Abstract
Purpose
The CCR5/CCL5 axis is essential for interactions between malignant cells and microenvironment components, promoting tumor progression in oral squamous cell carcinoma (OSCC). This study aims to evaluate the association of CCL5 and CCR5 with the behavior of oral cancer and assess the therapeutic potential of a CCR5 antagonist.
Methods
A retrospective study to analyze CCR5 and CCL5 expression on paraffin-embedded tissues was performed. In cell lines, rhCCL5 was added to induce CCR5-related pathways, and Maraviroc and shRNA against CCR5 were used to neutralize the receptor. Finally, an in vivo murine orthotopic xenograft model of tongue cancer was used to evaluate Maraviroc as an oncologic therapy. After 15 days, the mice were killed, and the primary tumors and cervical lymph nodes were analyzed.
Results
The expression of CCR5 was associated with clinical stage and metastasis, and CCL5 was related to overall survival. Adding rhCCL5 induced cell proliferation, while shRNA and Maraviroc reduced it in a dose-dependent manner. Maraviroc treatment also increased apoptosis and modified cytoskeletal organization. In vivo, Maraviroc reduced neck metastasis.
Conclusions
The effects of CCR5 antagonists in OSCC have been poorly studied, and this study reports in vitro and in vivo evidence for the effects of Maraviroc in OSCC. Our results suggest that the CCR5/CCL5 axis plays a role in oral cancer behavior, and that its inhibition is a promising new therapy alternative.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00432-023-05443-1.
Keywords: Oral cancer, CCR5 receptor, CCL5 chemokine, Survival, Prognosis, Metastasis
Background
Oral squamous cell carcinoma (OSCC) is the most common head and neck cancer, comprising 2–4% of all human malignancies (Sung et al. 2021). It mainly affects men over 45 years old and is caused by risk factors including tobacco, alcohol, betel chewing, poor diet, and human papillomavirus (Lin et al. 2020). Low income and education levels are also associated with OSCC (Capote-Moreno et al. 2020; Neckel et al. 2020). An increase in cases of young patients and women over 60 years old has been reported in recent decades (Satgunaseelan et al. 2020; Stepan et al. 2023). The diagnosis involves a biopsy, and the primary treatment is surgery with coadjuvant radiotherapy or chemotherapy (Madera et al. 2018). Unfortunately, OSCC has a low survival rate of 40–50% after five years (Dantas et al. 2016), due to late diagnosis and its invasive and metastatic behavior (Yang et al. 2021). Although the clinical stage (TNM stage) determines treatment and prognosis, patients with the same stage may experience different survival rates and recurrence. Better prognostic biomarkers are crucial for proper treatment.
Lymph node metastasis is a predictor for poor OSCC survival (Yang et al. 2021). Understanding the molecular processes involved in lymph node metastasis, such as epithelial-mesenchymal transition, cell invasion, extracellular matrix degradation, migration and proliferation, cell–cell interactions, and angiogenesis, is critical for improving therapeutic options. Accurate lymph node metastatic disease diagnosis is challenging, and over or under-treatment may negatively impact patients´ quality of life (Baba et al. 2019; Deo et al. 2012). Several molecular biomarkers have been studied as predictors, but the complexity of the metastatic process results in inconsistent clinical correlations.
Interaction between OSCC cells and cancer-associated fibroblasts/myofibroblasts in the primary tumor and lymph node is crucial for tumor invasion and metastasis (Sobral et al. 2011; Wang et al. 2021). In this scenario, chemokines, which are heparin-binding proteins that operate through selective membrane-bound GPCRs that act as regulatory molecules in leukocyte maturation, play a fundamental role in attracting cancer cells to the metastatic site (da Silva et al. 2016; Li et al. 2022a, b). The CCR5/CCL5 chemokine axis has been reported to play a role in the pathogenesis of various malignancies such as pancreatic cancer (Singh et al. 2018), colorectal cancer (Ucuncu et al. 2019), hepatocellular cancer (Singh et al. 2020), and head and neck carcinomas (Domingueti et al. 2019; Gonzalez-Arriagada et al. 2018) and lymphomas (Manfroi et al. 2021). In OSCC, the CCR5/CCL5 axis is associated with regional lymph node metastasis (Domingueti et al. 2019; Gonzalez-Arriagada et al. 2018; Hemmatazad and Berger 2021), and genetic polymorphisms of CCR5 and CCL5 have been related to the initiation and progression of the disease Weng et al. 2010). CCR5 is an HIV-related chemokine receptor, and drugs such as Maraviroc (CCR5 antagonist) have demonstrated efficacy in suppressing cancer cell proliferation, migration, and metastasis (Kodama et al. 2020; Velasco-Velazquez et al. 2012). Blocking this axis is an alternative complementary therapeutic option to improve outcomes in tumor that resist oncologic therapy. The two main chemokines that bind to CCR5 are CCL3 and CCL5 (Kodama et al. 2020; Singh et al. 2018, 2020). It was recently shown that pre-metastatic lymph nodes secrete CCL5 (also known as RANTES) and promote the recruitment of breast cancer cells expressing CCR5 (Jin et al. 2018).
Although CCR5 antagonists have been suggested as a potential oncologic therapy, their efficacy in treating OSCC is still unclear (Gonzalez-Arriagada et al. 2022). Our study aimed to investigate CCR5 inhibition as an alternative treatment for metastatic OSCC. Our findings obtained using in vitro and in vivo models suggest that Maraviroc may be a potential therapy for OSCC. The CCR5/CCL5 axis is correlated with a poor outcome, and its proteins could serve as a biomarker.
Methods
Immunohistochemical expression of CCR5 and CCL5 in primary OSCC tumors from patients with and without a history of metastasis
Patient selection
A retrospective study analyzed paraffin-embedded tissues of OSCC primary tumors (n = 59) from the Anatomic Pathology Laboratory in Carlos van Buren Hospital, Valparaíso. Clinicopathological data were collected from patients’ files, including gender, age, tumor size, location, treatment, and survival (Supplementary Table I). We assessed the immunohistochemical expression of CCR5 and CCL5 in OSCC in specimens displaying the front of the primary tumor invasion.
Immunohistochemistry
The immunohistochemical analysis utilized anti-CCR5 (PA5-29,011, Invitrogen™, USA) and anti-CCL5 (701,030, Invitrogen™, USA) antibodies. Tissue sections, 3 μm thick, were subjected to antigenic recovery with citrate buffer prior to treatment with 3% hydrogen peroxide to block endogenous peroxidase. Slides were incubated with normal goat serum for 30 min at room temperature, followed by incubation at 4 °C with primary antibodies diluted in PBS1X. The EnVision™ Dual FLEX + system kit (Dako, Denmark) was used according to the instructions from the manufacturer, and the immunostaining was visualized by 3′ 3-diaminobenzidine tetrahydrochloride. Hematoxylin was used for counterstaining, and sections were mounted with DPX.
A semiquantitative scoring system was used to analyze the slides based on previously published methods (Gonzalez-Arriagada et al. 2018). The percentage of positive (PP) cells was categorized into 0 (negative), 1 (1–25%), 2 (26–50%), 3 (51–75%), and 4 (76–100% positive cells), while staining intensity (SI) was divided into 0 (negative), 1 (weak), 2 (moderate), and 3 (intensive staining). The final immunoreactive score (IRS) was obtained by multiplying the PP and SI values. The scores were then classified into two groups: low expression (0–4) and high expression (5–12).
Characterization of CCL5 and CCR5 expression within cell lines
Cell lines
The different cell lines used in this study were cultured in a 5% CO2 atmosphere at 37ºC. Normal oral keratinocytes (NOK) were cultured in a fetal bovine serum (FBS) free culture medium, with reduced levels of calcium, specific supplements (Gibco’s Keratinocyte-SFM, Invitrogen™, USA) and antibiotics. Human tongue cancer cell lines including SCC9 (CRL-1629), SCC15 (CRL-1623), SCC25 (CRL-1628), and CAL27 (CRL-2095) were acquired from American Type Culture Collection (ATCC, USA), while the HSC-3 cell line comes from a metastatic tongue SCC (Japan Health Science Research Resources Bank, JRCB 0623). These cell lines were cultured in DMEM/F-12 medium (Invitrogen™, USA) supplemented with 10% FBS and antibiotics.
RT-qPCR analysis
RNA was isolated with an RNeasy kit (Qiagen, Germany), and cDNA was synthesized with SuperScript IV RT (Invitrogen™, USA). The concentration of the resulting cDNA was assessed using NanoDrop™ (Thermo Fisher Scientific Inc., USA). Real-time PCR reactions were carried out in triplicate using TaqMan™ probes (Applied Biosystems™, USA) specific for CCR1 (Assay ID: Hs00928897_s1), CCR3 (Assay ID: Hs00266213_s1), CCR4 (Assay ID: Hs00747615_s1), CCR5 (Assay ID: Hs99999149_s1), CCL3 (Assy ID: Hs00234142_m1) and CCL5 (Assay ID: Hs00982282_m1) and the PCR Master Mix (Invitrogen™, USA) in a final volume of 25 μl. The reactions were performed in 96-well plates (MicroAmp™ Optical, Applied Biosystems™, USA) using the StepOnePlus Real Time PCR System (Applied Biosystems™, USA). The relative Ct values were normalized using the GADPH gene (Assay ID: Hs02786624_g1, Invitrogen™, USA). Data analysis was done with the 2−ΔΔCt formula, where ΔCt = (Ctgene − CtGADPH).
ELISA (Enzyme-Linked Immunosorbent Assay)
CCL3 and CCL5 were quantified in a cell culture medium using ELISA. The medium was collected, centrifuged, and stored at – 80 °C until use. The specific kits for human CCL3 (MIP-1a Human Instant ELISA™ Kit, Invitrogen™, USA) and human CCL5 (RANTES Human Instant ELISA™ Kit, Invitrogen™, USA) were used following the instructions from the manufacturer.
Western blot
For western blot, the cells were lysed in RIPA lysis buffer (NaCl 150 mM, EDTA 5 mM, pH 8.0, Tris 50 mM, pH 8.0, NP-40 (IGEPAL CA-630) 1.0%, sodium deoxycholate 0.5%, SDS 0.1%) containing protease and phosphatase inhibitors and sonicated using a stem sonicator. 30–60 μg of total protein were loaded in a 10% polyacrylamide gel (SDS-PAGE) and were transferred to nitrocellulose membranes (Hybond, GE) and blocked with 3% fat-free milk (m/v) diluted in TBS-T buffer (0.25 M Tris/HCl, 0.192 M glycine, 0.1% Tween 20) for 2 h at 4 °C. The membranes were incubated with primary antibodies against CCR1 (1:500, PA524785, Invitrogen™, USA), CCR3 (1:500, PA527168, Invitrogen™, USA), CCR4 (1:500, PA599885, Invitrogen™, USA) and CCR5 (1:500, PA529011, Invitrogen™, USA) diluted in TBS for 2 h at 4 °C. After washing with TBS-T, the incubation with the secondary antibody conjugated to peroxidase enzyme was done for 1 h, and the reveal was performed with an ECL chemiluminescence kit (GE Healthcare, USA), as per manufacturer instructions. The iBright FL1000 (Invitrogen™, USA) imaging system was used to visualize the reaction.
Treatment with recombinant human CCL5 (rhCCL5)
Cell lines expressing low levels of CCL5 but which expressed CCR5 were treated with recombinant human CCL5 (278-RN-050, R&D Systems, Inc., USA) at concentrations of 5 and 20 ng/ml in 0.1% FBS culture medium for 0, 24, 48, and 72 h. The impact of events associated with aggressive behavior was assessed.
Treatment with maraviroc
Cell lines with high CCR5 levels were treated with Maraviroc (PZ0002, Sigma-Aldrich, USA) at increasing concentrations (50–100 ng/ml) for varying periods (0, 24, 48, and 72 h). The effects of aggressive behavior-related events were assessed.
Neutralization of CCR5
RNA interference (RNAi) was applied to neutralize gene expression using short hairpin oligonucleotides. Lentiviral vectors were produced by Invitrogen (Invitrogen™, USA) containing short hairpin RNA (shRNA) targeting human CCR5 with the sequence (Top_CCR5: 5’TGCTGAATAGAGCCCTGTTAAGAGTTGTTTTGGCCACTGACTGACAACTCTTAAGGGCTCTATT3’, and Bottom_CCR5: 5’CCTGAATAGAGCCCTTAAGAGTTGTCAGTCAGTGGCCAAAACAACTCTTAACAGGGCTCTATTC3’). The strategy was the transduction of lentivirus particles carrying shRNA against CCR5 to select specific clones with reduced expression using the BLOCK-iT™ Pol II miR RNAi Expression Vector Kit, following manufacturer specifications (Invitrogen™, USA). All the vectors were transformed into Escherichia coli DH5α competent cells and purified using PureLink™ Hi Pure Plasmid Miniprep (Invitrogen™, USA). Cells were incubated with a culture medium containing lentivirus particles with a multiplicity of infection of 1.5 (MOI = 1.5) when cells were at 50% confluence. Transfection was performed using Lipofectamine 3000 (Invitrogen™, USA) (Onyeisi et al. 2020). After 16 h of incubation, the medium was substituted with a complete medium, and cells were cultured for an additional 48 h. The transfected cells were selected by adding blasticidin (6 μg/mL) (R21001, Gibco, USA) for 10 days. Neutralization efficiency was determined by sequencing (Macrogen Inc., Korea).
Analysis of phenotypes associated with aggressive tumor behavior
Experiments were conducted to investigate the role of CCL5 and CCR5 in OSCC cell lines’ aggressive behavior following the aforementioned treatments.
Cell viability assessed using the MTS test (CellTiter 96® AQueous Non-Radioactive Cell Proliferation Assay, Promega, USA) in line with the instructions from the manufacturer (Dourado et al. 2022).
Cell proliferation evaluated by assessing PCNA expression (P8825, Sigma-Aldrich, USA) (Xu et al. 2017).
Apoptosis measured using ApoDETECT Annexin V-FITC Kit (331,200, Invitrogen™, USA) staining of cell membranes. Anexin V identifies cells in the early stages of apoptosis (Somaida et al. 2020).
Morphology and Organization of Cytoskeleton assessed using Phalloidin conjugated to rhodamine (R415, Invitrogen™, USA) and fluorescent nuclear DAPI stain (D1306, Invitrogen™, USA), respectively (Borges et al. 2020). Negative controls were included. Confocal microscopy (Leica Microsystems, Germany) was used to acquire images.
Animal studies
All mouse studies were performed at the Universidad de los Andes animal facility, in accordance with protocols revised and approved by the Institutional Animal Care and Use Committee of Universidad de los Andes. NOD-scid IL2Rgnull-3/GM/SF (NSG-SGM3) mice were purchased from Jackson Laboratories (Bar Harbor, ME, USA).
Xenograft tumors were established in athymic NSG mice by injecting 0.5 × 106 HSC3 cells in 40 ml of PBS at the lateral border of the tongue using a 27G needle. In vivo study was conducted three times, with 5–6 animals per group. The mice were monitored daily, with tumor dimensions being measured with a pachymeter and expressed in mm3. Animals were euthanized if they lost significant weight (≤ 20%) or if the tumors interfered with feeding. The animals were anesthetized with an intraperitoneal injection of ketamine/xylazine (70–100/10–20 mg/kg) for each procedure.
Treatment with Maraviroc and evaluation of metastasis
Mice injected with HSC3 cells were divided into two groups. One group received Maraviroc treatment (10 mg/kg, i.p., in 20 ml of PBS) for 14 days starting on the third-day post-inoculation, while the other group received no treatment. Cells were stained with a fluorescent dye, DIR (1,1#-Dioctadecyltetram, Biotiom, Gentaur Ltd., UK), and the Odyssey NIR fluorescence imaging system was used (LI-COR, Lincoln, NE). Tumor formation, regional lymph node and distant metastasis were monitored. After 15 days, animals were euthanized for histological processing of primary tumors, submandibular glands, neck lymph nodes, lungs, and liver. Weight (in grams) and tumor size (in cm2, measured with an Iwanson caliper) were recorded, and animal welfare was monitored. In cases where the animal suffered any inconvenience, saline solution and tramadol were available for rehydration and pain management.
Statistical analysis
The association of CCR5 and CCL5 expression with clinicopathological features was analyzed with the Chi-squared test. Survival analyses including 5-year overall survival (OS), 5-year disease-specific survival (DSS), and 5-year disease-free survival (DFS) were performed using Kaplan–Meier curves, univariate and multivariate Cox regression. Covariates included in the regression models were selected using backward elimination. The significance level was set at 5% (p ≤ 0.05).
In vitro assays were carried out a minimum of three times. A Mann–Whitney U test or one-way ANOVA test was applied, with a significance level set at 5% (p ≤ 0.05).
Results
CCR5 and CCL5 overexpression is associated with lymph node metastasis
The immunostaining for CCR5 and CCL5 revealed a cytoplasmic pattern with varying intensity and distribution among tumoral cells (Fig. 1). For CCR5, the most significant findings were the associations of CCR5 with lymph node metastasis (p = 0.04), perineural invasion (p = 0.009), and margin status (p = 0.04) (Table 1). However, the univariate analysis (Supplementary Table II) did not show CCR5 as a predictor for OS (p = 0.18), DSS (p = 0.17), or DFS (p = 0.49). Kaplan–Meier survival curves also did not demonstrate a statistically significant worse prognosis in patients with high CCR5 expression (Supplementary Fig. 1), which remained true for DSS (Supplementary Fig. 2) and DFS (Supplementary Fig. 3).
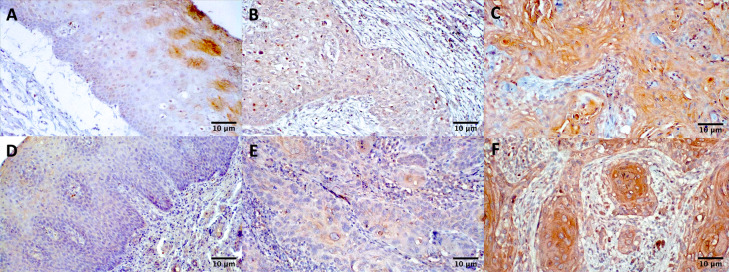
Fig. 1.
Staining for CCR5 and CCL5 revealed a higher expression in primary tumors of squamous cell carcinoma compared to normal mucosa (Magnification 200x). Immunohistochemistry analysis of CCR5 in a normal mucosa tissue (A), primary OSCC with low expression (B) and primary OSCC with high expression (C), and CCL5 in a normal mucosa tissue (D), primary OSCC with low expression (E) and primary OSCC with high expression (F)
Table 1.
Association of CCR5 and CCL5 expression with the clinicopathological features of the patients included in the study (chi-square test)
| CCR5 | CCL5 | |||||||
|---|---|---|---|---|---|---|---|---|
| Low expression | High expression | Total | p-value | Low expression | High expression | Total | p-value | |
| n (%) | n (%) | n | n (%) | n (%) | n | |||
| Age | ||||||||
| ≤ 64 years | 10 (34.5) | 19 (65.5) | 29 | 0.51 | 14 (50) | 14 (50) | 28 | 0.49 |
| ≥ 64 years | 12 (42.9) | 16 (57.1) | 28 | 11 (40.7) | 16 (59.3) | 27 | ||
| Sex | ||||||||
| Male | 14 (48.3) | 15 (51.7) | 29 | 0.12 | 12 (42.9) | 16 (57.1) | 28 | 0.69 |
| Female | 8 (28.6) | 20 (71.4) | 28 | 13 (48,1) | 14 (51,9) | 27 | ||
| Smoking | ||||||||
| No | 12 (41.4) | 17 (58.6) | 29 | 0.66 | 14 (50) | 14 (50) | 28 | 0.49 |
| Yes | 10 (35.7) | 18 (64.3) | 28 | 11 (40.7) | 16 (59.3) | 27 | ||
| Drinking | ||||||||
| No | 14 (42.4) | 19 (57.6) | 33 | 0.48 | 16 (50) | 16 (50) | 32 | 0.42 |
| Yes | 8 (33.3) | 16 (66.7) | 24 | 9 (39.1) | 14 (60.9) | 23 | ||
| Lymph node metastasis | ||||||||
| No | 16 (50) | 16 (50) | 32 | 0.04 | 17 (56.7) | 13 (43.3) | 30 | 0.06 |
| Yes | 6 (24) | 19 (76) | 25 | 8 (32) | 17 (68) | 25 | ||
| Tumor size | ||||||||
| T1–2 | 13 (41.9) | 18 (58.1) | 31 | 0.57 | 16 (53.3) | 14 (46.7) | 31 | 0.19 |
| T3–4 | 9 (34,6) | 17 (65.4) | 26 | 9 (36) | 16 (64) | 25 | ||
| Clinical stage | ||||||||
| I | 5 (50) | 5 (50) | 10 | 0.57 | 6 (60) | 4 (40) | 10 | 0.203 |
| II | 6 (50) | 6 (50) | 12 | 7 (63.6) | 4 (36.4) | 11 | ||
| III | 6 (31.6) | 13 (68.4) | 19 | 5 (27.8) | 13 (72.2) | 18 | ||
| IV | 5 (31.3) | 11 (68.8) | 16 | 7 (43.8) | 9 (56.3) | 16 | ||
| Histopathological differentiation | ||||||||
| Well-differentiated | 15 (45.5) | 18 (54.5) | 33 | 0.21 | 18 (56.3) | 14 (43.8) | 32 | 0.29 |
| Moderate/Poor differentiation | 7 (29.9) | 17 (70.8) | 24 | 9 (37.5) | 15 (62.5) | 24 | ||
| WPOI | ||||||||
| Less infiltrative | 2 (66.7) | 1 (33.3) | 3 | 0.32 | 1 (33.3) | 2 (66.7) | 3 | 0.56 |
| Highly infiltrative | 20 (37) | 34 (63) | 54 | 24 (46.2) | 28 (53.8) | 52 | ||
| Bud | ||||||||
| No | 10 (55.6) | 8 (44.4) | 18 | 0.07 | 8 (47.1) | 9 (52.9) | 17 | 0.87 |
| Yes | 12 (30.8) | 27 (69.2) | 39 | 17 (44.7) | 21 (55.3) | 38 | ||
| PNI | ||||||||
| No | 16 (55.2) | 13 (44.8) | 29 | 0.009 | 17 (63) | 10 (37) | 27 | 0.10 |
| Yes | 6 (21.4) | 22 (78.6) | 28 | 8 (28.6) | 20 (71.4) | 28 | ||
| Margin status | ||||||||
| Compromised | 0 (0) | 6 (100) | 6 | 0.04 | 2 (40) | 3 (60) | 5 | 0.58 |
| Free | 22 (43.1) | 29 (56.9) | 51 | 23 (46) | 27 (54) | 50 | ||
| Recurrence | ||||||||
| No | 14 (38.9) | 22 (61.1) | 36 | 0.95 | 17 (50) | 17 (50) | 34 | 0.38 |
| Yes | 8 (38.1) | 13 (61.9) | 21 | 8 (38.1) | 13 (61.9) | 21 | ||
| Patient status | ||||||||
| Alive | 11 (40.7) | 16 (59.3) | 27 | 0.47 | 14 (56) | 11 (44) | 25 | 0.18 |
| Dead due to OSCC | 8 (32) | 17 (68) | 25 | 8 (32) | 17 (68) | 25 | ||
| Dead, non-related to OSCC | 3 (60) | 2 (40) | 5 | 3 (60) | 2 (40) | 5 | ||
| Total # of patients | 22 | 35 | 57 | 25 | 30 | 55 | ||
Statistically significant values are in bold
The immunohistochemical expression of CCL5 showed a tendency towards association with lymph node metastasis (p = 0.06) (Table 1). In the univariate analysis, CCL5 was almost statistically significant in predicting OS (p = 0.06) and DSS (p = 0.07) (Supplementary Table II). In the multivariate analysis, CCL5 was included along with other prognostic factors for OS and DSS, including size (OS, p = 0.07; DSS, p = 0.01), lymph node metastasis (OS, p = 0.004; DSS, p = 0.005), clinical stage (OS, p = 0.03; DSS, p = 0.01), perineural invasion (OS, p = 0.04; DSS, p = 0.07), and recurrence (OS, p = 0.002; DSS, p = 0.002). Finally, considering clinical stage and recurrence (Table 2), CCL5 appeared as a predictor factor for OS (p = 0.014) and DSS (0.01). High CCL5 expression was associated with a worse prognosis for OS (p = 0.05) and lymph node metastasis, PNI, alcohol consumption, clinical stage, and recurrence (p < 0.05) based on Kaplan–Meier survival curves (Supplementary Fig. 1). For DSS, CCL5 showed an association with lymph node metastasis, alcohol consumption, clinical stage, and recurrence (p < 0.02) (Supplementary Fig. 2). No association was observed with Kaplan–Meier curves for DFS (Supplementary Fig. 3).
Table 2.
Multivariate analysis for overall survival and disease-specific survival in OSCC
| Overall survival | Disease-specific survival | |||
|---|---|---|---|---|
| HR (95% CI) | p value | HR (95% CI) | p-value | |
| Clinical stage | ||||
| I | 1 | 0.02 | 1 | 0.006 |
| II | 0.59 (0.14–2.50) | 0.47 | 1.45 (0.24–8.76) | 0.68 |
| III | 1.05 (0.34–3.21) | 0.92 | 1.99 (0.40–9.77) | 0.39 |
| IV | 3.26 (1.07–9.88) | 0.03 | 8.14 (1.71–38.75) | 0.008 |
| Recurrence | ||||
| No | 1 | 1 | ||
| Yes | 2.93 (1.33–6.45) | 0.007 | 3.24 (1.34–7.84) | 0.009 |
| CCL5 | ||||
| Low expression | 1 | 1 | ||
| High expression | 2.99 (1.25–7.17) | 0.014 | 3.56 (1.32–9.59) | 0.01 |
Statistically significant values are in bold
CCL5 is more expressed than CCL3 in OSCC cell lines
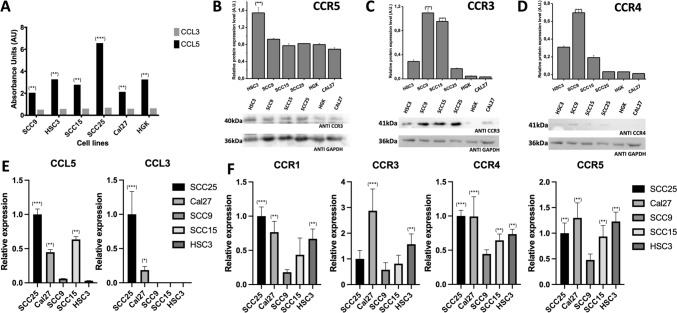
Cells were initially characterized for the CCR5 ligands (CCL3 and CCL5) and the chemokine receptors CCR1, CCR3, CCR4 and CCR5. CCL5 expression was higher than CCL3 in all cell lines (Fig. 2A), and lower levels of CCL5 mRNA were exhibited in HSC3 and SCC9 (Fig. 2E).
Fig. 2.
CCR5 and CCL5 are overexpressed in OSCC-derived cell lines. A The expression (ELISA) in the CCL5 medium is significantly higher than in CCL3. B The HSC3 cell line has a higher expression of CCR5 (Western Blot). GADPH was used as the housekeeping control. C The expression (Western Blot) of CCR3 was lower than CCR5, except in SCC15 and SCC9 cell lines. D The expression (Western Blot) of CCR4 was lower than CCR5, except in SCC9 cell lines. E Total RNAs from the cell lines were converted into cDNA to carry out a qPCR. The CCL3 and CCL5 mRNA levels were significantly higher in SCC25 and lower in HSC3 and SCC9, coinciding with ELISA results. F The mRNA levels of CCR1 and CCR4 were lower than CCR3 and CCR5. The highest expression of the receptor CCR5 was in SCC25, Cal27 and HSC3 cell lines, while the highest expressions of mRNA levels of CCR3 are notoriously higher than other cell lines. According to these results, the cell lines HSC3 (high expression of CCR5 and low expression of CCL5) and SCC25 (high expression of CCR5 and also high expression of CCL5) were selected. *p < 0.01, **p < 0.001, ***p < 0.0001
HSC3 showed the highest CCR5 expression (Fig. 2B), and lower expression of other receptors (Fig. 2C, D). Real-time PCR revealed similar results (Fig. 2F). In contrast, the cell line Cal27 showed a very high expression of CCR3 (Fig. 2F). Based on these results, we selected HSC3 (high CCR5, low CCL5 expression) and SCC25 (high CCR5 and CCL5 expression) for further experiments.
CCL5 induces proliferation and aggressive behavior in OSCC cell lines
HSC3 and SCC25 cells were treated with rhCCL5, which increased cell viability in a dose- and time-dependent manner (Fig. 3A, B). The treatment also decreased apoptosis (Fig. 3C), which was more pronounced at lower doses of rhCCL5 (5 ng/mL). Additionally, rhCCL5 treatment promoted higher cell viability (Fig. 3G), cell proliferation by PCNA expression, and cytoskeleton organization as revealed by phalloidin staining (Fig. 3H).
Fig. 3.
Cell lines (HSC3 and SCC25) become more aggressive with rhCCR5 and less aggressive with the Maraviroc treatment. A, B Both cell lines increase their viability (MTS) after applying rhCCR5, which is more evident in the HSC3 cell line. C, D Both cell lines reduced their viability (MTS) after the treatment with Maraviroc. E Annexin V results in HSC3 showed that apoptosis is reduced by rhCCL5. F Apoptosis in HSC3 cells, measured with Annexin V, is significantly increased with the Maraviroc treatment. G Immunofluorescence revealed that the expression of phalloidin in the cytoskeleton and PCNA is increased after applying rhCCL5 and reduced in transfected cells with shCCR5, and a higher reduction is observed with Maraviroc. H Cell viability, measured with MTS, is increased with rhCCL5 and reduced with the Maraviroc treatment, even in the presence of rhCCL5 and in transfected cells with shCCR5. I Quantification of the fluorescence intensity of actin fibers, showing a significantly higher intensity when cells are stimulated with rhCCL5 J Quantification of PCNA positive cells showing a significantly higher expression when are stimulated with rhCCL5. *p < 0.01, **p < 0.001, ***p < 0.0001
The inhibition of CCR5 reduces proliferation and other parameters related to the aggressive behavior of OSCC cell lines
Maraviroc reduced cell viability and increased apoptosis in HSC3 and SCC25 cells in a dose- and time-dependent manner (Fig. 3D–F). CCR5-transfected cells responded to rhCCL5; however, this response is reduced by Maraviroc (Fig. 3G). Analysis of lamellopodia formation reveals that blocking CCR5 with shCCR5 and Maraviroc promoted both lower cell proliferation and less cytoskeleton organization compared to CCL5-stimulated cells analyzing lamellipodia formation (Fig. 3H).
Maraviroc reduces the metastatic formation of tongue squamous cell carcinoma in vivo
Injecting HSC3 cells resulted in highly infiltrative cancer with perineural invasion (Fig. 4A) and lymph node neck metastasis (Fig. 4B). Maraviroc treatment resulted in smaller tumor sizes (Fig. 4C); however, the difference was not statistically significant (p = 0.16). The untreated group showed a reduction in weight (p = 0.04) (Fig. 4D, E). After two weeks of treatment with DIR-labelled cells, the treatment group showed a lower number of metastatic lymph nodes (Fig. 4F and Supplementary Fig. 4), although this difference was not statistically significant (p = 0.08).
Fig. 4.
Results of the murine xenograft model of OSCC treated with Maraviroc. A Hematoxylin and Eosin-stained image showing highly infiltrative neoplasia with perineurial invasion. Nerves indicated by *. (High power field, 40X). B Hematoxylin and Eosin-stained image showing a cervical lymph node with intracapsular metastasis adjacent to the salivary gland. Lymph node indicated by *. (Low power field, 10X). C The mean tumor size of the experiments is lower in treated mice than in untreated mice, although the p-value did not reach statistical significance. D Mean weight measurements of the experiments show that the difference between treated and untreated mice is higher with time. E Graph representing weight loss values, which shows a significant difference between the treated and untreated groups. F A representative figure of near-infrared image to detect cervical metastases after two weeks of treatment shows the metastatic foci in the neck of untreated mice versus the absence of foci in the neck of treated mice. White arrows indicate neck metastatic foci. The treatment group exhibited a tendency towards a lower number of metastatic lymph nodes compared to the control group (p = 0.08)
Discussion
OSCC has high mortality and morbidity rates, primarily due to late diagnosis, treatment delays, and aggressive behavior linked to high invasiveness and metastasis (Kolegova et al. 2022; Ling et al. 2021). The CCR5/CCL5 axis plays a role in lymphocyte migration and immune modulation (Zeng et al. 2022), but its involvement in cancer progression is increasingly recognized (Gozález-Arriagada et al. 2022; Singh et al. 2020). CCR5 expression has been linked to lymph node metastasis in OSCC (da Silva et al. 2016; Domingueti et al. 2019; Gonzalez-Arriagada et al. 2018), highlighting its inhibition as a potential therapeutic target (Hemmatazad and Berger 2021). Maraviroc, a CCR5 inhibitor, has shown promise in suppressing cell proliferation and migration, potentially reducing metastasis (Pervaiz et al. 2019; Velasco-Velazquez et al. 2012). Some authors suggested it could be an adjuvant for immunotherapy (Gong and Ren 2020; Haag et al. 2022). This study was aimed to evaluate CCR5 and CCL5 as prognostic biomarkers and assess the potential of Maraviroc as a therapeutic alternative for metastatic tumors in OSCC patients.
The findings of the current study demonstrated that the overexpression of CCR5/CCL5 axis proteins in OSCC patients is associated with poor outcomes, including regional lymph node metastasis and low overall survival, consistent with previous studies about OSCC or other head and neck carcinomas (Domingueti et al. 2019; Li et al. 2022a, b). However, we did not observe any association with recurrence or disease-free survival, although larger sample sizes are needed to study these less frequent outcomes more comprehensively (Szturz and Vermorken 2020).
CCL5 is reportedly secreted by pre-metastatic lymph nodes to attract CCR5 + cancer cells (Jin et al. 2018). Our results in patient tissues suggest that CCL5 may promote OSCC metastasis, and we found that CCL5 is more highly expressed than CCL3 in OSCC cells (Chuang et al. 2009; Guan et al. 2023; Singh et al. 2018; 2020). The immunohistochemical expression of CCL5 was associated with clinical staging and lymph node metastasis, considering that the CCR5/CCL5 axis activation triggers intracellular pathways that promote invasion and migration. High CCL5 expression has been linked to poor survival (Bai et al. 2020; Cao et al. 2011; Chen et al. 2019; Huang et al. 2021; Qiu et al. 2022), and our survival analyses identified CCL5 as a predictor of poor outcomes in OS and DSS, both results which remain significant in multivariate analysis as an independent factor. Our results were obtained through immunohistochemistry of paraffin-embedded tissues, but further research is needed to study the expression of CCL5 as a circulating biomarker in OSCC, as has been done in other cancer types (Wang et al. 2016; Willenbrock et al. 2021). In OSCC cell lines, rhCCL5 induced proliferation and cytoskeleton organization while reducing apoptosis. These effects are dose- and time-dependent and were more prominent in cell lines with lower self-releasing of CCL5.
The association of high CCR5 expression with lymph node metastasis and perineural invasion was previously reported in OSCC and other types of cancer (Cao et al. 2011; Gao et al. 2018; Gonzalez-Arriagada et al. 2018; Kalpana et al. 2019; Wang et al. 2022). The current findings suggest that CCR5 can stimulate perineural invasion, which is considered a clinical predictor of metastasis and poor survival. Survival analyses using CCR5 showed a weak association with OS, although it has been previously linked to poor survival outcomes (Gonzalez-Arriagada et al. 2018; Wang et al. 2022), but its relationship with metastasis support it as a probable biomarker for poor prognosis.
CCL5 can bind to CCR5 and CCR3, and both receptors’ expression has been associated with lower survival in gastric cancer (Sugasawa et al. 2008), suggesting that targeting therapies could be more effective if both receptors are inhibited. In cell lines, we observed a higher expression of CCR5 than other receptors, except in Cal27, where CCR3 is also highly expressed (Chuang et al. 2009; Gonzalez-Arriagada et al. 2018).
Maraviroc was used to inhibit CCR5, reducing cell proliferation and increasing apoptosis in OSCC cell lines in a time- and dose-dependent manner, similar to reports in other cancer types (Huang et al. 2020; Pervaiz et al. 2015; 2019). Long-term Maraviroc treatment has been reported as safe in patients (Gulick et al. 2014; Piconi et al. 2019). Interestingly, cells transfected to inhibit CCR5 still responded to CCL5 stimulation, possibly because CCL5 can bind CCR3 (Guan et al. 2023; Kodama et al. 2020), which may increase lymphocytes activity during Maraviroc treatment (Hunt et al. 2013). Furthermore, even transfected cells responded positively to Maraviroc and reduced cell proliferation, indicating that it can possibly inhibit other chemokine receptors.
In vivo experiments exhibited highly infiltrative tumors with perineural invasion. We observed a trend suggesting that Maraviroc treatment reduces the number of metastatic foci, although it did not reach statistical significance. Additionally, treated mice exhibited better conditions for feeding and weight. These findings align with previous studies on gastric cancer (Halvorsen et al. 2016; Mencarelli et al. 2013; Velasco-Velazquez et al. 2012). Maraviroc treatment led to a smaller tumor size, though this difference did not attain statistical significance. Additionally, the treated group exhibited higher body weight compared to the control group. Our results suggest that CCR5 inhibition is a promising alternative for managing OSCC.
Conclusion
In conclusion, Maraviroc could be considered as a therapeutical alternative for OSCC, and CCR5/CCL5 axis proteins may serve as a biomarker of poor prognosis. Further research on the CCR5/CCL5 axis is needed to validate our findings as clinical biomarkers and to improve treatment, increasing survival rates of OSCC patients.
Supplementary Information
Below is the link to the electronic supplementary material.
Author contributions
WAGA, RDC, IEG: Study concept and design. WAGA, JM, CG, BGP, RMF, SOP, IEG: Performed research and analyzed data. WAGA, CLB, FAM, IEG: Resource provision. WGA, RDC, IEG: Writing-original draft. All authors: Writing-review and editing.
Funding
This work was supported by grants from Agencia Nacional de Investigación y Desarrollo (ANID) de Chile: Fondecyt Regular 1190775 to WAGA, Fondecyt Regular 1230875 to FAM, Fondecyt de Iniciación 11180531 to IEG, and ANID-Basal funding for Scientific and Technological Center of Excellence, IMPACT, #FB210024 to FAM & WAGA.
Data availability
All analyzed and raw derivative data are available on request.
Declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethical approval
For research using human specimens, the study was approved by Research Ethics Committee of the Universidad de los Andes (CEC2022020) and the Research Ethics Committee of the Universidad de Valparaíso (CEC195-19), following the Helsinki Declaration and its later amendments. Approval for the animal experiments was granted by the Bioethics Committee for Animal Studies of Universidad de Valparaíso (BEA135-19) and Universidad de los Andes (CEC2022020). The study was also approved by the Universidad de Valparaíso Biosecurity Committee (BS0001-19).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
3/16/2024
The original version of the article was revised to correct the error in the article title.
References
- Baba A, Okuyama Y, Ikeda K, Kozakai A, Suzuki T, Saito H, Ogane S, Yamazoe S, Yamauchi H, Ogino N, Seto Y, Kobashi Y, Mogami T, Ojiri H (2019) Undetectability of oral tongue cancer on magnetic resonance imaging; clinical significance as a predictor to avoid unnecessary elective neck dissection in node negative patients. Dentomaxillofac Radiol 48(3):20180272. 10.1259/dmfr.20180272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai S, Wu Y, Yan Y, Kang H, Zhang J, Ma W, Gao Y, Hui B, Zhang X, Ren J (2020) The effect of CCL5 on the immune cells infiltration and the prognosis of patients with kidney renal clear cell carcinoma. Int J Med Sci 17(18):2917–2925. 10.7150/ijms.51126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borges GA, Elias ST, Amorim B, de Lima CL, Coletta RD, Castilho RM, Squarize CH, Silva EN (2020) Curcumin downregulates the PI3K-AKT-mTOR pathway and inhibits growth and progression in head and neck cancer cells. Phytother Res 34(12):3311–3324. 10.1002/ptr.6780 [DOI] [PubMed] [Google Scholar]
- Cao Z, Xu X, Luo X, Li L, Huang B, Li X, Tao D, Hu J, Gong J (2011) Role of RANTES and its receptor in gastric cancer metastasis. J Huazhong Univ Sci Technolog Med Sci 31(3):342–347. 10.1007/s11596-011-0378-3 [DOI] [PubMed] [Google Scholar]
- Capote-Moreno A, Brabyn P, Muñoz-Guerra MF, Sastre-Perez J, Escorial-Hernandez V, Rodríguez-Campo FJ, García T, Naval-Gías L (2020) Oral squamous cell carcinoma: epidemiological study and risk factor assessment based on a 39-year series. Int J Oral Maxillofac Surg 49(12):1525–1534. 10.1016/j.ijom.2020.03.009 [DOI] [PubMed] [Google Scholar]
- Chen M, Yang X, Yang M, Zhang W, Li L, Sun Q (2019) Identification of a novel biomarker-CCL5 using antibody microarray for colorectal cancer. Pathol Res Pract 215(5):1033–1037. 10.1016/j.prp.2019.02.011 [DOI] [PubMed] [Google Scholar]
- Chuang JY, Yang WH, Chen HT, Huang CY, Tan TW, Lin YT, Hsu CJ, Fong YC, Tang CH (2009) CCL5/CCR5 axis promotes the motility of human oral cancer cells. J Cell Physiol 220(2):418–426. 10.1002/jcp.21783 [DOI] [PubMed] [Google Scholar]
- da Silva JM, Soave DF, Moreira TP, Batista AC, Russo RC, Teixeira MM, da Silva TA (2016) Significance of chemokine and chemokine receptors in head and neck squamous cell carcinoma: a critical review. Oral Oncol 56:8–16. 10.1016/j.oraloncology.2016.02.016 [DOI] [PubMed] [Google Scholar]
- Dantas TS, de Barros PG, Sousa EF, da Cunha MDP, de Aguiar ASW, Costa FWG, Mota MRL, Alves AP, Sousa FB (2016) Influence of educational level, stage, and histological type on survival of oral cancer in a Brazilian population: a retrospective study of 10 years observation. Med 95(3):e2314. 10.1097/MD.0000000000002314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deo SV, Shukla NK, Jha D, Khanna P, Pandit A, Thulkar S (2012) Are We over-treating neck in buccal & alveolo-buccal cancers: experience from a tertiary cancer care center. Indian J Surg Oncol 3(4):272–275. 10.1007/s13193-012-0173-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domingueti CB, Janini JBM, Paranaíba RLM, Lozano-Burgos C, Olivero P, González-Arriagada WA (2019) Prognostic value of immunoexpression of CCR4, CCR5, CCR7 and CXCR4 in squamous cell carcinoma of tongue and floor of the mouth. Med Oral Patol Oral Cir Bucal 24(3):e354–e363. 10.4317/medoral.22904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dourado MR, Elseragy A, da Costa BC, Téo FH, Guimarães GN, Machado RA, Risteli M, Wahbi W, Gurgel CA, Ribeiro LM, González-Arriagada WA, da Silva SD, Carrinho AL, Rocha M, Rossa C, Salo T, Coletta RD (2022) Stress induced phosphoprotein 1 overexpression controls proliferation, migration and invasion and is associated with poor survival in oral squamous cell carcinoma. Front Oncol 12:1085917. 10.3389/fonc.2022.1085917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao T, Shen Z, Ma C, Li Y, Kang X, Sun M (2018) The CCL5/CCR5 chemotactic pathway promotes perineural invasion in salivary adenoid cystic carcinoma. J Oral Maxillofac Surg 76(8):1708–1718. 10.1016/j.joms.2018.02.009 [DOI] [PubMed] [Google Scholar]
- Gong R, Ren H (2020) Targeting chemokines/chemokine receptors: a promising strategy for enhancing the immunotherapy of pancreatic ductal adenocarcinoma. Signal Transduct Target Ther 5:149. 10.1038/s41392-020-00267-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Arriagada WA, Lozano-Burgos C, Zuniga-Moreta R, Gonzalez-Diaz P, Coletta RD (2018) Clinicopathological significance of chemokine receptor (CCR1, CCR3, CCR4, CCR5, CCR7 and CXCR4) expression in head and neck squamous cell carcinomas. J Oral Pathol Med 47(8):755–763. 10.1111/jop.12736 [DOI] [PubMed] [Google Scholar]
- Gonzalez-Arriagada WA, Garcia IE, Martinez-Flores R, Morales-Pison S, Coletta RD (2022) Therapeutic perspectives of HIV-associated chemokine receptor (CCR5 and CXCR4) antagonists in carcinomas. Int J Mol Sci 24(1):478. 10.3390/ijms24010478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan B, Li H, Yao J, Guo J, Yu F, Li G, Wan B, Ma J, Huang D, Sun L, Chen Y (2023) CCL3-CCR5 axis promotes cell migration and invasion of colon adenocarcinoma via Akt signaling pathway. Environ Toxicol 38(1):172–184. 10.1002/tox.23675 [DOI] [PubMed] [Google Scholar]
- Gulick RM, Fatkenheuer G, Burnside R, Hardy WD, Nelson MR, Goodrich J, Mukwaya G, Portsmouth S, Heera JR (2014) Five-year safety evaluation of maraviroc in HIV-1-infected treatment-experienced patients. J Acquir Immune Defic Syndr 65(1):78–81. 10.1097/QAI.0b013e3182a7a97a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haag GM, Springfeld C, Grün B, Apostolidis L, Zschäbitz S, Dietrich M, Berger AK, Weber TF, Zoernig I, Schaaf M, Waberer L, Müller DW, Al-Batran SE, Niels H, Jaeger D (2022) Pembrolizumab and maraviroc in refractory mismatch repair proficient/microsatellite-stable metastatic colorectal cancer—the PICCASSO phase I trial. Eur J Cancer 167:112–122. 10.1016/j.ejca.2022.03.017 [DOI] [PubMed] [Google Scholar]
- Halvorsen EC, Hamilton MJ, Young A, Wadsworth BJ, LePard NE, Lee HN, Firmino N, Collier JL, Bennewith KL (2016) Maraviroc decreases CCL8-mediated migration of CCR5(+) regulatory T cells and reduces metastatic tumor growth in the lungs. Oncoimmunology 5(6):e1150398. 10.1080/2162402X.2016.1150398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemmatazad H, Berger MD (2021) CCR5 is a potential therapeutic target for cancer. Expert Opin Ther Targets 25(4):311–327. 10.1080/14728222.2021.1902505 [DOI] [PubMed] [Google Scholar]
- Huang H, Zepp M, Georges RB, Jarahian M, Kazemi M, Eyol E, Berger MR (2020) The CCR5 antagonist maraviroc causes remission of pancreatic cancer liver metastasis in nude rats based on cell cycle inhibition and apoptosis induction. Cancer Lett 474:82–93. 10.1016/j.canlet.2020.01.009 [DOI] [PubMed] [Google Scholar]
- Huang R, Guo L, Gao M, Li J, Xiang S (2021) Research trends and regulation of CCL5 in prostate cancer. Onco Targets Ther 14:1417–1427. 10.2147/OTT.S279189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt PW, Shulman NS, Hayes TL, Dahl V, Somsouk M, Funderburg NT, McLaughlin B, Landay AL, Adeyemi O, Gilman LE, Clagett B, Rodriguez B, Martin JN, Schacker TW, Shacklett BL, Palmer S, Lederman MM, Deeks SG (2013) The immunologic effects of maraviroc intensification in treated HIV-infected individuals with incomplete CD4+ T-cell recovery: a randomized trial. Blood 121(23):4635–4646. 10.1182/blood-2012-06-436345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin K, Pandey NB, Popel AS (2018) Simultaneous blockade of IL-6 and CCL5 signaling for synergistic inhibition of triple-negative breast cancer growth and metastasis. Breast Cancer Res 20:54. 10.1186/s13058-018-0981-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalpana G, Figy C, Yeung M, Yeung KC (2019) Reduced RhoA expression enhances breast cancer metastasis with a concomitant increase in CCR5 and CXCR4 chemokines signaling. Sci Rep 9:16351. 10.1038/s41598-019-52746-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodama T, Koma YI, Arai N, Kido A, Urakawa N, Nishio M, Shigeoka M, Yokozaki H (2020) CCL3-CCR5 axis contributes to progression of esophageal squamous cell carcinoma by promoting cell migration and invasion via Akt and ERK pathways. Lab Invest 100:1140–1157. 10.1038/s41374-020-0441-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolegova ES, Patysheva MR, Larionova IV, Fedorova IK, Kulbakin DE, Choinzonov EL, Denisov EV (2022) Early-onset oral cancer as a clinical entity: aetiology and pathogenesis. Int J Oral Maxillofac Surg 51(12):1497–1509. 10.1016/j.ijom.2022.04.005 [DOI] [PubMed] [Google Scholar]
- Li C, Chen S, Liu C, Mo C, Gong W, Hu J, He M, Xie L, Hou X, Tang J, Ou M (2022a) CCR5 as a prognostic biomarker correlated with immune infiltrates in head and neck squamous cell carcinoma by bioinformatic study. Hereditas 159:37. 10.1186/s41065-022-00251-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Wu M, Zhao X (2022b) Role of chemokine systems in cancer and inflammatory diseases. MedComm 3(2):e147. 10.1002/mco2.147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin NC, Hsien SI, Hsu JT, Chen MYC (2020) Impact on patients with oral squamous cell carcinoma in different anatomical subsites: a single-center study in Taiwan. Sci Rep 11:15446. 10.1038/s41598-021-95007-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling Z, Cheng B, Tao X (2021) Epithelial-to-mesenchymal transition in oral squamous cell carcinoma: challenges and opportunities. Int J Cancer 148(7):1548–1561. 10.1002/ijc.33352 [DOI] [PubMed] [Google Scholar]
- Madera MV, Franco JV, Merchan-Galvis AM, Gallardo CR, Bonfill X (2018) Quality assessment of clinical practice guidelines on treatments for oral cancer. Cancer Treat Rev 65:47–53. 10.1016/j.ctrv.2018.03.001 [DOI] [PubMed] [Google Scholar]
- Manfroi B, De Grandis M, Moreaux J, Tabruyn S, Mayol JF, Quintero M, Righini C, Sturm N, Aurrand-Lions M, Huard B (2021) The microenvironment of DLBCL is characterized by noncanonical macrophages recruited by tumor-derived CCL5. Blood Adv 5(21):4338–4351. 10.1182/bloodadvances.2021004203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mencarelli A, Graziosi L, Renga B, Cipriani S, D’Amore C, Francisci D, Bruno A, Baldelli F, Donini A, Fiorucci S (2013) CCR5 antagonism by maraviroc reduces the potential for gastric cancer cell dissemination. Transl Oncol 6(6):784–793. 10.1593/tlo.13499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neckel N, Michael M, Troeltzsch D, Wüster J, Koerdt S, Doll C, Jöhrens K, Neumann K, Heiland M, Raguse JD (2020) Rediscussing the role of traditional risk factors in young adults with oral squamous cell carcinoma. Anticancer Res 40(12):6987–6995. 10.21873/anticanres.14723 [DOI] [PubMed] [Google Scholar]
- Onyeisi JOS, Pernambuco PCA, Mesquita APS, Azevedo LC, Nader HB, Lopes CC (2020) Effects of syndecan-4 gene silencing by micro RNA interference in anoikis resistant endothelial cells: Syndecan-4 silencing and anoikis resistance. Int J Biochem Cell Biol 128:105848. 10.1016/j.biocel.2020.105848 [DOI] [PubMed] [Google Scholar]
- Pervaiz A, Ansari S, Berger MR, Adwan H (2015) CCR5 blockage by maraviroc induces cytotoxic and apoptotic effects in colorectal cancer cells. Med Oncol 32(5):158. 10.1007/s12032-015-0607-x [DOI] [PubMed] [Google Scholar]
- Pervaiz A, Zepp M, Mahmood S, Ali DM, Berger MR, Adwan H (2019) CCR5 blockage by maraviroc: a potential therapeutic option for metastatic breast cancer. Cell Oncol (dordr) 42(1):93–106. 10.1007/s13402-018-0415-3 [DOI] [PubMed] [Google Scholar]
- Piconi S, Foschi A, Malagoli A, Carli F, Zona S, Milic J, Ricci ED, Rizzardini G, Guaraldi G (2019) Impact of prolonged maraviroc treatment on non-AIDS-related comorbidities in HIV-positive patients: a retrospective cohort study. J Antimicrob Chemother 74(9):2723–2731. 10.1093/jac/dkz227 [DOI] [PubMed] [Google Scholar]
- Qiu J, Xu L, Zeng X, Wu H, Liang F, Lv Q, Du Z (2022) CCL5 mediates breast cancer metastasis and prognosis through CCR5/Treg cells. Front Oncol 12:972383. 10.3389/fonc.2022.972383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satgunaseelan L, Allanson BM, Asher R, Reddy R, Low HTH, Veness M, Iyer NG, Smee RI, Palme CE, Gupta R, Clark JR (2020) The incidence of squamous cell carcinoma of the oral tongue is rising in young non-smoking women: an international multi-institutional analysis. Oral Oncol 110:104875. 10.1016/j.oraloncology.2020.104875 [DOI] [PubMed] [Google Scholar]
- Singh SK, Mishra MK, Eltoum IA, Bae S, Lillard JW Jr, Singh R (2018) CCR5/CCL5 axis interaction promotes migratory and invasiveness of pancreatic cancer cells. Sci Rep 8(1):1323. 10.1038/s41598-018-19643-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh SK, Mishra MK, Rivers BM, Gordetsky JB, Bae S, Singh R (2020) Biological and clinical significance of the CCR5/CCL5 axis in hepatocellular carcinoma. Cancers (basel) 12(4):883. 10.3390/cancers12040883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobral LM, Bufalino A, Lopes MA, Graner E, Salo T, Coletta RD (2011) Myofibroblasts in the stroma of oral cancer promote tumorigenesis via secretion of activin A. Oral Oncol 47(9):840–846. 10.1016/j.oraloncology.2011.06.011 [DOI] [PubMed] [Google Scholar]
- Somaida A, Tariq I, Ambreen G, Abdelsalam AM, Ayoub AM, Wojcik M, Dzoyem JP, Bakowsky U (2020) Potent cytotoxicity of four cameroonian plant extracts on different cancer cell lines. Pharm 13(11):357. 10.3390/ph13110357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepan KO, Mazul AL, Larson J, Shah P, Jackson RS, Pipkorn P, Kang SY, Puram SV (2023) Changing epidemiology of oral cavity cancer in the United States. Otolaryngol Head Neck Surg 168(4):761–768. 10.1177/01945998221098011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugasawa H, Ichikura T, Tsujimoto H, Kinoshita M, Morita D, Ono S, Chochi K, Tsuda H, Seki S, Mochizuki H (2008) Prognostic significance of expression of CCL5/RANTES receptors in patients with gastric cancer. J Surg Oncol 97(5):445–450. 10.1002/jso.20984 [DOI] [PubMed] [Google Scholar]
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F (2021) Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71(3):209–249. 10.3322/caac.21660 [DOI] [PubMed] [Google Scholar]
- Szturz P, Vermorken JB (2020) Management of recurrent and metastatic oral cavity cancer: raising the bar a step higher. Oral Oncol 101:104492. 10.1016/j.oraloncology.2019.104492 [DOI] [PubMed] [Google Scholar]
- Ucuncu M, Serilmez M, Sari M, Bademler S, Karabulut S (2019) The diagnostic significance of PDGF, EphA7, CCR5, and CCL5 levels in colorectal cancer. Biomol 9(9):464. 10.3390/biom9090464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velasco-Velazquez M, Jiao X, De La Fuente M, Pestell TG, Ertel A, Lisanti MP, Pestell RG (2012) CCR5 antagonist blocks metastasis of basal breast cancer cells. Cancer Res 72(15):3839–3850. 10.1158/0008-5472.CAN-11-3917 [DOI] [PubMed] [Google Scholar]
- Wang T, Wei Y, Tian L, Song H, Ma Y, Yao Q, Feng M, Wang Y, Gao M, Xue Y (2016) C-C motif chemokine ligand 5 (CCL5) levels in gastric cancer patient sera predict occult peritoneal metastasis and a poorer prognosis. Int J Surg 32:136–142. 10.1016/j.ijsu.2016.07.008 [DOI] [PubMed] [Google Scholar]
- Wang L, Li L, Zhu G (2021) Role of extracellular vesicles on cancer lymphangiogenesis and lymph node metastasis. Front Oncol 11:721785. 10.3389/fonc.2021.721785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Tao Z, Tian Z, Jin J, Dong J, Dai Y, Yu W, Tang B, Hu S (2022) CCR5 as a prognostic factor in lower-grade glioma is involved in the remodeling of the tumor microenvironment. Front Genet 13:874896. 10.3389/fgene.2022.874896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng CJ, Chien MH, Lin CW, Chung TT, Zavras AI, Tsai CM, Mk C, Yang SF (2010) Effect of CC chemokine ligand 5 and CC chemokine receptor 5 genes polymorphisms on the risk and clinicopathological development of oral cancer. Oral Oncol 46(10):767–772. 10.1016/j.oraloncology.2010.07.011 [DOI] [PubMed] [Google Scholar]
- Willenbrock F, Cox CM, Parkes EE, Wilhelm-Benartzi CS, Abraham AG, Owens R, Sabbagh A, Jones CM, Hughes DLI, Maughan T, Hurt CN, O’Neill EE, Mukherjee S (2021) Circulating biomarkers and outcomes from a randomised phase 2 trial of gemcitabine versus capecitabine-based chemoradiotherapy for pancreatic cancer. Br J Cancer 124(3):581–586. 10.1038/s41416-020-01120-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Wu W, Cheng G, Qian M, Hu K, Yin G, Wang S (2017) Enhancement of proliferation and invasion of gastric cancer cell by KDM5C via decrease in p53 expression. Technol Cancer Res Treat 16(2):141–149. 10.1177/1533034616629261 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Yang H, Son NH, Lee SH, Kim D, Kim HJ, Cha IH, Nam W (2021) Predictive modelling of level IIb lymph node metastasis in oral squamous cell carcinoma. Sci Rep 11(1):17562. 10.1038/s41598-021-96827-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng Z, Lan T, Wei Y, Wei X (2022) CCL5/CCR5 axis in human diseases and related treatments. Genes Dis 9(1):12–27. 10.1016/j.gendis.2021.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All analyzed and raw derivative data are available on request.