Abstract
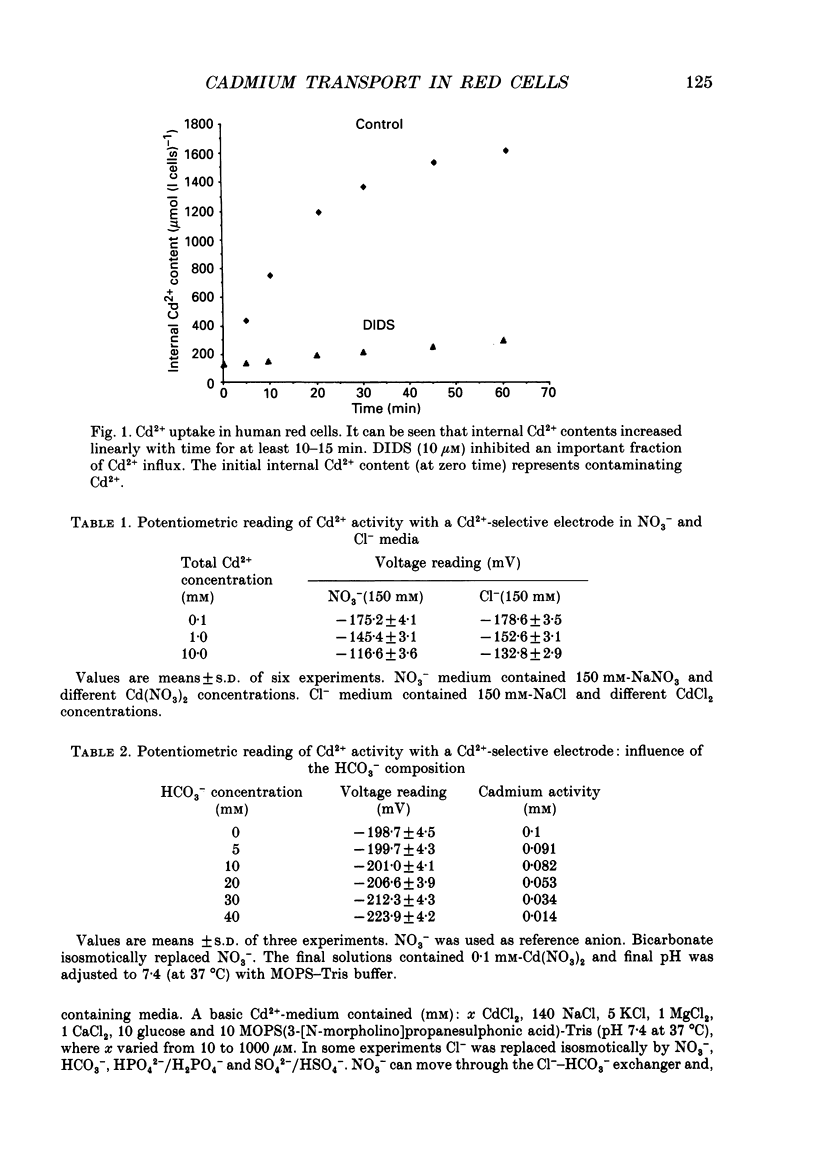
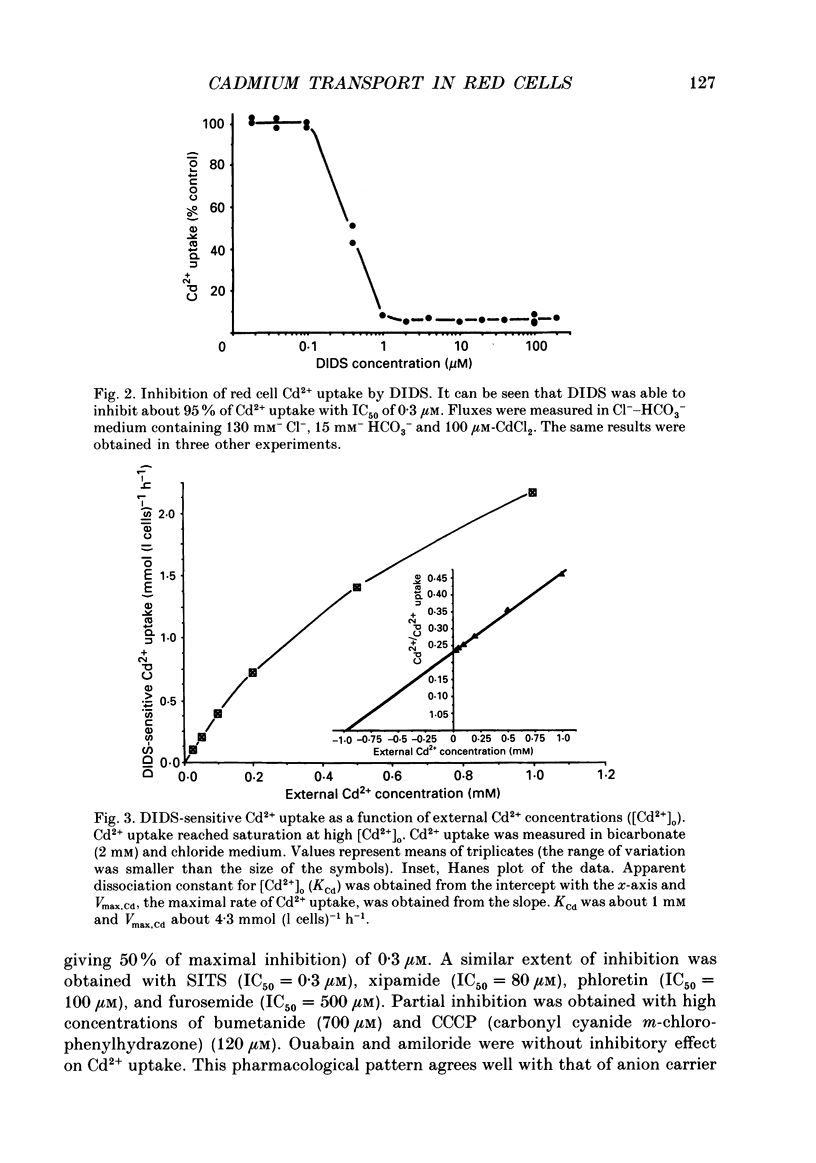
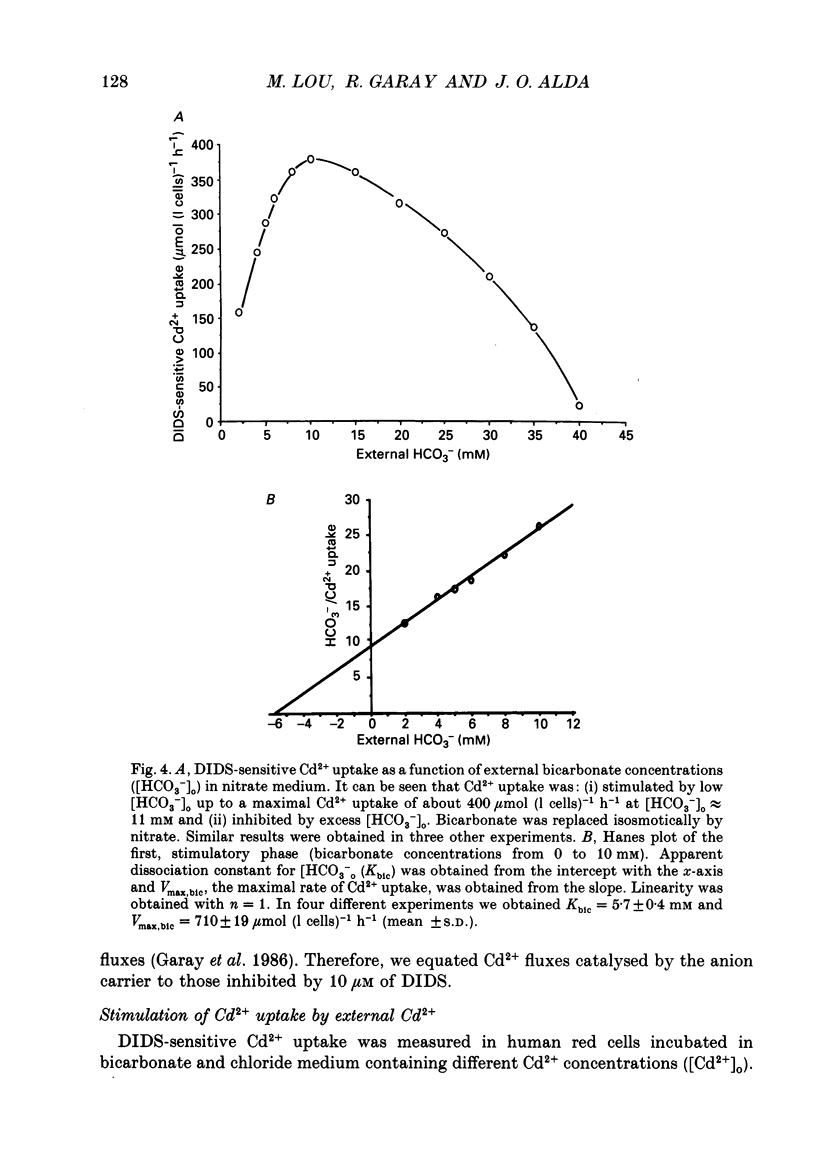
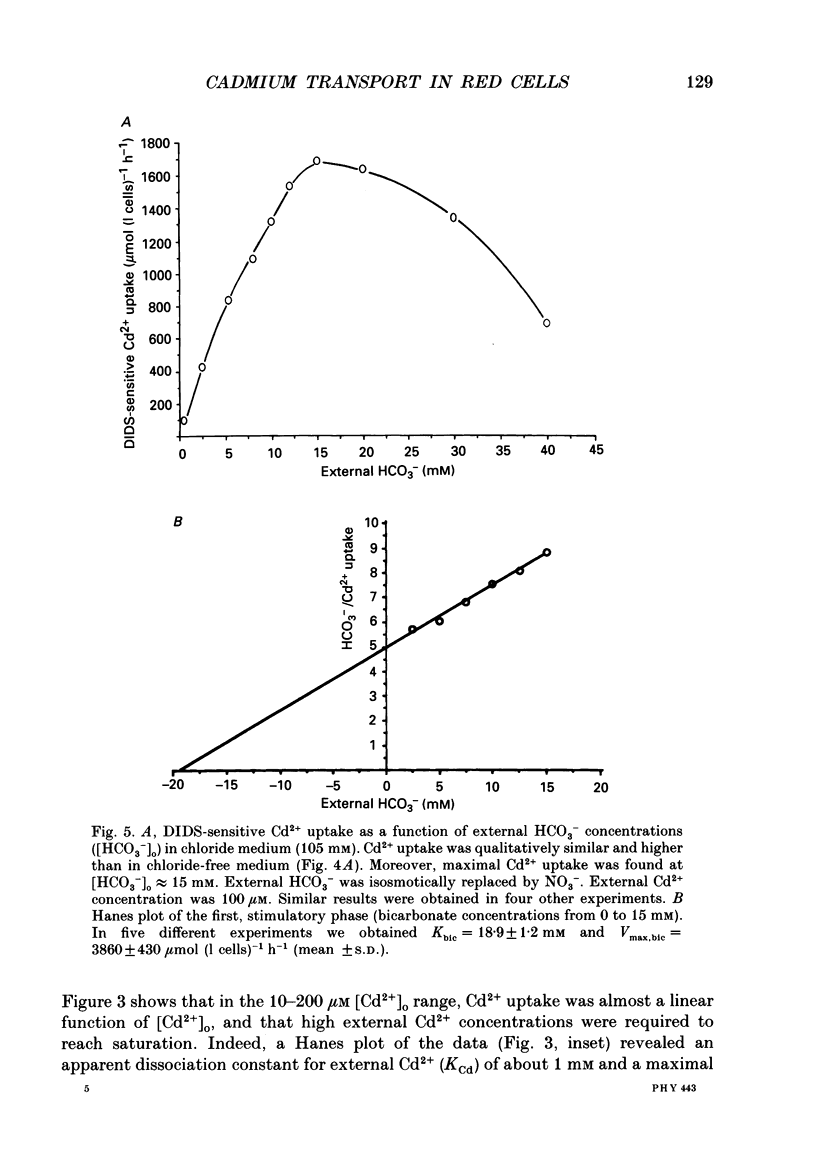
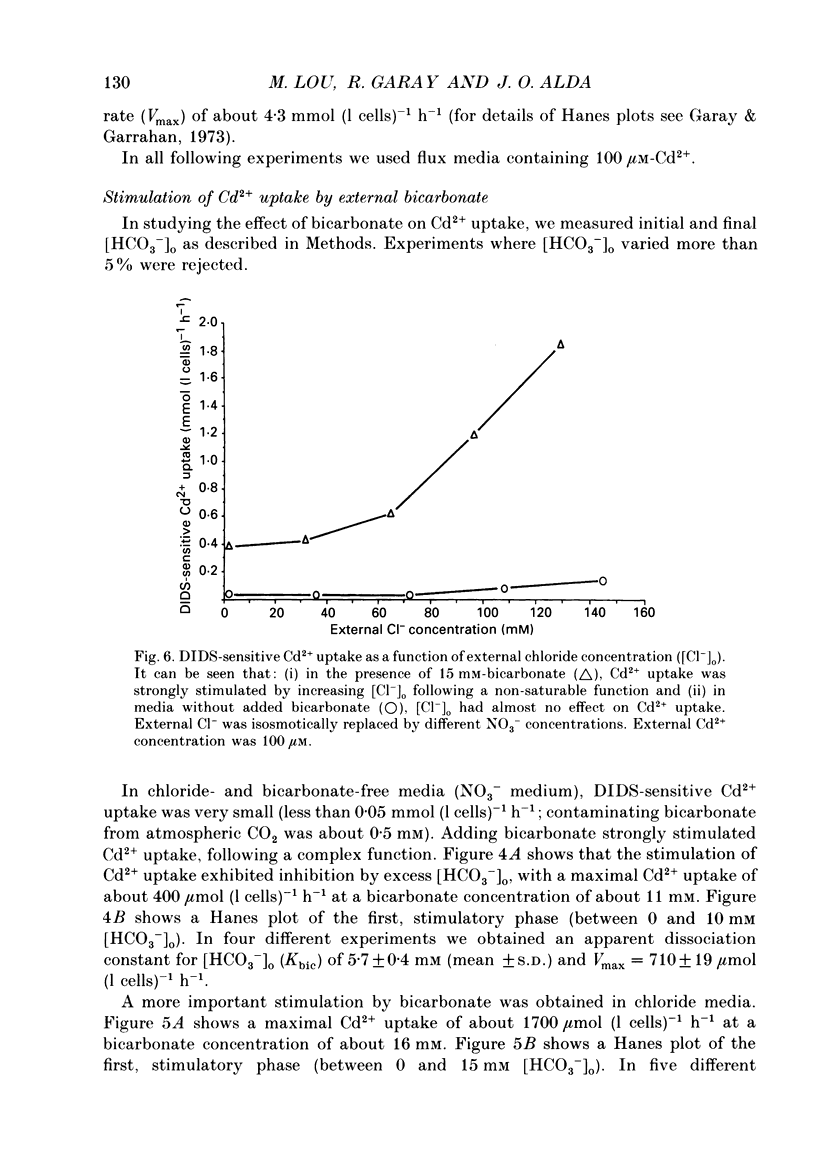
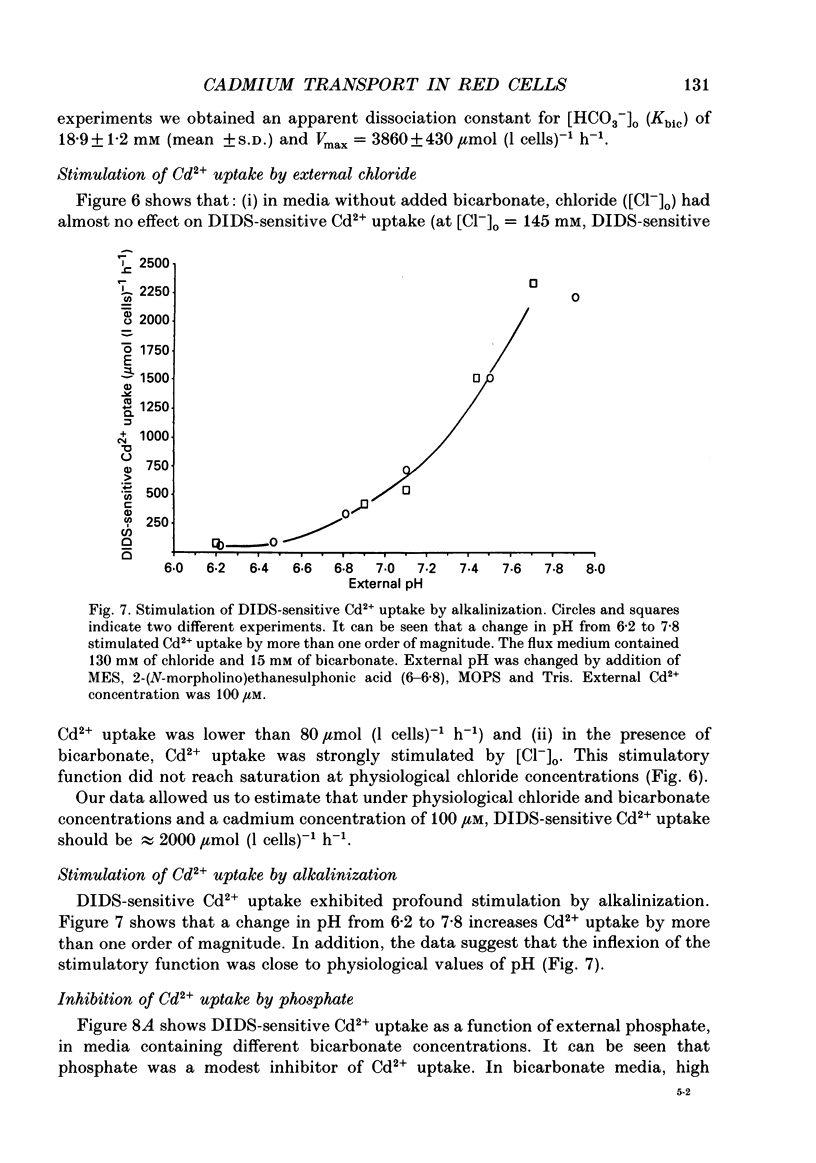
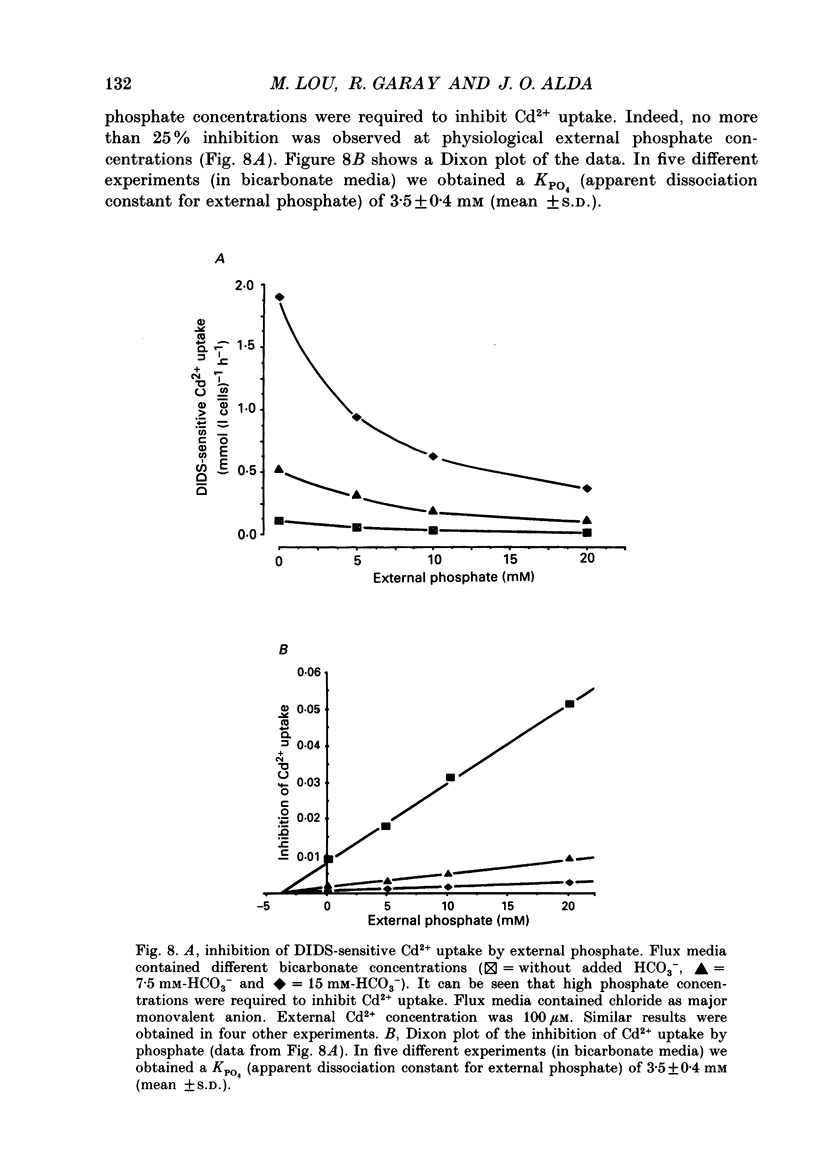
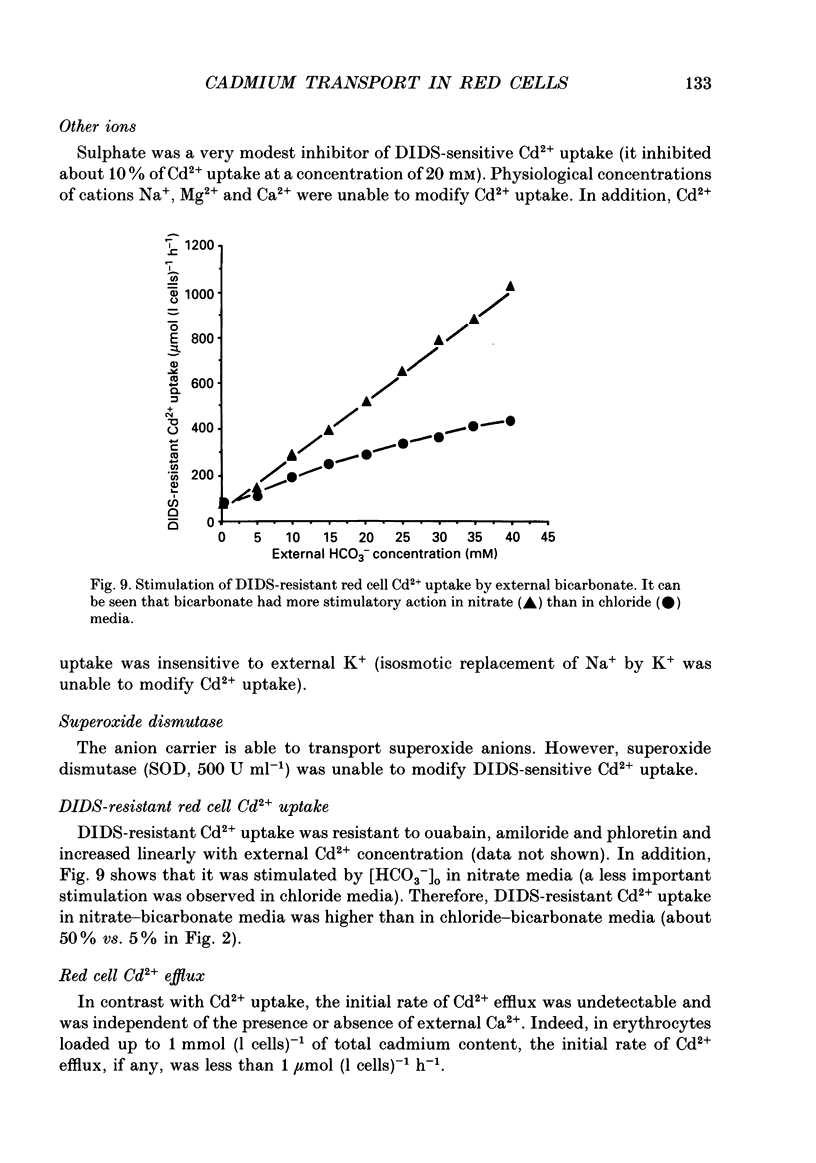
1. The initial rate of Cd2+ uptake in human red cells was measured by atomic absorption spectrophotometry. 2. About 96% of Cd2+ uptake was inhibited by DIDS (4,4'-diisothiocyanatostilbene-2,2'-disulphonic acid) with IC50 (concentration giving 50% of maximal inhibition) of 0.3 microM and by furosemide with IC50 of 500 microM and was resistant to ouabain and amiloride. This indicates the implication of the [Cl(-)-HCO3-] anion exchanger in Cd2+ uptake. 3. DIDS-sensitive Cd2+ uptake required the presence of external HCO3-. HCO3- ions had a biphasic effect on Cd2+ uptake. Low bicarbonate concentrations were stimulatory, suggesting formation of translocating bicarbonate-cadmium complexes. Higher bicarbonate concentrations were inhibitory, suggesting further bicarbonate complexation with formation of non-translocating species. Depending on the presence or absence of external Cl-, a maximal Cd2+ uptake of 1.7 or 0.37 mmol (l cells)-1 h-1 was observed at bicarbonate concentrations of 15.6 or 11 mM respectively. 4. In the presence of bicarbonate, external Cl- ions strongly stimulated Cd2+ uptake, with linear increase between 70 and 125 mM. This suggests that one translocating species may have chloride as ligand. 5. DIDS-sensitive Cd2+ uptake was modestly inhibited by physiological concentrations of external phosphate and was resistant to external K+, Mg2+ and Ca2+. 6. In conclusion, the anion exchanger is the major transport mechanism for red cell cadmium uptake. Translocating species appear to be monovalent anion complexes of cadmium with HCO3- such as [Cd(OH)(HCO3)2]- and [Cd(OH)(HCO3)Cl]-.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alda J. O., Garay R. Chloride (or bicarbonate)-dependent copper uptake through the anion exchanger in human red blood cells. Am J Physiol. 1990 Oct;259(4 Pt 1):C570–C576. doi: 10.1152/ajpcell.1990.259.4.C570. [DOI] [PubMed] [Google Scholar]
- Becker B. F., Duhm J. Evidence for anionic cation transport of lithium, sodium and potassium across the human erythrocyte membrane induced by divalent anions. J Physiol. 1978 Sep;282:149–168. doi: 10.1113/jphysiol.1978.sp012454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enger M. D., Hildebrand C. E., Stewart C. C. Cd2+ responses of cultured human blood cells. Toxicol Appl Pharmacol. 1983 Jun 30;69(2):214–224. doi: 10.1016/0041-008x(83)90302-2. [DOI] [PubMed] [Google Scholar]
- Foulkes E. C. On the mechanism of transfer of heavy metals across cell membranes. Toxicology. 1988 Nov 30;52(3):263–272. doi: 10.1016/0300-483x(88)90131-x. [DOI] [PubMed] [Google Scholar]
- Funder J. Alkali metal cation transport through the human erythrocyte membrane by the anion exchange mechanism. Acta Physiol Scand. 1980 Jan;108(1):31–37. doi: 10.1111/j.1748-1716.1980.tb06497.x. [DOI] [PubMed] [Google Scholar]
- Funder J., Wieth J. O. Combined effects of digitalis therapy and of plasma bicarbonate on human red cell socium and potassium. Scand J Clin Lab Invest. 1974 Oct;34(2):153–160. [PubMed] [Google Scholar]
- Funder J., Wieth J. O. Human red cell sodium and potassium in metabolic alkalosis. Scand J Clin Lab Invest. 1974 Sep;34(1):49–59. doi: 10.3109/00365517409061821. [DOI] [PubMed] [Google Scholar]
- Garay R. P., Garrahan P. J. The interaction of sodium and potassium with the sodium pump in red cells. J Physiol. 1973 Jun;231(2):297–325. doi: 10.1113/jphysiol.1973.sp010234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garay R. P., Hannaert P. A., Nazaret C., Cragoe E. J., Jr The significance of the relative effects of loop diuretics and anti-brain edema agents on the Na+,K+,Cl- cotransport system and the Cl-/NaCO3- anion exchanger. Naunyn Schmiedebergs Arch Pharmacol. 1986 Oct;334(2):202–209. doi: 10.1007/BF00505823. [DOI] [PubMed] [Google Scholar]
- Garty M., Bracken W. M., Klaassen C. D. Cadmium uptake by rat red blood cells. Toxicology. 1986 Dec 15;42(2-3):111–119. doi: 10.1016/0300-483x(86)90002-8. [DOI] [PubMed] [Google Scholar]
- Garty M., Wong K. L., Klaassen C. D. Redistribution of cadmium to blood of rats. Toxicol Appl Pharmacol. 1981 Jul;59(3):548–554. doi: 10.1016/0041-008x(81)90309-4. [DOI] [PubMed] [Google Scholar]
- Jennings M. L. Kinetics and mechanism of anion transport in red blood cells. Annu Rev Physiol. 1985;47:519–533. doi: 10.1146/annurev.ph.47.030185.002511. [DOI] [PubMed] [Google Scholar]
- Kalfakakou V., Simons T. J. Anionic mechanisms of zinc uptake across the human red cell membrane. J Physiol. 1990 Feb;421:485–497. doi: 10.1113/jphysiol.1990.sp017957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey G. N., Sarkadi B., Haas M., Gunn R. B., Davis J. M., Tosteson D. C. Lithium transport pathways in human red blood cells. J Gen Physiol. 1978 Aug;72(2):233–247. doi: 10.1085/jgp.72.2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schild L., Giebisch G., Karniski L., Aronson P. S. Chloride transport in the mammalian proximal tubule. Pflugers Arch. 1986;407 (Suppl 2):S156–S159. doi: 10.1007/BF00584945. [DOI] [PubMed] [Google Scholar]
- Simons T. J. The role of anion transport in the passive movement of lead across the human red cell membrane. J Physiol. 1986 Sep;378:287–312. doi: 10.1113/jphysiol.1986.sp016220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K., Min K. S., Onosaka S., Fukuhara C., Ueda M. The origin of metallothionein in red blood cells. Toxicol Appl Pharmacol. 1985 Mar 30;78(1):63–68. doi: 10.1016/0041-008x(85)90305-9. [DOI] [PubMed] [Google Scholar]
- Torrubia J. O., Garay R. Evidence for a major route for zinc uptake in human red blood cells: [Zn(HCO3)2Cl]- influx through the [Cl-/HCO3-] anion exchanger. J Cell Physiol. 1989 Feb;138(2):316–322. doi: 10.1002/jcp.1041380214. [DOI] [PubMed] [Google Scholar]
- Yost K. J. Cadmium, the environment and human health: an overview. Experientia. 1984 Feb 15;40(2):157–164. doi: 10.1007/BF01963579. [DOI] [PubMed] [Google Scholar]


