Abstract
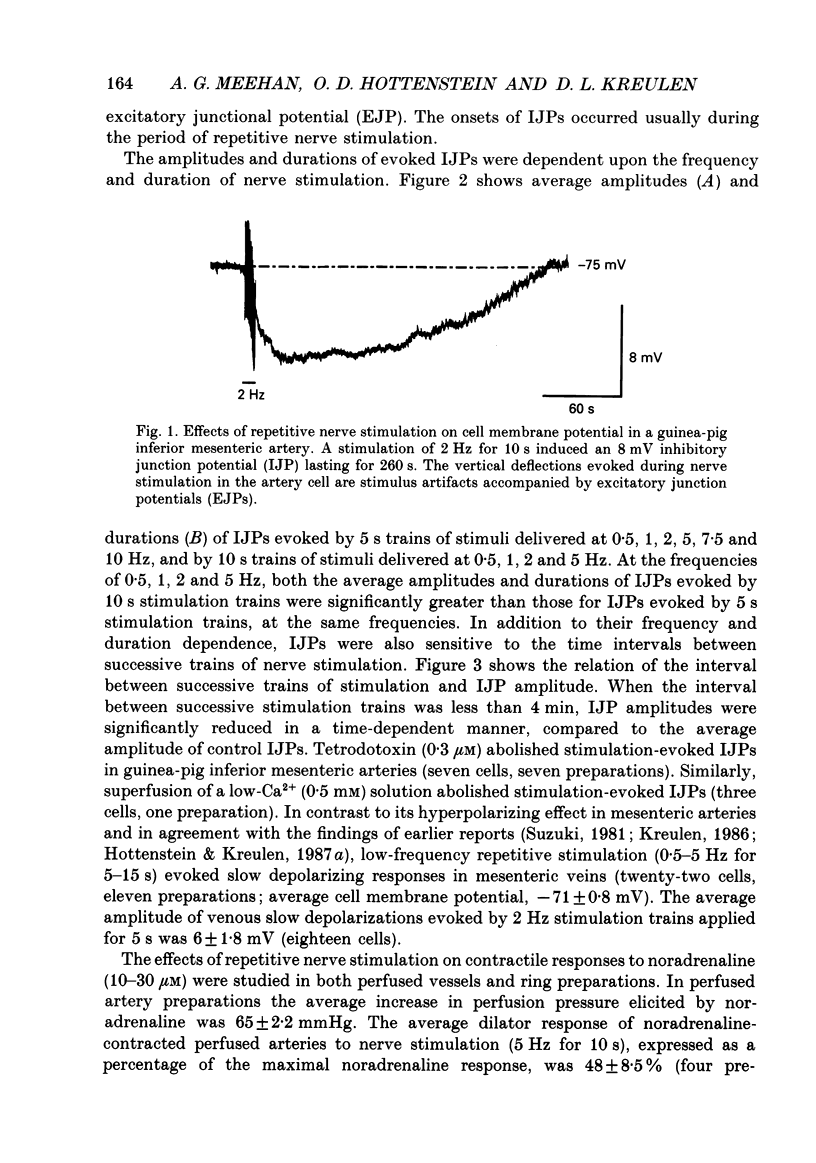
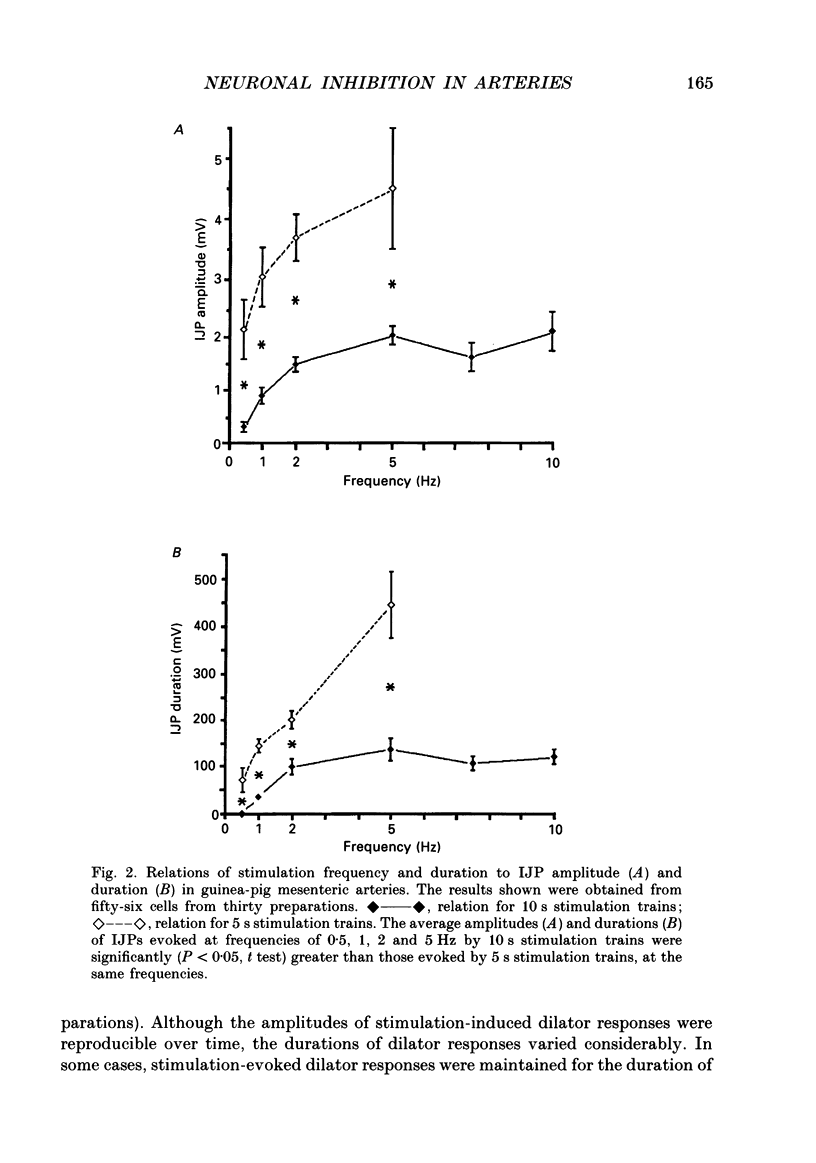
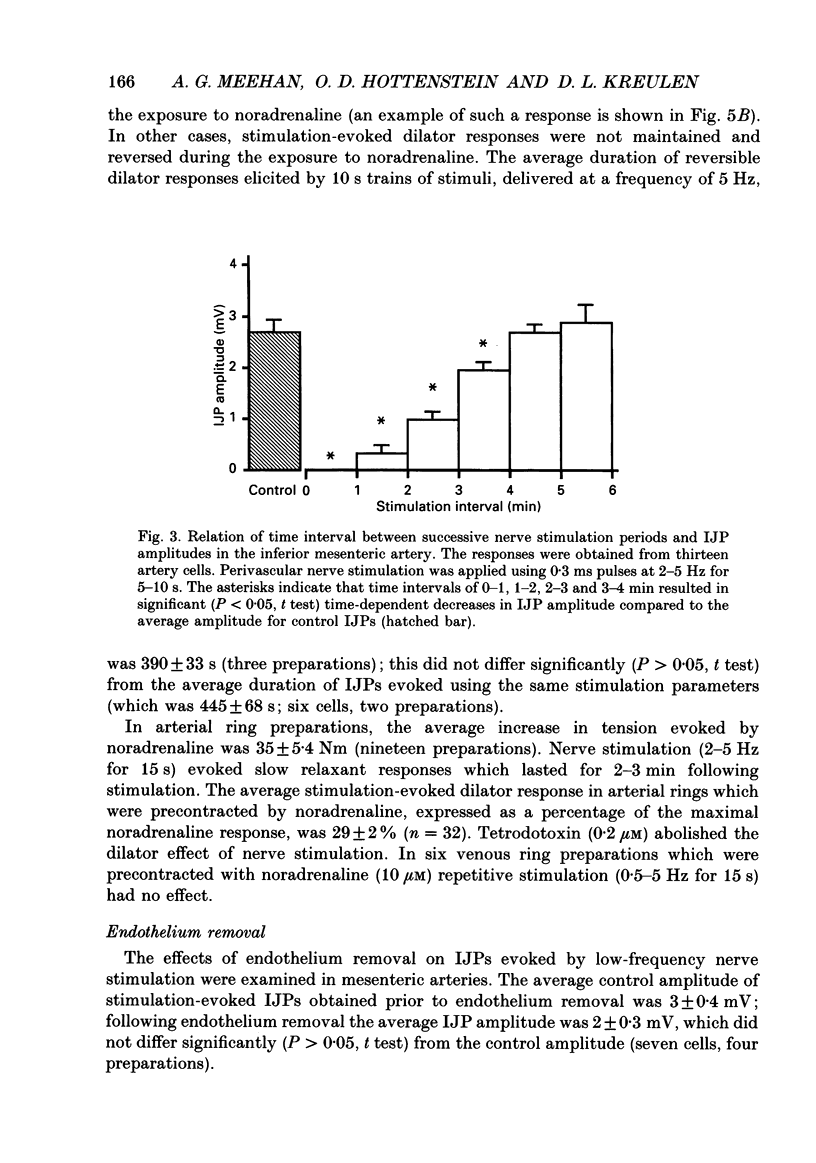
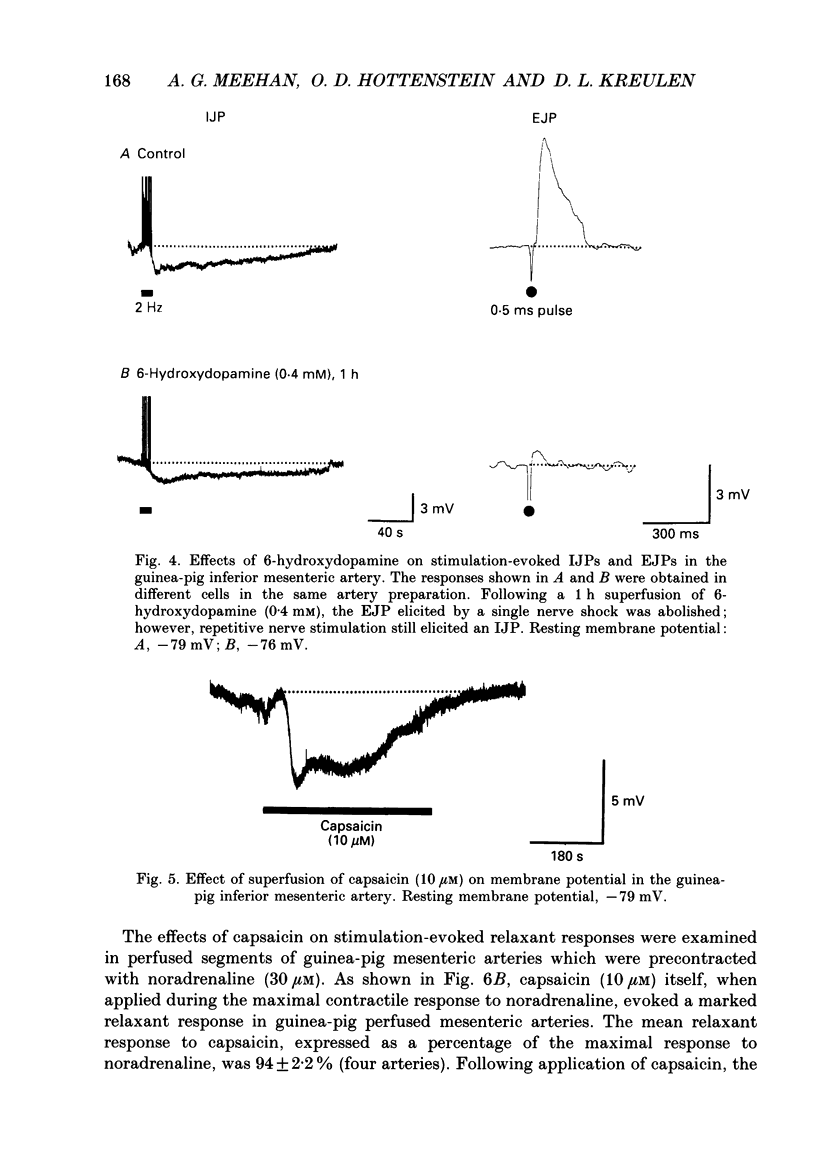
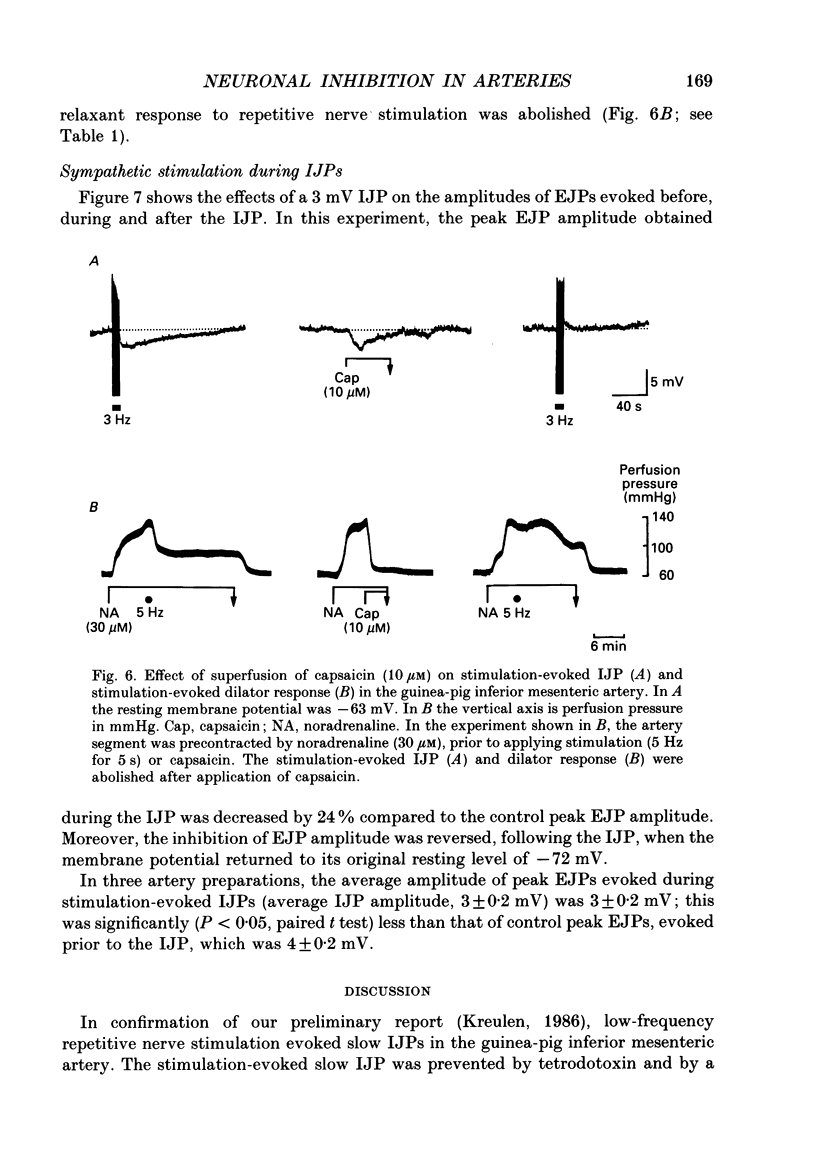
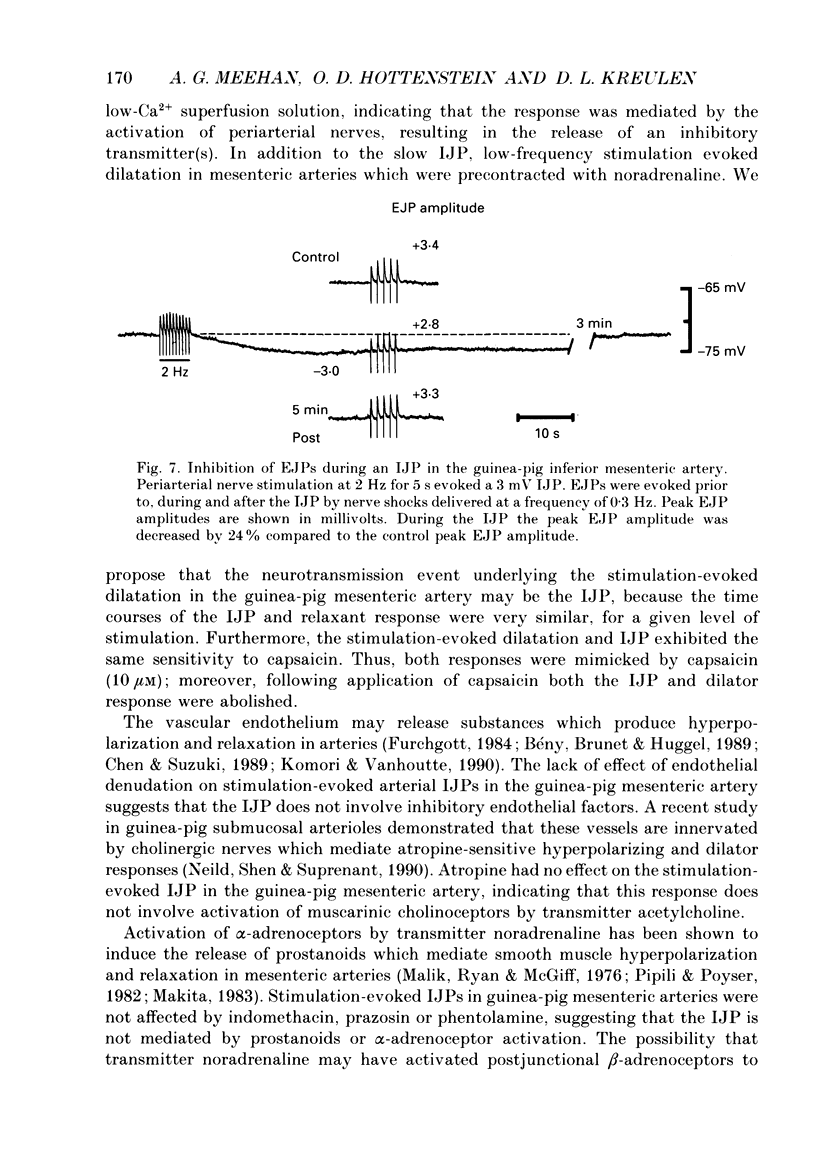
1. The present study examined the effects of repetitive nerve stimulation on membrane potential and on contractile responses to noradrenaline in the guinea-pig inferior mesenteric artery and its distal branches. 2. Repetitive stimulation of perivascular nerves evoked slow inhibitory junction potentials (IJPs) and dilator responses. Individual nerve shocks elicited excitatory junction potentials (EJP)s. 3. Stimulation-evoked IJPs were abolished in the presence of tetrodotoxin (0.3 microM) or a low-Ca2+ (0.5 mM) superfusion solution. 4. The amplitudes and durations of IJPs were dependent on the frequency and duration of repetitive nerve stimulation. Nerve stimulation delivered at 5 Hz for 5 s induced IJPs which had an average amplitude of 2 mV and an average duration of 130 s. When the time interval between successive stimulation periods was less than 4 min, the amplitudes of IJPs were reduced in a time-dependent manner. 5. Stimulation-evoked IJPs were unaffected following endothelium removal. Furthermore, stimulation-evoked IJPs were not affected by atropine (1 microM), indomethacin (20 microM), prazosin (0.5 microM), phentolamine (10 microM), propranolol (0.5 microM) or alpha,beta-methylene ATP (0.2 microM). 6. Pre-treatment of arteries with guanethidine (30 microM) or 6-hydroxydopamine (0.4 mM) abolished stimulation-evoked EJPs but had no effect on stimulation-evoked IJPs. 7. In a similar manner to repetitive nerve stimulation, capsaicin (10 microM) itself induced membrane hyperpolarization and dilatation in mesenteric arteries. Moreover, following application of capsaicin (10 microM), stimulation-evoked IJPs and dilator responses were abolished. 8. EJPs evoked during stimulation-induced IJPs were reduced in amplitude, compared to EJPs evoked under resting conditions. 9. These findings suggest that, in addition to an excitatory sympathetic innervation, mesenteric arteries receive an inhibitory, capsaicin-sensitive innervation which is activated by low-frequency repetitive stimulation.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Buck S. H., Burks T. F. The neuropharmacology of capsaicin: review of some recent observations. Pharmacol Rev. 1986 Sep;38(3):179–226. [PubMed] [Google Scholar]
- Bény J. L., Brunet P. C., Huggel H. Effects of substance P, calcitonin gene-related peptide and capsaicin on tension and membrane potential of pig coronary artery in vitro. Regul Pept. 1989 Apr;25(1):25–36. doi: 10.1016/0167-0115(89)90245-0. [DOI] [PubMed] [Google Scholar]
- Chen G., Suzuki H. Some electrical properties of the endothelium-dependent hyperpolarization recorded from rat arterial smooth muscle cells. J Physiol. 1989 Mar;410:91–106. doi: 10.1113/jphysiol.1989.sp017522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furness J. B., Papka R. E., Della N. G., Costa M., Eskay R. L. Substance P-like immunoreactivity in nerves associated with the vascular system of guinea-pigs. Neuroscience. 1982 Feb;7(2):447–459. doi: 10.1016/0306-4522(82)90279-2. [DOI] [PubMed] [Google Scholar]
- Han S. P., Naes L., Westfall T. C. Inhibition of periarterial nerve stimulation-induced vasodilation of the mesenteric arterial bed by CGRP (8-37) and CGRP receptor desensitization. Biochem Biophys Res Commun. 1990 Apr 30;168(2):786–791. doi: 10.1016/0006-291x(90)92390-l. [DOI] [PubMed] [Google Scholar]
- Holzer P. Local effector functions of capsaicin-sensitive sensory nerve endings: involvement of tachykinins, calcitonin gene-related peptide and other neuropeptides. Neuroscience. 1988 Mar;24(3):739–768. doi: 10.1016/0306-4522(88)90064-4. [DOI] [PubMed] [Google Scholar]
- Hottenstein O. D., Kreulen D. L. Comparison of the frequency dependence of venous and arterial responses to sympathetic nerve stimulation in guinea-pigs. J Physiol. 1987 Mar;384:153–167. doi: 10.1113/jphysiol.1987.sp016448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hottenstein O. D., Pawlik W. W., Remak G., Jacobson E. D. Capsaicin-sensitive nerves modulate resting blood flow and vascular tone in rat gut. Naunyn Schmiedebergs Arch Pharmacol. 1991 Feb;343(2):179–184. doi: 10.1007/BF00168607. [DOI] [PubMed] [Google Scholar]
- Jacobson E. D. Physiology of the mesenteric circulation. Physiologist. 1982 Oct;25(5):439–443. [PubMed] [Google Scholar]
- Kawasaki H., Takasaki K., Saito A., Goto K. Calcitonin gene-related peptide acts as a novel vasodilator neurotransmitter in mesenteric resistance vessels of the rat. Nature. 1988 Sep 8;335(6186):164–167. doi: 10.1038/335164a0. [DOI] [PubMed] [Google Scholar]
- Komori K., Vanhoutte P. M. Endothelium-derived hyperpolarizing factor. Blood Vessels. 1990;27(2-5):238–245. doi: 10.1159/000158815. [DOI] [PubMed] [Google Scholar]
- Kreulen D. L. Activation of mesenteric arteries and veins by preganglionic and postganglionic nerves. Am J Physiol. 1986 Dec;251(6 Pt 2):H1267–H1275. doi: 10.1152/ajpheart.1986.251.6.H1267. [DOI] [PubMed] [Google Scholar]
- Lonart G., Gyorgy L., Doda M., Vizi E. S. Evidence that dopaminergic innervation is not involved in the vasodilatation of cat superior mesenteric arterial bed: the role of beta-adrenoceptors and circulating catecholamines. Circ Res. 1988 Jun;62(6):1134–1137. doi: 10.1161/01.res.62.6.1134. [DOI] [PubMed] [Google Scholar]
- Lundgren O. Role of splanchnic resistance vessels in overall cardiovascular homeostasis. Fed Proc. 1983 Apr;42(6):1673–1677. [PubMed] [Google Scholar]
- MCGREGOR D. D. THE EFFECT OF SYMPATHETIC NERVE STIMULATION OF VASOCONSTRICTOR RESPONSES IN PERFUSED MESENTERIC BLOOD VESSELS OF THE RAT. J Physiol. 1965 Mar;177:21–30. doi: 10.1113/jphysiol.1965.sp007572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makita Y. Effects of prostaglandin I2 and carbocyclic thromboxane A2 on smooth muscle cells and neuromuscular transmission in the guinea-pig mesenteric artery. Br J Pharmacol. 1983 Mar;78(3):517–527. doi: 10.1111/j.1476-5381.1983.tb08811.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik K. U., Ryan P., McGiff J. C. Modification by prostaglandins E1 and E2, indomethacin, and arachidonic acid of the vasoconstrictor responses of the isolated perfused rabbit and rat mesenteric arteries to adrenergic stimuli. Circ Res. 1976 Aug;39(2):163–168. doi: 10.1161/01.res.39.2.163. [DOI] [PubMed] [Google Scholar]
- Neild T. O., Shen K. Z., Surprenant A. Vasodilatation of arterioles by acetylcholine released from single neurones in the guinea-pig submucosal plexus. J Physiol. 1990 Jan;420:247–265. doi: 10.1113/jphysiol.1990.sp017910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson M. T., Huang Y., Brayden J. E., Hescheler J., Standen N. B. Arterial dilations in response to calcitonin gene-related peptide involve activation of K+ channels. Nature. 1990 Apr 19;344(6268):770–773. doi: 10.1038/344770a0. [DOI] [PubMed] [Google Scholar]
- Nilsson H., Ljung B., Sjöblom N., Wallin B. G. The influence of the sympathetic impulse pattern on contractile responses of rat mesenteric arteries and veins. Acta Physiol Scand. 1985 Mar;123(3):303–309. doi: 10.1111/j.1748-1716.1985.tb07592.x. [DOI] [PubMed] [Google Scholar]
- Pipili E., Poyser N. L. Differential effects of prazosin and rauwolsin on the release of prostaglandins I2 and E2 from the perfused mesenteric arterial bed of the rabbit following nerve stimulation. Prostaglandins. 1982 Mar;23(3):299–309. doi: 10.1016/0090-6980(82)90075-2. [DOI] [PubMed] [Google Scholar]
- Ralevic V., Burnstock G. Actions mediated by P2-purinoceptor subtypes in the isolated perfused mesenteric bed of the rat. Br J Pharmacol. 1988 Oct;95(2):637–645. doi: 10.1111/j.1476-5381.1988.tb11686.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remak G., Hottenstein O. D., Jacobson E. D. Sensory nerves mediate neurogenic escape in rat gut. Am J Physiol. 1990 Mar;258(3 Pt 2):H778–H786. doi: 10.1152/ajpheart.1990.258.3.H778. [DOI] [PubMed] [Google Scholar]
- Rozsa Z., Jacobson E. D. Capsaicin-sensitive nerves are involved in bile-oleate-induced intestinal hyperemia. Am J Physiol. 1989 Mar;256(3 Pt 1):G476–G481. doi: 10.1152/ajpgi.1989.256.3.G476. [DOI] [PubMed] [Google Scholar]
- Rózsa Z., Sharkey K. A., Jancsó G., Varró V. Evidence for a role of capsaicin-sensitive mucosal afferent nerves in the regulation of mesenteric blood flow in the dog. Gastroenterology. 1986 Apr;90(4):906–910. doi: 10.1016/0016-5085(86)90866-8. [DOI] [PubMed] [Google Scholar]
- Suzuki H. Effects of endogenous and exogenous noradrenaline on the smooth muscle of guinea-pig mesenteric vein. J Physiol. 1981 Dec;321:495–512. doi: 10.1113/jphysiol.1981.sp013999. [DOI] [PMC free article] [PubMed] [Google Scholar]


