Abstract
Introduction
The best first-line treatment for patients with advanced hepatocellular carcinoma (HCC) and Child–Pugh (CP) class B remains unknown. The aim of the present study was to perform a real-world analysis on a large sample of patients with unresectable HCC with CP B treated with atezolizumab plus bevacizumab Vs Lenvatinib.
Methods
The study population included patients affected by advanced (BCLC-C) or intermediate (BCLC-B) HCC patients not suitable for locoregional therapies from both the Western and Eastern world (Italy, Germany, Republic of Korea and Japan), who received atezolizumab plus bevacizumab or Lenvatinib as first-line treatment. All the study population presented a CP class of B. The primary endpoint of the study was the overall survival (OS) of CP B patients treated with Lenvatinib compared to atezolizumab plus bevacizumab. Survival curves were estimated using the product-limit method of Kaplan–Meier. The role of stratification factors was analyzed with log-rank tests. Finally, an interaction test was performed for the main baseline clinical characteristics.
Results
217 CP B HCC patients were enrolled in the study: 65 (30%) received atezolizumab plus bevacizumab, and 152 (70%) received lenvatinib. The mOS for patients receiving Lenvatinib was 13.8 months (95% CI: 11.6–16.0), compared to 8.2 months (95% CI 6.3–10.2) for patients receiving atezolizumab plus bevacizumab as first-line treatment (atezolizumab plus bevacizumab Vs Lenvatinib: HR 1.9, 95% CI 1.2–3.0, p = 0.0050). No statistically significant differences were highlighted in terms of mPFS. The multivariate analysis confirmed that patients receiving Lenvatinib as first-line treatment have a significantly longer OS compared to patients receiving atezolizumab plus bevacizumab (HR 2.01; 95% CI 1.29–3.25, p = 0.0023). By evaluating the cohort of patients who received atezolizumab plus bevacizumab, we found that Child B patients with ECOG PS 0, or BCLC B stage or ALBI grade 1 were those who had benefited from the treatment thus showing survival outcomes no significantly different compared to those receiving Lenvatinib.
Conclusion
The present study suggests for the first time a major benefit from Lenvatinib compared to atezolizumab plus bevacizumab in a large cohort of patients with CP B class HCC.
Keywords: Advanced HCC, Atezolizumab, Bevacizumab, Real Word
Introduction
With over 830,000 annual deaths worldwide, hepatocellular carcinoma (HCC) represents the third leading cause of cancer death worldwide (Llovet et al. 2021). Multi-kinase inhibitors (MKIs) constituted the milestones for treatments of unresectable stages for many years. In 2008 Sorafenib was approved as first-line standard of care (Llovet et al. 2008; Cheng et al. 2009), followed by Lenvatinib, whose no inferiority was demonstrated in the REFLECT trial (Kudo et al. 2018). Recently, the therapeutic paradigm has completely changed thanks to the advent of immunotherapy for unresectable HCC patients. The results from the IMbrave150 trial led to the approval of the combination of the anti- Programmed death ligand-1 (anti-PDL1) atezolizumab with the monoclonal antibody directed against the vascular endothelial growth factor (VEGF) bevacizumab as new first-line standard of care (Finn et al. 2020). In particular, the combination of atezolizumab plus bevacizumab demonstrated a survival benefit over sorafenib in terms of both OS (19.2 vs 13.4 months; p < 0.001) and PFS (6.9 vs 4.3 months; p < 0.001). The phase III HIMALAYA trial investigates the combination of a single priming dose of the anti-cytotoxic T-lymphocyte antigen 4 (CTLA-4) tremelimumab plus the anti-PD-L1 durvalumab compared to sorafenib (Abou-Alfa et al. 2022), thus reporting a significant improvement in OS, while the phase III COSMIC-312 trial showed improved PFS with the combination cabozantinib plus atezolizumab over sorafenib, with no improvement in terms of OS (Kelley et al. 2022). Finally, results from the phase III LEAP-002 trial were recently presented: no superiority of the combination Lenvatinib plus the anti-PD-1 Pembrolizumab were reported in terms of both OS and PFS compared to Lenvatinib alone as first-line treatment (Finn, et al. 2022), whereas another anti-PD-1 antibody Camrelizumab has demonstrated to improve survival outcomes when received in combination with the anti-antiangiogenic MKI Rivoceranib as first-line treatment in a cohort of patients with unresectable HCC (Qin et al. 2022). Due to the dramatic expansion of the therapeutic options for patients affected by unresectable HCC, the definition of the candidacy criteria for immunotherapy rather than MKIs outside clinical trials has become an urgent need. A critic point in the HCC field concerns the treatment of patients with an impaired liver function (Child Pugh class B) since those patients are normally excluded from randomized clinical trials. In clinical practice, patients with a Child Pugh class B could be a candidate for systemic treatment after multidisciplinary discussion as a likely consequence of the good results in terms of both survival and improvement of quality of life reached by the main randomized clinical trials; nevertheless, only a few data are already available on safety and efficacy of the most used first-line therapies (Lenvatinib and atezolizumab plus bevacizumab) in patients with Child-Pugh B in real-world setting. Consequently, the best therapeutic approach in first-line setting for this extended and heterogeneous group of patients remains unknown. The aim of the present study was to perform a real-world analysis on a large sample of patients with unresectable HCC with CP B treated with atezolizumab plus bevacizumab Vs Lenvatinib Vs sorafenib as first-line treatment.
Materials and methods
Study population and procedures
The study population included patients affected by advanced (BCLC-C) or intermediate (BCLC-B) HCC patients not suitable for locoregional therapies from 45 institutions from both the Western and Eastern world (Italy, Germany, Republic of Korea and Japan). Patients accrued were all treated in tertiary centers with high volume and high expertise in HCC treatment. Baseline characteristics, including gender, age, underlying liver disease, body mass index (BMI), ECOG performance status, liver function classified according to the ALBI grade, neutrophil–lymphocyte ratio (NLR), and tumor-specific characteristics, including disease stage according to BCLC classification, presence of portal vein thrombosis, presence of extrahepatic disease, alpha-fetoprotein levels and subsequent systemic treatments were collected. Patients underwent treatment with atezolizumab plus bevacizumab between December 2018 and May 2022, or Lenvatinib between June 2018 and August 2021 or sorafenib between September 2009 and December 2019 as first-line treatment. Eligible patients had Child–Pugh B (B7–B8) histologically confirmed or clinically confirmed diagnosis of HCC according to international guidelines, and no previous systemic therapy.
All patients received Lenvatinib until Atezolizumab plus bevacizumab approval. Then, the choice between the two treatments was left to the treating physician. Lenvatinib was administered according to the REFLECT trial (Kudo et al. 2018), thus patients received 12 mg if baseline bodyweight was ≥ 60 kg or 8 mg if baseline bodyweight was < 60 kg, once daily orally. Atezolizumab plus bevacizumab was administered as reported in the IMbrave150 trial, thus all patients received 1200 mg of atezolizumab plus 15 mg/kg of body weight of bevacizumab intravenously every 3 weeks (Freites-Martinez et al. 2021). Treatment ‘decision was taken according to the physicians’ choice and after a multidisciplinary board. Treatment response was evaluated by computed tomography or magnetic resonance imaging, and categorized as complete response (CR), partial response (PR), stable disease (SD) or progression disease (PD) by local review according to the modified Response Evaluation Criteria in Solid Tumors (mRECIST) 1.1 (Lencioni and Llovet 2010 Feb).
AEs were graded using the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE) version 5.0 (Freites-Martinez et al. 2021). Treatment interruptions and/or dose reductions were allowed to manage adverse events (AEs).
The present study was approved by Ethics Committee at each center, complied with the provisions of the Good Clinical Practice guidelines and the Declaration of Helsinki and local laws, and fulfilled the Regulation (EU) 2016/679 of the European Parliament and of the Council of 27 April 2016 on the protection of natural persons with regard to the processing of personal data.
Statistical analysis
Categorical variables were compared using Fisher exact test, whereas continuous variables were compared using the t-test.
The primary endpoint of the study was the overall survival (OS) of CP B patients treated with Lenvatinib compared to atezolizumab plus bevacizumab.
The secondary endpoints included the progression-free survival (PFS), the objective response rate (ORR) and the safety profile experienced in Child B patients receiving Lenvatinib versus atezolizumab plus bevacizumab.
OS was defined as the time from the start date of the first-line treatment to the date of death. PFS was defined as the time from the start date of first-line treatment to the date of progression or death or last follow-up whichever occurred first. OS and PFS were reported as median values expressed in months, with 95% confidence interval (CI).
ORR was defined as the proportion of patients who achieved a CR or a PR; disease control rate (DCR) was defined as the proportion of patients who achieved an ORR or a SD.
Survival curves were estimated using the product-limit method of Kaplan–Meier. The role of stratification factors was analyzed with log-rank tests.
Unadjusted and adjusted hazard ratios (HRs) by baseline characteristics were calculated using the Cox proportional hazards model.
Finally, an interaction test was performed for the main baseline clinical characteristics to define the eventual predictor factor of response to atezolizumab plus bevacizumab in Child B patients.
A p value < 0.05 was considered statistically significant.
A MedCalc package (MedCalc® version 16.8.4) was used for statistical analysis.
Results
Study population
Overall, 217 Child Pugh B HCC patients were enrolled in the study: 65 (30%) received atezolizumab plus bevacizumab, and 152 (70%) received lenvatinib. Median age was 70 (45–90) in the group of patients receiving atezolizumab plus bevacizumab, and 71 (40–91) in the group of patients receiving Lenvatinib. The two groups of patients were almost homogeneous, without significant differences in terms of baseline characteristics (Table 1). In particular, the reserving liver function was similar between the two treatment ‘arms, as well as the tumor load, with a proportion of patients presenting with extra-hepatic disease without significant differences. Overall, the proportion of patients with a viral etiology was significantly higher compared to those with a NASH/NAFLD etiology (47 Vs 20%). Interestingly, a significant proportion of the whole cohort of patients presented a BCLC C stage at the initiation of systemic therapy, which was reflected and comparable in the two arms of patients, those receiving atezolizumab plus bevacizumab and those receiving Lenvatinib (71 Vs 64%, p = 0.351). Moreover, 42, 36 and 60% of the entire cohort was diagnosed with an ECOG PS ≥ 1, AFP > 400 and ALBI grade > 1, respectively. No statistically significant differences were found in terms of these baseline clinical characteristics in the two arms of treatment. Finally, 11% of patients who received atezolizumab plus bevacizumab and the 26% of patients who received Lenvatinib was treated with a subsequent anticancer systemic therapy after progression to the first line (p = 0.119).
Table 1.
Patients' characteristics
| Whole population N = 217 | Pts treated with Atezo/Beva N = 65 (%) | Pts treated with lenvatinib N = 152 (%) | p | |
|---|---|---|---|---|
| Gender | ||||
| Male | 173 (80) | 58 (89) | 115 (76) | 0.027 |
| Female | 44 (20) | 7 (11) | 37 (24) | |
| Age | ||||
| > 70 years | 116 (53) | 36 (55) | 80 (53) | 0.767 |
| ≤ 70 years | 101 (47) | 29 (45) | 72 (47) | |
| Ethnia | ||||
| European | 22 (10) | 15 (23) | 7 (5) | 0.000105 |
| Asiatic | 195 (90) | 50 (77) | 145 (95) | |
| Etiology | ||||
| Viral | 103 (47) | 29 (45) | 74 (49) | 0.772 |
| (HCV + HBV) | 44 (20) | 15 (23) | 29 (19) | |
| NASH/NAFLD | 70 (33) | 21 (32) | 49 (32) | |
| Other | ||||
| Surgery | ||||
| Yes | 43 (20) | 14 (21.5) | 29 (19) | |
| No | 174 (80) | 51 (78.5) | 123 (81) | 0.712 |
| BCLC | ||||
| B | 74 (34) | 19 (29) | 55 (36) | |
| C | 143 (66) | 46 (71) | 97 (64) | 0.351 |
| ECOG PS | ||||
| 0 | 132 (51) | 38 (58.5) | 94 (62) | 0.651 |
| ≥ 1 | 85 (42) | 27 (41.5) | 58 (38) | |
| Portal Vein | ||||
| Thrombosis | ||||
| Yes | 59 (27) | 19 (29) | 40 (26) | 0.739 |
| No | 158 (73) | 46 (71) | 112 (74) | |
| Extrahepatic | ||||
| Disease | ||||
| Yes | 68 (31) | 25 (38) | 43 (28) | 0.152 |
| No | 149 (69) | 40 (62) | 109 (72) | |
| AFP | ||||
| > 400 | 78 (36) | 24 (37) | 54 (36) | 0.529 |
| ≤ 400 | 131 (60) | 35 (54) | 96 (63) | |
| NA | 8 (4) | 6 (9) | 2 (1) | |
| NLR | ||||
| > 3 | 91 (42) | 32 (49) | 59 (39) | 0.871 |
| ≤ 3 | 75 (35) | 28 (43) | 47 (31) | |
| NA | 51 (23) | 5 (8) | 46 (30) | |
| Bilirubin | ||||
| > 2 mg/dl | 40 (18) | 16 (24.5) | 24 (16) | 0.131 |
| ≤ 2 mg/dl | 173 (80) | 48 (74) | 125 (82) | |
| NA | 4 (2) | 1 (1.5) | 3 (2) | |
| ALBI | ||||
| 1 | 81 (37) | 27 (41.5) | 54 (36) | 0.439 |
| > 1 | 131 (60) | 36 (55.5) | 95 (62) | |
| NA | 5 (3) | 2 (3) | 3 (2) | |
| Subsequent antidrug therapies | ||||
| Yes | 46 (21) | 7 (11) | 39 (26) | 0.119 |
| No | 67 (31) | 4 (6) | 63 (41) | |
| NA | 104 (48) | 54 (83) | 50 (33) | |
Patients’ baseline characteristics of the whole cohort, of patients receiving atezolizumab plus bevacizumab and patients receiving Lenvatinib as first-line treatment are reported in Table 1.
Efficacy
The median follow-up in our analysis for the entire population was 11.3 months (95% CI 7.2–20.7). The median follow-up for patients receiving Lenvatinib was 12.2 months (95% CI 10.1–33.2); the median follow-up for patients receiving atezolizumab plus bevacizumab was 10.9 (95% CI 6.2–20.7).
In the whole population median mOS was 12.6 months (95% CI: 10.8–14.5) and median mPFS was of 8.1 months (95% CI 6.6–9.7). Overall, ORR was 30%, and DCR was 62% (Table 2).
Table 2.
Efficacy outcomes
| Overall population (N = 217) (%) | Lenvatinib arm (N = 152) (%) | Atezo + Beva Arm (N = 65) (%) | p | |
|---|---|---|---|---|
| CR | 8 (4) | 6 (4) | 2 (3) | 1.0000 |
| PR | 57 (26) | 47 (31) | 10 (15) | 0.0185 |
| ORR | 65 (30) | 53 (35) | 12 (18) | 0.0158 |
| DCR | 135 (62) | 99 (65) | 36 (55) | 0.2213 |
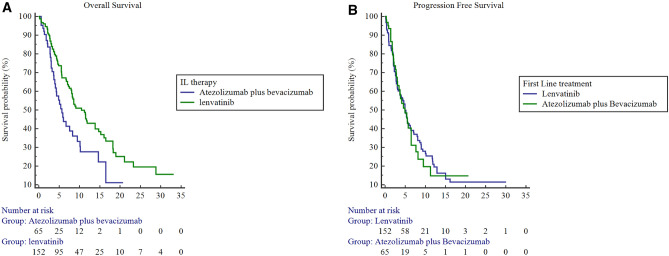
After splitting the population in the two treatment arms, we highlighted a mOS for patients receiving Lenvatinib 0f 13.8 months (95% CI: 11.6–16.0), compared to 8.2 months (95% CI 6.3–10.2) for patients receiving atezolizumab plus bevacizumab as first line treatment (atezolizumab plus bevacizumab Vs Lenvatinib: HR 1.9, 95% CI 1.2–3.0, p = 0.005) (Fig. 1A). In terms of PFS, no statistically significant differences were found between the two treatment arms since patients receiving Lenvatinib experienced an mPFS of 8.2 months (95% CI 6.5–9.9) compared to 6.9 months (95% CI:4.8–9.0) in patients receiving atezolizumab plus bevacizumab (atezolizumab plus bevacizumab Vs Lenvatinib: HR 1.0, 95% CI 0.7–1.5; p = 0.8443) (Fig. 1B). Statistically differences were reported in patients treated with lenvatinib compared to atezolizumab plus bevacizumab in terms of ORR (35 Vs 18%, p = 0.0185), but not in terms of DCR (65 Vs 55%, p = 0.2213 (Table 2).
Fig. 1.
Kaplan Meier for OS (A) and PFS (B) in patients with Child Pugh class B treated with Lenvatinib versus Atezolizumab plus Bevacizumab
At the univariate analysis, beyond the kind of systemic treatment (atezolizumab plus bevacizumab versus Lenvatinib), ECOG PS (0 versus > 0), NLR (≥ 3 versus < 3), basal total bilirubin level (> 2 versus ≤ 2 mg/dl), and ALBI grade (2 versus 1) resulted to have a statistically significant impact on OS in the whole population (Table 3).
Table 3.
Univariate and multivariate analysis
| Covariate | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p | HR | 95% CI | p | |
| Gender | ||||||
| Male | 1 | 1 | 0.92–2.60 | 0.0988 | ||
| Female | 1.15 | 0.72–1.81 | 0.5627 | 1.55 | ||
| Age | ||||||
| < 70 | 1.12 | 0.78–1.61 | 0.5373 | 1.09 | 0.71–1.68 | 0.6964 |
| ≥ 70 | 1 | 1 | ||||
| Etiology | ||||||
| Viral | 1.20 | 0.74–1.94 | 0.4659 | |||
| Non Viral | 1 | |||||
| Surgery | ||||||
| Yes | 1.12 | 0.69–1.81 | 0.6544 | |||
| No | 1 | |||||
| RFA | ||||||
| No | 1.15 | 0.76–1.72 | 0.5077 | |||
| Yes | 1 | |||||
| TACE | ||||||
| No | 1.36 | 0.93–1.97 | 0.1103 | |||
| Yes | 1 | |||||
| BCLC | ||||||
| C | 1.31 | 0.91–1.91 | 0.1508 | |||
| B | 1 | |||||
| ECOG PS | ||||||
| > 0 | 1.65 | 1.12–2.43 | 0.0114 | 1.41 | 0.92–2.18 | 0.1172 |
| 0 | 1 | 1 | ||||
| AFP | ||||||
| ≥ 400 | 1.38 | 0.93–2.03 | 0.1106 | |||
| < 400 | 1 | |||||
| NLR | ||||||
| ≥ 3 | 1.62 | 1.07–2.48 | 0.0230 | 1.50 | 0.98–2.31 | 0.0643 |
| < 3 | 1 | 1 | ||||
| EHD | ||||||
| Yes | 1.39 | 0.92–2.09 | 0.1197 | |||
| No | 1 | |||||
| Bilirubin | ||||||
| > 2 | 2.44 | 1.36–4.37 | 0.0028 | |||
| ≤ 2 | 1 | |||||
| ALBI | ||||||
| 2 | 1.62 | 1.12–2.34 | 0.0100 | 1.46 | 0.93–2.30 | 0.1005 |
| 1 | 1 | |||||
| IL Therapy | ||||||
| Atezolizumab | 1.9 | 1.21–2.99 | 0.0050 | 2.01 | 1.29–3.25 | 0.0023 |
| Plus | ||||||
| Bevacizumab | ||||||
| Lenvatinib | 1 | 1 | ||||
Following adjustment for clinical covariates positive at univariate analysis, multivariate analysis confirmed that patients receiving Lenvatinib as first-line treatment has a significantly longer OS compared to patients receiving atezolizumab plus bevacizumab (HR 2.01; 95% CI 1.29–3.25, p = 0.0023).
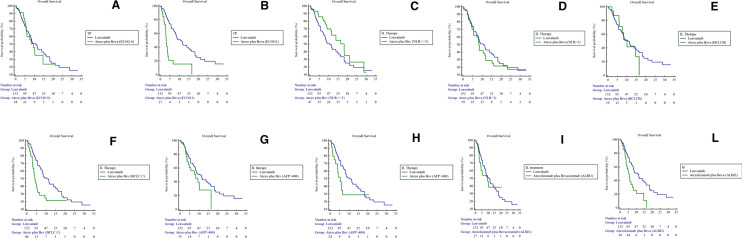
With the aim to find the clinical characteristics able to define which Child B patients could benefit from treatment with atezolizumab plus bevacizumab, we stratified patients in the atezolizumab plus bevacizumab arm on the basis of the main clinical covariates known to be related to survival in this setting of patients (ECOG PS, NLR, BCLC, AFP, ALBI grade). By evaluating the cohort of patients who received atezolizumab plus bevacizumab, we found that Child B patients with ECOG PS 0, or BCLC B stage or ALBI grade 1 were those who had benefit from the treatment thus showing survival outcomes no significantly different compared to those receiving Lenvatinib (Fig. 2). In particular, patients with ECOG 0 who received atezolizumab plus bevacizumab as first-line treatment showed a mOS of 10.6 months compared to 13.8 months in patients receiving Lenvatinib (HR 1.2, 95% CI 0.7–2.0, p = 0.6067); contrarily, patients with an ECOG PS 1 treated with atezolizumab plus bevacizumab as first-line treatment showed a mOS 4.2 months compared to 13.8 months for patients receiving Lenvatinib (HR 14.1, 95% CI 5.1–38.6, p < 0.0001). Patients with BCLC B who received atezolizumab plus bevacizumab as first-line treatment showed a mOS of 10.5 months compared to 13.8 months in patients receiving Lenvatinib (HR 1.1, 95% CI 0.5–2.3, p = 0.7655); contrarily, patients with an BCLC C treated with atezolizumab plus bevacizumab as first-line treatment showed a mOS 7.5 months compared to 13.8 months for patients receiving Lenvatinib (HR 2.7, 95% CI 1.5–4.6, p = 0.0005). Finally, patients with ALBI 1 who received atezolizumab plus bevacizumab as first-line treatment showed a mOS of 11.0 months compared to 13.8 months in patients receiving Lenvatinib (HR 1.2, 95% CI 0.6–2.4, p = 0.5210); contrarily, patients with an ALBI 2 treated with atezolizumab plus bevacizumab as first-line treatment showed a mOS 6.9 months compared to 13.8 months for patients receiving Lenvatinib (HR 2.7, 95% CI 1.5–5.0, p = 0.0008).
Fig. 2.
Kaplan Meier for OS in patients treated with Lenvatinib versus atezolizumab plus bevacizumab according to the ECOG PS (A, B), NLR (C, D), BCLC (E, F), AFP (G, H), ALBI score (I, L)
Then, we evaluated the predictive role of the main baseline clinical characteristics. Interaction test highlighted no prognostic role for ALBI, ECOG PS and BCLC in favor of the atezolizumab plus bevacizumab arm.
Safety
A safety analysis was conducted of the 217 patients included in our cohort (N = 65 treated with lenvatinib, and N = 152 treated with atezolizumab plus bevacizumab) (Table 4).
Table 4.
Adverse events
| Adverse events | Whole population N = 217 |
Pts treated with atezo/beva N = 65 (%) |
Pts treated with lenvatinib N = 152 (%) |
p |
|---|---|---|---|---|
| Any kind of AEs’ incidence | 95 (11) | 26 (10) | 69 (11.5) | |
| HSFR | ||||
| Yes | 27 (12) | 0 (0) | 27 (18) | 0.000058 |
| No | 190 (88) | 65 (100) | 125 (82) | |
| NA | 0 (0) | 0 (0) | 0 (0) | |
| Diarrhea | ||||
| Yes | 29 (13) | 8 (12) | 21 (14) | 0.831 |
| No | 188 (87) | 57 (88) | 131 (86) | |
| NA | 0 (0) | 0 (0) | 0 (0) | |
| Hypertension | ||||
| Yes | 30 (14) | 9 (14) | 21 (14) | 1 |
| No | 187 (86) | 56 (86) | 131 (86) | |
| NA | 0 (0) | 0 (0) | 0 (0) | |
| Immune-related toxicities | ||||
| Yes | 9 (4) | 9 (14) | 0 (0) | 0.000013 |
| No | 208 (96) | 56 (86) | 152 (100) | |
| NA | 0 (0) | 0 (0) | 0 (0) | |
The overall incidence of drug-related AEs was 44%. No statistically differences were reported in terms of drug-related AEs’ incidence in patients treated with atezolizumab plus bevacizumab and Lenvatinib (40 Vs 45%, respectively). Overall, the most frequent AEs highlighted in our cohort of patients were diarrhea (13%) and hypertension (14%). The quality of drug-related AEs experienced in the two treatment arms was quite different. As expected, patients receiving atezolizumab plus bevacizumab experienced a higher incidence of immune-related toxicities compared to patients receiving Lenvatinib (14 Vs 0%, respectively; p = 0.000013). On the other hand, patients receiving Lenvatinib experienced a significantly higher incidence of HFSR compared to patients receiving atezolizumab plus bevacizumab (18 Vs 0%, respectively; p = 0.000058). No statistically significant differences were reported in terms of the incidence of diarrhea and hypertension in patients included in the two treatment arms.
Discussion
The present analysis demonstrated for the first time that patients affected by unresectable HCC with a Child Pugh B may benefit more from Lenvatinib compared to the new standard of care atezolizumab plus bevacizumab. This result is even more significative if we focus on the comparable median follow-up in the two treatment arms (12.2 months Vs 10.9 months). Of note, no statistically differences were reported between the two treatment ‘arms in terms of liver function and tumor load at the baseline, including the extrahepatic disease. In the last years, several new treatment options have been investigated and approved for patients affected by unresectable HCC. Unfortunately, patients with a Child Pugh class B were excluded in the most important randomized control trials, thus treatment recommendations for this setting of patients are still lacking. Only a few treatments have been investigated in patients with Child B in retrospective studies, but sample sizes were small and limited endpoints were evaluated. Previous studies suggested that patients with a mildly impaired liver function defined as Child Pugh class B may benefit from systemic treatments, including both Lenvatinib and atezolizumab plus bevacizumab, even if with shorter survival and at a price of a higher incidence of adverse events compared to patients with Child–Pugh A. Huynh and collaborators recently performed a post hoc analysis of REFLECT trial to evaluate safety and efficacy of Lenvatinib in patients who deteriorated to Child Pugh B compared to those who remained Child Pugh class A within 8 weeks after randomization (Huynh et al. 2022). The analysis confirmed that patients experiencing a deterioration of liver function during treatment may continue treatment since no significative differences were reported in terms of PFS and no new safety signals were reported in patients with Child Pugh B compared to patients with Child Pugh A (Huynh et al. 2022). Survival outcomes reported in patients progressed to Child Pugh B in the overmentioned analysis experienced worse survival outcomes if compared with our cohort of patients, but a different selection of patients between the present work and the previous has to be considered. Huynh and colleagues analyzed patients progressed to Child Pugh B during treatment, thus including patients whose liver disfunction could be partially related to treatment. Contrarily, we decided to include in the study patients with a Child Pugh class of B at the baseline, which means that the liver disfunction could be ascribed to the hepatopathy and/or to cancer, thus making these patients more likely to benefit from anticancer treatment. On the other hand, our results resulted to be consistent with those of Welland and collaborators, whose sample included a significant subgroup of Child Pugh B patients (Welland et al. 2022). Concerning the treatment with atezolizumab plus bevacizumab, D’Alessio and collaborators performed one of the first retrospective real-life experience of the use of the combination treatment by including in the sample also 48 patients with a Child Pugh class B (D'Alessio et al. 2022). Interestingly, the survival outcomes reported in the analysis of D’Alessio were absolutely consistent with those reported in the present analysis: the mOS was 6.7 months (compared to 8,2 months in the present study) and mPFS was 6.8 months (compared to 6.9 months in the present study). Moreover, the ORR reported by D’Alessio et al. was 21%, once again a result online with those reported in the present analysis (18%) (D'Alessio et al. 2022). Since the survival outcomes of atezolizumab plus bevacizumab reported are consistent with previous real-life experience in the same setting of patients, the benefit resulted in the present analysis with Lenvatinib could not be ascribed to a less performance of atezolizumab plus bevacizumab. In this era of new emerging therapeutic options for patients affected by advanced HCC, the definition of the best first-line treatment for patients with compromised liver function is an unresolve issue: prognostic and predictor factors able to define which patients could respond better to treatment rather than another one in this setting are urgently needed. To the best of our knowledge, the present analysis highlighted for the first time a positive prognostic role of treatment with Lenvatinib in patients with mildly impaired liver function. Of note, all patients were treated in tertiary centers and all therapeutic decisions were taken in a multidisciplinary board context with specialists of high expertise, thus acquiring particular importance. The reasons underlying the better survival outcomes of Lenvatinib compared to atezolizumab plus bevacizumab in this setting of patients remain unknown. Nevertheless, few hypotheses could be done. Several previous real-world studies of Lenvatinib reported better survival outcomes compared to those reported in the registration trial (Casadei-Gardini et al. 2022, 2021; Rimini et al. 2021, 2022a; Burgio et al. 2021; Rapposelli et al. 2021). Furthermore, in the recently presented LEAP-002 trial the median OS reached with Lenvatinib in monotherapy was remarkable, and significantly higher compared to those reported in the REFLECT trial. It is convincible that the long-lasting experience on TKIs (previously sorafenib, and then both sorafenib and Lenvatinib) might have a role in the better management of patients receiving Lenvatinib compared to those receiving atezolizumab plus bevacizumab. Indeed, atezolizumab plus bevacizumab constitutes the first treatment combination including an immunotherapy used in the advanced HCC field, thus clinicians are fronting the first time this kind of therapy, with its peculiar side effects. The results reported in the present analysis could be partially ascribed to this consideration, but further investigations are needed. Another significant result from our analysis focused on the subgroups of patients who were highlighted to better respond to the combination atezolizumab plus bevacizumab, thus reporting survival outcomes comparable to those receiving Lenvatinib. Patients with the best clinical baseline characteristics, particularly with ECOG PS 0, BCLC stage of B and ALBI 1, were demonstrated to benefit from atezolizumab plus bevacizumab. Of note, all the characteristics reported have been previously demonstrated to have a significant prognostic impact on patients affected by unresectable HCC (Rimini et al. 2021, 2022a; Hiraoka et al. 2019). Probably, the dismal prognosis conferred by the unfavorable clinical characteristics in this setting of patients could prevent to highlight the clinical benefit obtained with anticancer treatment. Further studies are needed to verify our ideas. A final consideration focused on the adverse events reported in our sample could be done. The safety profile of both treatments (Lenvatinib and atezolizumab plus bevacizumab) is qualitatively consistent with previous works, including the registration trials, thus meaning that no new adverse events were reported in patients with an impaired liver function. In previous real-world studies focused on both Lenvatinib and atezolizumab plus bevacizumab, no significant differences were reported in terms of the incidence of adverse events in patients with Child Pugh class B compared to Child Pugh class A. Nevertheless, the incidence of adverse events reported in the present analysis resulted to be significantly lower compared to the results from previous works, probably due to an underestimation which could be ascribed to the retrospective and multicenter nature of the present analysis.
Our work accounts for several limitations. First of all, the retrospective nature of the present analysis could not exclude possible selection bias related to the same nature of the work. Thus, our exploratory findings could be considered only hypothesis-generating and could not substitute level I evidence derived from prospective trials. A prospective validation of the present results is mandatory to transfer these finding into clinical practice. Secondly, the present study was conducted on a large sample of patients treated in different centers in different countries, thus a centralized imaging review was not performed as well as the criteria for tumor assessments were defined by an internal protocol of each center. In the same way, the evaluation of adverse events resents from the subjective clinical evaluation of each physician, which could explicate the underestimation in terms of incidence. Another important limitation of the present study could derive from the lack of the Child Pugh score, since only the Child Pugh class was considered in the patients’ selection. Patients included in Child Pugh class B have already been defined as a significantly heterogeneous population, since different scores could be related to different clinical conditions. Indeed, patients with a Child Pugh score of 7 (B7) normally present an impairment of liver function which directly derives from the tumor burden; for this reason, this subgroup of patients could be more likely to respond to systemic treatment and to experience a survival benefit. Conversely, patients with a B9 score are normally affected by a significant cirrhosis and liver function impairment, which affects the prognosis more that the oncologic diagnosis. As a result, patients with B9 scores are normally unlike to benefit from systemic anticancer treatment, whereas best supportive care along with the hepatologic medical treatment constitute the most effective therapeutic strategy. Patients with a score of B8 could be included in both clinical situations since in these patients liver function impairment could be related to both tumor burden and the underlying cirrhosis: in this setting, the clear identification of the source of liver disfunction is crucial to define patients who may benefit from an anticancer treatment rather than only best supportive care. Finally, our analysis did not consider a dynamic evaluation of liver function: we stratified patients on the basis of the baseline characteristics but no further evaluation of clinical and bio-humoral characteristics has been collected, thus data about the liver function deterioration during treatment are lacking. Despite the overmentioned limits, the present study suggests for the first time a major benefit from Lenvatinib compared to atezolizumab plus bevacizumab in a large cohort of patients with mildly impaired liver function (Child Pugh class B). If the current tendence in clinical practice is to treat patients with Child Pugh B after an adequate selection performed by the multidisciplinary team, further prospective investigations focused on this subgroup of patients are needed to optimize treatment in a such fragile population.
Author contributions
Conception and design: ACG, MR. Acquisition of data (acquired and managed patients): All authors. Analysis and interpretation of data: AC-G, MR. Writing, review, and/or revision of the manuscript: AC-G, MR. Final approval of manuscript: All authors.
Funding
The present work received no financial support.
Data availability
Data are available on request from the authors.
Declarations
Conflict of interest
MK has received Lecture Fee from Bayer, Eisai, MSD, EA Pharma, Chugai; received Research Grant from Chugai, Otsuka, Takeda, Taiho, Sumitomo Dainippon, Daiichi Sankyo, MSD, Eisai, Bayer, AbbVie, Medico’s Hirata, Astellas Pharma, Bristol-Myers Squibb; received Advisory Consulting fee from MSD, BMS, Taiho, Eisai, Ono pharmaceutical, Chugai. F. F. has received travel support from Ipsen, and speaker’s fees from AbbVie, MSD, Ipsen, Esai and Fresenius. K. M.S. has received grants and personal fees from MERCK, MSD, Servier, EISAI, Amgen. A.C.G. has received grants and personal fees from MSD, Eisai, Bayer, and is an advisor for MSD, Eisai, Bayer, Bristol-Myers Squibb, AstraZeneca and GSK. The other coauthors have no conflict of interest. A.C.G. has received grants and personal fees from MSD, Eisai, Bayer, and is an advisor for MSD, Eisai, Bayer, Bristol-Myers Squibb, AstraZeneca and GSK. The other coauthors have no conflict of interest.
Institutional review board
The Ethical Review Board of each Institutional Hospital approved the present study. This study was performed in line with the principles of the Declaration of Helsinki.
Informed consent
Written informed consent for treatment was obtained for all patients.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abou-Alfa GK, Chan SL, Kudo M et al (2022) Phase 3 randomized, open-label, multicenter study of tremelimumab and durvalumab as first-line therapy in patients with unresectable hepatocellular carcinoma: HIMALAYA. J Clin Oncol 40:379. 10.1200/JCO.2022.40.4_suppl.379- [Google Scholar]
- Burgio V, Iavarone M, Di Costanzo GG, Marra F, Lonardi S, Tamburini E, Piscaglia F, Masi G, Celsa C, Foschi FG, Silletta M, Amoruso DC, Rimini M, Bruccoleri M, Tortora R, Campani C, Soldà C, Viola MG, Forgione A, Conti F, Salani F, Catanese S, Giacchetto CM, Fulgenzi C, Coppola C, Lampertico P, Pellino A, Rancatore G, Cabibbo G, Ratti F, Pedica F, Della Corte A, Colombo M, De Cobelli F, Aldrighetti L, Cascinu S, Casadei-Gardini A (2021) Real-life clinical data of lenvatinib versus sorafenib for unresectable hepatocellular carcinoma in Italy. Cancer Manag Res 24(13):9379–9389. 10.2147/CMAR.S330195. (PMID:34992463;PMCID:PMC8713715) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadei-Gardini A, Scartozzi M, Tada T, Yoo C, Shimose S, Masi G, Lonardi S, Frassineti LG, Nicola S, Piscaglia F, Kumada T, Kim HD, Koga H, Vivaldi C, Soldà C, Hiraoka A, Bang Y, Atsukawa M, Torimura T, Tsuj K, Itobayashi E, Toyoda H, Fukunishi S, Rimassa L, Rimini M, Cascinu S, Cucchetti A (2021) Lenvatinib versus sorafenib in first-line treatment of unresectable hepatocellular carcinoma: an inverse probability of treatment weighting analysis. Liver Int 41(6):1389–1397. 10.1111/liv.14817. (Epub 2021 Feb 20 PMID: 33547848) [DOI] [PubMed] [Google Scholar]
- Casadei-Gardini A, Rimini M, Kudo M, Shimose S, Tada T, Suda G, Goh MJ, Jefremow A, Scartozzi M, Cabibbo G, Campani C, Tamburini E, Tovoli F, Ueshima K, Aoki T, Iwamoto H, Torimura T, Kumada T, Hiraoka A, Atsukawa M, Itobayashi E, Toyoda H, Sakamoto N, Sho T, Kang W, Siebler J, Neurath MF, Burgio V, Cascinu S (2022) REal life study of LEnVAtiNib therapy for HepAtocellular carcinoma: RELEVANT study. Liver Cancer. 10.1159/000525145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, Luo R, Feng J, Ye S, Yang TS, Xu J, Sun Y, Liang H, Liu J, Wang J, Tak WY, Pan H, Burock K, Zou J, Voliotis D, Guan Z (2009) Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol 10(1):25–34. 10.1016/S1470-2045(08)70285-7. (Epub 2008 Dec 16 PMID: 19095497) [DOI] [PubMed] [Google Scholar]
- D’Alessio A, Fulgenzi CAM, Nishida N, Schönlein M, von Felden J, Schulze K, Wege H, Gaillard VE, Saeed A, Wietharn B, Hildebrand H, Wu L, Ang C, Marron TU, Weinmann A, Galle PR, Bettinger D, Bengsch B, Vogel A, Balcar L, Scheiner B, Lee PC, Huang YH, Amara S, Muzaffar M, Naqash AR, Cammarota A, Personeni N, Pressiani T, Sharma R, Pinter M, Cortellini A, Kudo M, Rimassa L, Pinato DJ (2022) Preliminary evidence of safety and tolerability of atezolizumab plus bevacizumab in patients with hepatocellular carcinoma and Child-Pugh A and B cirrhosis: a real-world study. Hepatology 76(4):1000–1012. 10.1002/hep.32468. (Epub 2022 Apr 8 PMID: 35313048) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, Kudo M, Breder V, Merle P, Kaseb AO, Li D, Verret W, Xu DZ, Hernandez S, Liu J, Huang C, Mulla S, Wang Y, Lim HY, Zhu AX, Cheng AL, IMbrave150 Investigators (2020) Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N Engl J Med 382(20):1894–1905. 10.1056/NEJMoa1915745 [DOI] [PubMed] [Google Scholar]
- Finn R et al (2022) Primary results from the phase 3 LEAP-002 study: lenvatinib plus pembrolizumab versus lenvatinib as first-line (1L) therapy for advanced hepatocellular carcinoma (aHCC). ESMO Congress, LBA34
- Freites-Martinez A, Santana N, Arias-Santiago S, Viera A (2021) Using the common terminology criteria for adverse events (CTCAE - Version 5.0) to evaluate the severity of adverse events of anticancer therapies. Actas Dermosifiliogr (Engl Ed) 112(1):90–92. 10.1016/j.ad.2019.05.009. (Epub 2020 Sep 3 PMID: 32891586) [DOI] [PubMed] [Google Scholar]
- Hiraoka A, Kumada T, Atsukawa M, Hirooka M, Tsuji K, Ishikawa T, Takaguchi K, Kariyama K, Itobayashi E, Tajiri K, Shimada N, Shibata H, Ochi H, Tada T, Toyoda H, Nouso K, Tsutsui A, Nagano T, Itokawa N, Hayama K, Imai M, Joko K, Koizumi Y, Hiasa Y, Michitaka K, Kudo M, Real-life Practice Experts for HCC (RELPEC) Study Group, HCC 48 Group (hepatocellular carcinoma experts from 48 clinics in Japan) (2019) Prognostic factor of lenvatinib for unresectable hepatocellular carcinoma in real-world conditions-Multicenter analysis. Cancer Med 8(8):3719–3728. 10.1002/cam4.2241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huynh J, Cho MT, Kim EJ, Ren M, Ramji Z, Vogel A (2022) Lenvatinib in patients with unresectable hepatocellular carcinoma who progressed to Child-Pugh B liver function. Ther Adv Med Oncol 24(14):17588359221116608. 10.1177/17588359221116608. (PMID:36051472;PMCID:PMC9425881) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley RK, Rimassa L, Cheng AL, Kaseb A, Qin S, Zhu AX, Chan SL, Melkadze T, Sukeepaisarnjaroen W, Breder V, Verset G, Gane E, Borbath I, Rangel JDG, Ryoo BY, Makharadze T, Merle P, Benzaghou F, Banerjee K, Hazra S, Fawcett J, Yau T (2022) Cabozantinib plus atezolizumab versus sorafenib for advanced hepatocellular carcinoma (COSMIC-312): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol 23(8):995–1008. 10.1016/S1470-2045(22)00326-6. (Epub 2022 Jul 4 PMID: 35798016) [DOI] [PubMed] [Google Scholar]
- Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F et al (2018) Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet 391:1163–1173 [DOI] [PubMed] [Google Scholar]
- Lencioni R, Llovet JM (2010) Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis 30(1):52–60. 10.1055/s-0030-1247132. (Epub 2010 Feb 19 PMID: 20175033) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A, Schwartz M, Porta C, Zeuzem S, Bolondi L, Greten TF, Galle PR, Seitz JF, Borbath I, Häussinger D, Giannaris T, Shan M, Moscovici M, Voliotis D, Bruix J, SHARP Investigators Study Group (2008) Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 359(4):378–390. 10.1056/NEJMoa070885 [DOI] [PubMed] [Google Scholar]
- Llovet JM, Kelley RK, Villanueva A, Singal AG, Pikarsky E, Roayaie S, Lencioni R, Koike K, Zucman-Rossi J, Finn RS (2021) Hepatocellular Carcinoma. Nat Rev Dis Primers 7(1):6. 10.1038/s41572-020-00240-3. (PMID: 33479224) [DOI] [PubMed] [Google Scholar]
- Qin S et al (2022) Camrelizumab (C) plus rivoceranib (R) vs. sorafenib (S) as first-line therapy for unresectable hepatocellular carcinoma (uHCC): A randomized, phase III trial. ESMO Congress 2022, LBA35
- Rapposelli IG, Shimose S, Kumada T, Okamura S, Hiraoka A, Di Costanzo GG, Marra F, Tamburini E, Forgione A, Foschi FG, Silletta M, Lonardi S, Masi G, Scartozzi M, Nakano M, Shibata H, Kawata K, Pellino A, Vivaldi C, Lai E, Takata A, Tajiri K, Toyoda H, Tortora R, Campani C, Viola MG, Piscaglia F, Conti F, Fulgenzi CAM, Frassineti GL, Rizzato MD, Salani F, Astara G, Torimura T, Atsukawa M, Tada T, Burgio V, Rimini M, Cascinu S, Casadei-Gardini A (2021) Identification of lenvatinib prognostic index via recursive partitioning analysis in advanced hepatocellular carcinoma. ESMO Open 6(4):100190. 10.1016/j.esmoop.2021.100190. (Epub 2021 Jun 16. PMID: 34144271; PMCID: PMC8219999) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimini M, Kudo M, Tada T, Shigeo S, Kang W, Suda G, Jefremow A, Burgio V, Iavarone M, Tortora R, Marra F, Lonardi S, Tamburini E, Piscaglia F, Masi G, Cabibbo G, Foschi FG, Silletta M, Kumada T, Iwamoto H, Aoki T, Goh MJ, Sakamoto N, Siebler J, Hiraoka A, Niizeki T, Ueshima K, Sho T, Atsukawa M, Hirooka M, Tsuji K, Ishikawa T, Takaguchi K, Kariyama K, Itobayashi E, Tajiri K, Shimada N, Shibata H, Ochi H, Yasuda S, Toyoda H, Fukunishi S, Ohama H, Kawata K, Tani J, Nakamura S, Nouso K, Tsutsui A, Nagano T, Takaaki T, Itokawa N, Okubo T, Arai T, Imai M, Joko K, Koizumi Y, Hiasa Y, Cucchetti A, Ratti F, Aldrighetti L, Cascinu S, Casadei-Gardini A (2021) Nonalcoholic steatohepatitis in hepatocarcinoma: new insights about its prognostic role in patients treated with lenvatinib. ESMO Open 6(6):100330. 10.1016/j.esmoop.2021.100330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimini M, Shimose S, Lonardi S, Tada T, Masi G, Iwamoto H, Lai E, Burgio V, Hiraoka A, Ishikawa T, Soldà C, Shirono T, Vivaldi C, Takaguchi K, Shimada N, Astara G, Koga H, Nouso K, Joko K, Torimura T, Hiasa Y, Salani F, Scartozzi M, Cascinu S, Casadei-Gardini A (2021) Lenvatinib versus Sorafenib as first-line treatment in hepatocellular carcinoma: a multi-institutional matched case-control study. Hepatol Res 51(12):1229–1241. 10.1111/hepr.13718. (Epub 2021 Oct 21 PMID: 34591334) [DOI] [PubMed] [Google Scholar]
- Rimini M, Kang W, Burgio V, Persano M, Aoki T, Shimose S, Tada T, Kumada T, Sho T, Lai E, Celsa C, Campani C, Tonnini M, Tamburini E, Hiraoka A, Takaguchi K, Nishida N, Iwamoto H, Itobayashi E, Tsuji K, Sakamoto N, Ishikawa T, Toyoda H, Kudo M, Kawaguchi T, Hatanaka T, Nouso K, Suda G, Cabibbo G, Marra F, Della Corte A, Ratti F, Pedica F, De Cobelli F, Aldrighetti L, Scartozzi M, Cascinu S, Casadei-Gardini A (2022a) Validation of the easy-to-use lenvatinib prognostic (LEP) index to predict prognosis in advanced hepatocellular carcinoma patients treated with Lenvatinib. Hepatol Res. 10.1111/hepr.13824 [DOI] [PubMed] [Google Scholar]
- Rimini M, Rimassa L, Ueshima K, Burgio V, Shigeo S, Tada T, Suda G, Yoo C, Cheon J, Pinato DJ, Lonardi S, Scartozzi M, Iavarone M, Di Costanzo GG, Marra F, Soldà C, Tamburini E, Piscaglia F, Masi G, Cabibbo G, Foschi FG, Silletta M, Pressiani T, Nishida N, Iwamoto H, Sakamoto N, Ryoo BY, Chon HJ, Claudia F, Niizeki T, Sho T, Kang B, D’Alessio A, Kumada T, Hiraoka A, Hirooka M, Kariyama K, Tani J, Atsukawa M, Takaguchi K, Itobayashi E, Fukunishi S, Tsuji K, Ishikawa T, Tajiri K, Ochi H, Yasuda S, Toyoda H, Ogawa C, Nishimur T, Hatanaka T, Kakizaki S, Shimada N, Kawata K, Tanaka T, Ohama H, Nouso K, Morishita A, Tsutsui A, Nagano T, Itokawa N, Okubo T, Arai T, Imai M, Naganuma A, Koizumi Y, Nakamura S, Joko K, Iijima H, Hiasa Y, Pedica F, De Cobelli F, Ratti F, Alrighetti L, Kudo M, Cascinu S, Casadei-Gardini A (2022b) Atezolizumab plus bevacizumab versus lenvatinib or sorafenib in non-viral unresectable hepatocellular carcinoma: an international propensity score matching analysis. ESMO Open 7(6):100591. 10.1016/j.esmoop.2022.100591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welland S, Leyh C, Finkelmeier F, Jefremow A, Shmanko K, Gonzalez-Carmona MA, Kandulski A, Jeliazkova P, Best J, Fründt TW, Djanani A, Pangerl M, Maieron A, Greil R, Fricke C, Sookthai D, Günther R, Schmiderer A, Wege H, Venerito M, Ehmer U, Müller M, Strassburg CP, Weinmann A, Siebler J, Waidmann O, Lange CM, Saborowski A, Vogel A (2022) Real-world data for Lenvatinib in hepatocellular carcinoma (ELEVATOR): a retrospective multicenter study. Liver Cancer 11(3):219–232. 10.1159/000521746. (PMID:35949288;PMCID:PMC9218621) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available on request from the authors.