Abstract
Plants have multiple potassium (K+) uptake and efflux mechanisms that are expressed throughout plant tissues to fulfill different physiological functions. Several different classes of K+ channels and carriers have been identified at the molecular level in plants. K+ transporters of the HKT1 superfamily have been cloned from wheat (Triticum aestivum), Arabidopsis, and Eucalyptus camaldulensis. The functional characteristics as well as the primary structure of these transporters are diverse with orthologues found in bacterial and fungal genomes. In this report, we provide a detailed characterization of the functional characteristics, as expressed in Xenopus laevis oocytes, of two cDNAs isolated from E. camaldulensis that encode proteins belonging to the HKT1 superfamily of K+/Na+ transporters. The transport of K+ in EcHKT-expressing oocytes is enhanced by Na+, but K+ was also transported in the absence of Na+. Na+ is transported in the absence of K+ as has been demonstrated for HKT1 and AtHKT1. Overall, the E. camaldulensis transporters show some similarities and differences in ionic selectivity to HKT1 and AtHKT1. One striking difference between HKT1 and EcHKT is the sensitivity to changes in the external osmolarity of the solution. Hypotonic solutions increased EcHKT induced currents in oocytes by 100% as compared with no increased current in HKT1 expressing or uninjected oocytes. These osmotically sensitive currents were not enhanced by voltage and may mediate water flux. The physiological function of these osmotically induced increases in currents may be related to the ecological niches that E. camaldulensis inhabits, which are periodically flooded. Therefore, the osmosensing function of EcHKT may provide this species with a competitive advantage in maintaining K+ homeostasis under certain conditions.
Potassium (K+) plays an important role in a wide range of physiological and biochemical functions and is essential for plant growth and development (Kochian and Lucas, 1988; Schroeder et al., 1994; Maathuis and Sanders, 1996). K+ is acquired by roots from soil and is transported and recirculated to other parts of the plant via the xylem and phloem (Marschner, 1995). Because of the importance of K+ in many aspects of plant growth and adaptation to the environment, plants require multiple mechanisms for K+ acquisition (Schachtman, 2000), translocation (Jeschke et al., 1987; Marten et al., 1999; Lacombe et al., 2000), and for maintaining cellular K+ homeostasis (Leigh and Wyn Jones, 1984; Walker et al., 1996).
Many specialized transport processes that must be tightly regulated (Glass, 1983) are involved in uptake, translocation, and in maintaining cellular K+ homeostasis. Transport mechanisms such as channels and symports are known to be involved in K+ uptake across the plasma membrane (Gassmann et al., 1993; Kochian and Lucas, 1993). K+ transport into and out of the vacuole is important in maintaining cellular homeostasis and it appears that both channels (Ward and Schroeder, 1994) and antiports (E. Blumwald, personal communication) may be involved in vacuolar K+ transport. The molecular identity of K+ transport mechanisms is known in only a few of thousands of plant species. Several different genes encoding channels that facilitate both inward and outward K+ fluxes have been cloned from Arabidopsis (Amtmann and Sanders, 1999; Schachtman, 2000). Two types of K+ carriers (KUP and HKT families) have also been identified at the molecular level from Arabidopsis and from cereals (Schachtman, 2000). Although the physiological roles of some of these K+ transport mechanisms have been identified (Schroeder et al., 1994; Nakamura et al., 1995; Gaymard et al., 1998; Hirsch et al., 1998; Zimmermann and Sentenac, 1999; Rigas et al., 2001), the primary physiological role of others is poorly understood including: HKT (Schachtman and Schroeder, 1994; Rubio et al., 1995; Gassmann et al., 1996), KUP/HAK (Quintero and Blatt, 1997; Santa-Maria et al., 1997; Fu and Luan, 1998; Kim et al., 1998; Rubio et al., 2000), KCO (Czempinski et al., 1997), and LCT (Schachtman et al., 1997; Clemens et al., 1998).
The physiological function of HKT1 has been difficult to unravel, but it does not appear to be involved in ion acquisition (Box and Schachtman, 2000), because sodium does not significantly stimulate growth or potassium uptake in long- or short-term experiments. Homologs of this transporter have been found in a wide range of bacterial, fungal, and plant genomes (Schachtman and Liu, 1999). HKT1-like proteins from a monocot (Schachtman and Schroeder, 1994), a dicot (Uozumi et al., 2000), and a woody perennial (Fairbairn et al., 2000) have been cloned. Only the function of the monocot HKT1 has been fully characterized (Gassmann et al., 1996). The wheat (Triticum aestivum) HKT1 transports K+ and Na+ (Rubio et al., 1995) and has been classified as a high-affinity K+ transporter. In contrast, the Arabidopsis homologue (AtHKT1) complements an Escherichia coli mutant deficient in K+ uptake, but the cDNA induces Na+ and not K+ currents when expressed in oocytes (Uozumi et al., 2000) and therefore has been classified as a Na+ transporter. This major difference between HKT1 and AtHKT1 highlights the functional diversity within these transporters in two different plant species. The functional diversity found within this superfamily across phyla is very large. For example, it is known that members of this superfamily are energized by Na+ in some organisms (Tholema et al., 1999) and by protons in other organisms (Bihler et al., 1999). Therefore, detailed functional characterization of these proteins from different plant species could uncover new and potentially useful functions for the improvement of plant performance.
In this study, we characterized the function of two transporters from Eucalyptus camaldulensis. E. camaldulensis is one of the most widely distributed Eucalypt species in Australia (Eldridge et al., 1993), which is the driest continent on earth. To survive and grow in the range of harsh environments, this species has been able to develop specialized physiological adaptations (Gibson et al., 1994). E. camaldulensis inhabits river beds that are dry for most of the year and flooded during other parts of the year. Such an adaptable and successful plant family is likely to possess unique mechanisms for adaptation to dry environments, which include drought and salinity tolerance (Zubrinich et al., 2000). In contrast to the short life cycle of the Arabidopsis model system (6–12 weeks), E. camaldulensis may grow in the same place for 250 years (Williams and Brooker, 1997). Therefore, the adaptation to drought and salinity that this species possesses is most likely different from species that rapidly complete their lifecycle under stress. In our characterization of the two HKT1 homologs from E. camaldulensis, we found similarities and differences to the homologs from wheat and Arabidopsis. The most striking new functional characteristic we discovered was the protein's intrinsic capability to sense changes in the external osmolarity of the solution.
RESULTS
K+ Currents Are Enhanced by Na+
We demonstrated previously that 1 mm Na+ plus 10 mm K+ resulted in a significant increase of inward currents in both EcHKT1- and EcHKT2-expressing oocytes compared with 10 mm K+ alone (Fairbairn et al., 2000), indicating that K+ transport by EcHKT1 and EcHKT2 is stimulated by Na+. To investigate whether other monovalent cations stimulate K+ transport, we compared the currents in EcHKT1- or EcHKT2-expressing oocytes in the presence of 1 mm of the ion plus 10 mm K+.
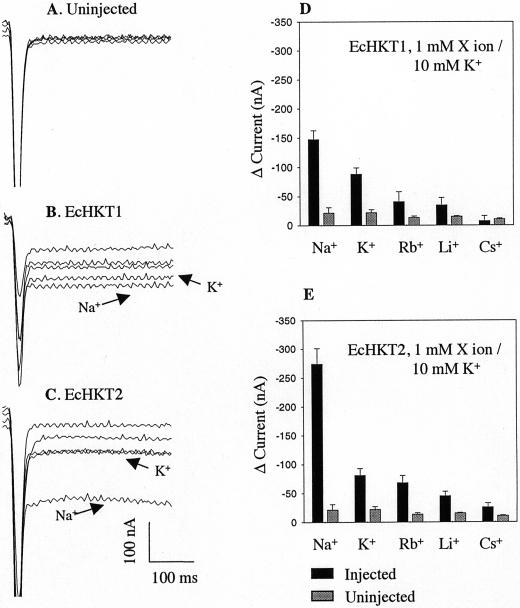
Figure 1 shows the representative currents for an uninjected oocyte (Fig. 1A) and both EcHKT1- and EcHKT2-expressing oocytes (Fig. 1, B and C) measured at −120 mV from a holding potential of −40 mV. Uninjected oocytes in a bath solution perfused with 1 mm Na+ or other monovalent cations in solutions containing 10 mm K+ had the same low steady-state background currents regardless of the monovalent cation added to the bath (Fig. 1A). Inward currents were measured at −120 mV from solutions without Na+ or K+ and subtracted from currents measured in solutions containing 10 mm K+ and 1 mm of a range of monovalent cations to obtain changes in current. The addition of 1 mm Na+ to solutions containing 10 mm K+ increased the inward current more than the addition of 1 mm K+, Rb+, Li+, and Cs+ in both the EcHKT1- and EcHKT2-expressing oocytes (Fig. 1, D and E). The percentage increase in currents due to the addition of Na+ was larger in EcHKT2-expressing oocytes (Fig. 1, C and E). The current change induced by bathing oocytes in 11 mm K+ (with no Na+) at −120 mV was similar for oocytes expressing either EcHKT1 and EcHKT2. This result indicates that K+ currents induced by EcHKT1 and EcHKT2 in oocytes are enhanced by Na+ to a different extent.
Figure 1.
Effects of 1 mm Na+, Rb+, Li+, and Cs+ on K+ currents in EcHKT1- and EcHKT2-expressing and uninjected Xenopus laevis oocytes. Current traces recorded at −120 mV from an uninjected (A), EcHKT1-expressing (B), and EcHKT2-expressing (C) oocyte. Traces with K+ and Na+ in bath have been labeled and the rest are traces with Rb+, Li+, and Cs+ that correspond to histograms. Current changes (D and E) in EcHKT1(n = 4 oocytes), EcHKT2 (n = 4), and uninjected oocytes (n = 4) measured in the presence of 1 mm X ion plus 10 mm K+ in bath solution. Current difference (Δ current) is the difference between the currents recorded at 0 mm monovalent cations and 1 mm X ion/10 mm K+.
In oocytes expressing EcHKT1, the currents measured in solutions containing 1 mm Rb+, Li+, or Cs+ and 10 mm K+ were smaller than at 11 mm (Fig. 1D) K+ alone, indicating these monovalent ions partially (Rb+ and Li+) or completely (Cs+) blocked K+ influx through EcHKT1. In EcHKT2-expressing oocytes, Li+ and Cs+ but not Rb+ similarly blocked K+ influx (Fig. 1E).
Cation Selectivity in the Presence of 1 mm Na+
To test whether Na+ could enhance the influx of other cations in EcHKT1- and EcHKT2-expressing oocytes, currents at −120 mV were measured with 1 mm Na+ plus 10 mm of other alkali cations.
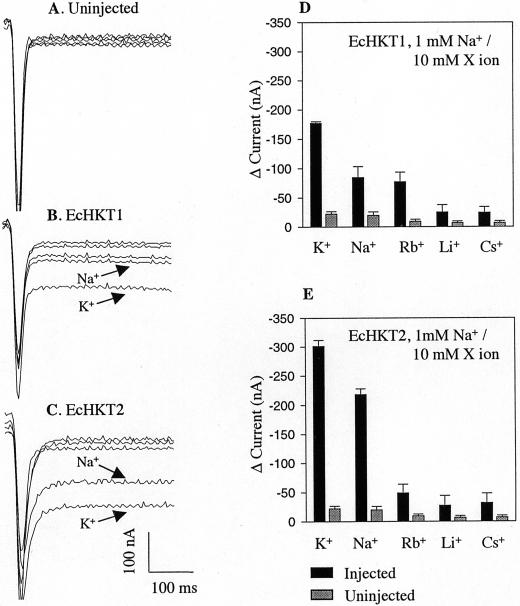
The addition of 1 mm Na+ and 10 mm of any monovalent cation tested did not have a significant effect on uninjected oocytes (Fig. 2, D and E). However, in both EcHKT1- and EcHKT2-expressing oocytes, exposure to 1 mm Na+ plus 10 mm K+ led to the largest inward currents as compared with the currents in 1 mm Na+ plus other monovalent cations (Fig. 2, B–E). EcHKT2-expressing oocytes mediated larger inward currents than EcHKT1 in the presence of 1 mm Na+ plus 10 mm K+ (Figs. 1 and 2) or Na+ alone (10 mm Na+ in Fig. 3 and 11 mm Na+ in Fig. 2). This suggests that EcHKT2 may be more highly expressed in oocytes than EcHKT1, or EcHKT2 could have a larger conductance pathway than EcHKT1. EcHKT1 and to a lesser extent EcHKT2 also mediated a small amount of Rb+ influx at 1 mm Na+ plus 10 mm Rb+ (Fig. 2, D and E).
Figure 2.
Effects of 1 mm Na+ on K+, Rb+, Li+, and Cs+ in EcHKT1- and EcHKT2-expressing and uninjected X. laevis oocytes. Current traces recorded at −120 mV from an uninjected (A), EcHKT1-expressing (B), and EcHKT2-expressing (C) oocyte. Traces with K+ and Na+ in bath have been labeled and the rest are traces with Rb+, Li+, and Cs+ that correspond to histograms. Current changes (D and E) measured in EcHKT1(n = 4 oocytes), EcHKT2 (n = 4), and uninjected oocytes (n = 4) in the presence of 1 mm Na+ plus 10 mm X ion in bath solution. Current difference (Δ current) is the difference between the currents recorded at 0 mm monovalent cations and 1 mm Na+/10 mm X ion.
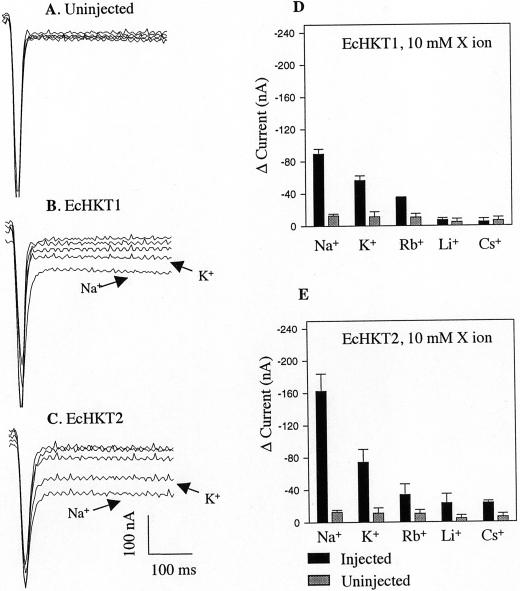
Figure 3.
Effects of bath solutions containing the single monovalent cations Na+, Rb+, Li+, and Cs+ on EcHKT1- and EcHKT2-expressing and uninjected X. laevis oocytes. Current traces recorded at −120 mV from an uninjected (A), EcHKT1-expressing (B), and EcHKT2-expressing (C) oocyte. Traces with K+ and Na+ in bath have been labeled and the rest are traces with Rb+, Li+, and Cs+ that correspond to histograms. Current changes (D and E) measured in EcHKT1 (n = 4 oocytes), EcHKT2 (n = 4), and uninjected oocytes (n = 4) were measured in the presence of single monovalent cation ion (10 mm) in bath solution. Current difference (Δ current) is the difference between the currents recorded at 0 mm monovalent cations and 10 mm cation ion.
Selectivity in the Presence of a Single Ion
To further evaluate the selectivity of the E. camaldulensis HKT1 homologs, currents induced in EcHKT1- or EcHKT2-expressing oocytes were measured in the presence of single ions including K+, Na+, Rb+ Li+, and Cs+ at a concentration of 10 mm for each.
A range of monovalent cations at 10 mm induced only small steady-state background currents in uninjected oocytes. In EcHKT1-expressing oocytes, inward currents were observed in the presence of 10 mm Na+, K+, or Rb+, exhibiting a permeability sequence of Na+ > K+ > Rb+ > Li+ = Cs+, as estimated by the change in current level (Fig. 3D). The same permeability sequence was observed in EcHKT2 expressing oocytes, but EcHKT2 was also slightly permeable to Li+ and Cs+ in comparison with EcHKT1 (Fig. 3, D and E). When a single monovalent cation was present in the bath solution, EcHKT2 was more permeable to Na+ than EcHKT1 (Fig. 2, B–E, and Fig. 3, B–E). These results indicate that both EcHKT1 and EcHKT2 mediate alkali cation uptake and are mainly permeable to Na+, K+, or Rb+.
Kinetics of Na+- and K+-Induced Currents
To evaluate the transport kinetics of the steady-state currents, EcHKT1- and EcHKT2-expressing oocytes were exposed to bath solutions containing different external Na+ and K+ concentrations. To measure the Na+ and K+ affinities of these transporters in their cotransporter mode, oocytes were bathed in solutions containing fixed concentrations of Na+ and variable K+ concentrations and in fixed concentrations of K+ and variable Na+ concentrations. Inward currents mediated by EcHKT1 and EcHKT2 increased as a function of external Na+ or K+ concentrations. Although EcHKT1- and EcHKT2-induced currents responded to changes in K+ from 0.3 to 10 mm, it was not possible to fit Michaelis-Menten curves to these changes to derive the Km for K+ (data not shown). In the cotransporter mode, we were able to obtain Michaelis-Menten curves at a fixed K+ concentration of 1 mm by varying Na+ concentrations. Figure 4A shows the relationship between the steady-state currents at −120 mV and external Na+ concentrations at 1 mm K+. The Km was derived using Equation 1:
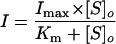 |
1 |
where I is the steady current difference between 0 mm Na+ or K+ and a series of Na+ or K+ concentrations, Imax is the maximum current at saturating external Na+ concentration, Km is the external Na+ concentration that gives the half value of Imax, and [S]o denotes either external Na+ or K+ concentration. Michaelis-Menten curves were fitted and drawn using Sigma Plot 4.0 based on the experimentally determined Km and Imax from Equation 1.
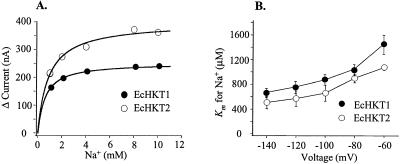
Figure 4.
Kinetics of inward currents mediated by EcHKT1 or EcHKT2 as a function of external Na+ concentration. A, The relationship between Na+ concentration and current at 1 mm external K+ with curves fitted to the Michaelis-Menten equation. B, The voltage dependence at 1 mm K+.
At −120 mV, for EcHKT1-expressing oocytes at external K+ concentrations of 1 mm, Km was 753 ± 109 μm (n = 3) and for EcHKT2 at 1 mm, Km was 569 ± 109 μm (n = 3; Fig. 4A).
The Km was voltage dependent (Fig. 4B) in EcHKT1 and EcHKT2. Therefore, the Km of EcHKT1 and EcHKT2 decreased as membrane potentials became more negative (from −140 to −60 mV) and there was no significant difference between the Km of EcHKT1 or EcHKT2 across all voltages.
Co-expression of EcHKT1/EcHKT2
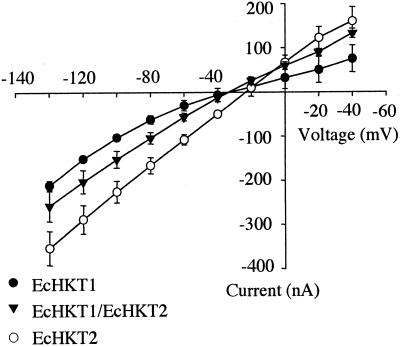
The presence of two different isoforms of HKT1 in E. camaldulensis is intriguing because it is possible that overlapping expression patterns (Fairbairn et al., 2000) may result in interactions between isoforms of the proteins. Therefore, EcHKT1/EcHKT2 co-expressing oocytes were tested in solutions containing 1 mm Na+ plus 10 mm K+. The current amplitudes induced by co-expression of EcHKT1/EcHKT2 were less than the current in EcHKT2-expressing oocytes, but slightly more than in oocytes expressing EcHKT1 (Fig. 5). Therefore, according to the change in amplitude that was midway between EcHKT1- and EcHKT2-expressing oocytes, it appears that co-injected oocytes display a mixture of currents arising from EcHKT1 and EcHKT2.
Figure 5.
Current-voltage (I-V) relationship in EcHKT1-expressing (n = 6), EcHKT2-expressing (n = 5), and EcHKT1/EcHKT2 co-expressing oocytes (n = 8). Solutions contained 1.8 mm CaCl2, 10 mm MES[2-(N-morpholino)-ethanesulfonic acid]-Tris, 200 mm sorbitol, 1 mm Na+, and 10 mm K+ (pH 6.5).
Response to Osmotic Changes
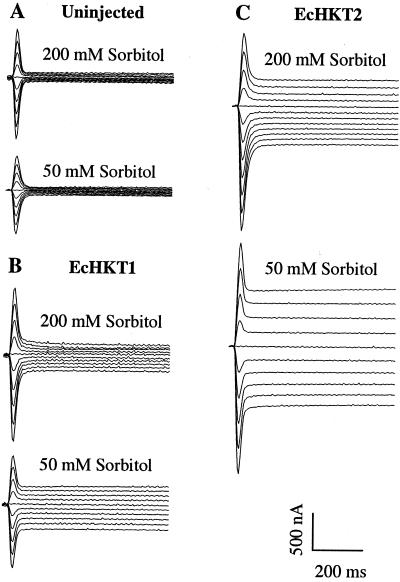
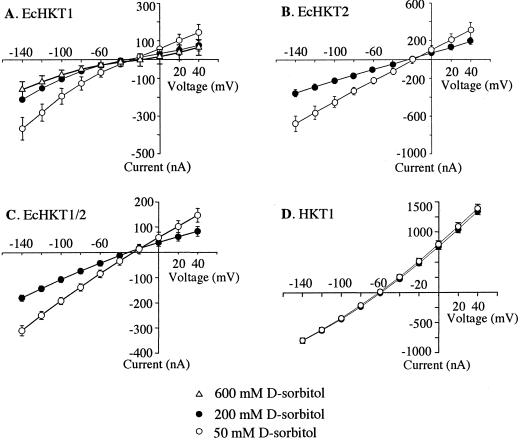
In most experiments, EcHKT1- and EcHKT2-expressing oocytes were recorded in solutions containing 1 mm Na+ plus 10 mm K+ and 200 mm sorbitol. However, we also tested the response of injected and uninjected oocytes to a hypotonic solution (50 mm sorbitol) that contained the same concentration of cations. Exposure to hypotonic solution led to increased inward currents in EcHKT1-and EcHKT2-expressing oocytes as compared with currents at 200 mm sorbitol in the bath solution (Fig. 6). This effect was only partially reversible upon perfusion back to 200 mm sorbitol (data not shown). EcHKT2-expressing oocytes had larger currents than EcHKT1-expressing oocytes, as has been shown in previous experiments. At −120 mV, the current increase due to hypotonic solution in EcHKT1 expressing oocytes was −129 ± 40 nA (n = 6) and −271 ± 35 nA (n = 8) in EcHKT2-expressing oocytes. The percentage increase in currents from 200 to 50 mm was approximately the same (100%) for both transporters. Increased currents were most evident at more positive and negative membrane potentials and reversal potentials were not shifted by changes in the osmolarity of the solution (Fig. 7). HKT1-expressing oocytes were also tested for response to changes in the osmolarity of the solution, but the HKT1-induced currents were not responsive to hypotonic solution, showing an increase of only −8 ± 1 nA at −120 mV (Fig. 7D). Oocytes co-expressing EcHKT1/EcHKT2 also showed increases in currents in response to hypotonic solutions of −137 ± 8 nA at −120 mV (Fig. 7C).
Figure 6.
Hypotonic solutions induced increased inward currents in EcHKT1- and EcHKT2-expressing oocytes. Each oocyte tested was bathed in solution containing 200 mm and then the chamber was perfused with solution containing 50 mm sorbitol. Solutions also included 1.8 mm CaCl2, 10 mm MES-Tris, 1 mm Na+, 10 mm K+ (pH 6.5), and uninjected oocyte (A), EcHKT1-expressing oocyte (B), and EcHKT2-expressing (C) oocyte. Holding potential was −40 mV and the current traces corresponded to the membrane potentials of −140 mV to +40 mV with 20-mV increments.
Figure 7.
I-V relationships in isoosmotic (200 mm sorbitol), hypotonic (50 mm sorbitol), or hypertonic (600 mm sorbitol) solutions. I-V curves from oocytes expressing EcHKT1 (A; n = 6), EcHKT2 (B; n = 8), EcHKT1 and EcHKT2 (C; n = 7), and HKT1 (D; n = 7). Solutions also contained 1.8 mm CaCl2, 10 mm MES-Tris, 200 mm sorbitol, 1 mm Na+, and 10 mm K+ (pH 6.5).
We tested the effect of hypertonic solutions on currents generated by EcHKT1-expressing oocytes. Hypertonic solutions (600 mm sorbitol) did not significantly affect the current levels (Fig. 7A) in EcHKT1-injected oocytes.
To test whether EcHKT1- or EcHKT2-mediated inward currents in response to hypotonic solution were coupled to an inward water flux, we measured the diameter change of EcHKT1- or EcHKT2-expressing oocytes incubated in hypotonic solution (50 mm sorbitol) containing 1 mm Na+ plus 10 mm K+. An increase of oocyte diameter was observed during the 60-min incubation. The increase was 11.6% ± 0.7% (n = 6) in uninjected oocytes, 7.3% ± 1.7% (n = 8) in EcHKT1-expressing oocytes, and 11.0% ± 0.6% (n = 6) in EcHKT2 oocytes. The observed increases in diameter under hypotonic conditions, between uninjected, EcHKT1-, or EcHKT2-expressing oocytes were not significantly different. We also tested whether water flux could be enhanced under conditions where the membrane potential was more negative. Holding the oocyte membrane potential at more negative voltages (−120 mV) for 30 or 60 min in hypotonic solutions did not result in changes in oocyte diameter that were different from those observed in oocytes that were not voltage clamped.
To compare more directly with oocytes expressing a known water channel (Kammerloher et al., 1994) and with previously used experimental conditions (Maurel et al., 1995), we bathed oocytes expressing EcHKT1 and PIP2a 3 d after injection and uninjected oocytes in Barth solution that had been diluted five times with deionized water. Under these experimental conditions, the PIP2a-expressing oocytes swelled and burst after approximately 5 min (n = 12) as compared with EcHKT1-expressing oocytes, which burst after 45 min (n = 12). Of the 11 control uninjected oocytes tested, four burst after 45 min, whereas the other seven remained intact for at least 60 min.
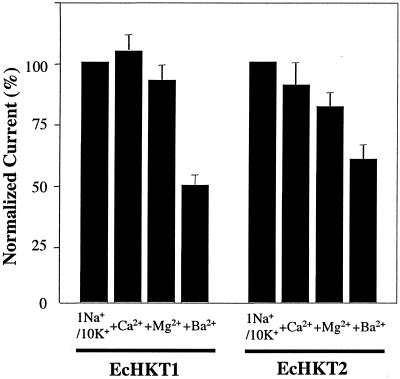
Effects of Divalent Cations on Transport Currents
The effects of divalent cations on EcHKT1- and EcHKT2-mediated currents were investigated because Mg2+ appears to stimulate HKT1-induced currents in oocytes (D. Hayes, D.P. Schachtman, and N.A. Walker, unpublished data). Currents were measured in bath solutions containing 1 mm Na+ plus 10 mm K+ with and without 2 mm Ca2+, Mg2+, or Ba2+. Normalized currents measured at −120 mV in EcHKT1- or EcHKT2-expressing oocytes exposed to different ionic conditions revealed that Ca2+ or Mg2+ did not have significant effects on EcHKT1- or EcHKT2-mediated inward currents (P > 0.05; Fig. 8). However, 2 mm Ba2+ significantly reduced both EcHKT1- and EcHKT2-mediated currents (P < 0.05). These results confirm the findings of Fairbairn et al. (2000), who showed that bacterial cells deficient in K+ transport grew poorly when EcHKT was expressed and low concentrations of Ba2+ were added. The reason for the poor growth of E. coli in previous experiments has been confirmed in these experiments with oocytes to be due to the blockage of K+ uptake by Ba2+.
Figure 8.
Effects of divalent cations on EcHKT1- or EcHKT2-mediated inward currents. Currents at −120 mV and 1 mm Na+/10 mm K+ without divalent cations were used to normalize the currents at −120 mV measured at 1 mm Na+/10 mm K+ with 2 mm Ca2+, Mg2+, and Ba2 (EcHKT1, n = 4; EcHKT2, n = 6).
DISCUSSION
Although we are now entering a new era in which a complete plant genome sequence is available for use in understanding fundamental questions in plant biology (Arabidopsis Initiative, 2000), much future work will be required to understand gene function. In the plant kingdom, we can expect to discover great diversity in the functions of paralogous proteins because of the many different environmental niches that plants inhabit. Examples of natural variation in genome composition and protein function have been found widely in bacteria that are adapted to survive in extreme environments such as those species that are tolerant of very high temperatures, radiation, and high salinity (Nelson et al., 2000). These proteins from bacterial extremophiles have many unique properties that are useful for scientific and industrial applications. In this study, we have described the function of two cation symporters that have a similar function to HKT1 from wheat, but exhibit a slightly different cation permeability and a unique ability to sense changes in the solute concentration of the external solution.
Functional and Structural Comparisons
HKT1 from wheat (Schachtman and Schroeder, 1994), AtHKT1 from Arabidopsis (Uozumi et al., 2000), and EcHKT1/2 from E. camaldulensis (Fairbairn et al., 2000) all are sufficiently similar to be classified into the same superfamily (>40% amino acid identity), but exhibit functional differences in transport characteristics when measured in X. laevis oocytes. All of these transporters appear to transport Na+ when this single cation is in the bath solution. In oocytes expressing either EcHKT1 or EcHKT2, we measured the largest currents with a single ion present when Na+ was in the bath solution. The mechanism of Na+ uptake is not certain, but the model proposed by Gassmann, Rubio, and Schroeder (Gassmann et al., 1996) suggests that Na+ moves through two different binding sites in the protein. In the case of HKT1, Na+ may be transported at rapid rates via a series of binding sites in the “pore” similar to an ion channel (Gassmann et al., 1996). Na+ also stimulates the uptake of K+ in EcHKT1/2 and HKT1, but does not stimulate the uptake of other monovalent cations such as Li+ or Cs+. Overall, the largest currents that we measured in EcHKT1- and EcHKT2-expressing oocytes were when both Na+ and K+ were in the bath solution.
One functional difference between EcHKT1/EcHKT2 and HKT1 or AtHKT1 is that the transporters in E. camaldulensis mediate K+ fluxes in the absence of other monovalent cations. The mechanism of K+ transport in the absence of other monovalent cations is not known, but this type of transport can be blocked by the addition of other monovalent cations such as Rb+, Li+, and Cs+. The functional differences in cation permeability between EcHKT and HKT1 may be the result of differences in single or groups of amino acids. Alignments of HKT1 with EcHKT1 and EcHKT2 show many regions that are very similar and also very different between the proteins (Fairbairn et al., 2000). Although the overall amino acid identity is low, these proteins share a high degree of identity in specific regions.
HKT1 exhibits the greatest sensitivity to blockade by Cs+ and Rb+ with some inhibition by Li+ (Gassmann et al., 1996). Although EcHKT1 is permeable to Rb+, the K+ uptake pathway is also partially blocked by Rb+, whereas in EcHKT2 the K+ uptake pathway is not blocked by Rb+. As in the case of HKT1, EcHKT1 and EcHKT2 were both most sensitive to blockade by Cs+. Although small background currents were observed in oocytes expressing EcHKT1 and EcHKT2 with Cs+ in the bath solution, this level of current was not significantly different from zero. The composition of a predicted large hydrophilic loop located between transmembrane domains three and four has been shown to be important in K+/Na+ selectivity of the transporter (Liu et al., 2000). The composition and length of this loop differs in EcHKT and HKT1. Many of the charged residues that are important to the function of the high-affinity K+ binding site in HKT1 are absent in EcHKT. Many other amino acid residues have been shown to be involved in salt-tolerant phenotypes and ionic selectivity of HKT1 as expressed in yeast (Saccharomyces cerevisiae; Rubio et al., 1995, 1999), but most of these residues are conserved between EcHKT and HKT1.
Osmosensing is a functional characteristic that differs between the HKT1 and EcHKT proteins. Although it is difficult to predict which domains of the protein are involved in this function, because 60% of the amino acid sequence differs, it should be possible to construct chimeras between HKT1 and EcHKT to localize the structural features involved in osmosensing.
Osmosensing
All organisms have mechanisms that provide them with the flexibility to survive and grow under hypo- and hyperosmotic conditions. Osmosensing targets are found in plants, microbes, and mammals and include receptors, signaling molecules, and ion transport mechanisms. The E. camaldulensis HKT1 transporters show intrinsic sensitivity to changes in the solute concentrations of the external medium. Osmosensitivity was only in response to hypo-osmotic solutions and was not found in the wheat HKT1. Although oocytes have mechanosensitive channels (Yang and Sachs, 1986) we did not observe increased currents under hypotonic conditions in uninjected oocytes. The absence of mechanosensitive currents in oocytes under hypotonic conditions has also been reported recently (Saitou et al., 2000). There are few reports on changes in plant ion transport processes in response to changes in osmolarity, which is surprising because plants often encounter large changes in environmental conditions. In plants, the guard cell inward K+ channels are activated by hypotonic solutions and outward K+ channels are activated by hypertonic solution (Liu and Luan, 1998). A feedback model for enhancement of guard cell swelling and shrinkage was proposed to explain the differential stimulation and inhibition of these channels by changes in external osmolarity. In addition to these guard cell K+ channels, it is also known that plants have specialized receptors that may sense and transduce salinity, drought, and cold (Urao et al., 1999, 2000). For example, one receptor is most highly expressed in roots, functions as a His kinase, and gene expression is up-regulated by salt, dehydration, and cold. The in planta function of this receptor is not yet clear, but it complements a yeast mutant that is unable to grow under high osmolarity by activating the HOG1 MAPK cascade (Urao et al., 1999).
In plants, microbes, and mammals, ion transport mechanisms that respond to changes in osmolarity may fulfill different physiological roles for the whole organism. In mammals, channels that are targets for osmosensing are important not only in response to changes in osmolarity (Nilius et al., 1996), but also in response to touch (Walker et al., 2000) and chemosensing. Ionic current through volume regulated anion channels are stimulated by hypo-osmotic conditions and as cells swell, anions are released to aid the cell in maintaining volume homeostasis (Nilius et al., 1996). The reduced intracellular ionic strength due to swelling and not membrane pressure appears to be the trigger for activation of these channels (Voets et al., 1999). The kidney, liver, and heart also express the OTRPC4 gene encoding a nonselective cation channel that is stimulated by decreases in extracellular osmolarity (Strotmann et al., 2000), similar to what we observed in EcHKT1/2-injected oocytes. OTRPC4 functions as a nonselective cation channel that is thought to fulfill a signaling function by increasing Ca2+ influx, thereby triggering regulatory volume decreases in mammalian cells. In E. coli, mechanosensitive channels have been well characterized at the molecular level and are thought to provide the cell with a “release valve” when the external solute concentrations suddenly decrease (Blount and Moe, 1999). Decreases in external solution concentration of 250 mm may increase intracellular pressure by more than 0.6 MPa, which creates the force for opening these mechanosensitive channels (Blount and Moe, 1999).
The enhancement of ionic currents due to changes in tonicity are easily understood in terms of the shrinking and swelling of guard cells (Liu and Luan, 1998). However, it is not certain why K+ transporters EcHKT1 and EcHKT2 expressed in roots and in other parts of the plant would be stimulated by hypotonic conditions. The cation currents in EcHKT1/2-expressing oocytes clearly are stimulated by hypotonic solutions, but under these conditions if osmotic adjustment was important, root cells would expel solutes. It is well known that plant cells efflux K+ under hypotonic conditions and this response was shown to be more rapid than under hypertonic stress (Felix et al., 2000). In terms of the whole plant, this stimulation of K+ uptake may be understood because for growth under flooded conditions that are often encountered by E. camaldulensis, it will be necessary for a plant to continue to take up K+. Under conditions where the soil solution ion concentrations are diluted, it may be necessary for plants to use some type of carrier mechanism for uptake. Therefore, EcHKT in the roots may either provide the plant with a source of K+ or may help to maintain cellular homeostasis by taking up K+ that is lost from root cells during stress. This suggestion rests on the assumption that EcHKT1 functions in the plasma membrane, but because of the difficulties in producing antigenic antibodies we and to our knowledge others have not been able to conclusively demonstrate the membrane in which these transporters are localized. Therefore, we speculate that in E. camaldulensis the increased K+ uptake via EcHKT1 and EcHKT2 may provide the plant with a mechanism for maintaining K+ homeostasis when soil solution K+ concentrations are diluted by floods.
We found that the enhanced ion uptake was not accompanied by increased water flux as has been shown for some solute transporters (Loo et al., 1996) under hypotonic conditions where external solutions contained 50 mm sorbitol. This suggested that EcHKT-mediated inward currents are not coupled with water influx. This fits with a proposed physiological role of this mechanism because increased water flux could further dilute cellular K+ concentrations. However, under different experimental conditions using diluted Barth solutions, oocytes expressing EcHKT1 swelled and burst. Because the two sets of swelling experiments gave different results, the question of water flux through EcHKT under hypotonic conditions will need to be more thoroughly investigated in the future.
The unique and new functions that we have identified in EcHKT1 and EcHKT2 provide new insights into the function of this widespread K+ and Na+ transporter. The novel osmosensing functional characteristic highlights the importance of studying the function of proteins from plant species that exhibit unique adaptations to harsh environmental conditions.
MATERIALS AND METHODS
cRNA Transcription
The full-length cDNAs of EcHKT1 and EcHKT2 were cloned from pGEM-T (Promega, Madison, WI) plasmid libraries of Eucalyptus camaldulensis (Fairbairn et al., 2000). The pGEM-T-containing EcHKT1 was linearized by digestion with NotI. EcHKT2 cDNA (NotI fragment) was subcloned from pGEM-T into pYES2 (Invitrogen, San Deigo) and the resulting construct was linearized with XbaI. Capped cRNAs of both EcHKT1 and EcHKT2 were synthesized in vitro with T7 RNA polymerase using the mMESSAGE mMACHINE kit (Ambion Inc., Austin, TX).
Oocyte Preparation
Oocytes were isolated from frogs (n = 9) using standard techniques (Schachtman and Schroeder, 1994) and incubated for 1 to 2 d before injection with cRNA. Oocytes were kept at 18°C in ND96 solution containing 96 mm NaCl, 2 mm KCl, 1.8 mm CaCl2, 1 mm MgCl2, and 5 mm HEPES [4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid]-NaOH (pH 7.5) and supplemented with gentamycin (50 mg mL−1). Stage V or VI oocytes were chosen for injection with 50 nL cRNA at a concentration of 0.5 ng μL−1. Electrophysiology experiments and swelling assays were performed 1 to 3 d after cRNA injection. All data points represent experiments from oocytes taken from at least two different frogs.
Electrophysiology
Oocyte currents were measured using the two microelectrode voltage-clamp method with a Cornerstone TEV-200 Voltage Clamp amplifier (Dagan, Minneapolis) connected to a computer via the LM-12 Laboratory Interface (Dagan). Oocytes were impaled with electrodes filled with 3 m KCl with resistance ranging from 0.5 to 1 MΩ. Only oocytes with an initial resting potential more negative than −30 mV, in recording solutions without Na+ or K+ ions, were used in the experiments. In experiments where low concentrations of alkali cations were used, the base solution containing 1.8 mm CaCl2 and 10 mm MES-Tris (pH 6.5) was supplemented with 200 mm d-sorbitol. K+ or Na+ were added as Glu salts. In experiments with Rb+, Cs+, and Li+ chloride salts were used. In some experiments to test for osmosensitivity, the 200 mm d-sorbitol was replaced with 50 and 600 mm sorbitol. In all experiments, individual oocytes were bathed in solutions that were changed by perfusion of the recording chamber and currents were recorded from the same oocyte in multiple solutions. In most cases, data is presented as the mean of a number of oocytes in each solution.
Voltage-clamping step pulses and data acquisitions were performed using pCLAMP6.0 (Axon Instruments, Inc., Foster City, CA). Under voltage-clamped conditions, I-V relationships were obtained with the appropriate pulse protocol. In most experiments, the holding potential was −40 mV, step pulses were between +40 mV and −160 mV with 10-mV increments, and each pulse lasted 1,000 ms with a 500-ms interval between pulses. Currents were low-pass filtered at 1 KHz, and analyzed with Clampfit. Steady state currents were obtained by averaging a current trace, which did not contain the capacitance current component. The measured steady currents were used to construct the I-V curves.
For kinetic analysis, steady current differences between 0 mm Na+ or K+ and a series of Na+ or K+ concentrations were obtained at potentials from −80 to −140 mV and fitted to the Michaelis-Menten equation (Eq. 1).
Swelling Assays of Oocytes in Hypotonic Solution
Oocyte swelling was estimated by measuring the vertical and horizontal diameter. The uninjected oocytes and EcHKT1- or EcHKT2-expressing oocytes were incubated in solution containing 1.8 mm CaCl2, 10 mm MES-Tris, 1.0 mm NaCl, 10 mm KCl (pH 6.5), and 200 sorbitol. To mimic the electrophysiological experiments, the diameter was measured in the 200 mm sorbitol and the oocytes were then incubated in 50 mm sorbitol with the same cation and anion concentrations as above. The change was measured after incubation for 60 min. All experiments were performed at room temperature (23°C).
Oocytes injected with EcHKT1 were also compared with oocytes injected with PIP2a (Kammerloher et al., 1994) and uninjected oocytes. In these experiments, oocytes bathed in Barth solution were transferred to Barth solution that had been diluted five times with deionized water (Maurel et al., 1995). Oocytes were incubated for up to 60 min at room temperature.
ACKNOWLEDGMENTS
We thank Christophe Maurel (Centre National de la Recherche Scientifique, Montpellier, France) for providing the PIP2a clone and for advice on the oocyte swelling assays. We also thank Mark Thomas and colleagues at CSIRO-Plant Industry Horticulture Unit for their support in all aspects of the work.
Footnotes
This work was funded by the Australian Research Council (grant to D.P.S.).
LITERATURE CITED
- Amtmann A, Sanders D. Mechanisms of Na+ uptake by plant cells. Adv Bot Res. 1999;29:75–112. [Google Scholar]
- Arabidopsis Initiative. Analysis of the genome sequence of the flowering plant Arabidopsis. Nature. 2000;408:796–815. doi: 10.1038/35048692. [DOI] [PubMed] [Google Scholar]
- Bihler H, Gaber RF, Slayman CL, Bertl A. The presumed potassium carrier Trk2p in Saccharomyces cerevisiae determines an H+-dependent, K+-independent current. FEBS Lett. 1999;447:115–120. doi: 10.1016/s0014-5793(99)00281-1. [DOI] [PubMed] [Google Scholar]
- Blount P, Moe PC. Bacterial mechanosensitive channels: integrating physiology, structure and function. Trends Microbiol. 1999;7:420–424. doi: 10.1016/s0966-842x(99)01594-2. [DOI] [PubMed] [Google Scholar]
- Box S, Schachtman DP. The effect of low concentrations of sodium on potassium uptake and growth of wheat. Aust J Plant Physiol. 2000;27:175–182. [Google Scholar]
- Clemens S, Antosiewicz DM, Ward JM, Schachtman DP, Schroeder JI. The plant transporter LCT1 mediates the uptake of cadmium and calcium. Proc Natl Acad Sci USA. 1998;95:12043–12048. doi: 10.1073/pnas.95.20.12043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czempinski K, Zimmerman S, Ehrhardt T, Müller-Röber B. New structure and function in plant K+ channels: KC01, an outward rectifier with a steep Ca2+ dependency. EMBO J. 1997;16:2565–2575. doi: 10.1093/emboj/16.10.2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldridge K, Davidson J, Harwood C, VanWyk G. Eucalypt Domestication and Breeding. Oxford: Oxford University Press; 1993. [Google Scholar]
- Fairbairn DJ, Liu WH, Schachtman DP, Gomez-Gallego S, Day SR, Teasdale RD. Characterization of two distinct HKT1-like potassium transporters from Eucalyptus camaldulensis. Plant Mol Biol. 2000;43:515–525. doi: 10.1023/a:1006496402463. [DOI] [PubMed] [Google Scholar]
- Felix G, Regenass M, Boller T. Sensing of osmotic pressure changes in tomato cells. Plant Physiol. 2000;124:1169–1179. doi: 10.1104/pp.124.3.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu H, Luan S. AtKUP1: a dual affinity K+ transporter from Arabidopsis. Plant Cell. 1998;10:63–73. doi: 10.1105/tpc.10.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gassmann W, Rubio F, Schroeder JI. Alkali cation selectivity of the wheat root high-affinity potassium transporter HKT1. Plant J. 1996;10:869–882. doi: 10.1046/j.1365-313x.1996.10050869.x. [DOI] [PubMed] [Google Scholar]
- Gassmann W, Ward JM, Schroeder JI. Physiological roles of inward-rectifying K+ channels. Plant Cell. 1993;5:1491–1493. doi: 10.1105/tpc.5.11.1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaymard F, Pilot F, Lacombe B, Bouchez D, Bruneau D, Boucherez J, Michaux-Ferriere N, Thibaud JB, Sentenac H. Identification and disruption of a plant shaker-like outward channel involved in K+ release into the xylem sap. Cell. 1998;94:647–655. doi: 10.1016/s0092-8674(00)81606-2. [DOI] [PubMed] [Google Scholar]
- Gibson A, Bachelard EP, Hubrick KT. Growth strategies of Eucalyptus camaldulensis at three sites in northern Australia. Aust J Plant Physiol. 1994;21:653–662. [Google Scholar]
- Glass ADM. The regulation of ion transport. Annu Rev Plant Physiol. 1983;34:311–326. [Google Scholar]
- Hirsch RE, Lewis BD, Spalding EP, Sussman MR. A role for the AKT1 potassium channel in plant nutrition. Science. 1998;280:918–921. doi: 10.1126/science.280.5365.918. [DOI] [PubMed] [Google Scholar]
- Jeschke WD, Pate JS, Atkins CA. Partitioning of K+, Na+, Mg2+, and Ca2+ through xylem and phloem to component organs of nodulated white lupin under mild salinity. J Plant Physiol. 1987;128:77–93. [Google Scholar]
- Kammerloher W, Fischer U, Piechottka GP, Schaffner AR. Water channels in the plant plasma membrane cloned by immunoselection from a mammalian expression system. Plant J. 1994;6:187–199. doi: 10.1046/j.1365-313x.1994.6020187.x. [DOI] [PubMed] [Google Scholar]
- Kim EJ, Kwak JM, Uozumi N, Schroeder JI. AtKUP1: an Arabidopsis gene encoding high-affinity potassium transport activity. Plant Cell. 1998;10:51–62. doi: 10.1105/tpc.10.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochian LV, Lucas WJ. Potassium transport in roots. In: Callow JA, editor. Advances in Botanical Research. London: Academic Press Ltd.; 1988. pp. 93–178. [Google Scholar]
- Kochian LV, Lucas WJ. Can K+ channels do it all. Plant Cell. 1993;5:720–721. doi: 10.1105/tpc.5.7.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacombe B, Pilot G, Michard E, Gaymard F, Sentenac H, Thibaud JB. A shaker-like K+ channel with weak rectification is expressed in both source and sink phloem tissues of Arabidopsis. Plant Cell. 2000;12:837–851. doi: 10.1105/tpc.12.6.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leigh RA, Wyn Jones RG. A hypothesis relating critical potassium concentrations for growth to the distribution and functions of this ion in the plant cell. New Phytol. 1984;97:1–13. [Google Scholar]
- Liu K, Luan S. Voltage-dependent K+ channels as targets of osmosensing in guard cells. Plant Cell. 1998;10:1957–1970. doi: 10.1105/tpc.10.11.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Schachtman DP, Zhang W. Partial deletion of a loop region in the high affinity K+ transporter HKT1 changes ionic permeability leading to increased salt tolerance. J Biol Chem. 2000;275:27924–27932. doi: 10.1074/jbc.M002056200. [DOI] [PubMed] [Google Scholar]
- Loo DDF, Seuthen T, Chandy G, Wright EM. Cotransport of water by the Na+/glucose cotransporter. Proc Natl Acad Sci USA. 1996;93:13367–13370. doi: 10.1073/pnas.93.23.13367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maathuis FJM, Sanders D. Mechanisms of potassium absorption by higher plant roots. Physiol Plant. 1996;96:158–168. [Google Scholar]
- Marschner H. Mineral Nutrition of Higher Plants. San Diego: Academic Press; 1995. [Google Scholar]
- Marten I, Hoth S, Deeken R, Ache P, Ketchum KA, Hoshi T, Hedrich R. AKT3, a phloem-localized K+ channel, is blocked by protons. Proc Nat Acad Sci USA. 1999;96:7581–7586. doi: 10.1073/pnas.96.13.7581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurel C, Kado RT, Guern J, Chrispeels MJ. Phosphorylation regulates the water channel activity of the seed-specific aquaporin alpha-TIP. EMBO J. 1995;14:3028–3035. doi: 10.1002/j.1460-2075.1995.tb07305.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura RL, McKendree WL, Jr, Hirsch RE, Sedbrook JC, Gaber RF, Sussman MR. Expression of an Arabidopsis potassium channel gene in guard cells. Plant Physiol. 1995;109:371–374. doi: 10.1104/pp.109.2.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson KE, Paulsen IT, Heilelberg JF, Fraser CM. Status of genome projects for nonpathogenic bacteria and archaea. Nat Biotech. 2000;18:1049–1054. doi: 10.1038/80235. [DOI] [PubMed] [Google Scholar]
- Nilius B, Eggermont J, Voets T, Droogmans G. Volume regulated Cl− channels. Gen Pharmacol. 1996;27:1131–1140. doi: 10.1016/s0306-3623(96)00061-4. [DOI] [PubMed] [Google Scholar]
- Quintero FJ, Blatt MR. A new family of K+ transporters from Arabidopsis that are conserved across phyla. FEBS Lett. 1997;415:206–211. doi: 10.1016/s0014-5793(97)01125-3. [DOI] [PubMed] [Google Scholar]
- Rigas S, Devrosses G, Haralampidis K, Vicente-Agullo F, Feldman KA, Grabov A, Dolam L, Hatzopoulos P. TRH1 encodes a potassium transporter required for tip growth in Arabidopsis roots hairs. Plant Cell. 2001;13:139–151. doi: 10.1105/tpc.13.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubio F, Gassmann W, Schroeder JI. Sodium-driven potassium uptake by the plant potassium transporter HKT1 and mutations conferring salt tolerance. Science. 1995;270:1660–1663. doi: 10.1126/science.270.5242.1660. [DOI] [PubMed] [Google Scholar]
- Rubio F, Santa-Maria GE, Rodriguez-Navarro A. Cloning of Arabidopsis and barley cDNAs encoding HAK potassium transporters in root and shoot cells. Physiol Plant. 2000;109:34–43. [Google Scholar]
- Rubio F, Schwarz M, Gassmann W, Schroeder JI. Genetic selection of mutations in the high affinity K+ transporter HKT1 that define functions of a loop site for reduced Na+ permeability and increased Na+ tolerance. J Biol Chem. 1999;274:6839–6847. doi: 10.1074/jbc.274.11.6839. [DOI] [PubMed] [Google Scholar]
- Saitou T, Ishikawa T, Obara K, Nakayama K. Characterization of whole-cell currents elicited by mechanical stimulation of Xenopus oocytes. Pflugers Arch Eur J Physiol. 2000;440:858–865. doi: 10.1007/s004240000337. [DOI] [PubMed] [Google Scholar]
- Santa-Maria GE, Rubio F, Dubcovsky J, Rodriguez-Navarro A. The HAK1 gene of barley is a member of a large gene family and encodes a high-affinity potassium transporter. Plant Cell. 1997;9:2281–2289. doi: 10.1105/tpc.9.12.2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachtman DP. Molecular insights into the structure and function of plant K+ transport mechanisms. Biochim Biophys Acta Biomembr. 2000;1465:127–139. doi: 10.1016/s0005-2736(00)00134-6. [DOI] [PubMed] [Google Scholar]
- Schachtman DP, Kumar R, Schroeder JI, Marsh EL. The structure and function of a novel cation transporter (LCT1) in higher plants. Proc Natl Acad Sci USA. 1997;94:11079–11084. doi: 10.1073/pnas.94.20.11079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachtman DP, Liu W. Piecing together the puzzle of the interaction between potassium and sodium uptake in plants. Trends Plant Sci. 1999;4:281–287. doi: 10.1016/s1360-1385(99)01428-4. [DOI] [PubMed] [Google Scholar]
- Schachtman DP, Schroeder JI. Structure and transport mechanism of a high-affinity potassium uptake transporter from higher plants. Nature. 1994;370:655–658. doi: 10.1038/370655a0. [DOI] [PubMed] [Google Scholar]
- Schroeder JI, Ward JM, Gassmann W. Perspectives on the physiology and structure of inward-rectifying K+ channels in higher plants: biophysical implications for K+ uptake. Annu Rev Biophys Biomol Struct. 1994;23:441–471. doi: 10.1146/annurev.bb.23.060194.002301. [DOI] [PubMed] [Google Scholar]
- Strotmann R, Harteneck C, Nunnenmacher K, Schultz G, Plant TD. OTRPC4, a nonselective cation channel that confers sensitivity to extracellular osmolarity. Nat Cell Biol. 2000;2:695–702. doi: 10.1038/35036318. [DOI] [PubMed] [Google Scholar]
- Tholema N, Bakker EP, Suzuki A, Nakamura T. Change to alanine of one out of four selectivity filter glycines in KtrB causes a two orders of magnitude decrease in the affinities for both K+ and Na+ of the Na+ dependent K+ uptake system KtrAB from Vibrio alginolyticus. FEBS Lett. 1999;450:217–220. doi: 10.1016/s0014-5793(99)00504-9. [DOI] [PubMed] [Google Scholar]
- Uozumi N, Kim EJ, Rubio F, Yamaguchi T, Muto S, Tsuboi A, Bakker EP, Nakamura T, Schroeder JI. The Arabidopsis HKT1 gene homolog mediates inward Na+ currents in Xenopus laevis oocytes and Na+ uptake in Saccharomyces cerevisiae. Plant Physiol. 2000;122:1249–1259. doi: 10.1104/pp.122.4.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urao T, Yakubov B, Satoh R, Yamaguchi-Shinozaki K, Seki M, Hirayama T, Shinozaki K. A transmembrane hybrid-type kinase in Arabidopsis functions as an osmosensor. Plant Cell. 1999;11:1743–1754. doi: 10.1105/tpc.11.9.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urao T, Yamaguchi-Shinozaki K, Shinozaki K. Two-component system in plant signal transduction. Trends Plant Sci. 2000;5:67–74. doi: 10.1016/s1360-1385(99)01542-3. [DOI] [PubMed] [Google Scholar]
- Voets T, Droogmans G, Raskin G, Eggermont J, Nilius B. Reduced intracellular ionic strength as the initial trigger for activation of endothelial volume-regulated anion channels. Proc Natl Acad Sci USA. 1999;96:5298–5303. doi: 10.1073/pnas.96.9.5298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker DJ, Leigh RA, Miller AJ. Potassium homeostasis in vacuolate plant cells. Proc Natl Acad Sci USA. 1996;93:10510–10514. doi: 10.1073/pnas.93.19.10510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker RG, Willingham AT, Zuker CS. A Drosophila mechanosensory transduction channel. Science. 2000;287:2229–2234. doi: 10.1126/science.287.5461.2229. [DOI] [PubMed] [Google Scholar]
- Ward JM, Schroeder JI. Calcium-activated K+ channels and calcium-induced calcium release by slow vacuolar ion channels in guard cell vacuoles implicated in the control of stomatal closure. Plant Cell. 1994;6:669–683. doi: 10.1105/tpc.6.5.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JE, Brooker IH. Eucalypts: an introduction. In: Williams J, Woinarski J, editors. Eucalypt Ecology. Cambridge, UK: Cambridge University Press; 1997. pp. 1–15. [Google Scholar]
- Yang XC, Sachs F. Block of stretch-activated ion channels in Xenopus oocytes by gadolinium and calcium ions. Science. 1986;243:1068–1071. doi: 10.1126/science.2466333. [DOI] [PubMed] [Google Scholar]
- Zimmermann S, Sentenac H. Plant ion channels: from molecular structure to physiological functions. Curr Opin Plant Biol. 1999;2:477–482. doi: 10.1016/s1369-5266(99)00020-5. [DOI] [PubMed] [Google Scholar]
- Zubrinich TM, Loveys B, Gallasch S, Seekamp JV, Tyerman SD. Tolerance of salinized floodplain conditions in a naturally occurring Eucalyptus hybrid related to lowered plant water potential. Tree Physiol. 2000;20:953–963. doi: 10.1093/treephys/20.14.953. [DOI] [PubMed] [Google Scholar]