Abstract
1. Isometric twitch and tetanic tensions were recorded from whole muscles and single motor units in fourth deep lumbrical muscles isolated from young adult (60 days) rats. Muscles were superfused with oxygenated Ringer solution at 25 degrees C except where stated otherwise. 2. It was confirmed that the muscle is supplied most commonly by eleven motor axons, nine via the lateral plantar nerve (LPN), and two via the sural nerve (SN). Motor units whose axons were isolated from either LPN or SN were studied. There was no difference in mean motor unit size. 3. In their unfused tetani most units showed 'sag' and some 'no sag', with no segregation between LPN and SN. 'No sag' units were always small (unit tetanic tension less than 8% whole-muscle tetanic tension), tended to be relatively slowly contracting and relaxing during an isometric twitch, and tended to have relatively low twitch:tetanus ratios. Units showing sag ranged from large to small. 4. In some motor units muscle fibres were depleted of their glycogen by repetitive stimulation at 30 degrees C in glucose-free Ringer solution, and the muscle and its unstimulated control frozen and sectioned. Neighbouring sections were stained for glycogen and for binding of two myosin-specific antibodies, one specific for slow myosin and the other for type IIA myosin. Myosin ATPase and succinic dehydrogenase histochemistry were also carried out in some muscles. 5. Serial reconstructions showed that all or virtually all extrafusal fibres in the muscle were present in a midbelly section, and that the myosin type of individual fibres did not change significantly along their length. Spindle profiles were seen frequently and in two muscles eight and twelve spindles were identified. 6. Of twenty-six motor units examined twenty contained almost exclusively muscle fibres of the recently described type IIX. All these units showed sag in their isometric tetani. 7. Six units each contained 50% or more of slow myosin-containing fibres (IIC and a few type I). The remaining fibres in these units were IIA. All these units were therefore of mixed fibre composition, and are discussed as IIC/IIA units. In whole muscles slow-myosin-containing fibres were generally distributed evenly (non-randomly) throughout the muscle cross-section. 8. Whole muscles contained on average 970 fibres (S.D. +/- 70) of which 82 (+/- 9) were slow-myosin-containing. A few muscles from older rats (3-24 months) contained very few such fibres.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
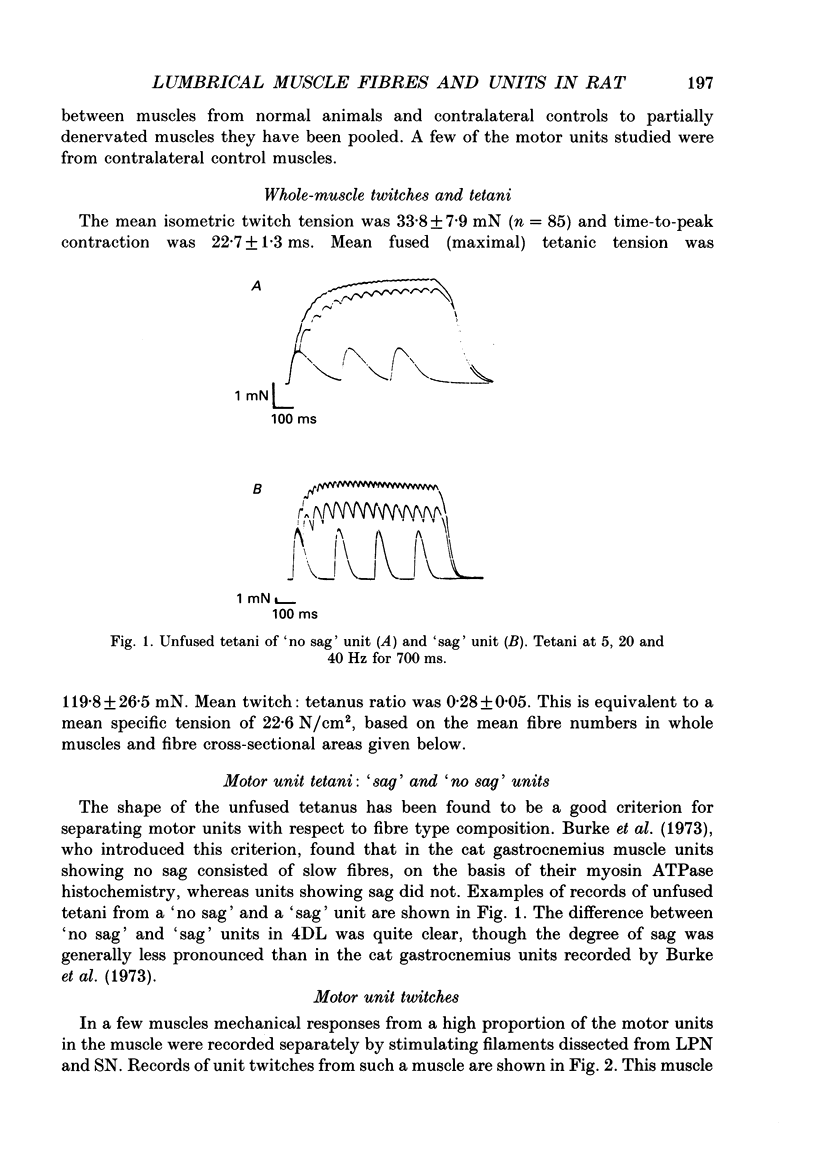
PDF



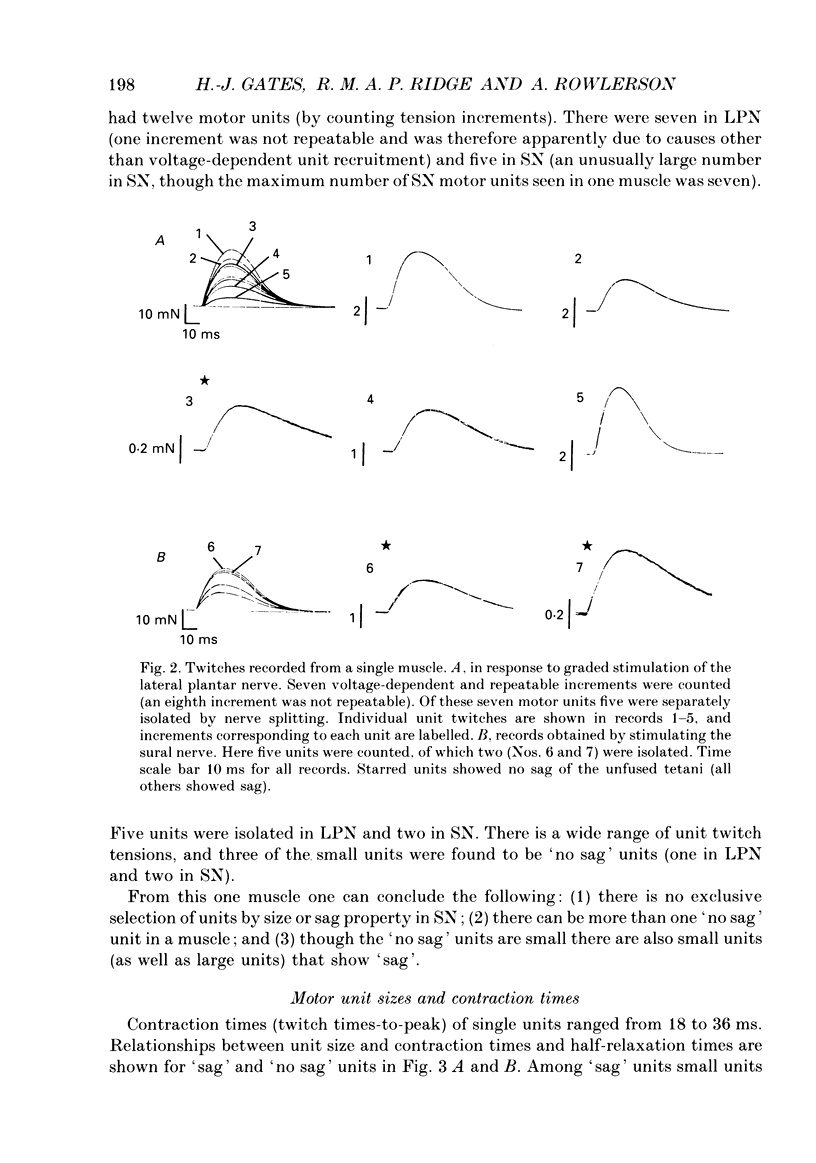


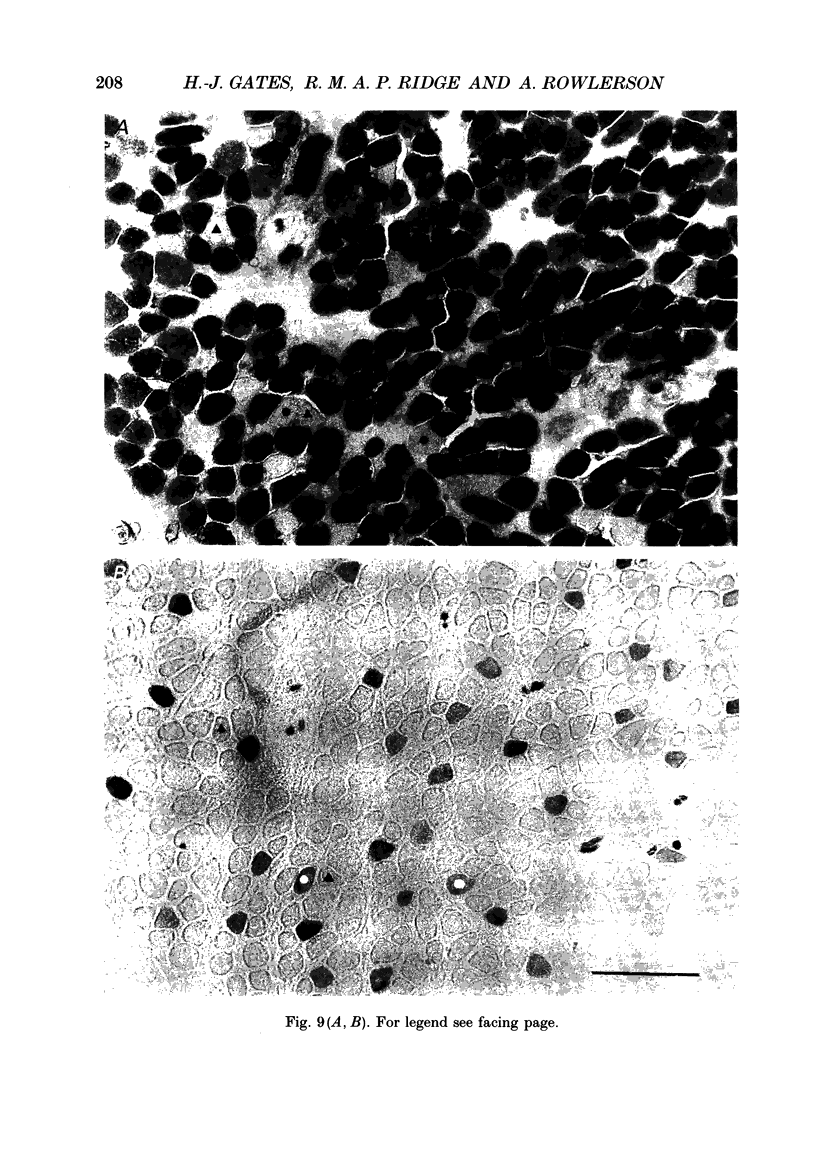
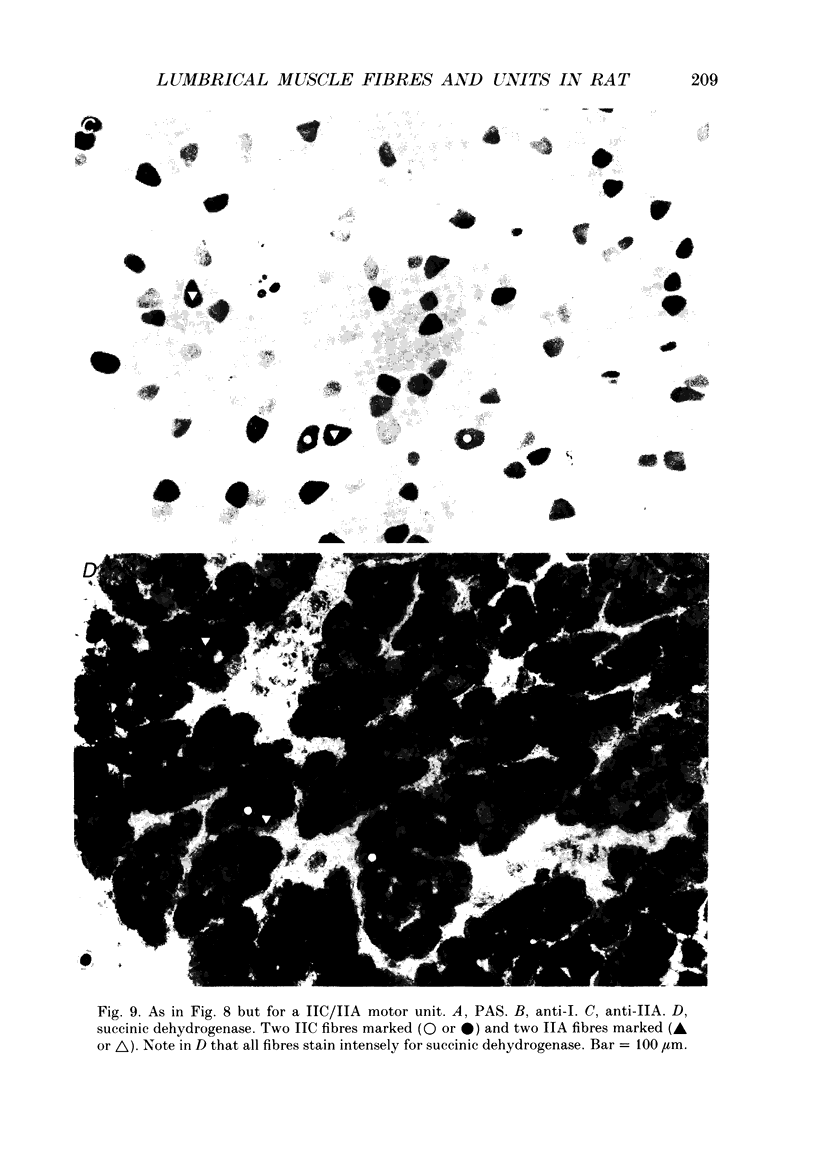
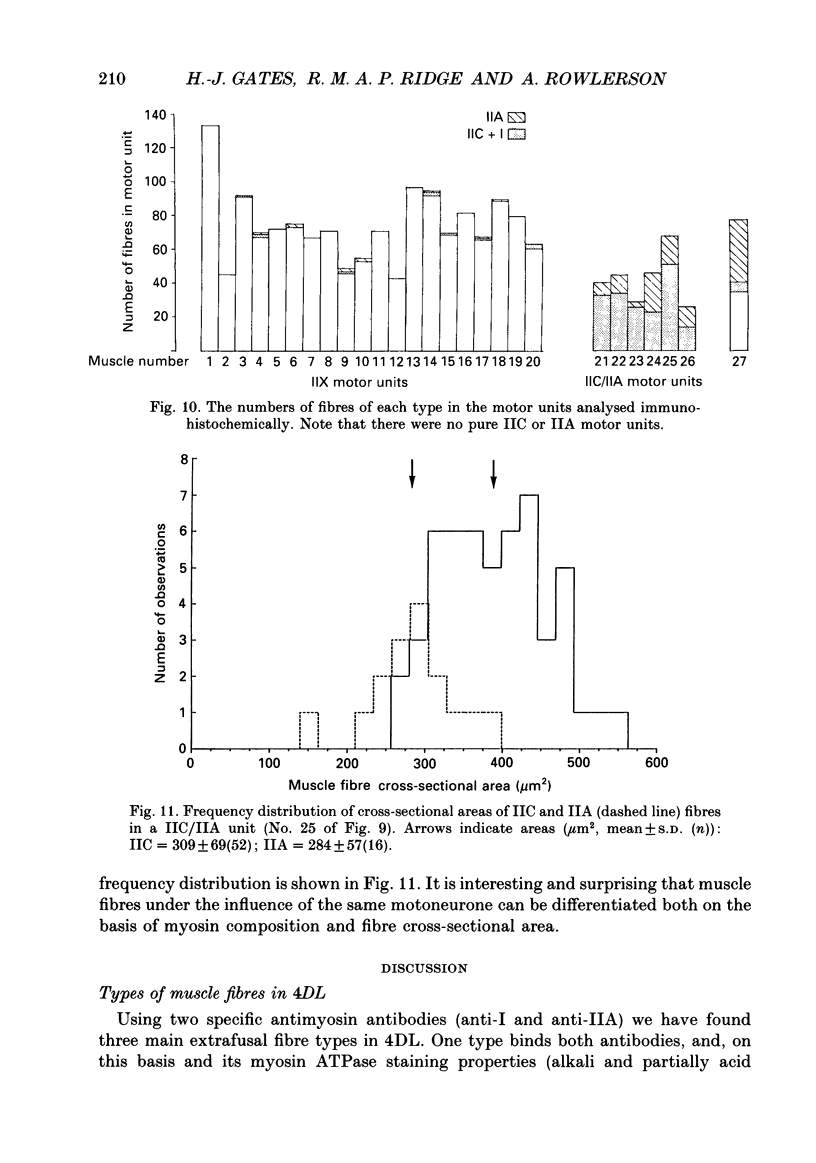





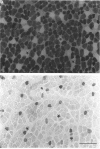
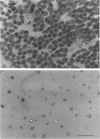
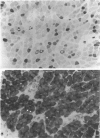
Images in this article
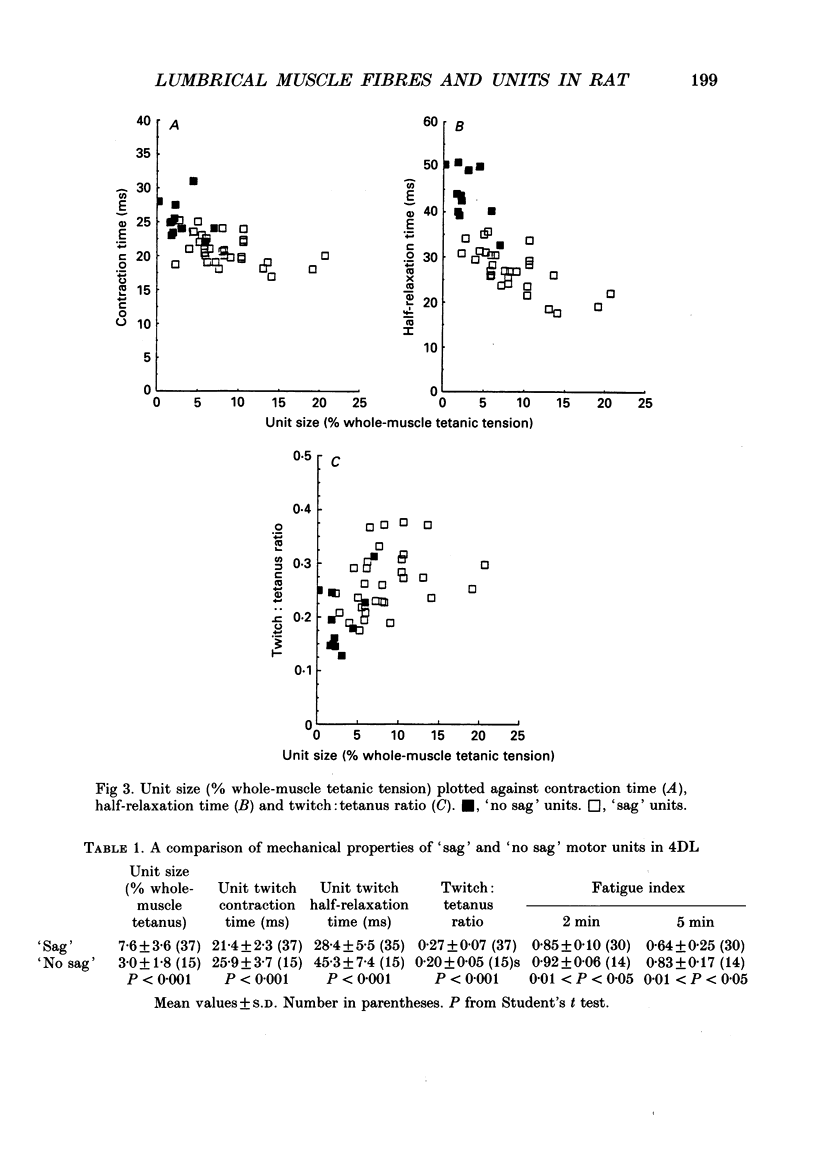
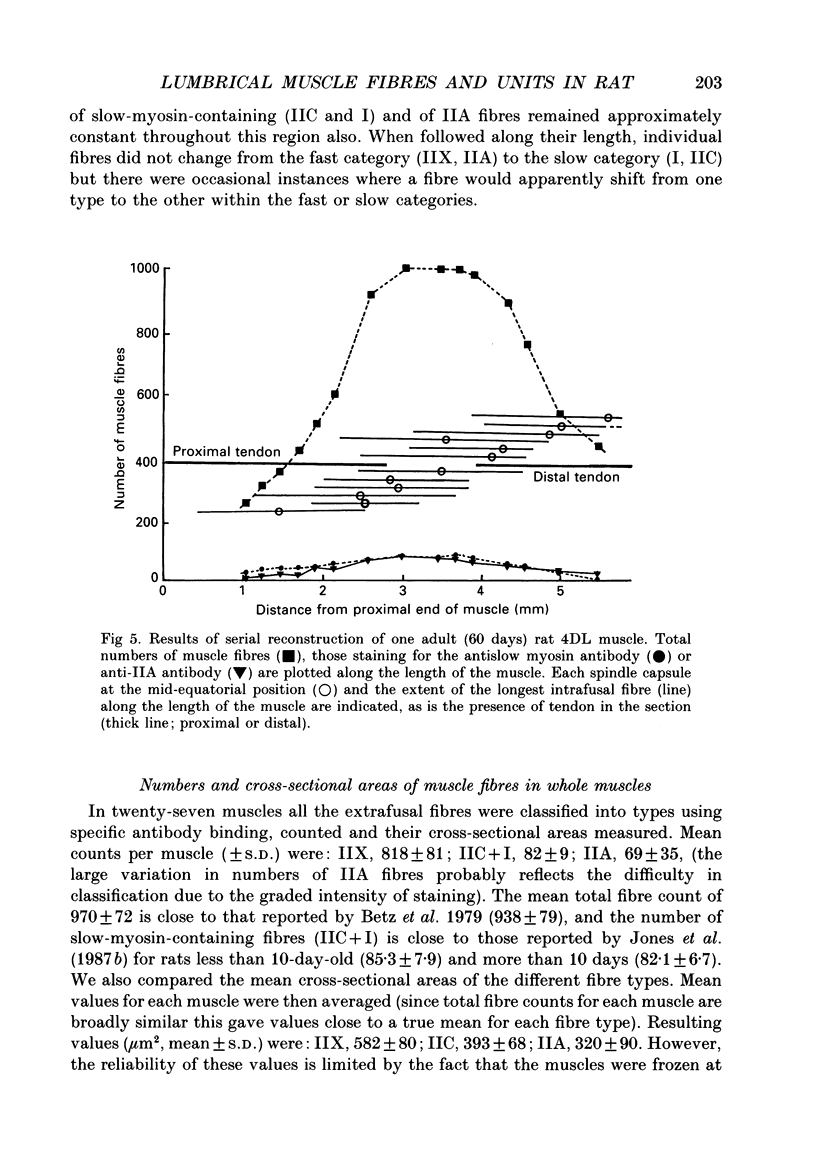
Selected References
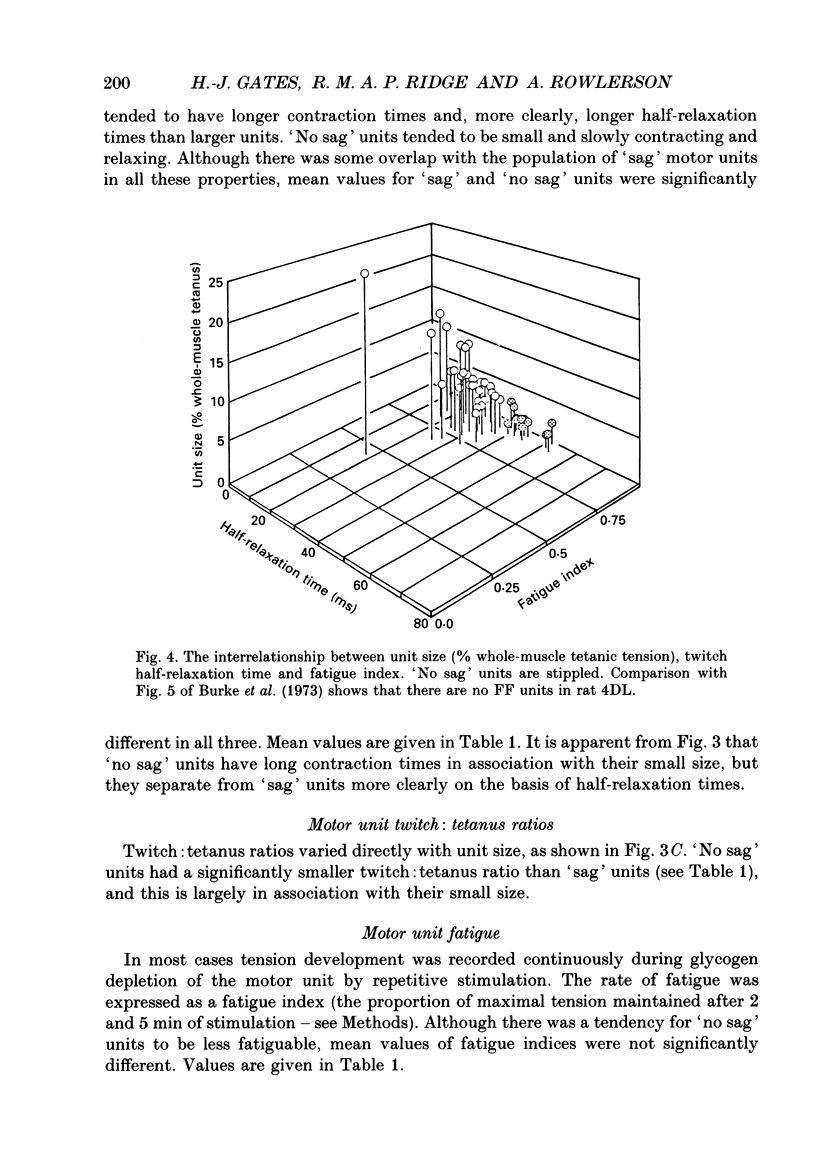
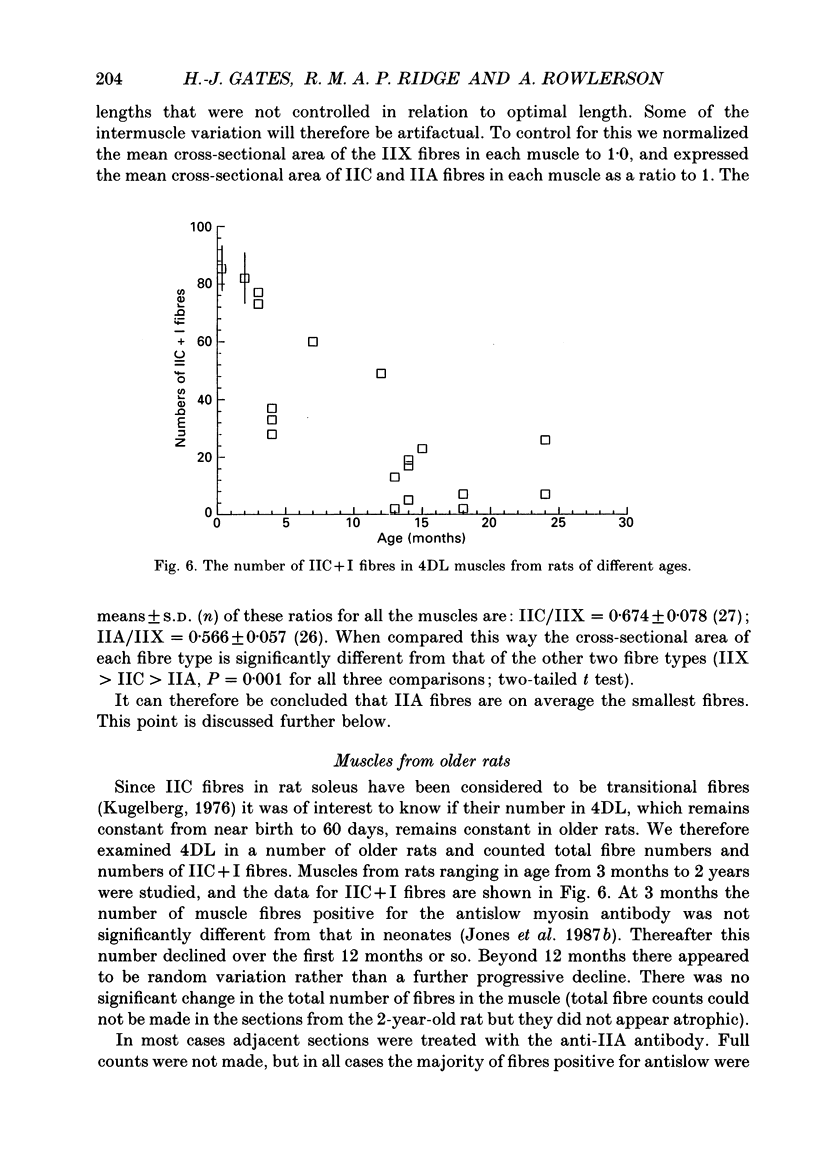
These references are in PubMed. This may not be the complete list of references from this article.
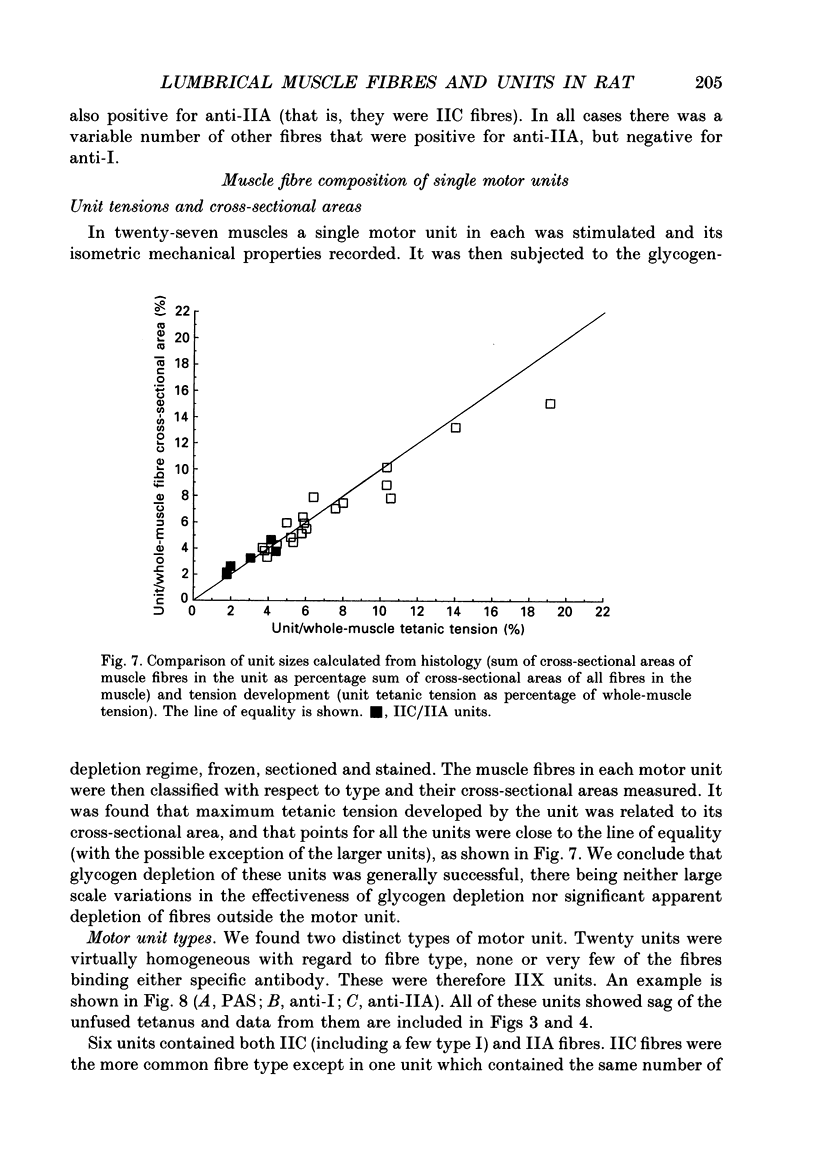
- Andrew B. L., Part N. J. The division of control of muscle spindles between fusimotor and mixed skeletofusimotor fibres in a rat caudal muscle. Q J Exp Physiol Cogn Med Sci. 1974 Oct;59(4):331–349. doi: 10.1113/expphysiol.1974.sp002277. [DOI] [PubMed] [Google Scholar]
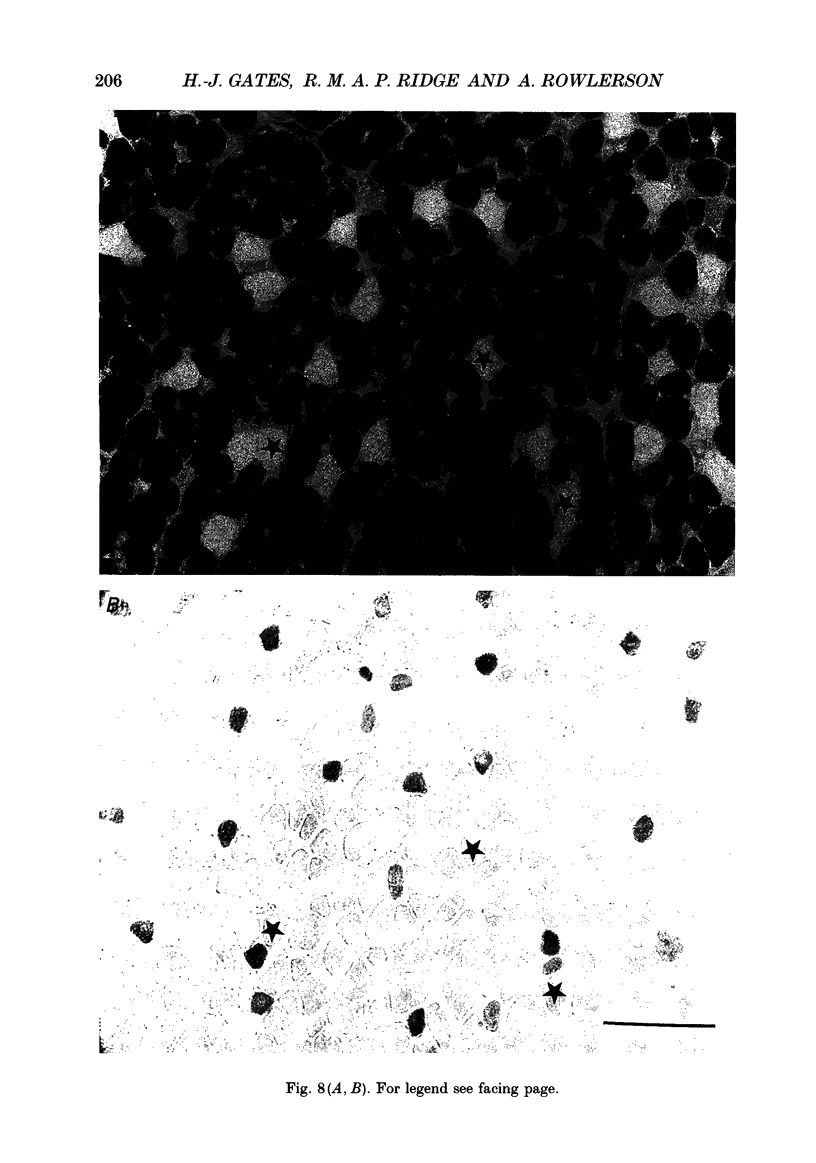
- Ausoni S., Gorza L., Schiaffino S., Gundersen K., Lømo T. Expression of myosin heavy chain isoforms in stimulated fast and slow rat muscles. J Neurosci. 1990 Jan;10(1):153–160. doi: 10.1523/JNEUROSCI.10-01-00153.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balice-Gordon R. J., Thompson W. J. Synaptic rearrangements and alterations in motor unit properties in neonatal rat extensor digitorum longus muscle. J Physiol. 1988 Apr;398:191–210. doi: 10.1113/jphysiol.1988.sp017038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betz W. J., Caldwell J. H., Ribchester R. R. The size of motor units during post-natal development of rat lumbrical muscle. J Physiol. 1979 Dec;297(0):463–478. doi: 10.1113/jphysiol.1979.sp013051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betz W. J., Ribchester R. R., Ridge R. M. Competitive mechanisms underlying synapse elimination in the lumbrical muscle of the rat. J Neurobiol. 1990 Jan;21(1):1–17. doi: 10.1002/neu.480210102. [DOI] [PubMed] [Google Scholar]
- Brooke M. H., Kaiser K. K. Muscle fiber types: how many and what kind? Arch Neurol. 1970 Oct;23(4):369–379. doi: 10.1001/archneur.1970.00480280083010. [DOI] [PubMed] [Google Scholar]
- Brown M. C., Jansen J. K., Van Essen D. Polyneuronal innervation of skeletal muscle in new-born rats and its elimination during maturation. J Physiol. 1976 Oct;261(2):387–422. doi: 10.1113/jphysiol.1976.sp011565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke R. E., Levine D. N., Tsairis P., Zajac F. E., 3rd Physiological types and histochemical profiles in motor units of the cat gastrocnemius. J Physiol. 1973 Nov;234(3):723–748. doi: 10.1113/jphysiol.1973.sp010369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenè E., Rowlerson A., Veggetti A., Mascarello F. Preparation of type-specific antimyosin antibodies and determination of their specificity by biochemical and immunohistochemical methods. Ital J Biochem. 1982 Sep-Oct;31(5):329–341. [PubMed] [Google Scholar]
- Chamberlain S., Lewis D. M. Contractile characteristics and innervation ratio of rat soleus motor units. J Physiol. 1989 May;412:1–21. doi: 10.1113/jphysiol.1989.sp017601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Close R. Properties of motor units in fast and slow skeletal muscles of the rat. J Physiol. 1967 Nov;193(1):45–55. doi: 10.1113/jphysiol.1967.sp008342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condon K., Silberstein L., Blau H. M., Thompson W. J. Development of muscle fiber types in the prenatal rat hindlimb. Dev Biol. 1990 Apr;138(2):256–274. doi: 10.1016/0012-1606(90)90196-p. [DOI] [PubMed] [Google Scholar]
- Gorza L. Identification of a novel type 2 fiber population in mammalian skeletal muscle by combined use of histochemical myosin ATPase and anti-myosin monoclonal antibodies. J Histochem Cytochem. 1990 Feb;38(2):257–265. doi: 10.1177/38.2.2137154. [DOI] [PubMed] [Google Scholar]
- Harris A. J., Fitzsimons R. B., McEwan J. C. Neural control of the sequence of expression of myosin heavy chain isoforms in foetal mammalian muscles. Development. 1989 Dec;107(4):751–769. doi: 10.1242/dev.107.4.751. [DOI] [PubMed] [Google Scholar]
- Jones S. P., Ridge R. M. Motor units in a skeletal muscle of neonatal rat: mechanical properties and weak neuromuscular transmission. J Physiol. 1987 May;386:355–375. doi: 10.1113/jphysiol.1987.sp016538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones S. P., Ridge R. M., Rowlerson A. Rat muscle during post-natal development: evidence in favour of no interconversion between fast- and slow-twitch fibres. J Physiol. 1987 May;386:395–406. doi: 10.1113/jphysiol.1987.sp016540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones S. P., Ridge R. M., Rowlerson A. The non-selective innervation of muscle fibres and mixed composition of motor units in a muscle of neonatal rat. J Physiol. 1987 May;386:377–394. doi: 10.1113/jphysiol.1987.sp016539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kernell D., Eerbeek O., Verhey B. A. Motor unit categorization on basis of contractile properties: an experimental analysis of the composition of the cat's m. peroneus longus. Exp Brain Res. 1983;50(2-3):211–219. doi: 10.1007/BF00239185. [DOI] [PubMed] [Google Scholar]
- Kugelberg E. Adaptive transformation of rat soleus motor units during growth. J Neurol Sci. 1976 Mar;27(3):269–289. doi: 10.1016/0022-510x(76)90001-0. [DOI] [PubMed] [Google Scholar]
- Kugelberg E. Histochemical composition, contraction speed and fatiguability of rat soleus motor units. J Neurol Sci. 1973 Oct;20(2):177–198. doi: 10.1016/0022-510x(73)90029-4. [DOI] [PubMed] [Google Scholar]
- Kugelberg E., Lindegren B. Transmission and contraction fatigue of rat motor units in relation to succinate dehydrogenase activity of motor unit fibres. J Physiol. 1979 Mar;288:285–300. [PMC free article] [PubMed] [Google Scholar]
- Milburn A. Stages in the development of cat muscle spindles. J Embryol Exp Morphol. 1984 Aug;82:177–216. [PubMed] [Google Scholar]
- NACHLAS M. M., TSOU K. C., DE SOUZA E., CHENG C. S., SELIGMAN A. M. Cytochemical demonstration of succinic dehydrogenase by the use of a new p-nitrophenyl substituted ditetrazole. J Histochem Cytochem. 1957 Jul;5(4):420–436. doi: 10.1177/5.4.420. [DOI] [PubMed] [Google Scholar]
- Porayko O., Smith R. S. Morphology of muscle spindles in the rat. Experientia. 1968 Jun 15;24(6):588–589. doi: 10.1007/BF02153790. [DOI] [PubMed] [Google Scholar]
- Ross J. J., Duxson M. J., Harris A. J. Formation of primary and secondary myotubes in rat lumbrical muscles. Development. 1987 Jul;100(3):383–394. doi: 10.1242/dev.100.3.383. [DOI] [PubMed] [Google Scholar]
- Schiaffino S., Gorza L., Sartore S., Saggin L., Ausoni S., Vianello M., Gundersen K., Lømo T. Three myosin heavy chain isoforms in type 2 skeletal muscle fibres. J Muscle Res Cell Motil. 1989 Jun;10(3):197–205. doi: 10.1007/BF01739810. [DOI] [PubMed] [Google Scholar]
- Thompson W. J., Sutton L. A., Riley D. A. Fibre type composition of single motor units during synapse elimination in neonatal rat soleus muscle. Nature. 1984 Jun 21;309(5970):709–711. doi: 10.1038/309709a0. [DOI] [PubMed] [Google Scholar]