes a large number of economically important Brassica crops and the model plant Arabidopsis. In the present study, several lines of evidence are provided for phloem transport of glucosinolates in Arabidopsis. When radiolabeled p-hydroxybenzylglucosinolate (p-OHBG) and sucrose were co-applied to the tip of detached leaves, both tracers were collected in the phloem exudates at the petioles. Long-distance transport of [14C]p-OHBG was investigated in wild-type and transgenic 35S::CYP79A1 plants, synthesizing high amounts of p-OHBG, which is not a natural constituent of wild-type Arabidopsis. In both wild-type and 35S::CYP79A1 plants, radiolabeled p-OHBG was rapidly transported from the application site into the whole plant and intact p-OHBG was recovered from different tissues. The pattern of distribution of the radioactivity corresponded to that expected for transport of photoassimilates such as sucrose, and was consistent with translocation in phloem following the source-sink relationship. Radiolabeled p-OHBG was shown to accumulate in the seeds of wild-type and 35S::CYP79A1 plants, where p-OHBG had been either exogenously applied or endogenously synthesized from Tyr in the leaves. p-OHBG was found in phloem exudates collected from cut petioles of leaves from both wild-type and 35S::CYP79A1 plants. Phloem exudates were shown to contain intact glucosinolates, and not desulphoglucosinolates, as the transport form. It is concluded that intact glucosinolates are readily loaded into and transported by the phloem.
The phloem plays an essential role in the delivery of resources (photoassimilates, amino acids, and signaling molecules) to heterotrophic plant tissues. In angiosperms, the phloem is composed of sieve elements and their associated companion cells. Several cellular borders must be passed when photoassimilates and macromolecules are loaded into the phloem. These borders are the endodermis-vascular parenchyma interface, the phloem parenchyma-companion cell interface, and the companion cell-sieve element interface (Thompson and Schulz, 1999). Upon entering sieve elements, the molecules move in the osmotically driven translocation stream from source (sites of production and export) to sink (sites of import) tissues along the vascular pathway. The osmotic pressure gradient is created and maintained by loading and unloading of photoassimilates at the source and sink tissues, respectively.
Glucosinolates are naturally occurring organic anions characterized by having a thio-Glc and a sulfate moiety. Glucosinolates are found mainly in the order Capparales, where they coexist with thioglucosidases called myrosinases (EC 3.2.3.1). Tissue disruption brings glucosinolates into contact with myrosinases, resulting in the release of various compounds such as isothiocyanates, nitriles, and thiocyanates. These compounds have diverse biological activities, e.g. as deterrents and attractants in plant herbivore interactions (Halkier, 1999). Several observations suggest that glucosinolates are transported in planta. For example, high amounts of benzylglucosinolate, which were de novo synthesized in leaves of Tropaeolum majus, were found to accumulate in other tissues, such as developing seeds (Lykkesfeldt and Møller, 1993). Analysis of the glucosinolate profile in seeds and leaves of oilseed rape (Brassica napus) F1 hybrids from reciprocal crosses between oilseed rape cv Cobra and a synthetic line showed that the profile of the aliphatic glucosinolates in the seed was identical to the profile in the leaves of the maternal parent (Magrath and Mithen, 1993). This suggests that fully formed glucosinolates were transferred from maternal tissue into the developing seeds. In another study, in vivo feeding of radiolabeled Tyr to isolated seeds and intact siliques of Sinapis alba showed that although a low rate of de novo biosynthesis of p-hydroxybenzylglucosinolate (p-OHBG) took place in the seed, the majority of p-OHBG was de novo biosynthesized in the silique wall and subsequently transported to the seed (Du and Halkier, 1998). Brudenell et al. (1999) found that both glucosinolates and desulphoglucosinolates had physicochemical properties allowing phloem mobility. In support of this, aphid (Myzus persicae Sulz.) feeding experiments on black mustard (Brassica nigra) have shown that there was more than 10 mm sinigrin in phloem sap of young leaves, whereas there was only about 1 to 2 mm in mature, presenescent, and senescent leaves (Merritt, 1996). These data suggest that transport of glucosinolates follows the principle of assimilate transport and allocation, i.e. mass flow from source to sink.
In the present study, 35S::CYP79A1 plants (Bak et al., 1999) were used to study the transport of endogenously synthesized p-OHBG, which may mimic the transport of other glucosinolates. Cytochrome P450 CYP79A1 catalyzes the conversion of Tyr to p-hydroxyphenylacetaldoxime in the biosynthesis of cyanogenic glucoside dhurrin in Sorghum bicolor (Halkier, 1999). When CYP79A1 was overexpressed in Arabidopsis, p-hydroxyphenylacetaldoxime was channeled into the preexisting glucosinolate biosynthetic pathway, leading to production of high amounts of p-OHBG, not present in wild-type plants (Bak et al., 1999). Long-distance transport of glucosinolates was studied by feeding radiolabeled Tyr or p-OHBG to rosette leaves of 35S::CYP79A1 plants and wild-type Arabidopsis and by subsequently monitoring translocation and distribution of p-OHBG within the plants. Both exogenously applied and endogenously synthesized p-OHBG were shown to be readily loaded into and transported by the phloem, e.g. to the seeds. This suggests that the transport of p-OHBG mimics the events occurring in trafficking endogenous glucosinolates. Phloem exudates were shown to contain intact glucosinolates, and not desulphoglucosinolates, as the transport form.
RESULTS
Loading and Export of Radiolabeled p-OHBG
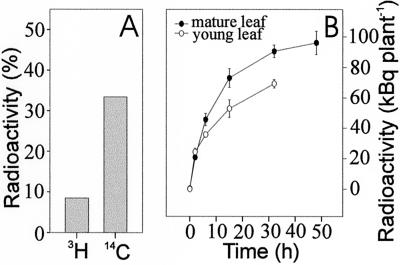
We used EDTA to enhance phloem exudation from cut petioles. EDTA chelates the Ca2+ required for callose formation and thereby prevents the sealing of sieve tubes in response to wounding (King and Zeevaart, 1974). When [3H]p-OHBG and [14C]Suc were co-applied on the adaxial surfaces of leaf tips, both radioactivities were recovered in the phloem exudates collected at the petioles (Fig. 1A). This indicates that p-OHBG has been taken up, loaded on to the phloem, and transported basipetally within the leaf, as is shown for [14C]Suc. The results from the single leaf experiments imply that glucosinolates move in the phloem.
Figure 1.
Loading and export of radiolabeled p-OHBG. A, [3H]p-OHBG and [14C]Suc were applied simultaneously to the tips of single detached leaves and the phloem exudates were subsequently collected by incubating the leaf petioles in 15 mm EDTA for 6 h. Values (±5%) represent the radioactivity of 3H or 14C in the phloem exudates as percentage of the total radioactivity applied. B, Time course of export of radioactivity after application of [3H]p-OHBG to a single either young or mature leaf of flowering plants. At given time points, the donor leaves were excised from four to six plants and the radioactivity in the remaining parts of the plant was measured.
[3H]p-OHBG was applied to the adaxial surface of a fully expanded rosette leaf (40–50 mm long) of plants at flowering stage and export of radioactivity from the leaf was analyzed. After 2 h, export could be detected (Fig. 1B). After 15 h, nearly 20% of the total [3H]p-OHBG taken up by the leaf had been exported to the rest of the plant, which indicates rapid mobilization of p-OHBG. After approximately 50 h incubation, approximately 80% of the radioactivity was retained in the leaf where the tracer was applied. When young rosette leaves (15–20 mm long) were analyzed in similar experiments, the total export of radioactivity after 15 h incubation accounted for only approximately 70% of that observed from fully expanded leaf (Fig. 1B). The difference in export between young and mature leaves indicates the source/sink status of the leaf.
Transport and Distribution of [14C]p-OHBG in Flowering Plants
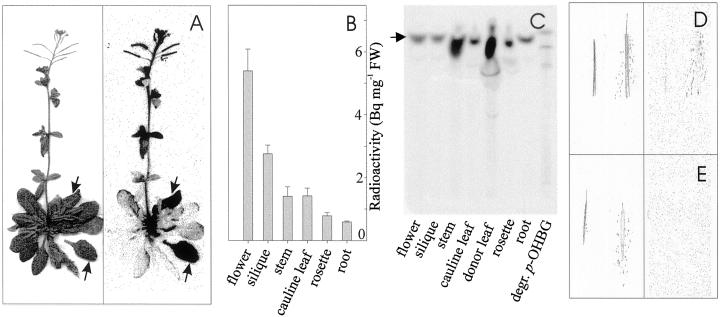
Glucosinolate transport and distribution patterns were studied by administration of [14C]p-OHBG to fully expanded rosette leaves of wild-type plants. After 24 h incubation, radioactivity was observed in roots, other rosette leaves, stem, cauline leaves, flower buds, and siliques (Fig. 2A). This shows that p-OHBG was taken up by the leaves and transported both basipetally and acropetally within the plant. Substantial amounts of radioactivity accumulated in young growing floral tissues (flowers and siliques) on per milligram fresh weight basis, indicating the sink strength of these tissues (Fig. 2B). The [14C]p-OHBG predominantly ended up in seeds (Fig. 2D). Young leaves, which are typical sink tissue, did not accumulate high amounts of radioactivity (Fig. 2A,B). A possible explanation is that floral tissues constitute the primary sinks at this stage of development, whereas at earlier developmental stages vegetative tissues may be equally important sinks (Oparka and Cruz, 2000). Relatively small amounts of radioactivity accumulated in the roots and mature leaves (Fig. 2, A and B). Thin-layer chromatography (TLC) analysis of an aliquot of methanol extracts of the different tissues showed that intact p-OHBG was recovered in all tissues analyzed (Fig. 2C). Except in the donor leaf, little degradation of p-OHBG was observed in other tissues, suggesting that no degradation occurred during and after transport.
Figure 2.
Long-distance transport and distribution of radioactivity in wild-type Arabidopsis 24 h after application of [14C]p-OHBG to mature rosette leaves of flowering plant. A, Photograph (left) and autoradiography (right) of Arabidopsis incubated for 24 h after application of [14C]p-OHBG to leaves (arrows). B, Measurement of radioactivity in methanol extracts of different tissues. Four plants were sampled and dissected. C, Qualitative TLC analysis of radiolabeled p-OHBG in methanol extracts of different tissues from a single plant. The position of intact p-OHBG is indicated (arrow). D, Photograph (left) and autoradiogram (right) of labeled Arabidopsis intact and crushed siliques. E, Non-labeled controls for D.
Long-Distance Transport of Exogenously Applied and Endogenously Synthesized Glucosinolates
Translocation and distribution of exogenously applied and endogenously synthesized p-OHBG were monitored by application of radiolabeled Tyr and p-OHBG to young rosette leaves of wild-type and 35S::CYP79A1 plants at vegetative stage. Three weeks after application, TLC analysis confirmed that all radiolabeled Tyr was metabolized (Fig. 3A, insert), all plants were transferred to 12-h light period. At flowering stage, the pattern of distribution of endogenously synthesized [14C]p-OHBG (data not shown) was similar to that observed for exogenously applied [14C]p-OHBG in wild-type flowering plants (Fig. 2).
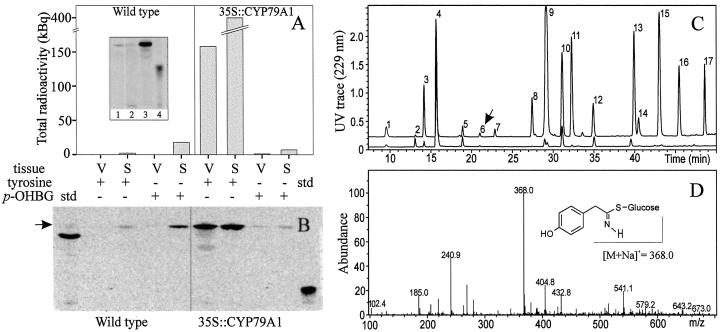
Figure 3.
The content of [14C]p-OHBG in seeds and vegetative tissues of wild-type and 35S::CYP79A1 plants. The plants were fed with either [14C]Tyr or [14C]p-OHBG at the vegetative stage and harvested at withering stage. A, Quantitative measurement of total radioactivity in methanol extracts of vegetative tissues (V) and seeds (S) of wild-type and 35S::CYP79A1 plants. Insert: 1, [14C]p-OHBG standard; 2, methanol extract of wild-type rosette leaves 3 weeks after application of [14C]Tyr; 3, methanol extract of 35S::CYP79A1 rosette leaves 3 weeks after application of [14C]Tyr; 4, [14C]Tyr standard. B, TLC analysis of radiolabeled desulpho p-OHBG in vegetative tissues (V) and seeds (S) of wild-type and 35S::CYP79A1 plants. Methanol extracts of vegetative tissues and seeds were applied to DEAE column and sulfatase treated (see “Materials and Methods”). Fifteen-microliter aliquots of 250 μL DEAE eluate were analyzed by TLC. The position of desulpho p-OHBG is indicated (arrow). C, LC-mass spectrometry (MS) UV trace (229 nm) of desulphoglucosinolates in seeds (upper trace) and vegetative tissues (lower trace) of wild-type plants fed with [14C]p-OHBG. The samples are identical to those in lane 4 and 5 in B. Arrow indicates the position of desulpho p-OHBG (D). Mass spectrum of peak 6 in C confirming the expected [M+Na]+ ion at m/z 368 corresponding to desulpho p-OHBG. The numbers correspond to the desulphoglucosinolates of the following glucosinolates: 1, 3-Hydroxypropylglucosinolate; 2, 3-methylsulphinylpropylglucosinolate (3MSOP); 3, 4-hydroxybutylglucosinolate; 4, 4-methylsulphinylbutylglucosinolate (4MSOB); 5, 5-methylsulphinylpentylglucosinolate; 6, p-OHBG; 7, 6-methylsulphinylhexylglucosinolate; 8, 7-methylsulphinylheptylglucosinolate; 9, 4-methylthiobutylglucosinolate; 10, indol-3-ylmethylglucosinolate; 11, 8-methylsulphinyloctylglucosinolate; 12, 4-methoxyindol-3-ylmethylglucosinolate; 13, 3-benzoyloxypropylglucosinolate; 14, 6-methylthiohexylglycosinolate; 15, 4-benzoyloxybutylglucosinolate; 16, 7-methylthioheptylglucosinolate; 17, 8-methylthiooctylglucosinolate.
At withering stage, the plants were harvested and dissected into mature seeds and vegetative tissues, and the glucosinolate profile was analyzed. Comparison of wild-type and 35S::CYP79A1 plants with foliarly applied [14C]p-OHBG revealed that wild-type plants accumulated more than twice the amount of [14C]p-OHBG in the seeds (Fig. 3A). This suggests that the exogenously applied p-OHBG in the 35S::CYP79A1 plants competes with endogenously synthesized p-OHBG for translocation into the seeds. Application of [14C]Tyr to 35S::CYP79A1 plants resulted in the accumulation of significant amounts of [14C]p-OHBG in both seeds and vegetative tissues (Fig. 3, A and B). The identity of p-OHBG was confirmed by mass spectrometry (MS; Fig. 3, C and D) in all samples. The presence of radiolabeled p-OHBG in the seeds shows that p-OHBG synthesized in the leaves has been transported to the seeds. However, more than one-third of the radiolabeled p-OHBG was retained in the vegetative tissue (Fig. 3A), implying competition with endogenous unlabeled p-OHBG for the seed sink. The data indicate that although seeds are a major sink for glucosinolates, they have a limited sink strength as has previously been indicated (Petersen et al., 2001a). Application of [14C]Tyr to wild-type plants resulted in the accumulation of trace amounts of [14C]p-OHBG in the seeds (Fig. 3, A and B). This indicates that an enzyme in wild-type Arabidopsis under the present conditions is able to convert Tyr into the corresponding oxime, leading to the formation of p-OHBG.
Comparison of glucosinolate content of seeds and vegetative tissues of both wild-type and 35S::CYP79A1 plants showed that the major glucosinolates, which accounted for approximately 90% to 95% of the total glucosinolate content in vegetative tissue, constituted about 98% of the total glucosinolate content in seeds (Table I). These data suggest that certain major glucosinolates in the vegetative tissues, such as 4MSOB, are transported to the seeds.
Table I.
Total glucosinolates in seeds and vegetative tissues of wild-type and 35S∷CYP79A1 plants fed with [14C]Tyr at the vegetative stage
| Glucosinolates | Concentration
|
|||
|---|---|---|---|---|
| Wild
type
|
35S∷CYP79A1
|
|||
| Seed | Vegetative tissue | Seed | Vegetative tissue | |
| μmol g−1 dry wt | ||||
| Aliphatic | 59.23 ± 7.36 | 2.04 ± 0.52 | 42.45 ± 2.66 | 1.03 ± 0.36 |
| Indole | 2.19 ± 0.17 | 0.18 ± 0.06 | 1.18 ± 0.24 | 0.16 ± 0.02 |
| p-OHBG | 0.13 ± 0.08 | 0.01 ± 0.01 | 24.23 ± 5.9 | 2.44 ± 0.25 |
Glucosinolates were divided into aliphatic, indole, and aromatic (p-OHBG) glucosinolates derived from chain-elongated Met, Trp, and Tyr, respectively.
Comparison of Glucosinolates in Phloem Exudates and Methanol Extracts of Rosette Leaves
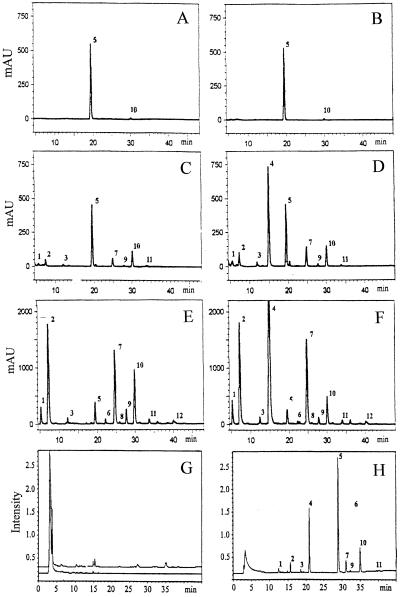
We analyzed by HPLC the composition and content of glucosinolates in phloem exudates and in methanol extracts of rosette leaves of both wild-type and 35S::CYP79A1 plants. Comparison of the composition and content of glucosinolates in phloem exudates (Fig. 4, C and D) with those in rosette leaves (Fig. 4, E and F) showed that not all glucosinolates present in leaves could be detected in phloem exudates, and that the relative amount of individual glucosinolates differed. Especially aliphatic glucosinolates, such as 3MSOP and 4MSOB, which were dominant in leaves, were present in low amounts in phloem exudates. It is interesting that indole glucosinolates turned out to be dominant in the phloem exudates (Fig. 4, C and D). As previously shown (Bak et al., 1999; Petersen et al., 2001a), the overall level of glucosinolates in the 35S::CYP79A1 plants increased 4-fold compared with wild-type and p-OHBG accounted for approximately 75% of the total glucosinolates (Fig. 4F). The level of p-OHBG in the phloem exudate was consistently about three times higher than the rest of glucosinolates (Fig. 4D). Incubation of leaf petioles in water revealed little exudation of glucosinolates (Fig. 4, A and B). This indicates that glucosinolates are present in low amounts, if at all, in the xylem.
Figure 4.
HPLC and LC-MS analysis of major glucosinolates in phloem exudates and methanol extracts of rosette leaves of wild-type and 35S::CYP79A1 plants. A through F, HPLC UV trace (229 nm). G and H, LC-MS UV-trace (229 nm). A, Glucosinolates in phloem exudates of wild-type leaves collected in distilled water. B, Glucosinolates in phloem exudates of 35S::CYP79A1 leaves collected in distilled water. C, Glucosinolates in phloem exudates of wild-type leaves collected in 15 mm EDTA. D, Glucosinolates in phloem exudates of 35S::CYP79A1 leaves collected in 15 mm EDTA. E, HPLC of glucosinolates in rosette leaves of wild-type plants. F, HPLC of glucosinolates in rosette leaves of 35S::CYP79A1 plants. G, LC-MS of phloem exudates without sulfatase treatment of both wild-type (lower trace) and 35S::CYP79A1 leaves (upper trace). H, LC-MS of sulfatase-treated phloem exudates of 35S::CYP79A1 leaves. The numbers correspond to the desulphoglucosinolates of the following glucosinolates: 1, 3MSOP; 2, 4MSOB; 3, 5-methylsulphinylpentylglycosinolate; 4, p-OHBG; 5, benzylglucosinolate, internal standard; 6, 7-methylsulphinylheptylglucosinolate; 7, indol-3-ylmethylglycosinolate; 8, 5-methylthiopentylglucosinolate; 9, 8-methylsulphinyloctylglucosinolate; 10, 4-methoxyindol-3-ylmethylglucosinolate; 11, 1-methoxyindol-3-ylmethylglucosinolate; 12, 8-methylthiooctylglucosinolate.
The question whether glucosinolates are transported as intact glucosinolates or as desulphoglucosinolates in the phloem was addressed by analyzing the phloem exudates for the presence of desulphoglucosinolates by liquid chromatography (LC)-MS. None of the peaks in the chromatogram had a mass equivalent to any expected desulphoglucosinolates in Arabidopsis (Fig. 4G). When sulfatase-treated phloem exudates were analyzed by LC-MS, several peaks representing desulphoglucosinolates were identified (Fig. 4F). This shows that intact glucosinolates are the dominant transport form in phloem.
DISCUSSION
In the present study, we have provided several lines of evidence for long-distance phloem transport of glucosinolates. First, we showed that radiolabeled p-OHBG and Suc upon application to detached leaves were loaded into to the phloem as evidenced by comigration of the tracers (Fig. 1A). Second, the speed of export from donor leaf (Fig. 1B) and the distribution patterns of radiolabeled p-OHBG (Fig. 2) were consistent with translocation of glucosinolates in the phloem. Third, long-distance transport was demonstrated by accumulation in the seeds of radiolabeled p-OHBG, which had been either exogenously applied to the leaves or de novo synthesized in the leaves (Fig. 3). Finally, the presence of intact glucosinolates in phloem exudates (Fig. 4) demonstrated that glucosinolates are present in the translocation stream of the phloem and that the dominant transport form is intact glucosinolates and not desul-phoglucosinolate.
Phloem Loading and Export of Glucosinolates
Phloem loading and export of radiolabeled p-OHBG applied exogenously to the leaves of flowering plants was shown by the presence of the tracer in phloem exudates and by the rapid translocation of the tracer from the application site to other parts of the plant, e.g. developing seeds (Figs. 1 and 2). The transport in vivo was also demonstrated by accumulation in the seeds of radiolabeled p-OHBG, which had been de novo synthesized in the leaves of 35S::CYP79A1 plants during vegetative growth (Fig. 3).
The mechanism of unloading and post-phloem transport is unknown. A growing body of evidence suggests that the pathway of phloem unloading of sugars and other phloem constituents is symplastic in rapidly developing sink tissues and in terminal sinks such as seeds (Oparka and Cruz, 2000). A carrier-mediated transport system for glucosinolates was first characterized in excised embryos of oilseed rape (Gijzen et al., 1989, 1994). We recently provided evidence of a proton-coupled glucosinolate transporter in the leaves of oilseed rape (Chen and Halkier, 2000a). A glucosinolate transporter may contribute to apoplastic phloem loading of glucosinolates and may also exist along the phloem pathway or in sink cells, where solute retrieval or uptake occurs continuously.
Glucosinolates in the Phloem
It was reported recently that both intact glucosinolates and desulphoglucosinolates have the physicochemical properties that satisfy the permeability criterion for phloem mobility (Brudenell et al., 1999). Identification of sulfotransferase activity in embryos has raised the question of whether the desulpho form was the transport form of glucosinolates (Rossiter and James, 1990; Toroser et al., 1995). The biosynthetic activity is, however, present in all tissues where intact glucosinolates are synthesized. In the present study, intact glucosinolates and not desulphoglucosinolates were detected in phloem exudates (Fig. 4, G and H), demonstrating that the intact glucosinolate is the transport form in the phloem.
The difference between the relative glucosinolate content in the phloem and the leaves (Fig. 4, C–H) suggests that there is a selection for specific glucosinolates to be loaded into the phloem. In general, the composition of phloem exudates (e.g. growth substances and Suc) has been shown to correlate with symplastic movement of compounds in the translocation stream (Gowan et al., 1995). Although the EDTA method is considered to be practical and reliable (King and Zeevaart, 1974; Hein et al., 1984; Gowan et al., 1995; Bourgis et al., 1999), it should be noted that phloem exudate collected by this method does not represent the entire translocation stream that would have moved from the cut part had it been left intact. Rather, it may represent an aliquot of the immediately available constituents in the transport stream (Hein et al., 1984). The relatively high level of indole glucosinolates in the exudate samples (Fig. 4, C and D) may be due to wounding response because indole glucosinolates are known to accumulate systemically in plants treated with jasmonate (Doughty et al., 1995; Bartlet et al., 1999).
Function of Glucosinolate Transport and Metabolism
The glucosinolate profile in the seeds (Table I) may reflect a combination of glucosinolates derived from long-distance transport and from de novo biosynthesis in silique walls (Du and Halkier, 1998). A quantitative survey of glucosinolate variation among 39 Arabidopsis ecotypes showed a significant positive correlation between the levels of aliphatic glucosinolates in leaves and seeds, suggesting that glucosinolates may be transported from leaves to seeds (Kliebenstein et al., 2001). Glucosinolates may be transported to sink cells other than those in the seeds, such as the recently discovered glucosinolate-rich S cells in Arabidopsis flower stalk (Koroleva et al., 2000).
The observed low concentration of glucosinolates in fully expanded leaves (Porter et al., 1991) and senescent vegetative parts (Table I; Fig. 3A) may be due to export. Export was clearly demonstrated in the present study for both exogenously applied and endogenously de novo synthesized p-OHBG. The absence of glucosinolate degradation products in the plant extracts (Fig. 2C) combined with the evidence for long-distance transport suggests that degradation of glucosinolates in intact tissues at later developmental stages is low.
In addition to possible nutritional functions of glucosinolates (Clossais-Besnard and Larher, 1991; Andreasson, 2000), the presence of glucosinolates in the phloem may provide means of defense against insects. Phloem-mediated transport of glucosinolates may enable coordination of de novo biosynthesis and use of defense compounds in different organs. Cloning of the transporter(s) will provide a valuable molecular tool for further studies of glucosinolate transport.
MATERIALS AND METHODS
Plant Material
Arabidopsis (ecotype Columbia) and 35S::CYP79A1 plants (Bak et al., 1999) were grown in potting mix (Enhetsjord K-jord, Weibulls, Sweden) at 22°C under a light period of 8 or 12 h for vegetative or reproductive growth, respectively, in Arabidopsis Chambers (Percival AR-60L, Boone, IA), where they were subjected to a photosynthetic flux of 100 to 120 μmol photons m−2 s−1 and 70% (v/v) relative humidity.
Application of Radiolabeled p-OHBG to Detached Rosette Leaves
Transport of radiolabeled p-OHBG in detached rosette leaves recut under water was investigated by applying 9 μL [3H]p-OHBG (259 mBq mmol−1 and 6.3 kBq μL−1) and 1 μL [14C]Suc (25 GBq mmol−1 and 7.4 kBq μL−1; Amersham, Buckinghamshire, UK) simultaneously to the adaxial surface of fully expanded leaves. The leaves were subsequently incubated for 6 h in 500 μL 15 mm EDTA (pH 7.5) in a chamber humidified to reduce xylem transpiration. Radioactivity in the solutions was quantified by a liquid scintillation counter. Radiolabeled p-OHBG was synthesized and purified as described previously (Chen and Halkier, 2000b).
Application of Radiolabeled p-OHBG and Tyr to Rosette Leaves of Whole Plants
Radiolabeled Tyr or p-OHBG was applied on the adaxial surface of expanded rosette leaves. The application site was slightly abraded with fine carborundum powder to increase uptake of the tracer. For total export studies, 50 μL [3H]p-OHBG was applied to a single rosette leaf of wild-type Arabidopsis at flowering stage. At given time points, four to six plants were analyzed. The leaves to which the tracer had been applied were cut off, and the remaining part of the plant was homogenized in liquid nitrogen and boiled in 70% (w/v) methanol for 8 min. Debris was spun down (3,500 rpm 10 min) and rinsed twice with methanol (about 50 mg tissue mL−1 methanol). Export of p-OHBG was estimated by liquid-scintillation counting of the combined methanol extracts.
For analysis of transport and distribution of p-OHBG in different tissues, 100 μL [14C]p-OHBG was applied to mature rosette leaves of wild-type plants at flowering stage. After 24 h of incubation, the majority of the plants were harvested and various tissues were extracted in methanol. The methanol extracts were analyzed by TLC (see below) and total radioactivity was quantified by liquid-scintillation counting. The remaining plants were left for approximately 2 weeks. Siliques with the characteristic yellow color at the tip were harvested and mature seeds were crashed out. Distribution of p-OHBG in different tissues of the whole plant was visualized by exposing them to phosphor screens at −20°C followed by analysis on a STORM 840 phosphorimager (Molecular Dynamics, Sunnyvale, CA).
Comparative studies on transport of exogenously applied and endogenously synthesized radiolabeled p-OHBG were carried out by application of a total of 300 μL [14C]Tyr (16.6 GBq mmol−1 and 1.9 kBq μL−1; Amersham) or 300 μL [14C]p-OHBG to rosette leaves of both wild-type and 35S::CYP79A1 plants at vegetative stage. The tracers were applied in 10-μL aliquots on 10 leaves three times with a 12-h interval. The plants were kept in 8-h light conditions until all the administered [14C]Tyr was metabolized as checked by TLC analysis every week. The plants were then switched to reproductive growth by being transferred into 12-h light period. Vegetative tissues and mature seeds were collected from the plants at withering stage and analyzed for content of radiolabeled p-OHBG.
Identification of [14C]p-OHBG by TLC
Methanol extracts were lyophilized and redissolved in 10 to 250 μL 70% (w/v) methanol, of which aliquots of 10 to 15 μL were analyzed by TLC on Silica Gel 60 F254 sheets (Merck, Darmstadt, Germany) using isopropanol:ethyl acetate:distilled water (7:1:2, v/v) as eluent. Radiolabeled bands on TLC plates were visualized by phosphorimager. p-OHBG was identified by comigration with authentic standard on TLC and by MS (see below). For analysis of p-OHBG degradation, a standard mixture of degradation products was generated by incubating 1 μL of [14C]p-OHBG with Arabidopsis leaf extract (approximately 2 μg of protein) in 20 mm sodium acetate buffer (pH 5) containing 0.3 mm ascorbate at 30°C for 30 min. Degradation of p-OHBG was monitored using the TLC system described above.
Collection of Phloem Exudates
Phloem exudate was collected from rosette leaves of both wild-type and 35S::CYP79A1 plants using the EDTA method (King and Zeevaart, 1974; Hein et al., 1984; Gowan et al., 1995). Rosette leaves (six–eight) were cut at the base of their petioles, recut under water, and subsequently rinsed to avoid contamination of cellular fluid. Each leaf was then incubated in 200 μL water or 15 mm EDTA (pH 7.5) for 4 h in a humid chamber at 20°C. Phloem exudates were lyophilized and stored at −80°C. The samples were analyzed for glucosinolate content and composition by both HPLC and LC-MS.
HPLC and LC-MS Analysis of Glucosinolates
The content and composition of glucosinolates in methanol extracts and in phloem exudates were determined by HPLC analysis as previously described (Petersen et al., 2001a). In brief, the glucosinolates were identified as desulphoglucosinolates after binding of methanol extracts on a DEAE anion-exchange column, followed by sulfatase treatment and elution with dH2O. Benzylglucosinolate (Merck) was added as internal standard at the start of the extraction procedure. Analyses were done in triplicates and individual desulphoglucosinolates were identified and quantified as previously described (Petersen et al., 2001b).
Samples of phloem exudates were subjected to LC-MS analysis before and after sulfatase treatment. LC-MS was done on a HP1100 LC coupled to a Bruker Esquire-LC ion trap mass spectrometer. The reversed-phase LC conditions were as follows: A C18 column (Chrompack Inertsil 3 ODS-3 S15x3 COL CP 29126) was used. The mobile phases were water doped with sodium acetate (A; 50 μm) and methanol (B). The flow was 0.25 mL min−1 and the gradient program was: isocratic 100% (w/v) A (0–2 min), linear gradient 0% to 60% (w/v) B (2–40 min), linear gradient 60% to 100% (w/v) B (40–45 min), and isocratic 100% (w/v) B (45–50 min). The mass spectrometer was run in positive ion mode. Twenty microliters of each sample was injected. The total ion chromatograms, reconstructed ion chromatograms and UV traces were used to locate peaks, and the [M+Na]+ adduct ions in conjunction with diode array UV spectra were used for identifications.
Footnotes
This work was supported by the Danish Scientific Research Council (to S.C.) and by the Danish National Research Foundation.
LITERATURE CITED
- Andreasson E. Structural and functional studies of the myrosinase-glucosinolate system in Arabidopsis thaliana and Brassica napus. PhD thesis. Uppsala: Swedish University of Agricultral Sciences; 2000. [Google Scholar]
- Bak S, Olsen CE, Petersen BL, Møller BL, Halkier BA. Metabolic engineering of p-hydroxybenzylglucosinolate in Arabidopsis by expression of the cyanogenic CYP79A1 from Sorghum bicolor. Plant J. 1999;20:663–672. doi: 10.1046/j.1365-313x.1999.00642.x. [DOI] [PubMed] [Google Scholar]
- Bartlet E, Kiddle G, Williams I, Wallsgrove RM. Wound-induced increases in the glucosinolate content of oilseed rape and their effect on subsequent herbivory by a crucifer specialist. Entomol Exp Appl. 1999;91:163–167. [Google Scholar]
- Bourgis F, Roje S, Nuccio ML, Fisher DB, Tarczynski MC, Li C, Herschbach C, Rennenberg H, Pimenta MJ, Shen T-L. S-Methylmethionine plays a major role in phloem sulfur transport and is synthesized by a novel type of methyltransferase. Plant Cell. 1999;11:1485–1498. doi: 10.1105/tpc.11.8.1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brudenell AJP, Griffiths H, Rossiter JT, Baker DA. The phloem mobility of glucosinolates. J Exp Bot. 1999;50:745–756. [Google Scholar]
- Chen S, Halkier BA. Characterization of glucosinolate uptake by leaf protoplasts of Brassica napus. J Biol Chem. 2000a;275:22955–22960. doi: 10.1074/jbc.M002768200. [DOI] [PubMed] [Google Scholar]
- Chen S, Halkier BA. In vivo synthesis and purification of radioactive p-hydroxybenzylglucosinolate (p-OHBG) in Sinapis alba. Phytochem Anal. 2000b;11:174–178. [Google Scholar]
- Clossais-Besnard N, Larher F. Physiological role of glucosinolate in Brassica napus: concentration and distribution pattern of glucosinolates among plant organs during a complete life cycle. J Sci Food Agric. 1991;56:25–38. [Google Scholar]
- Doughty KJ, Kiddle G, Pye BJ, Wallsgrove RM. Selective induction of glucosinolates in oilseed rape leaves by methyl jasmonate. Phytochemistry. 1995;38:347–350. [Google Scholar]
- Du L, Halkier BA. Biosynthesis of glucosinolates in the developing silique walls. Phytochemistry. 1998;48:1145–1150. [Google Scholar]
- Gijzen M, McGregor I, Seguin-Swartz G. Glucosinolate uptake by developing rapeseed embryos. Plant Physiol. 1989;89:260–263. doi: 10.1104/pp.89.1.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gijzen M, Seguin-Swartz G, McGregor I. Glucosinolate metabolism in rapeseed embryos: effect of feeding glucosinolate precursors and uptake of glucosinolate by different plant cultivars. J Plant Physiol. 1994;144:17–21. [Google Scholar]
- Gowan E, Lewis BA, Turgeon R. Phloem transport of antirrhinoside, an iridoid glycoside, in Asarina scandens. J Chem Ecol. 1995;21:1781–1788. doi: 10.1007/BF02033676. [DOI] [PubMed] [Google Scholar]
- Halkier BA. Glucosinolate. In: Ikan R, editor. Naturally Occurring Glycosides. New York: John Wiley & Sons, Inc.; 1999. pp. 193–223. [Google Scholar]
- Hein MB, Brenner ML, Brun WA. Effects of pod removal on the transport and accumulation of abscisic acid and indole-3-acetic acid in soybean leaves. Plant Physiol. 1984;76:955–958. doi: 10.1104/pp.76.4.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King RW, Zeevaart JAD. Enhancement of phloem exudation from cut petioles by chelating agents. Plant Physiol. 1974;53:96–103. doi: 10.1104/pp.53.1.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliebenstein DJ, Kroymann J, Brown P, Figuth A, Pedersen D, Gershenzon J, Mitchell-Olds T. Genetic control of natural variation in Arabidopsis thaliana glucosinolate accumulation. Plant Physiol. 2001;126:811–825. doi: 10.1104/pp.126.2.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koroleva OA, Davies A, Deeken R, Thorpe MR, Tomos AD, Hedrich R. Identification of a new glucosinolate-rich cell type in Arabidopsis flower stalk. Plant Physiol. 2000;124:599–608. doi: 10.1104/pp.124.2.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lykkesfeldt J, Møller BL. The synthesis of benzylglucosinolate in Tropaeolum majus: isothiocyanates as potent enzyme inhibitors. Plant Physiol. 1993;102:609–613. doi: 10.1104/pp.102.2.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magrath R, Mithen R. Maternal effects on the expression of individual aliphatic glucosinolates in seeds and seedlings of Brassica napus. Plant Breed. 1993;111:249–252. [Google Scholar]
- Merritt SZ. Within-plant variation in concentrations of amino acids, sugar, and sinigrin in phloem sap of black mustard, Brassica nigra (L.) Koch. J Chem Ecol. 1996;22:1133–1145. doi: 10.1007/BF02027950. [DOI] [PubMed] [Google Scholar]
- Oparka KJ, Cruz SS. The great escape: phloem transport and unloading of macromolecules. Annu Rev Plant Physiol Plant Mol Biol. 2000;51:323–347. doi: 10.1146/annurev.arplant.51.1.323. [DOI] [PubMed] [Google Scholar]
- Petersen BL, Andréasson E, Bak S, Agerbirk N, Halkier BA. Characterization of transgenic Arabidopsis thaliana with metabolically engineered high levels of p-hydroxybenzylglucosinolate. Planta. 2001a;212:612–618. doi: 10.1007/s004250000429. [DOI] [PubMed] [Google Scholar]
- Petersen BL, Chen S, Hansen CH, Olsen CE, Halkier BA (2001b) Composition and content of glucosinolates and expression of myrosinase in Arabidopsis thaliana. Planta (in press) [DOI] [PubMed]
- Porter AJR, Morton AM, Kiddle G, Doughty KJ, Wallsgrove RM. Variation in the glucosinolate content of oilseed rape (Brassica napus L.): I. Effects of leaf age and position. Ann Appl Biol. 1991;118:461–467. [Google Scholar]
- Rossiter JT, James DC. Biosynthesis of (R)-2-hydroxybut-3-enylglucosinolate (progoitrin) from [3,4-3H]but-3-enylglucosinolate in Brassica napus. J Chem Soc Perk Trans. 1990;1:1909–1913. [Google Scholar]
- Thompson GA, Schulz A. Macromolecular trafficking in the phloem. Trends Plant Sci. 1999;4:354–360. doi: 10.1016/s1360-1385(99)01463-6. [DOI] [PubMed] [Google Scholar]
- Toroser D, Wood C, Griffiths H, Thomas DR. Glucosinolate biosynthesis in oilseed rape (Brassica napus L.): studies with 35SO and glucosinolate precursors using oilseed rape pods and seeds. J Exp Bot. 1995;46:787–794. [Google Scholar]