Abstract
Although sugar has been suggested to promote floral transition in many plant species, growth on high concentrations (5% [w/v]) of sucrose (Suc) significantly delayed flowering time, causing an increase in the number of leaves at the time of flowering in Arabidopsis. The effect of high concentrations of Suc seemed to be metabolic rather than osmotic. The delay of floral transition was due to extension of the late vegetative phase, which resulted in a delayed activation of LFY expression. In addition, growth on low concentrations (1% [w/v]) of Suc slightly inhibited flowering in wild-type plants. This delay resulted from effects on the early vegetative phase. This inhibition was more pronounced in tfl1, an early flowering mutant, than in the wild type. Although 1% (w/v) Suc was reported to promote floral transition of late-flowering mutants such as co, fca, and gi, floral transition in these mutants was delayed by a further increase in Suc concentration. These results suggest that sugar may affect floral transition by activating or inhibiting genes that act to control floral transition, depending on the concentration of sugars, the genetic background of the plants, and when the sugar is introduced. Growth on 1% (w/v) Suc did not restore the reduced expression levels of FT and SOC1/AGL20 in co or fca mutants. Rather, expression of FT and SOC1/AGL20 was repressed by 1% (w/v) Suc in wild-type background. The possible effects of sugar on gene expression to promote floral transition are discussed.
Sugar plays a role as a signaling molecule that regulates a variety of genes (for review, see Koch, 1996). It probably affects various aspects of development in higher plants. For example, in Arabidopsis seedlings, high levels of sugar in the medium inhibits both hypocotyl elongation in the dark (Jang et al., 1997), and light-induced cotyledon opening (Dijkwel et al., 1997; Jang et al., 1997). Based on the analysis of transgenic plants in which sense or antisense RNA for two Arabidopsis hexokinase genes (AtHXK1 and AtHXK2) were overexpressed, it has been suggested that these hexokinases function as sugar sensors in the inhibition of hypocotyl elongation, and in light-induced cotyledon opening (Jang et al., 1997).
In addition to these developmental aspects that are affected by sugar, flowering also seems to be influenced by sugar. There has been a good amount of evidence suggesting that Suc promotes flowering in most species that have been examined (for review, see Bernier et al., 1993). After induction of flowering in a long-day (LD) plant, Sinapis alba, by either a single LD, or by a displaced short day (DSD), the concentration of Suc in the phloem reaching the shoot apex increases rapidly and transiently. This pulse of Suc translocation precedes the increase in cell division that normally is observed in the shoot apical meristem during floral evocation (Bernier et al., 1993). In Arabidopsis, induction of flowering in wild-type plants by either LD or DSD also causes an early and transient increase in Suc export from the leaves. The efficiency of floral induction by either LD or DSD is reflected by the amplitude of the increase in exported Suc (Corbesier et al., 1998).
The analysis of flowering time mutants provides further evidence that sugar promotes floral transition. A variety of mutants that flower either earlier (early flowering) or later (late flowering) than the wild type have been isolated and characterized in Arabidopsis. They have been categorized into several groups based on their responses to photoperiods and vernalization and their genetic relationships (Martínez-Zapater et al., 1994; Koornneef et al., 1998; Simpson et al., 1999). Mutations such as constans (co), fha, ft, fwa, and gigantea (gi) delay flowering under LD conditions but not under short-day conditions (Rédei, 1962; Koornneef et al., 1991), thus defining the photoperiod promotion pathway. Mutants of the second class (e.g. fca, fpa, fve, and luminidependens [ld]) flower later than wild type under both LD and short-day conditions, and the phenotype is generally rescued by vernalization (Martínez-Zapater and Somerville, 1990; Koornneef et al., 1991). These genes are placed in the autonomous promotion pathway. Araki and Komeda (1993) reported that co, gi, and, to some extent, ld, showed phenotypes similar to wild type with respect to both flowering time and leaf number when cultured in liquid medium containing 3% (w/v) Suc in the dark. Roldán et al. (1999) recently reported that in vitro culture of plants on vertically placed medium containing 1% (w/v) Suc in the dark or in the light, partially rescued the late-flowering phenotypes of co, gi, fca, fpa, and fve mutants. These results support the positive role of Suc in floral transition in Arabidopsis.
In contrast to the positive effects of 1% (w/v) Suc on floral transition, Zhou et al. (1998) reported that high levels of Glc in the medium significantly delayed flowering in Arabidopsis. Thus, high and low concentrations of sugar in the medium have been reported to affect floral transition in seemingly opposite manners. To fully understand how sugar regulates floral transition, detailed physiological experiments are still necessary. In this paper, we report analysis of the effects of sugar on floral transition. One percent Suc in the medium inhibited floral transition in wild-type plants, although it promoted floral transition in some late-flowering mutants such as co, fca, fha, gi, and ld. The inhibition observed in wild type was weak, but was more significant in terminal flower1 (tfl1), an early flowering mutant. The effect of 1% (w/v) Suc in the medium was observed mainly during the early vegetative phase in the 1st week of growth. In contrast, 5% (w/v) Suc in the medium delayed floral transition of all early and late-flowering mutants examined, including co, fca, fha, gi, and ld. This inhibition by 5% (w/v) Suc was effective mainly during the late vegetative phase and was due to its metabolic rather than its osmotic effects. These results and the previous reports led to the conclusion that sugar in the medium inhibits floral transition in at least two different ways and that the promotive and inhibiting effects of sugar on flowering depends on the concentration and time of addition of sugar and the genetic background of plants.
RESULTS
High Levels of Sugars Delayed Flowering in Arabidopsis
Wild-type Columbia (Col-0) plants grown on 5% (w/v) Suc flowered later than those grown on 2% (w/v) Suc, and they had more rosette leaves (Table I). The number of cauline leaves also increased (data not shown). To define the minimal period required to cause the Suc-dependent delay of flowering, plants were cultured for 1, 2, and 3 weeks on media with Suc and then transferred to soil. As shown in Table I, plants cultured for 2 or 3 weeks on media with 5% (w/v) Suc before transfer to soil flowered later than plants on media with 2% (w/v) Suc. The leaf number was comparable with that of plants grown continuously on the respective media until bolting. Preculture in vitro for 1 week with 5% (w/v) Suc resulted in flowering with leaf numbers similar to those of plants cultured with 2% (w/v) Suc. These results indicate that 2 weeks in culture was long enough to observe the negative effects of high levels of Suc on floral transition.
Table I.
Delay of flowering caused by high levels of Suc in the medium
| Period of in Vitro Culture | Suc in a Medium | No. of Rosette Leaves (±se) | Days to 1-cm Bolt (±se) | n |
|---|---|---|---|---|
| % | ||||
| 1 Week | 2 | 14.6 (±0.23) | n.d. | 36 |
| 5 | 15.5 (±0.15) | n.d. | 35 | |
| 2 Weeks | 2 | 14.3 (±0.18) | n.d. | 38 |
| 5 | 19.2 (±0.24) | n.d. | 37 | |
| 3 Weeks | 2 | 14.5 (±0.19) | n.d. | 38 |
| 5 | 20.0 (±0.32) | n.d. | 31 | |
| Until bolting | 2 | 14.3 (±0.16) | 23.0 (±0.23) | 40 |
| 5 | 21.0 (±0.20) | 31.2 (±0.40) | 33 |
Wild-type Col-0 plants were used in experiments. Bolting time was taken from the time seeds were sown. n, No. of plants tested.
To examine whether the negative effects of Suc on flowering were due to metabolic or osmotic effects, we looked at the effects of Glc analogs on flowering. When 110 mm 3-O-methyl-Glc was used to supplement the concentration of the basal medium with 2% (w/v) Suc (60 mm Suc), negative effects on flowering were not observed by the additional sugar (Table II). This Glc analog is transported into cells but not metabolized further, due to the non-phosphorylation of the 6-C of the molecule by hexose kinases. In contrast, when 2% (w/v) Suc was supplemented with either 110 mm Glc, Fru, or Gal, the number of rosette leaves was increased (Table II). Supplemented Suc only at 55 mm concentration had almost similar negative effects to those by 110-mm hexoses. Two- hundred-thirty-millimolar Glc alone also caused a similar increase in rosette leaf number; this molar concentration was much higher than that of Suc alone at 115 mm (Table II). The effects of mannitol, which serves to increase the osmotic pressure in the medium, were also investigated; however, the plants could not grow normally and showed abnormal phenotypes (data not shown). These results suggest that the inhibition of floral transition by sugars is due to metabolic effects rather than osmotic effects.
Table II.
Metabolic effects of sugar in the medium on floral transition
| Sugar in a Medium
|
Rosette Leaf No. (±se) | n | |
|---|---|---|---|
| 2% (w/v) (60 mm) Suc | Additional sugar | ||
| mm | |||
| + | – | 14.4 (±0.24) | 32 |
| + | 110 OMG | 13.1 (±0.15) | 45 |
| + | 110 Glc | 18.1 (±0.28) | 35 |
| + | 110 Fru | 19.1 (±0.39) | 30 |
| + | 110 Gal | 18.4 (±0.32) | 34 |
| + | 55 Suc | 17.5 (±0.24) | 35 |
| – | 230 Glc | 17.8 (±0.21) | 30 |
Wild-type Col-0 plants were cultured on the medium for 2 weeks and transferred to soil. Mean values (±se) of rosette leaf no. are shown. n, No. of plants tested. OMG, 3-O-Methyl-Glc.
To investigate whether sugars inhibit the floral transition by altering photosynthetic processes (Jang et al., 1997), chlorophyll levels were measured after culturing plants for 2 or 3 weeks. Photosynthesis seemed to increase due to the significant increase in leaf area at around 10 d after sowing (data not shown). Table III shows that when higher concentrations of Suc was used in growth medium, as compared with 1.5% (w/v) Suc, the chlorophyll content in the leaves increased. For plants cultured in media containing between 1.5% and 5% (w/v) Suc, RBCS transcript increased with higher Suc concentrations (data not shown). Accumulation of anthocyanin induced by Suc (Mita et al., 1997) was confirmed as a positive control for the Suc effect (Table III). These results suggest that the sugar inhibition of floral transition probably is not due to an inhibition of photosynthetic activity.
Table III.
Effects of Suc concentration on chlorophyll and anthocyanin accumulation and on floral transition
| Characteristics Affected by Suc | Culture Period | Suc in the Medium
|
|||||
|---|---|---|---|---|---|---|---|
| 1.5% | n | 3% | n | 5% | n | ||
| weeks | |||||||
| Chlorophyll (mg g−1 fresh wt)a | 2 | 1.75 (±0.10) | 15 | 2.17 (±0.06) | 15 | 2.35 (±0.05) | 15 |
| 3 | 1.98 (±0.04) | 16 | 2.48 (±0.04) | 15 | 3.01 (±0.05) | 15 | |
| Anthocyanin (Abs. g−1 fresh wt)b | 3 | 1.03 | 12 | 3.00 | 14 | 18.8 | 15 |
| Rosette leaf no.c | 3 | 13.3 (±0.2) | 33 | 15.4 (±0.2) | 38 | 19.0 (±0.3) | 39 |
| Total leaf no. | 17.3 (±0.3) | 20.2 (±0.3) | 25.1 (±0.4) | ||||
n, No. of plants examined.
Chlorophyll was extracted from overground parts of plants.
Plants were combined and anthocyanin was extracted. Abs, Absorbance.
Wild-type plants (Col-0) were cultured on the medium for 3 weeks and transferred to soil.
Sugar Affects the Duration of the Adult Vegetative Phase
The number and density of trichomes on the surface of rosette leaves changes with developmental time (Schultz and Haughn, 1993; Telfer et al., 1997). In the case of glabra1 (gl1) plants grown under LD conditions, trichomes are absent from the surfaces and from the margins of rosette leaves during the juvenile and the early adult vegetative phases, but are present on leaves in the late adult vegetative phase (Ray et al., 1996). On those leaves (both rosette and cauline leaves), trichomes are found only at the leaf margin. Thus, trichome on leaf margin of gl1 characterizes the developmental status of leaves. By using gl1, we next investigated whether high levels of sugars delay floral transition by extending all parts or only a part of the vegetative phase.
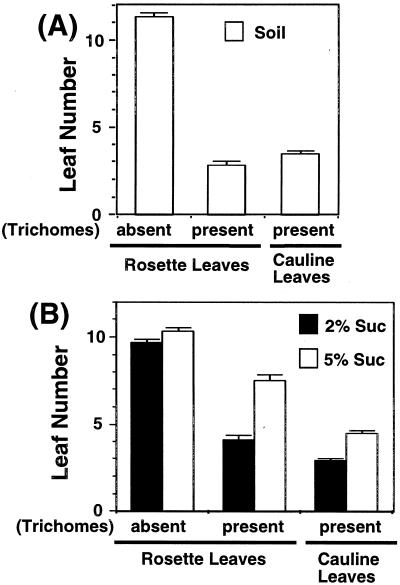
In one experiment, gl1-Columbia flowered on soil under continuous light (LL) conditions, with an average of 11.1 rosette leaves with no trichomes, 2.7 rosette leaves with trichomes, and 3.5 cauline leaves with trichomes (Fig. 1A). The density of trichomes per rosette leaf increased with the leaf position (data not shown). The numbers of leaves with and without trichomes were compared among gl1 plants cultured on media with low and high concentrations of Suc (Fig. 1B). Use of medium containing 2% or 5% (w/v) Suc had little, if any, effect on the number of leaves without trichomes. In contrast, the number of rosette leaves with trichomes increased significantly in plants grown on medium with high concentration of Suc. These results suggest that sugar mainly delays the progression of the late rosette phase.
Figure 1.
Suc affects a change in the identity of the lateral primordia in the shoot apex. A, gl1-Columbia plants were grown on soil under LL conditions. Numbers of rosette and cauline leaves were scored in terms of the absence or presence of trichomes on the leaf margins. The most typical results among four independent experiments are shown. B, gl1-Columbia plants were grown on media with either 2% (w/v) Suc or 5% (w/v) Suc under LL for 3 weeks and then transferred to soil. Numbers of rosette and cauline leaves were scored in terms of the absence or presence of trichomes on the leaf margins. Black bars indicate plants grown on media with 2% (w/v) Suc, and white bars indicate plants grown on media with 5% (w/v) Suc.
Effects of Sugars on LFY Expression
The change in the fate of the shoot apical meristem from a vegetative meristem producing rosette leaves, to an inflorescence meristem producing cauline leaves and flowers, is dependent on the activity of floral meristem identity genes, such as LEAFY (LFY) and APETALA1 (AP1; Weigel, 1995; Yanofsky, 1995). Both genes are expressed at high levels in the emerging flower primordium, but only LFY is also expressed in the leaf primordium before the transition to flowering (Blázquez, et al., 1997; Hempel et al., 1997). The level of LFY expression in lateral primordia increases with the age of the plant until apparently it reaches a threshold level. Once this level has been reached, a primordium that would otherwise develop as a leaf/paraclade becomes a flower (Blázquez, et al., 1997).
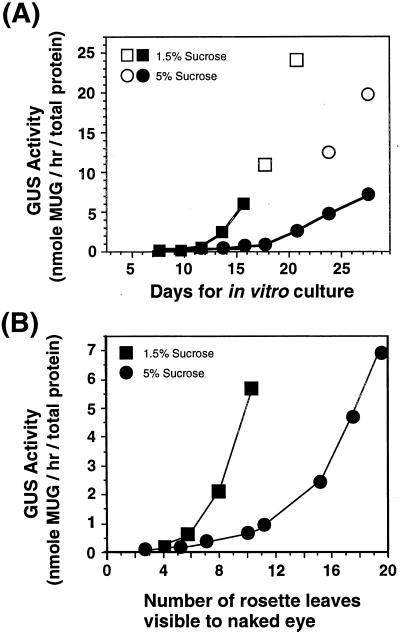
β-Glucuronidase (GUS) activity in plants carrying LFY::GUS was examined on a per-apex basis at different time points until flower buds became visible to the naked eye. GUS activity in plants grown on the medium with 1.5% (w/v) Suc increased very slowly until the 11th d (Fig. 2A). However, it began to rapidly increase after about the 13th d. In contrast, for plants grown on medium with 5% (w/v) Suc, GUS activity increases quite slowly until the 18th d and then increases significantly at the 21st d. GUS activity in visible flower buds of plants grown in the presence of either 1.5% or 5% (w/v) Suc were comparable, indicating that the level of LFY expression in the flower buds was not affected by the growth conditions. GUS activity also was plotted against the number of rosette leaves (Fig. 2B). The increase in GUS activity in plants grown on 1.5% (w/v) Suc was significant when seven or eight rosette leaves became visible, which corresponds to the 13th to 14th d after sowing. In contrast, the increase in the GUS activity in plants grown on 5% (w/v) Suc was not significant until 12 or more rosette leaves became visible, which corresponds to the 18th d, indicating that the delay of LFY up-regulation was not due to a reduced growth rate.
Figure 2.
Effects of Suc on LFY::GUS expression. A, Plants homozygous for LFY::GUS were grown on media with either 1.5% or 5% (w/v) Suc under LL, until flower buds were visible to the naked eye. Twenty-five to 30 plants were combined and used to measure GUS activity in shoot apices. Protein extracts were prepared as described in “Materials and Methods.” All plants on 1.5% (w/v) Suc medium had produced visible flower buds in the center of shoots on the 18th d, but the main stems had not bolted even by the 21st d. On 5% (w/v) Suc medium, 47% of plants showed visible flower buds by the 24th d and 82% by the 28th d. Black symbols indicate plants that had no flower buds visible to the naked eye at the end of the experiment, and white symbols indicate plants that had flower buds. B, Values of LFY::GUS activity are plotted against the mean values of the number of rosette leaves on plants that had no flower buds, harvested in experiment A. Symbols are the same as in A. GUS activity values are the mean of two measurements. MUG, 4-methylumbelliferyl-β-d-glucuronide. Similar results were obtained in two independent experiments.
The delay in LFY up-regulation opening the possibility that the delay in flowering might be a direct or indirect consequence of the inhibition of LFY expression by sugars. To examine this possibility, the effects of sugars on floral transition in lfy-1 mutant were investigated (Table IV). If sugar directly affects the activation of LFY to control flowering time, lfy mutants will not respond to high concentration of sugar in the medium, as compared with the low concentration. At 2% (w/v) Suc concentration, the lfy mutants flowered with many more cauline leaves than wild-type plants, but the number of rosette leaves was almost the same as that in the wild-type plants. lfy plants grown on medium with 5% (w/v) Suc had significantly more rosette leaves than those grown on 2% (w/v) Suc, indicating that the negative effect of Suc on flowering is not a direct consequence of the inhibition of LFY expression. Similar to wild-type plants, the increase in the number of cauline leaves in lfy mutants was less significant than that of the rosette leaves, supporting our findings that high levels of sugars extend the late rosette phase (Fig. 1).
Table IV.
Delay of flowering in lfy mutants caused by high levels of Suc
| Plants | Suc in a Medium | No. of Rosette Leaves | No. of Cauline Leaves | n |
|---|---|---|---|---|
| % | ||||
| WT (Col-0) | 2 | 14.5 (±0.15) | 4.4 (±0.10) | 48 |
| 5 | 20.6 (±0.32) | 6.3 (±0.13) | 48 | |
| lfy-1 | 2 | 15.0 (±0.18) | 11.9 (±0.22) | 27 |
| 5 | 20.6 (±0.37) | 12.7 (±0.27) | 28 |
Plants were cultured on the medium for 16 d and transferred to soil. Mean values (±se) of leaf no. are shown. lfy-1 plants carry the homozygous mutation. n, No. of plants tested.
Effects of Suc on Floral Transition of Flowering Time Mutants
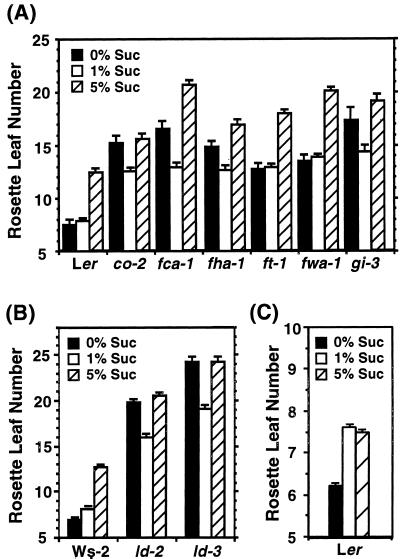
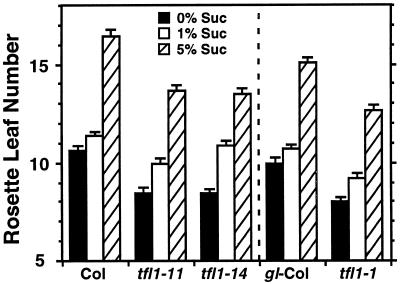
To understand how sugar-mediated pathways are related to various pathways that control floral transition, phenotypes of flowering time mutants were analyzed on media containing various concentrations of Suc under LL. There are several pathways that regulate floral transition in Arabidopsis. Therefore, we selected flowering time mutants representative of each of these pathways. Roldán et al. (1999) reported the effects of 1% (w/v) Suc on floral transition in some late-flowering mutants. In their experiments, plants were grown on solid medium in vertically placed petri dishes. However, it was difficult for us to grow plants on vertically placed media without sugar, probably because of the low efficiency of gas exchange caused by tightly sealing the petri dishes to prevent contamination. Therefore, we used horizontally placed culture pots that enabled most plants to grow autotrophically by photosynthesis and to set seeds (see “Materials and Methods”). co-2, fca-1, fha-1, ft-1, fwa-1, gi-3, ld-2, and ld-3 mutants maintained their late-flowering phenotypes on sugar-free medium, indicating that in vitro culture per se did not rescue the mutant phenotypes (Fig. 3, A and B). Addition of 1% (w/v) Suc to the medium decreased the number of rosette leaves in co-2, fca-1, fha-1, gi-3, ld-2, and ld-3 mutants. However, the delayed flowering time phenotype of these mutants was not completely rescued. By contrast, promotion of flowering by adding 1% (w/v) Suc was not observed in ft-1 and fwa-1 mutants or in wild-type plants. Rather, the number of rosette leaves was increased slightly in these three genotypes, most significantly in the wild type and least so in ft. Similar results were obtained in experiments using other alleles, such as co-1 and gi-2 in the Columbia background, and fca-2, fca-4, fha-2, ft-2, and fwa-2 in the Ler background (data not shown). tfl1 mutants such as tfl1-1, tfl1-11, and tfl1-14 flower early, with fewer leaves, on soil under LD conditions (Shannon and Meeks-Wagner, 1991), which we confirmed. They maintained the early flowering phenotype on sugar-free medium (Fig. 4) although the phenotype on the medium was less pronounced than that on soil (data not shown). Addition of 1% (w/v) Suc to the medium caused a delay in flowering time and an increase in rosette leaf number (Fig. 4). The inhibitory effect of 1% (w/v) Suc on flowering was more significant in tfl1 mutants than in wild type (Fig. 4).
Figure 3.
Effects of Suc on flowering in several late-flowering mutants. A, Wild-type plants, Lansberg erecta (Ler), and late-flowering mutants, co-2, fca-1, fd-1, fha-1, ft-1, fwa-1, and gi-3, were cultured on media with either 1% (w/v) Suc (1% Suc), or 5% (w/v) Suc (5% Suc), or without Suc (0% Suc) under LL. B, Wild-type plants, Wassilewskija-2, and late-flowering mutants, ld-2 and ld-3, were cultured on media with either 1% (w/v) Suc, 5% (w/v) Suc, or without Suc, under LL. C, Ler plants were grown on media with 0%, 1%, and 5% (w/v) Suc for 1 week, and then transferred to soil. Values are the average of 35 to 45 plants. The error bars indicate one se of the mean. Similar results were obtained in two independent experiments.
Figure 4.
Effects of Suc on leaf number in an early flowering mutant, tfl1. Early flowering mutants tfl1-11, tfl1-14 and tfl1-1 were grown on media with various concentrations of Suc under LL. Col-0 plants are the control for tfl1-11 and tfl1-14; gl1-Columbia for tfl1-1. Values are the average of 30 to 45 plants. The error bars indicate one se of the mean. Similar results were obtained in two independent experiments.
Using of 2% (w/v) Suc instead of 1% (w/v) Suc slightly reduced the positive effect of Suc on flowering in co, fca, fha, and gi mutants (data not shown). In contrast, when 5% (w/v) Suc was used, as compared with 1% (w/v) Suc, a delay in flowering time and an increase in number of rosette leaves were observed in all of the late-flowering mutants examined and in tfl1 mutants (Figs. 3, A and B, and 4). Therefore, the slight effect seen when comparing 0% with 5% (w/v) Suc does not infer that 5% (w/v) Suc had little effect on flowering in the late-flowering mutants. Similar effects of 5% (w/v) Suc were also observed with other alleles such as co-1, fca-2, fca-4, fha-2, ft-2, fwa-2, and gi-2.
A slight increase in rosette leaf number was observed in the presence of Suc when wild-type plants were transferred to soil after 1 week of culturing in vitro with 0%, 1%, and 5% (w/v) Suc (Fig. 3C). This indicates that the inhibitory effect of Suc is most likely exerted during the early vegetative phase.
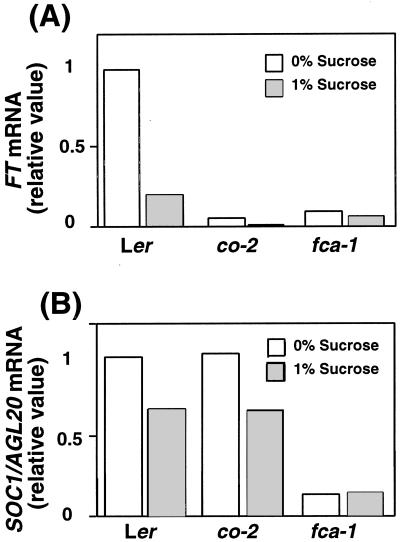
How does 1% (w/v) Suc promote floral transition in some late-flowering mutants? One possibility is that the promotion of flowering in late-flowering mutants may be mediated by the up-regulation of some gene by 1% (w/v) Suc. The candidate genes are postulated to be located downstream of two promotion pathways involving CO and FCA, respectively. FT, SUPPRESSOR OF OVEREXPRESSION OF CO 1 (SOC1)/AGAMOUS-LIKE20 (AGL20), and LFY are possible candidates (Kardailsky et al., 1999; Kobayashi et al., 1999; Lee et al., 2000; Samach et al., 2000; J.H. Ahn and D. Weigel, personal communication). Because 1% (w/v) Suc did not promote flowering in the mutant ft (Fig. 3; Roldán et al., 1997, 1999), FT is suggested to mediate signal(s) from sugar to promote floral transition. To investigate this possibility, levels of FT expression were first analyzed by reverse transcriptase (RT)-PCR in wild type, co, and fca grown on media with or without 1% (w/v) Suc for 5 d. FT expression levels were greatly reduced in co and fca mutants on media without Suc, which was consistent with the results of soil-grown plants of co (M. Ohto, Y. Kobayashi, and T. Araki, unpublished data) and fca (J.H. Ahn and D. Weigel, personal communication) under LD conditions. Contrary to our expectation, FT expression was not up-regulated by 1% (w/v) Suc in co or fca (Fig. 5A). Instead, FT expression levels were significantly reduced in wild-type plants on 1% (w/v) Suc (Fig. 5A). We next investigated the expression levels of SOC1/AGL20 and found that the expression levels were greatly reduced in fca, but not in co on a sugar-free medium, which was consistent with the results of soil-grown plants (Lee et al., 2000) under LD conditions. Similar to FT, SOC1/AGL20 expression was not up-regulated by 1% (w/v) Suc in fca (Fig. 5B). Instead, SOC1/AGL20 expression levels were reduced in wild type and co by 1% (w/v) Suc (Fig. 5B), but the extent of this repression was less significant than in the case with FT (Fig. 5A).
Figure 5.
Effects of Suc on the expression of FT and AGL20/SOC1. co-2, fca-1, and wild type were grown on media with or without 1% (w/v) Suc for 5 d. Fifty to 70 seedlings were used to extract total RNA for each treatment, and RT-PCR experiments were performed. The intensity of the bands of the corresponding signals was measured on an image analyzer. Values of FT (A) and SOC1/AGL20 (B) transcript levels were normalized to an internal control of AP2 transcript levels, and the relative values are shown. Similar results were obtained when Ubiquitin10 transcript was used as an internal control. Similar results were obtained in two independent experiments.
DISCUSSION
The delay in flowering time in Arabidopsis caused by high concentrations of Glc in the medium was previously reported (Zhou et al., 1998). In this report, we added several novel findings on the negative effects of sugar on floral transition.
First, metabolic rather than the osmotic effects of high sugar concentration may be responsible for the delay in flowering. The addition of a high concentration of Glc, Fru, or Gal, but not 3-O-methyl-Glc, into basal medium with 2% (w/v) Suc further delayed flowering (Table II). This may suggest that the delay in flowering is controlled by signal(s) originated from events such as the metabolism of hexoses or some upstream event, such as the phosphorylation of hexoses by hexokinase. Zhou et al. (1998) previously reported that inhibition of floral transition by a high concentration of Glc in the medium might be mediated by a pathway involving a sugar sensor, hexokinase, that is similar to the mechanism proposed for the sugar repression of photosynthetic gene expression. However, as suggested by Zhou et al. (1998), there may be hexokinase-independent pathway(s) in controlling sugar-responsive gene expression in higher plants. Therefore, the possibility remains that hexokinase-independent pathway(s) might be involved in the mechanism of the delay in flowering time by high concentration of sugar.
Second, sugar seems to affect a specific part of the vegetative phase, rather than extending all phases uniformly. Shift experiments from sugar-containing media to soil showed that 1 week of culturing with Suc was not enough to observe the negative effects on floral transition (Table I, Fig. 3C). This may mean differential sensitivity to high concentrations of sugar during the early and late parts of the vegetative phase. This notion was supported by experiments with gl1 plants, in which 5% (w/v) Suc significantly extended the adult vegetative phase. In gl1 plants, the adult vegetative phase is characterized by rosette leaves with trichomes at the leaf margin, whereas the juvenile vegetative phase is characterized by trichome-less rosette leaves (Fig. 1). These results suggest that sugar affects the transition from the adult vegetative phase to the reproductive phase. Under LD conditions, expression of LFY::GUS is rapidly up-regulated prior to the transition from the adult vegetative phase to the reproductive phase (Blázquez et al., 1997). Timing of LFY::GUS up-regulation was delayed by 8 to 10 d in plants on media containing 5% (w/v) Suc compared with plants on media containing 1.5% (w/v) Suc (Fig. 2). This raised the question of whether the negative effects of high sugar concentrations are due to the effect of sugar on LFY, which is a positive regulator of floral transition (Blázquez et al., 1997). However, in a loss-of-function mutant of LFY, lfy-1, floral transition was still delayed when cultured on media containing 5% (w/v) Suc (Table IV). Thus, it is unlikely that the delay in floral transition was a direct consequence of delayed LFY up-regulation. Alternatively, SHORT INTEGUMENT (SIN1) is proposed to mediate the negative effects of high concentration of sugar. sin1 may be a mutation in a meristem identity gene in the same sense as is the lfy mutation (Ray et al., 1996) because delay of flowering in sin1 was observed, in addition to the defects in ovule development. The delay of flowering in sin1/gl1 double mutant was characterized mainly by the increase in the number of rosette leaves with trichomes, as was the case of delay in flowering of gl1 by high concentration of sugar. To investigate this possibility, effects of high concentration of sugar on flowering in sin1 need to be analyzed.
Third, effects of sugar on floral transition differ depending on the concentration, genotype of plants, and when in the vegetative growth phase the treatment is given. As reported previously by Roldán et al. (1999), 1% (w/v) Suc promotes flowering in late-flowering mutants, such as co, fca, fha, gi, and ld (Fig. 3). However, as we clarified earlier, a further increase in concentration to 5% (w/v) delayed floral transition in these mutants (Fig. 3), suggesting that sugars promote or inhibit floral transition depending on the concentration. It is surprising that 1% (w/v) Suc slightly delayed floral transition in wild-type plants (Figs. 3 and 4). This negative effect was even more significant in early flowering tfl1 mutants (Shannon and Meeks-Wagner, 1991; Fig. 4), and was further exacerbated in both wild-type and tfl mutants when the Suc concentration was increased to 5% (w/v) (Fig. 4). However, if the exposure to Suc was limited to only the 1st week of growth, in the early rosette phase, both 1% (w/v) and 5% (w/v) Suc had similar effects in delaying floral transition (Fig. 3C). King and Bagnall (1996) reported previously that lower concentration of Suc in growth medium promoted flowering in wild-type plants, especially under suboptimal conditions for photosynthetic saturation. However, we consistently observed negative effects of 1% (w/v) Suc on flowering of wild-type plants in our experimental condition that are suboptimal for photosynthesis and in different genetic background as shown in Figures 3 and 4. The reason for the discrepancy between our results, which are consistent with the results by Roldán et al. (1999) and the results by King and Bagnall, are not clear.
Fourth, sugar may control floral transition by activating or by inhibiting genes that act to promote floral transition. Roldán et al. (1997, 1999) reported the promotive effects of 1% (w/v) Suc on vertically placed media in co, fca, fpa, fve, and gi in the floral promotion pathways. They believed that the improved availability of Suc via non-root tissues, such as the vegetative tissues neighboring the apex, might have been the reason for the promotive effects of Suc. However, the promotive effects of 1% (w/v) Suc on floral transition needs to be considered more carefully because Suc also has inhibitory effects on floral transition. An alternative interpretation is that 1% (w/v) Suc could complement mutations in the floral promotion pathways by activating gene(s) that act downstream of these pathways. It has been suggested recently that FT, SOC1/AGL20, and LFY represent such a class of genes (for review, see Araki, 2001). Because 1% (w/v) Suc was reported to activate LFY::GUS expression in the wild-type background, LFY was a candidate gene that might be activated by Suc to rescue the late-flowering phenotype of mutants in these floral promotion pathways (Blázquez et al., 1998). As we showed here, reduced levels of FT expression in the co and fca backgrounds, and of SOC1/AGL20 expression in the fca background, were not restored by 1% (w/v) Suc (Fig. 5, A and B). LFY is proposed to act in parallel with FT (Kardailsky et al., 1999; Kobayashi et al., 1999) and to act, at least in part, downstream of SOC1/AGL20 (Lee et al., 2000). In this respect, our results on FT and SOC1/AGL20 expression further support the hypothesis that LFY could act in complementing the late-flowering mutants in the presence of 1% (w/v) Suc. To confirm this possibility, the effects of 1% (w/v) Suc on co and fca mutants under a lfy mutant background need to be investigated.
One percent (w/v) Suc lowered the expression of FT in the wild-type background (Fig. 5A) and also lowered the expression of SOC1/AGL20 in the wild-type and co backgrounds (Fig. 5B). These results suggest that this inhibition of FT and SOC1/AGL20 might be responsible, at least in part, for the weak inhibition of floral transition by sugar during the early rosette phase. It has been reported that several physiological conditions such as day length, age, and temperature, affect floral transition by controlling the expression of flowering time and meristem identity genes (Blázquez et al., 1997; Kardailsky et al., 1999; Kobayashi et al., 1999; Lee et al., 2000: Samach et al., 2000). However, the positive as well as the negative effects of sugar on the expression of these genes, such as FT, SOC1/AGL20, and LFY, might be equally important in controlling floral transition.
Sugar signaling is becoming an important topic in the study of floral transition. As shown in this report, the effects of sugar on floral transition, whether promotive or inhibitive, are very pleiotropic. However, the genetic approach of characterizing mutants and their mutated genes should give important clues to understand the mechanism(s) involved in mediating responses to sugar and in controlling floral transition. The results presented in this paper should provide basic information needed for further analysis of the role of sugar in floral transition, and for the characterization of the genes that integrate sugar signals to control floral transition.
MATERIALS AND METHODS
Plant Materials and Culture Conditions
co-2, gi-3, fca-1, fca-2, fha-1, fha-2, fwa-1, fwa-2, ft-1, and ft-2, are all in the Ler background. co-1, gi-1, gl1-2 (gl1-Columbia), tfl1-11, tfl1-14, and lfy-1 are in the Columbia background. ld-2 and ld-3 are in the Wassilewskija-2 background. The transgenic plant carrying LFY::GUS is in the Nossen background. Seeds of wild-type plants, mutant lines, and LFY:GUS plants were obtained from the Arabidopsis Biological Resource Center (Ohio State University, Columbus). Sterilized seeds were imbibed, then treated in the dark at 4°C for 4 d before sowing. Plants were cultured on media that contained 80 mL of 0.5× or 1× Murashige and Skoog salts, with or without sugars, and solidified with 0.3% (w/v) Gellan Gum (Wako Pure Chemical Industries, Osaka) in plant culture pots (80-mm diameter, 70-mm height; Asahi Technoglass, Tokyo), or on soil, under cool-white fluorescent light at 22°C. When plants were cultured in vitro without sugars, 0.5× Murashige and Skoog salts were used, except the concentrations of KH2PO4, MgSO4, and CaCl2 in the media were adjusted to those in 1× Murashige and Skoog salts. Although 70% to 90% of the plants died during seedling growth due to the low efficiency of gas exchange when cultured in petri dishes (90-mm diameter, 20-mm height) without sugars, use of the plant culture pots enabled the plants to grow by photosynthesis and to produce siliques in vitro. The light intensity during plant growth was 50 μmol m−2 s−1 for in vitro-cultured plants under LL conditions, and 100 μmol m−2 s−1 for soil-grown plants under both LD (16 h light, 8 h dark) and LL conditions. Plants on soil were watered with 1:1,000 diluted liquid fertilizer 5-10-5 (Hyponex Japan, Osaka) for the first watering, and then with 1:6,000 diluted liquid fertilizer twice a week.
Analysis of the Flowering Time
Flowering time was measured by scoring the time when the main inflorescence shoot had elongated to over 1 cm. The numbers of rosette leaves and cauline leaves on the main shoot were counted either when the plants had the first flower, or when the plants had formed a terminal flower on the main shoot.
Quantitation of Chlorophyll and Anthocyanin
The level of anthocyanin was determined as previously described (Mita et al., 1997). To determine levels of chlorophyll, tissues were homogenized in 100× volume of methanol at 4°C for 1 d. After centrifugation, the absorbance of the supernatant at 625, 647, and 664 nm was measured and the amount of chlorophylls were calculated by fomulae reported by Moran (1982).
Analysis of LFY::GUS
Plants homozygous for the single locus LFY::GUS were established after a single backcross with wild-type plants. Mature rosette leaves were excised to reduce the volume of protein extraction buffer because GUS activity was present in newly emerging leaf primordia, but absent from older leaves (Blázquez et al., 1997). Total proteins were extracted with mortar and pestle, then used to measure total GUS activity per plant. 4-methylumbelliferyl-β-d-glucouronide was used as the substrate as previously described by Blázquez et al. (1997).
RT-PCR Procedure
Five-day-old seedlings were harvested and the total RNA was isolated. RT-PCR experiments were performed as described previously (Kobayashi et al., 1999) using the taq polymerase of Amplitaq Gold (PE Biosystems, Foster City, CA). The primers used for RT-PCR were as follows: for FT, 5′-TAC GAA AAT CCA AGT CCC ACT G-3′ and 5′-AAA CTC GCG AGT GTT GAA GTT C-3′ (Kobayashi et al., 1999); for SOC1/AGL20, 5′-CGA GCA AGA AAG ACT CAA GTG TTT AAG G-3′ and 5′-GAA GTG ACT GAG AGA GAG AGA GTG AG-3′ (Lee et al., 2000; Samach et al., 2000); for APETALA2 (AP2), 5′-CTC AAT GCC GAG TCA TCA GG-3′ and 5′-CAT GAG AGG AGG TTG GAA GC-3′ (Jofuku et al., 1994); and for UBIQUITIN10 (UBQ10), 5′-TTG CGT CTG CGT GGA GGT ATG-3′) and 5′-ACC ACC ACG AAG ACG CAG GAC-3′. PCR conditions were as follows: one cycle of 95°C (10 min); 27 cycles for FT, 23 cycles for SOC1/AGL20, 21 cycles for AP2, and 17 cycles for UBQ10 at 94°C (30 s), 60°C (30 s), and 72°C (30 s); and then one cycle of 72°C (2 min). Signals were detected by a Fujix BAS2000 image analyzer (Fuji Film, Tokyo) and the intensity of the radioactive bands was determined.
ACKNOWLEDGMENTS
The authors are thankful to Drs. Detlef Weigel and Ji Hoon Ahn (Salk Institute, La Jolla, CA) for their kind permission to cite their unpublished results on FT expression in fca background, which we confirmed in our experiments in this paper. We are grateful to Drs. Hirokazu Tsukaya and Yoshishige Inagaki (National Institute for Basic Biology, Okazaki, Aichi, Japan) for their useful comments and to Dr. John Harada (University of California, Davis) and his lab members for their useful comments to revise the manuscript. Thanks are also due to the Arabidopsis Biological Resource Center (Ohio State University) for materials and to Ms. Chieko Namba (National Institute for Basic Biology) for her excellent technical assistance.
Footnotes
This work was supported in part by the Ministry of Education, Culture, Sports, Science and Technology of Japan (Grants-in-Aid for Scientific Research on Priority Areas [A] nos. 09274225, 10170227, 11151229, and 12025229 to M.O; and Grant-in-Aid for Scientific Research on Priority Areas [A] no. 10182102 to K.N.) and by the “Basic Science” Program of The Sumitomo Foundation (grant no. 100305 to M.O.).
LITERATURE CITED
- Araki T. Transition from vegetative to reproductive phase. Curr Opin Plant Biol. 2001;4:63–68. doi: 10.1016/s1369-5266(00)00137-0. [DOI] [PubMed] [Google Scholar]
- Araki T, Komeda Y. Flowering in darkness in Arabidopsis thaliana. Plant J. 1993;4:801–811. [Google Scholar]
- Bernier G, Havelange A, Houssa C, Petitjean A, Lejeune P. Physiological signals that induce flowering. Plant Cell. 1993;5:1147–1155. doi: 10.1105/tpc.5.10.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blázquez MA, Green R, Nilsson O, Sussman MR, Weigel D. Gibberellins promote flowering of Arabidopsis by activating the LEAFY promoter. Plant Cell. 1998;10:791–800. doi: 10.1105/tpc.10.5.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blázquez MA, Soowal LN, Lee I, Weigel D. LEAFY expression and flower initiation in Arabidopsis. Development. 1997;124:3835–3844. doi: 10.1242/dev.124.19.3835. [DOI] [PubMed] [Google Scholar]
- Corbesier L, Lejeune P, Bernier G. The role of carbohydrates in the induction of flowering in Arabidopsis thaliana: comparison between the wild-type and a starchless mutant. Planta. 1998;206:131–137. doi: 10.1007/s004250050383. [DOI] [PubMed] [Google Scholar]
- Dijkwel PP, Huijser C, Weisbeek PJ, Chua N-H, Smeekens SCM. Sucrose control of phytochrome A signaling in Arabidopsis. Plant Cell. 1997;9:583–595. doi: 10.1105/tpc.9.4.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hempel FD, Weigel D, Mandel MA, Ditta G, Zambryski PC, Feldmann LJ, Yanofsky MF. Floral determination and expression of floral regulatory genes in Arabidopsis. Development. 1997;124:3845–3853. doi: 10.1242/dev.124.19.3845. [DOI] [PubMed] [Google Scholar]
- Jang J-C, León P, Zhou L, Sheen J. Hexokinase as a sugar sensor in higher plants. Plant Cell. 1997;9:5–19. doi: 10.1105/tpc.9.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jofuku KD, den Boer BGW, Van Montagu M, Okamuro JK. Control of Arabidopsis flower and seed development by the homeotic gene APETALA2. Plant Cell. 1994;6:1211–1225. doi: 10.1105/tpc.6.9.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kardailsky I, Shukla VK, Ahn JH, Dagenais N, Christensen SK, Nguyen JT, Chory J, Harrison MJ, Weigel D. Activation tagging of the floral inducer FT. Science. 1999;286:1962–1965. doi: 10.1126/science.286.5446.1962. [DOI] [PubMed] [Google Scholar]
- King R, Bagnall D. Photoreceptors and the photoperiodic response controlling flowering of Arabidopsis. Semin Cell Dev Biol. 1996;7:449–454. [Google Scholar]
- Kobayashi Y, Kaya H, Goto K, Iwabuchi M, Araki T. A pair of related genes with antagonistic roles in mediating flowering signals. Science. 1999;286:1960–1962. doi: 10.1126/science.286.5446.1960. [DOI] [PubMed] [Google Scholar]
- Koch KE. Carbohydrate-modulated gene expression in plants. Annu Rev Plant Physiol Plant Mol Biol. 1996;47:509–540. doi: 10.1146/annurev.arplant.47.1.509. [DOI] [PubMed] [Google Scholar]
- Koornneef M, Alonso-Blanco C, Blankestijn-de Vries H, Hanhart CJ, Peeters AJM. Genetic interactions among late flowering mutants of Arabidopsis. Genetics. 1998;148:885–892. doi: 10.1093/genetics/148.2.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koornneef M, Hanhart CJ, van der Veen JH. A genetic and physiological analysis of late flowering mutants in Arabidopsis thaliana. Mol Gen Genet. 1991;229:57–66. doi: 10.1007/BF00264213. [DOI] [PubMed] [Google Scholar]
- Lee H, Suh S-S, Park E, Cho E, Ahn JH, Kim S-G, Lee JS, Kwon YM, Lee I. The AGAMOUS-LIKE 20 MADS domain protein integrates floral inductive pathways in Arabidopsis. Genes Dev. 2000;14:2366–2376. doi: 10.1101/gad.813600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Zapater JM, Somerville CR. Effect of light quality and vernalization on late-flowering mutants of Arabidopsis thaliana. Plant Physiol. 1990;92:770–776. doi: 10.1104/pp.92.3.770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Zapater JM, Coupland G, Dean C, Koornneef M. The transition to flowering in Arabidopsis. In: Meyerowitz EM, Somerville CR, editors. Arabidopsis. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1994. pp. 403–433. [Google Scholar]
- Mita S, Murano N, Akaike M, Namamura K. Mutants of Arabidopsis thaliana with pleiotropic effects on the expression of the gene for β-amylase and on the accumulation of anthocyanin that are inducible by sugars. Plant J. 1997;11:841–851. doi: 10.1046/j.1365-313x.1997.11040841.x. [DOI] [PubMed] [Google Scholar]
- Moran P. Fomulae for determination of chlorophyllous pigments extracted with N,N-dimethylformamide. Plant Physiol. 1982;69:1376–1381. doi: 10.1104/pp.69.6.1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray A, Lang JD, Golden T, Ray S. SHORT INTEGUMENT (SIN1), a gene required for ovule development in Arabidopsis, also controls flowering time. Development. 1996;122:2631–2638. doi: 10.1242/dev.122.9.2631. [DOI] [PubMed] [Google Scholar]
- Rédei GP. Supervital mutants of Arabidopsis. Genetics. 1962;47:443–460. doi: 10.1093/genetics/47.4.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roldán M, Gómez-Mena C, Ruiz-García L, Martín-Trillo M, Salinas J, Martínez- Zapater JM. Effect of darkness and sugar availability to the apex on morphogenesis and flowering time of Arabidopsis. Flowering Newsl. 1997;24:18–24. [Google Scholar]
- Roldán M, Gómez-Mena C, Ruiz-García L, Salinas J, Martínez-Zapater JM. Sucrose availability on the aerial part of the plant promotes dark-morphogenesis and flowering in Arabidopsis. Plant J. 1999;20:581–590. doi: 10.1046/j.1365-313x.1999.00632.x. [DOI] [PubMed] [Google Scholar]
- Samach A, Onouchi H, Gold S, Ditta GS, Schwarz-Sommer Z, Yanofsky MF, Coupland G. Distinct roles of CONSTANS target genes in reproductive development of Arabidopsis. Science. 2000;288:1613–1616. doi: 10.1126/science.288.5471.1613. [DOI] [PubMed] [Google Scholar]
- Schultz EA, Haughn GW. Genetic analysis of the floral initiation process (FLIP) in Arabidopsis. Development. 1993;119:745–765. [Google Scholar]
- Shannon S, Meeks-Wagner DR. A mutation in the Arabidopsis TFL1 gene affects inflorescence meristem development. Plant Cell. 1991;3:771–781. doi: 10.1105/tpc.3.9.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson GG, Gendall AR, Dean C. When to switch to flowering. Annu Rev Cell Dev Biol. 1999;15:519–550. doi: 10.1146/annurev.cellbio.15.1.519. [DOI] [PubMed] [Google Scholar]
- Telfer A, Bollman KM, Poeting RS. Phase change and the regulation of trichome distribution in Arabidopsis thaliana. Development. 1997;124:645–654. doi: 10.1242/dev.124.3.645. [DOI] [PubMed] [Google Scholar]
- Weigel D. The genetics of flower development: from floral induction to ovule morphogenesis. Annu Rev Genet. 1995;29:19–39. doi: 10.1146/annurev.ge.29.120195.000315. [DOI] [PubMed] [Google Scholar]
- Yanofsky M. Floral meristems to floral organs: genes controlling early events in Arabidopsis flower development. Annu Rev Plant Physiol Plant Mol Biol. 1995;46:167–188. [Google Scholar]
- Zhou L, Jang J-C, Jones TL, Sheen J. Glucose and ethylene signal transduction crosstalk revealed by an Arabidopsis glucose-insensitive mutant. Proc Natl Acad Sci USA. 1998;95:10294–10299. doi: 10.1073/pnas.95.17.10294. [DOI] [PMC free article] [PubMed] [Google Scholar]