Abstract
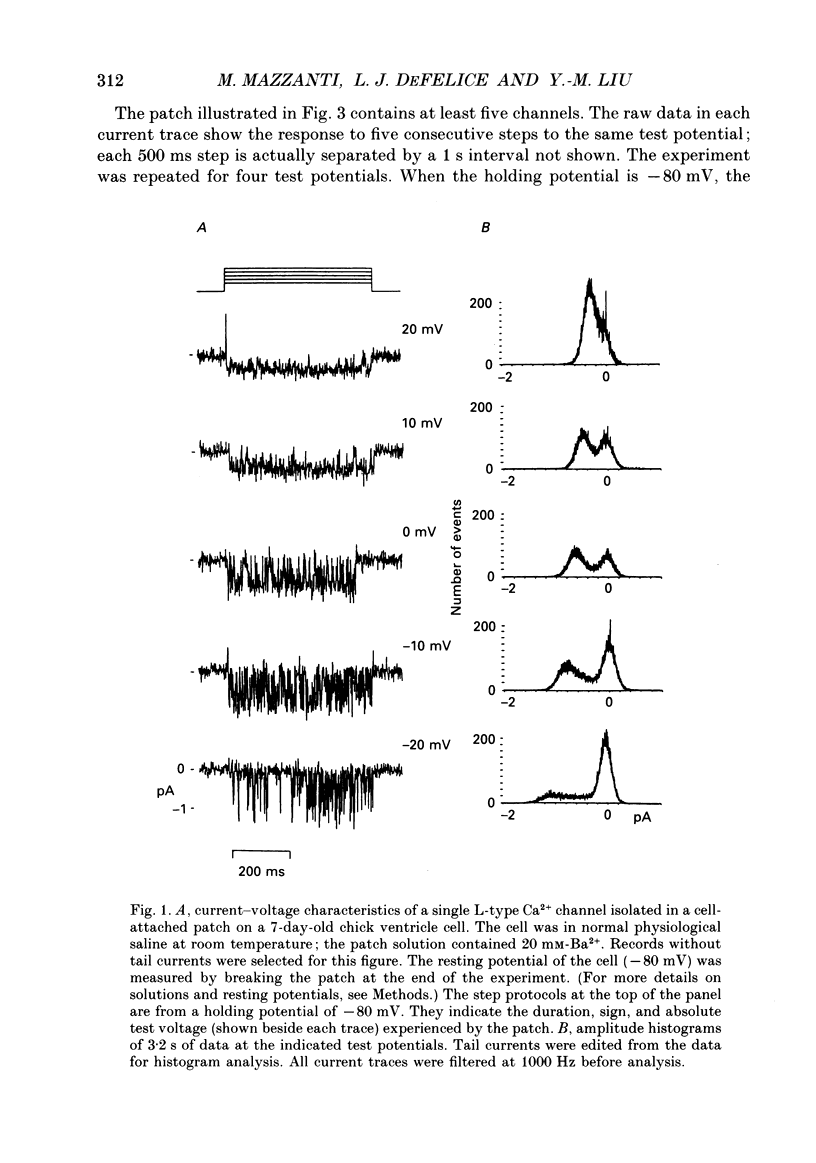
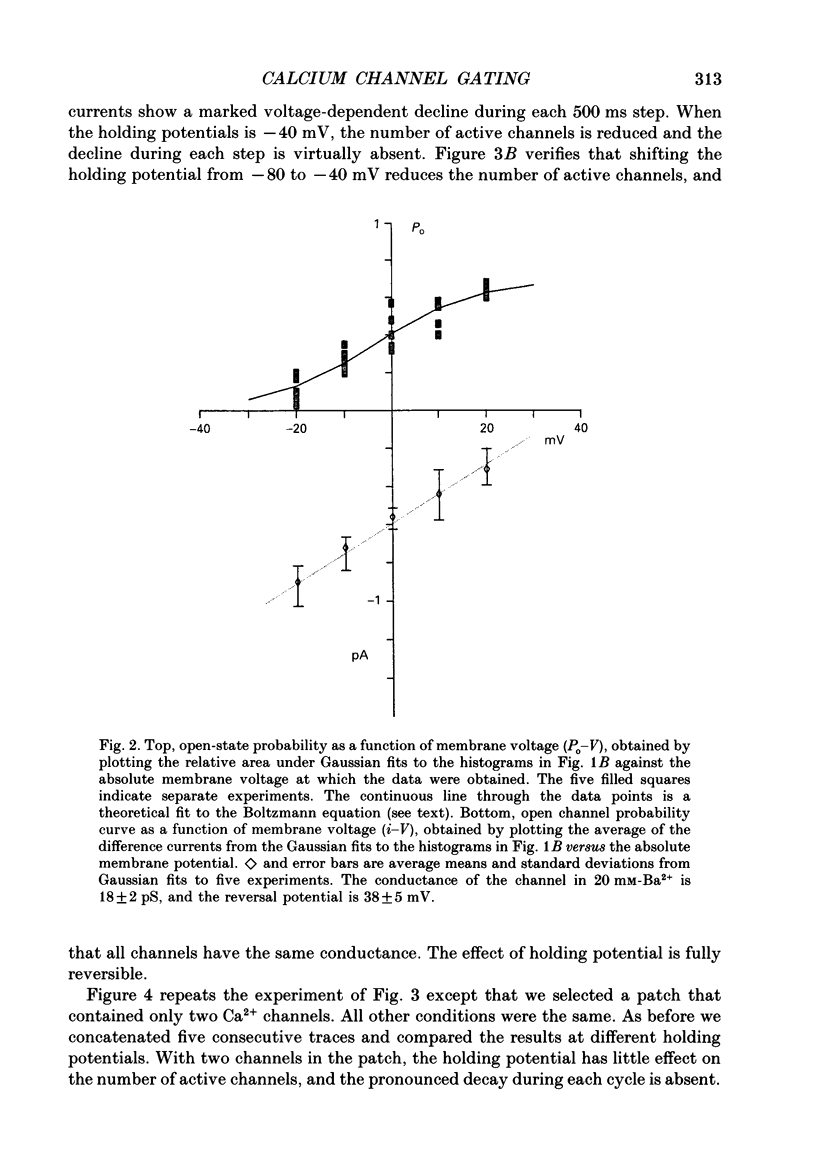
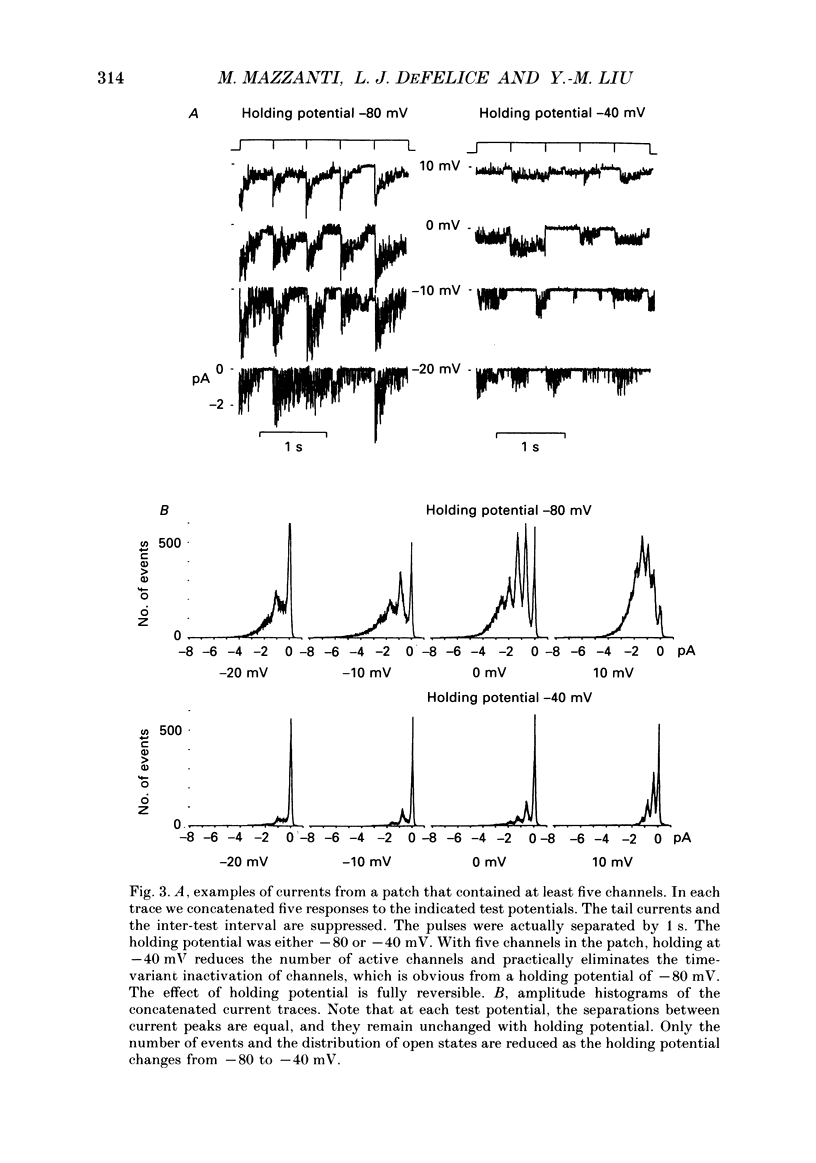
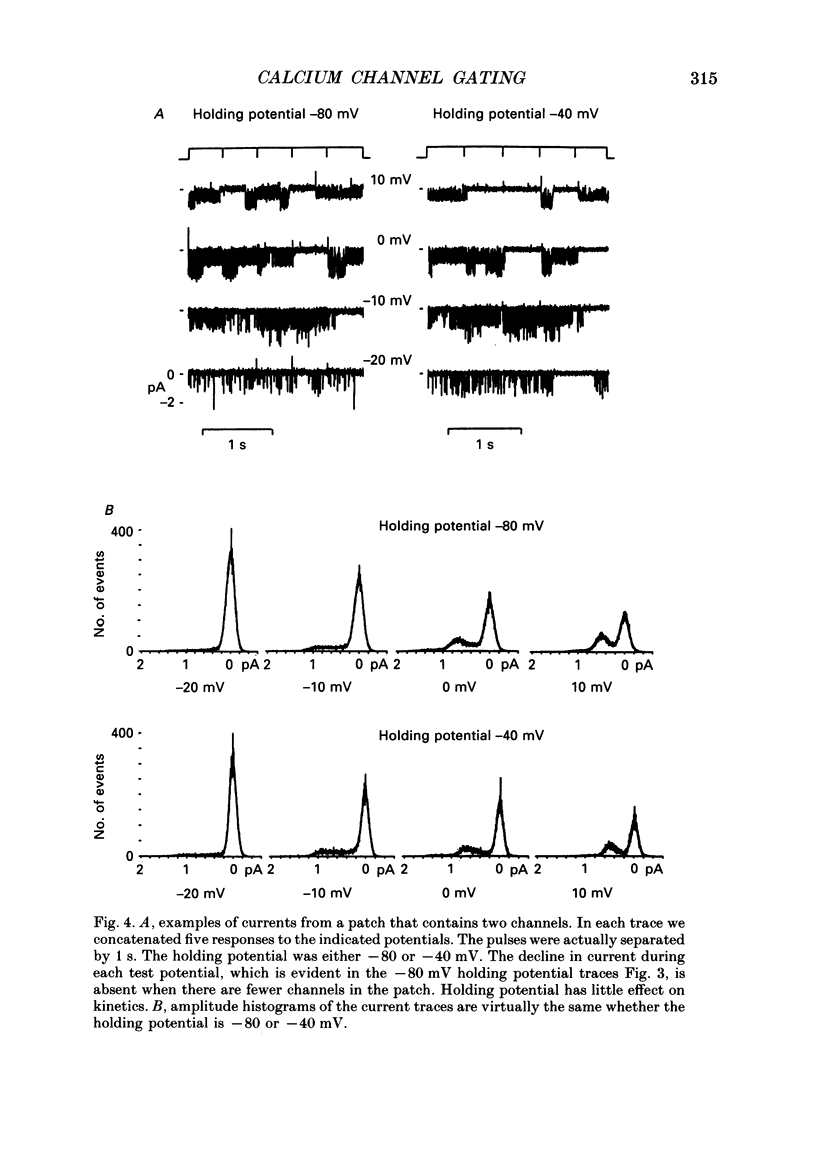
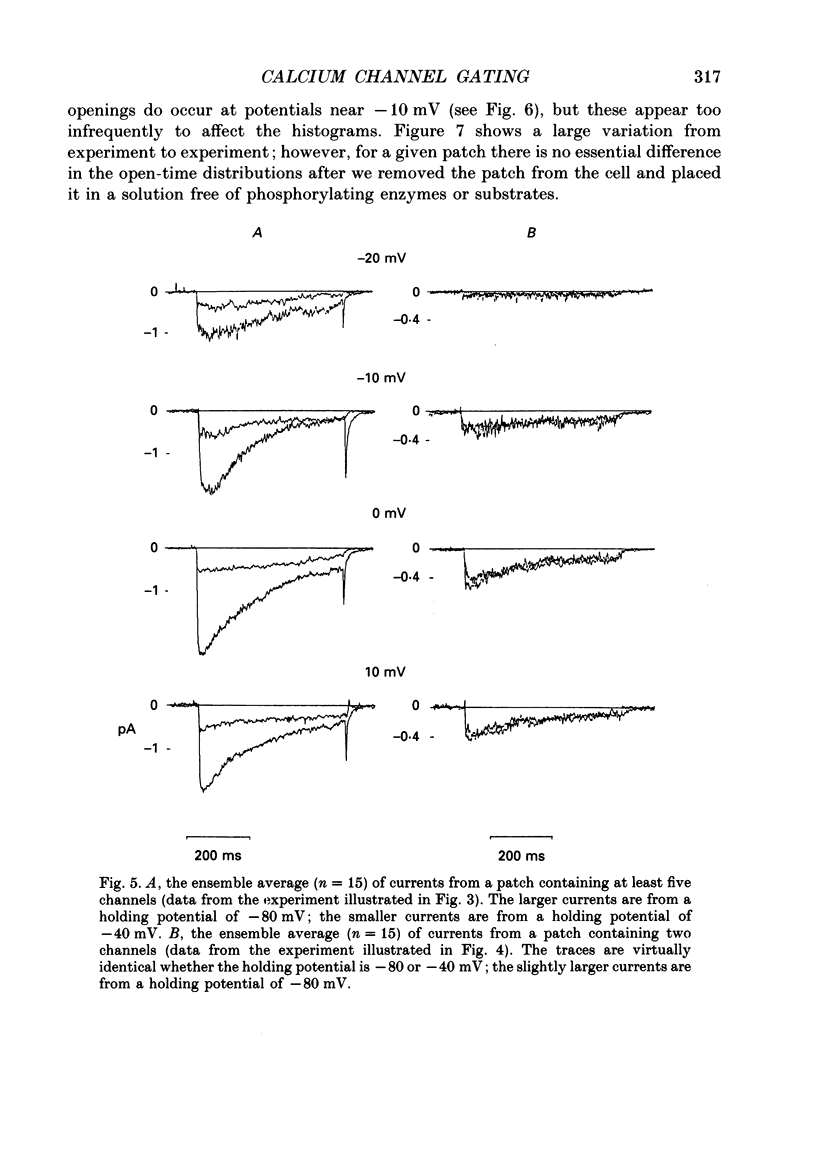
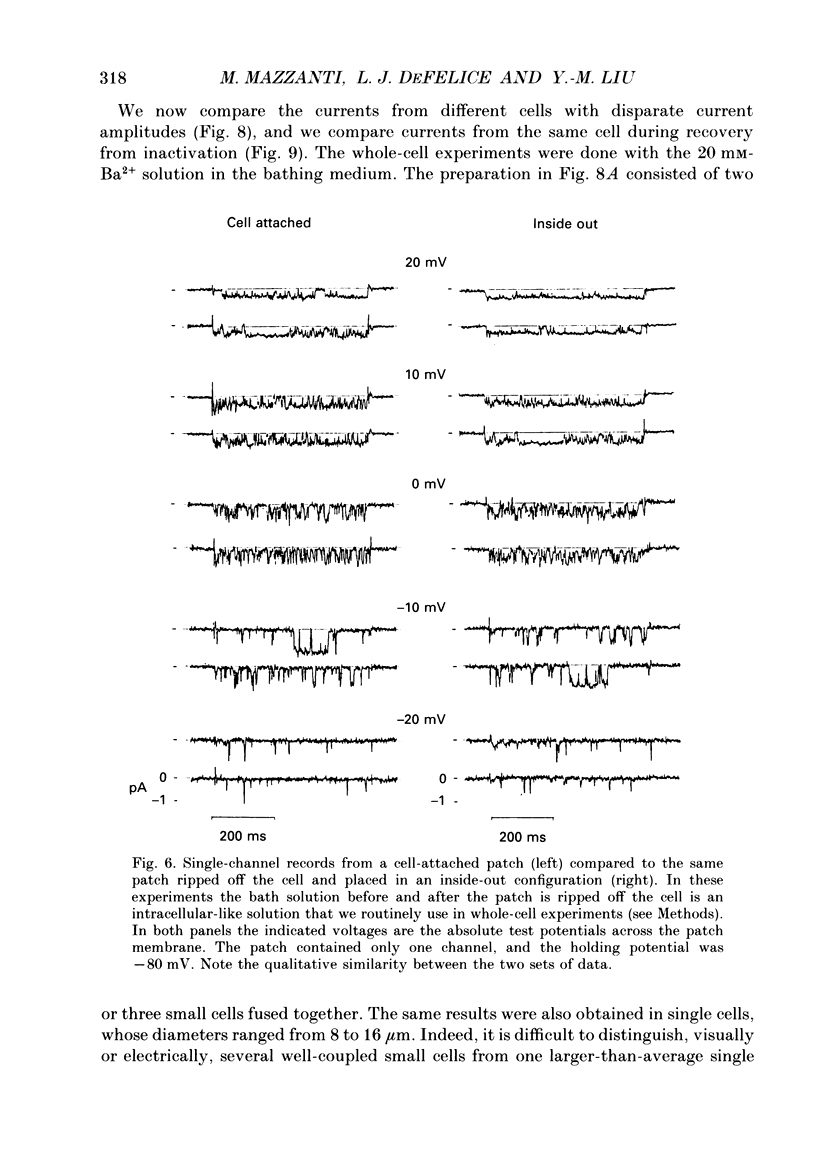
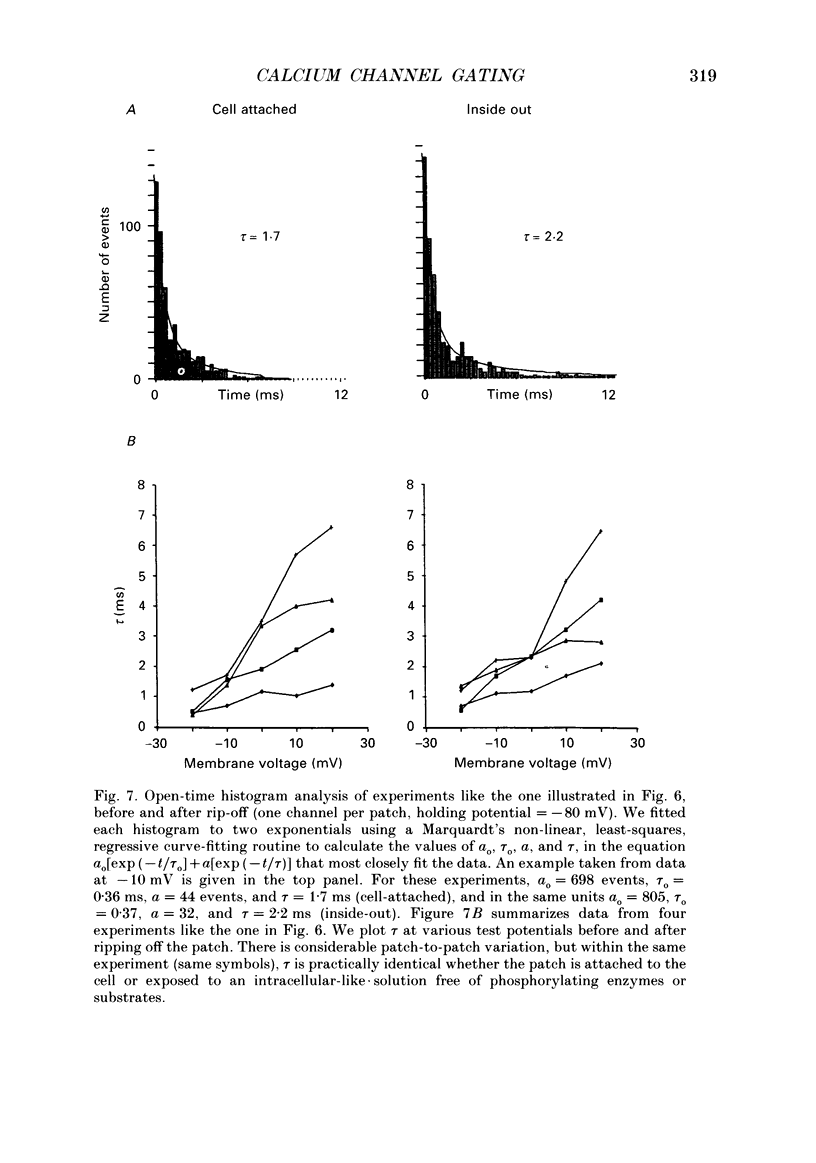
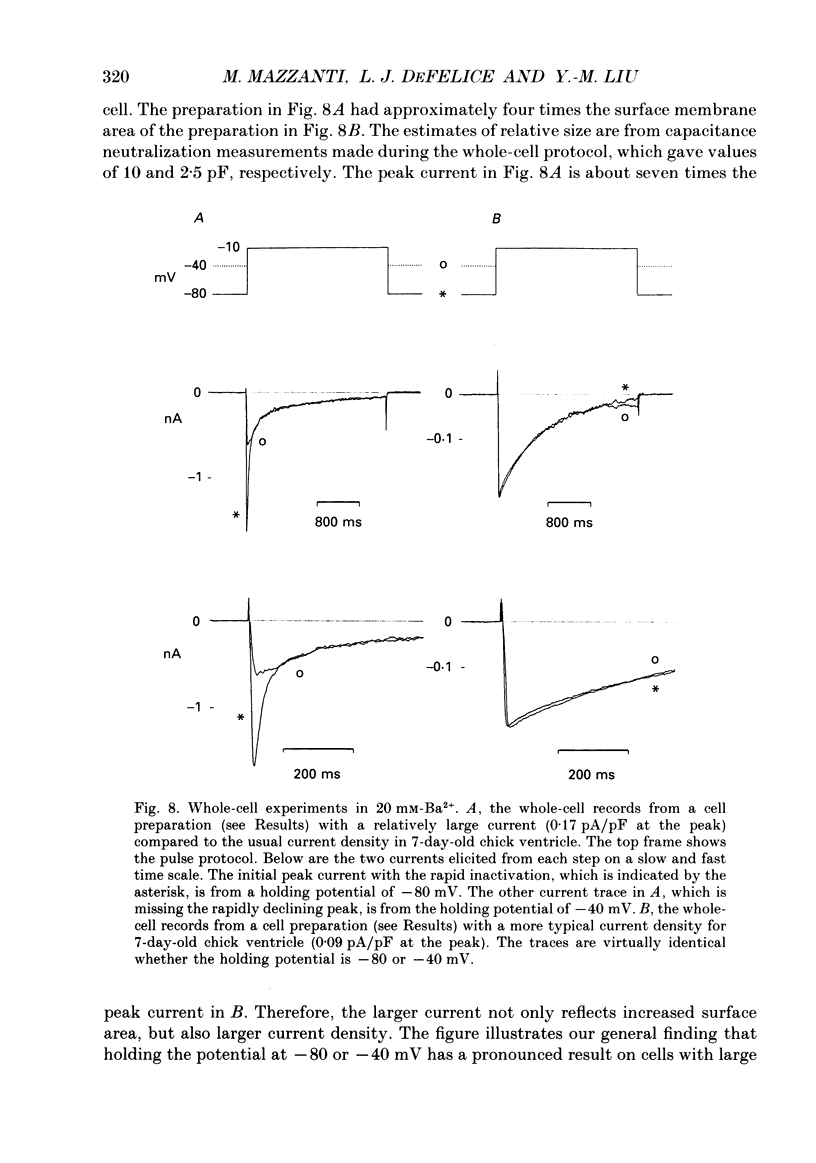
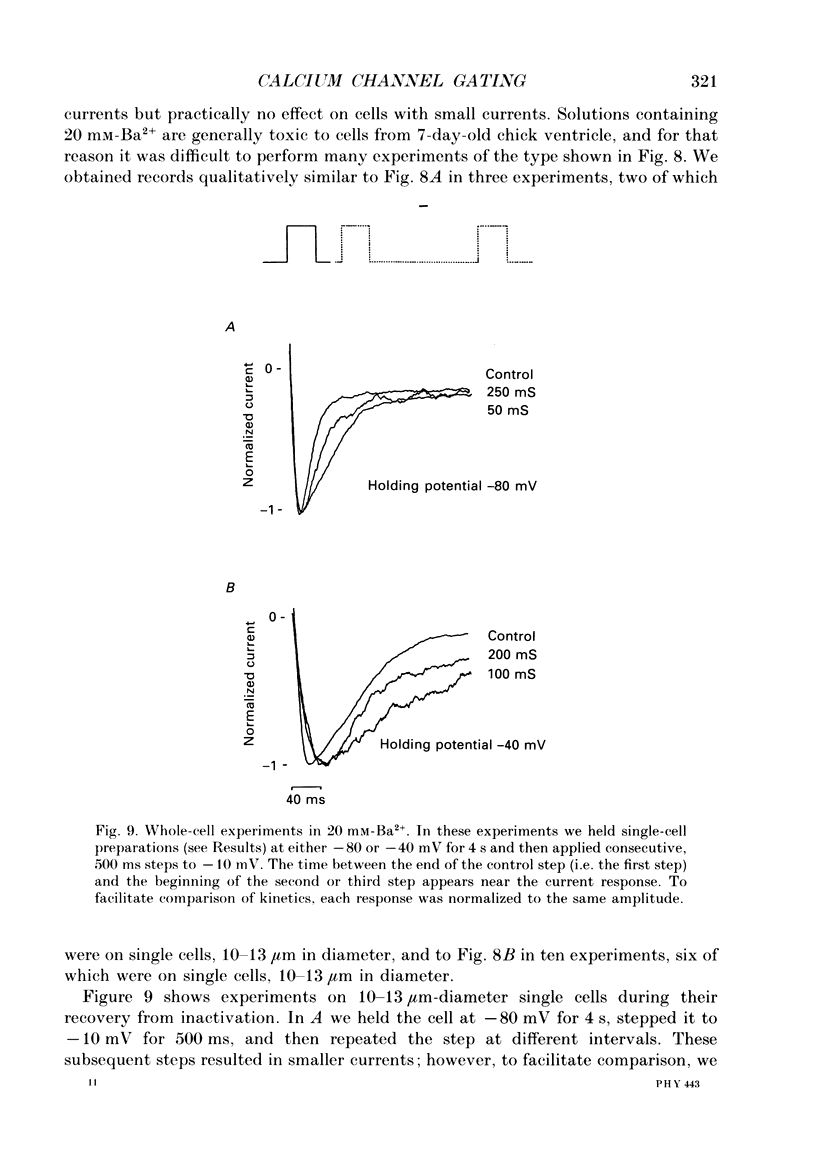
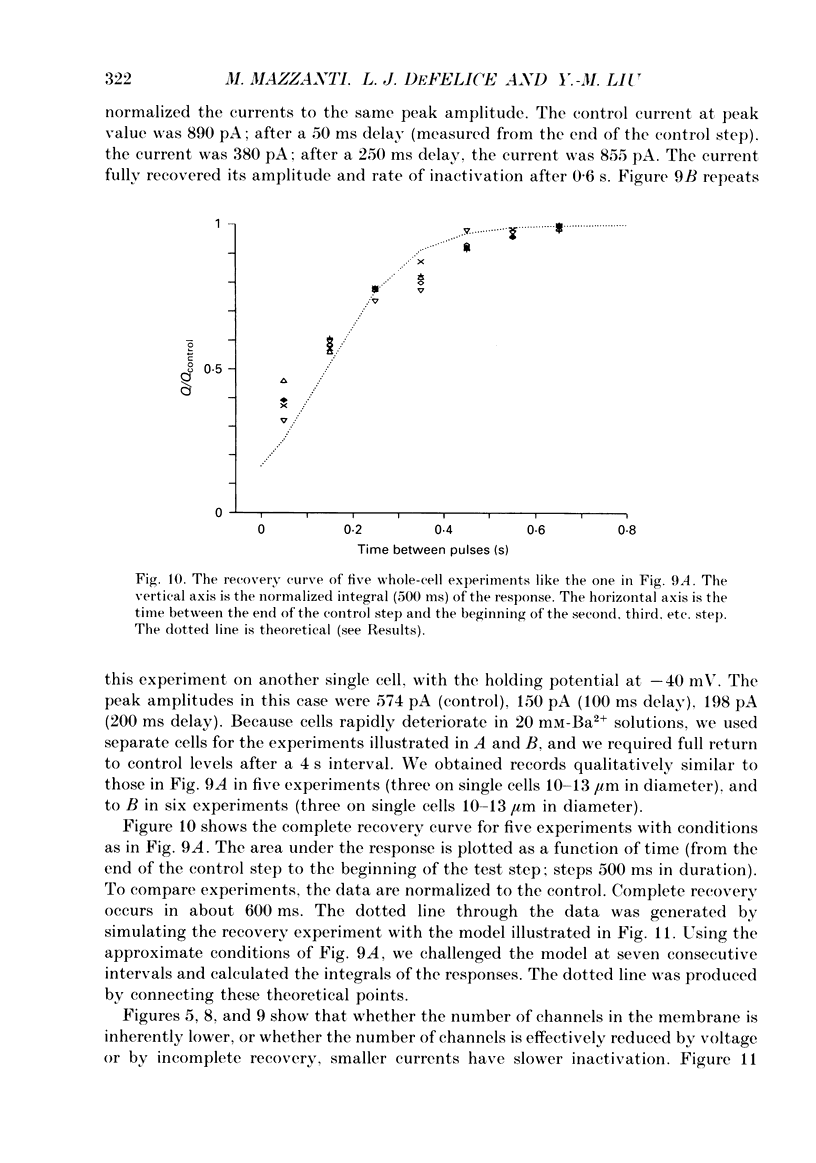
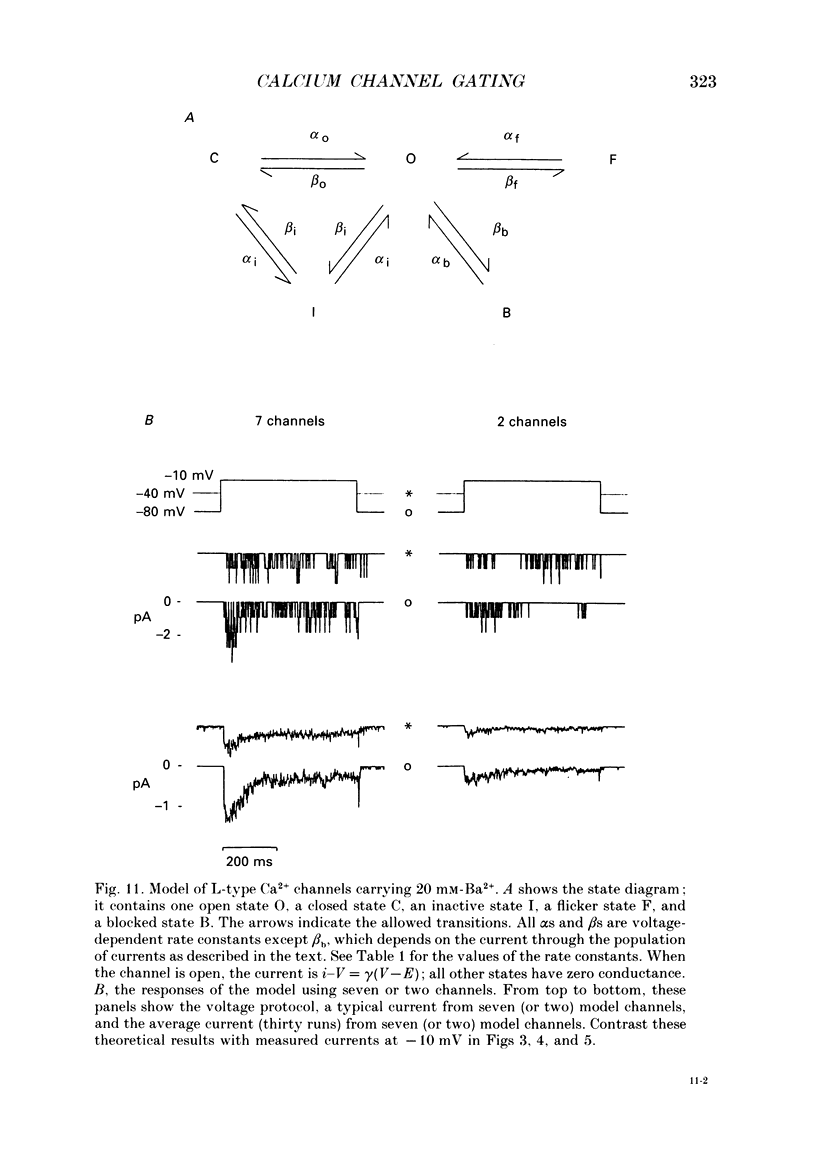
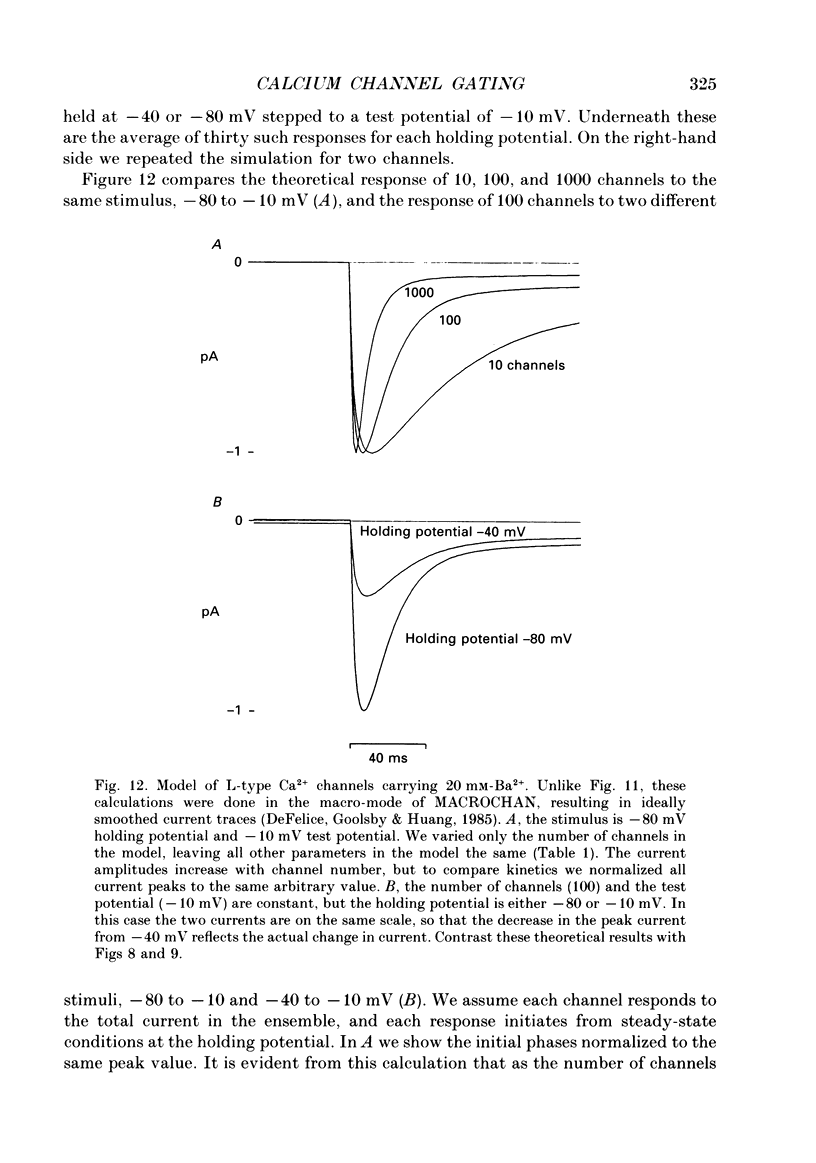
1. L-type calcium channels in embryonic chick heart ventricle have voltage-dependent, time-variant kinetics when they conduct inward currents carried by 20 mM-Ba2+. Depolarizing the membrane from -20 to 20 mV increases mean open time from 1.4 to 4.2 ms. Mean open time increases monotonically with voltage. The single-channel conductance, 18 +/- 2 pS, is approximately linear over this voltage range, and the extrapolated reversal potential is 38 +/- 5 mV. 2. In cell-attached patches with five or more L-type Ca2+ channels in the patch, the currents elicited by 500 ms depolarizing steps, from a -80 mV holding potential, inactivate rapidly and have large tail currents. In the same patch, currents from a -40 mV holding potential are smaller, inactivate more slowly, and have practically no tail currents. 3. In cell-attached patches containing one of two L-type Ca2+ channels, currents from -80 or -40 mV are virtually identical, and they are similar to the currents from multichannel patches held at -40 mV. 4. The voltage-dependent, time-variant kinetics of individual L-type Ca2+ channels are unaltered if the patch is removed from the cell and forms an inside-out configuration. In these experiments the internal membrane was bathed with an artificial, intracellular-like solution containing no phosphorylating enzymes or substrates. 5. Cells bathed in 20 mM-Ba2+ solutions and held at -80 mV have currents with an early phase that inactivates in tens of milliseconds, a late phase that inactivates in hundreds of milliseconds, and a large, slow tail current. Currents from -40 mV have only the late phase and practically no tails. However, if the maximum current is less than 0.1 pA pF-1, records from either -80 or -40 mV are virtually identical, and they are similar to currents from cells with higher channel density held at -40 mV. Furthermore, if cells are stimulated before full recovery from inactivation, the reduced current is accompanied by slower inactivation. 6. Whole-cell currents in 1.5 mM-Ca2+ solutions are entirely abolished by addition of 20 microM-nifedipine, and they are enhanced 2-3 times by addition of 30 microM-cyclic AMP and 3 mM-ATP to the whole-cell recording electrode. The whole-cell currents in 20 mM-Ba2+ solutions are also completely blocked by 20 microM-nifedipine, regardless of kinetics or holding potential. Thus, by definition, the cells we are studying contain only L-type channels.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Argibay J. A., Fischmeister R., Hartzell H. C. Inactivation, reactivation and pacing dependence of calcium current in frog cardiocytes: correlation with current density. J Physiol. 1988 Jul;401:201–226. doi: 10.1113/jphysiol.1988.sp017158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong D., Eckert R. Voltage-activated calcium channels that must be phosphorylated to respond to membrane depolarization. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2518–2522. doi: 10.1073/pnas.84.8.2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bean B. P., Nowycky M. C., Tsien R. W. Beta-adrenergic modulation of calcium channels in frog ventricular heart cells. 1984 Jan 26-Feb 1Nature. 307(5949):371–375. doi: 10.1038/307371a0. [DOI] [PubMed] [Google Scholar]
- Bean B. P., Rios E. Nonlinear charge movement in mammalian cardiac ventricular cells. Components from Na and Ca channel gating. J Gen Physiol. 1989 Jul;94(1):65–93. doi: 10.1085/jgp.94.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bean B. P. Two kinds of calcium channels in canine atrial cells. Differences in kinetics, selectivity, and pharmacology. J Gen Physiol. 1985 Jul;86(1):1–30. doi: 10.1085/jgp.86.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beeler G. W., Reuter H. Reconstruction of the action potential of ventricular myocardial fibres. J Physiol. 1977 Jun;268(1):177–210. doi: 10.1113/jphysiol.1977.sp011853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brehm P., Eckert R. Calcium entry leads to inactivation of calcium channel in Paramecium. Science. 1978 Dec 15;202(4373):1203–1206. doi: 10.1126/science.103199. [DOI] [PubMed] [Google Scholar]
- Brehm P., Eckert R., Tillotson D. Calcium-mediated inactivation of calcium current in Paramecium. J Physiol. 1980 Sep;306:193–203. doi: 10.1113/jphysiol.1980.sp013391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. M., Kunze D. L., Yatani A. The agonist effect of dihydropyridines on Ca channels. Nature. 1984 Oct 11;311(5986):570–572. doi: 10.1038/311570a0. [DOI] [PubMed] [Google Scholar]
- Campbell D. L., Giles W. R., Robinson K., Shibata E. F. Studies of the sodium-calcium exchanger in bull-frog atrial myocytes. J Physiol. 1988 Sep;403:317–340. doi: 10.1113/jphysiol.1988.sp017251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell D. L., Giles W. R., Shibata E. F. Ion transfer characteristics of the calcium current in bull-frog atrial myocytes. J Physiol. 1988 Sep;403:239–266. doi: 10.1113/jphysiol.1988.sp017248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalié A., Ochi R., Pelzer D., Trautwein W. Elementary currents through Ca2+ channels in guinea pig myocytes. Pflugers Arch. 1983 Sep;398(4):284–297. doi: 10.1007/BF00657238. [DOI] [PubMed] [Google Scholar]
- Cavalié A., Pelzer D., Trautwein W. Fast and slow gating behaviour of single calcium channels in cardiac cells. Relation to activation and inactivation of calcium-channel current. Pflugers Arch. 1986 Mar;406(3):241–258. doi: 10.1007/BF00640910. [DOI] [PubMed] [Google Scholar]
- Chad J. E., Eckert R. Calcium domains associated with individual channels can account for anomalous voltage relations of CA-dependent responses. Biophys J. 1984 May;45(5):993–999. doi: 10.1016/S0006-3495(84)84244-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischmeister R., Horackova M. Variation of intracellular Ca2+ following Ca2+ current in heart. A theoretical study of ionic diffusion inside a cylindrical cell. Biophys J. 1983 Mar;41(3):341–348. doi: 10.1016/S0006-3495(83)84445-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii S., Ayer R. K., Jr, DeHaan R. L. Development of the fast sodium current in early embryonic chick heart cells. J Membr Biol. 1988 Mar;101(3):209–223. doi: 10.1007/BF01872836. [DOI] [PubMed] [Google Scholar]
- Hagiwara S., Nakajima S. Effects of the intracellular Ca ion concentration upon the excitability of the muscle fiber membrane of a barnacle. J Gen Physiol. 1966 Mar;49(4):807–818. doi: 10.1085/jgp.49.4.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartzell H. C., White R. E. Effects of magnesium on inactivation of the voltage-gated calcium current in cardiac myocytes. J Gen Physiol. 1989 Oct;94(4):745–767. doi: 10.1085/jgp.94.4.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess P., Lansman J. B., Tsien R. W. Calcium channel selectivity for divalent and monovalent cations. Voltage and concentration dependence of single channel current in ventricular heart cells. J Gen Physiol. 1986 Sep;88(3):293–319. doi: 10.1085/jgp.88.3.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess P., Lansman J. B., Tsien R. W. Different modes of Ca channel gating behaviour favoured by dihydropyridine Ca agonists and antagonists. Nature. 1984 Oct 11;311(5986):538–544. doi: 10.1038/311538a0. [DOI] [PubMed] [Google Scholar]
- Hess P., Tsien R. W. Mechanism of ion permeation through calcium channels. 1984 May 31-Jun 6Nature. 309(5967):453–456. doi: 10.1038/309453a0. [DOI] [PubMed] [Google Scholar]
- Hirano Y., Fozzard H. A., January C. T. Characteristics of L- and T-type Ca2+ currents in canine cardiac Purkinje cells. Am J Physiol. 1989 May;256(5 Pt 2):H1478–H1492. doi: 10.1152/ajpheart.1989.256.5.H1478. [DOI] [PubMed] [Google Scholar]
- Hume J. R., Giles W. Ionic currents in single isolated bullfrog atrial cells. J Gen Physiol. 1983 Feb;81(2):153–194. doi: 10.1085/jgp.81.2.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephson I. R., Sanchez-Chapula J., Brown A. M. A comparison of calcium currents in rat and guinea pig single ventricular cells. Circ Res. 1984 Feb;54(2):144–156. doi: 10.1161/01.res.54.2.144. [DOI] [PubMed] [Google Scholar]
- Kass R. S., Sanguinetti M. C. Inactivation of calcium channel current in the calf cardiac Purkinje fiber. Evidence for voltage- and calcium-mediated mechanisms. J Gen Physiol. 1984 Nov;84(5):705–726. doi: 10.1085/jgp.84.5.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawano S., DeHaan R. L. Low-threshold current is major calcium current in chick ventricle cells. Am J Physiol. 1989 May;256(5 Pt 2):H1505–H1508. doi: 10.1152/ajpheart.1989.256.5.H1505. [DOI] [PubMed] [Google Scholar]
- Kohlhardt M., Krause H., Kübler M., Herdey A. Kinetics of inactivation and recovery of the slow inward current in the mammalian ventricular myocardium. Pflugers Arch. 1975 Mar 22;355(1):1–17. doi: 10.1007/BF00584795. [DOI] [PubMed] [Google Scholar]
- Lacerda A. E., Brown A. M. Nonmodal gating of cardiac calcium channels as revealed by dihydropyridines. J Gen Physiol. 1989 Jun;93(6):1243–1273. doi: 10.1085/jgp.93.6.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacerda A. E., Rampe D., Brown A. M. Effects of protein kinase C activators on cardiac Ca2+ channels. Nature. 1988 Sep 15;335(6187):249–251. doi: 10.1038/335249a0. [DOI] [PubMed] [Google Scholar]
- Lansman J. B., Hess P., Tsien R. W. Blockade of current through single calcium channels by Cd2+, Mg2+, and Ca2+. Voltage and concentration dependence of calcium entry into the pore. J Gen Physiol. 1986 Sep;88(3):321–347. doi: 10.1085/jgp.88.3.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lederer W. J., Niggli E., Hadley R. W. Sodium-calcium exchange in excitable cells: fuzzy space. Science. 1990 Apr 20;248(4953):283–283. doi: 10.1126/science.2326638. [DOI] [PubMed] [Google Scholar]
- Lee K. S., Marban E., Tsien R. W. Inactivation of calcium channels in mammalian heart cells: joint dependence on membrane potential and intracellular calcium. J Physiol. 1985 Jul;364:395–411. doi: 10.1113/jphysiol.1985.sp015752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lux H. D., Brown A. M. Single channel studies on inactivation of calcium currents. Science. 1984 Jul 27;225(4660):432–434. doi: 10.1126/science.6330896. [DOI] [PubMed] [Google Scholar]
- Mazzanti M., DeFelice L. J. Ca channel gating during cardiac action potentials. Biophys J. 1990 Oct;58(4):1059–1065. doi: 10.1016/S0006-3495(90)82448-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzanti M., DeFelice L. J. K channel kinetics during the spontaneous heart beat in embryonic chick ventricle cells. Biophys J. 1988 Dec;54(6):1139–1148. doi: 10.1016/S0006-3495(88)83048-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzanti M., DeFelice L. J. Na channel kinetics during the spontaneous heart beat in embryonic chick ventricle cells. Biophys J. 1987 Jul;52(1):95–100. doi: 10.1016/S0006-3495(87)83192-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzanti M., DeFelice L. J. Regulation of the Na-conducting Ca channel during the cardiac action potential. Biophys J. 1987 Jan;51(1):115–121. doi: 10.1016/S0006-3495(87)83316-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald T. F., Cavalié A., Trautwein W., Pelzer D. Voltage-dependent properties of macroscopic and elementary calcium channel currents in guinea pig ventricular myocytes. Pflugers Arch. 1986 May;406(5):437–448. doi: 10.1007/BF00583365. [DOI] [PubMed] [Google Scholar]
- Mentrard D., Vassort G., Fischmeister R. Calcium-mediated inactivation of the calcium conductance in cesium-loaded frog heart cells. J Gen Physiol. 1984 Jan;83(1):105–131. doi: 10.1085/jgp.83.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilius B., Hess P., Lansman J. B., Tsien R. W. A novel type of cardiac calcium channel in ventricular cells. Nature. 1985 Aug 1;316(6027):443–446. doi: 10.1038/316443a0. [DOI] [PubMed] [Google Scholar]
- Osaka T., Joyner R. W. Developmental changes in calcium currents of rabbit ventricular cells. Circ Res. 1991 Mar;68(3):788–796. doi: 10.1161/01.res.68.3.788. [DOI] [PubMed] [Google Scholar]
- Osterrieder W., Brum G., Hescheler J., Trautwein W., Flockerzi V., Hofmann F. Injection of subunits of cyclic AMP-dependent protein kinase into cardiac myocytes modulates Ca2+ current. Nature. 1982 Aug 5;298(5874):576–578. doi: 10.1038/298576a0. [DOI] [PubMed] [Google Scholar]
- Pelzer D., Pelzer S., McDonald T. F. Properties and regulation of calcium channels in muscle cells. Rev Physiol Biochem Pharmacol. 1990;114:107–207. doi: 10.1007/BFb0031019. [DOI] [PubMed] [Google Scholar]
- Pietrobon D., Hess P. Novel mechanism of voltage-dependent gating in L-type calcium channels. Nature. 1990 Aug 16;346(6285):651–655. doi: 10.1038/346651a0. [DOI] [PubMed] [Google Scholar]
- Reuter H. Calcium channel modulation by neurotransmitters, enzymes and drugs. Nature. 1983 Feb 17;301(5901):569–574. doi: 10.1038/301569a0. [DOI] [PubMed] [Google Scholar]
- Reuter H., Stevens C. F., Tsien R. W., Yellen G. Properties of single calcium channels in cardiac cell culture. Nature. 1982 Jun 10;297(5866):501–504. doi: 10.1038/297501a0. [DOI] [PubMed] [Google Scholar]
- Rosenberg R. L., Hess P., Tsien R. W. Cardiac calcium channels in planar lipid bilayers. L-type channels and calcium-permeable channels open at negative membrane potentials. J Gen Physiol. 1988 Jul;92(1):27–54. doi: 10.1085/jgp.92.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsien R. W., Bean B. P., Hess P., Nowycky M. Calcium channels: mechanisms of beta-adrenergic modulation and ion permeation. Cold Spring Harb Symp Quant Biol. 1983;48(Pt 1):201–212. doi: 10.1101/sqb.1983.048.01.023. [DOI] [PubMed] [Google Scholar]
- Tsien R. W., Giles W., Greengard P. Cyclic AMP mediates the effects of adrenaline on cardiac purkinje fibres. Nat New Biol. 1972 Dec 6;240(101):181–183. doi: 10.1038/newbio240181a0. [DOI] [PubMed] [Google Scholar]
- Wellis D. P., DeFelice L. J., Mazzanti M. Outward sodium current in beating heart cells. Biophys J. 1990 Jan;57(1):41–48. doi: 10.1016/S0006-3495(90)82505-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue D. T., Backx P. H., Imredy J. P. Calcium-sensitive inactivation in the gating of single calcium channels. Science. 1990 Dec 21;250(4988):1735–1738. doi: 10.1126/science.2176745. [DOI] [PubMed] [Google Scholar]
- Yue D. T., Herzig S., Marban E. Beta-adrenergic stimulation of calcium channels occurs by potentiation of high-activity gating modes. Proc Natl Acad Sci U S A. 1990 Jan;87(2):753–757. doi: 10.1073/pnas.87.2.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue D. T., Marban E. Permeation in the dihydropyridine-sensitive calcium channel. Multi-ion occupancy but no anomalous mole-fraction effect between Ba2+ and Ca2+. J Gen Physiol. 1990 May;95(5):911–939. doi: 10.1085/jgp.95.5.911. [DOI] [PMC free article] [PubMed] [Google Scholar]


