Abstract
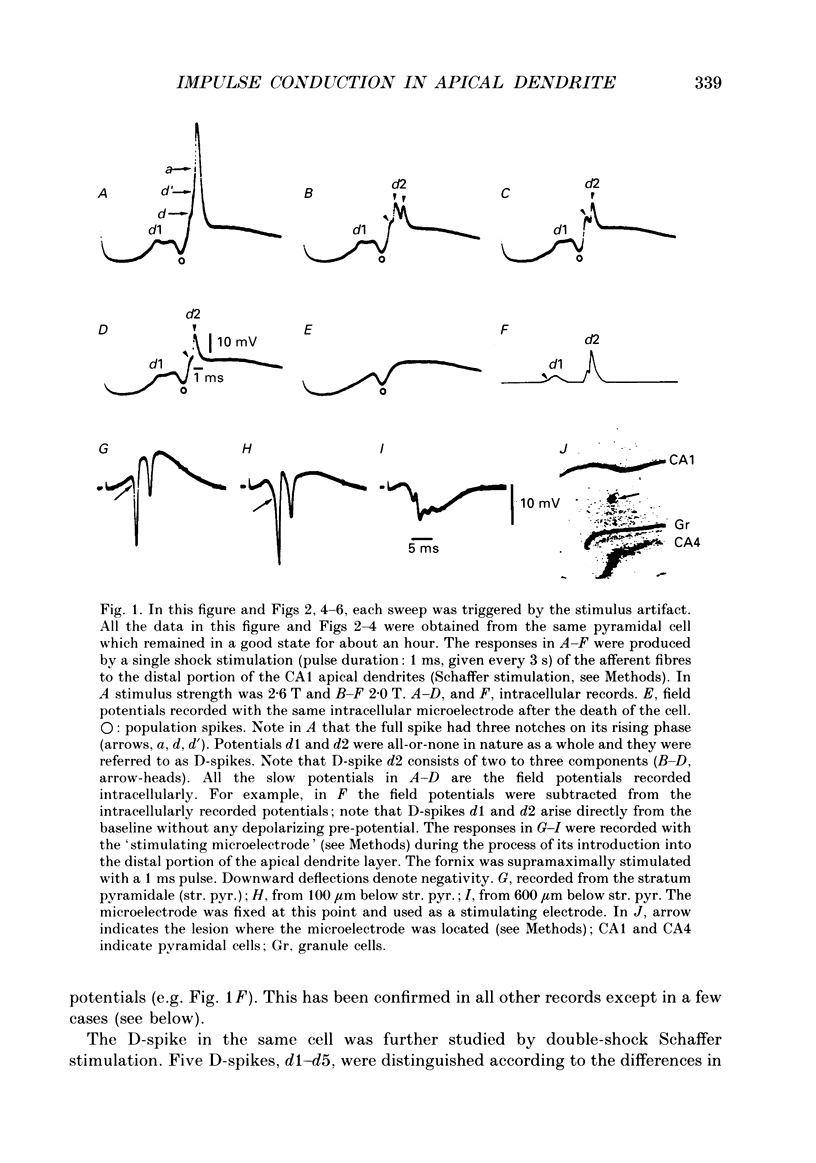
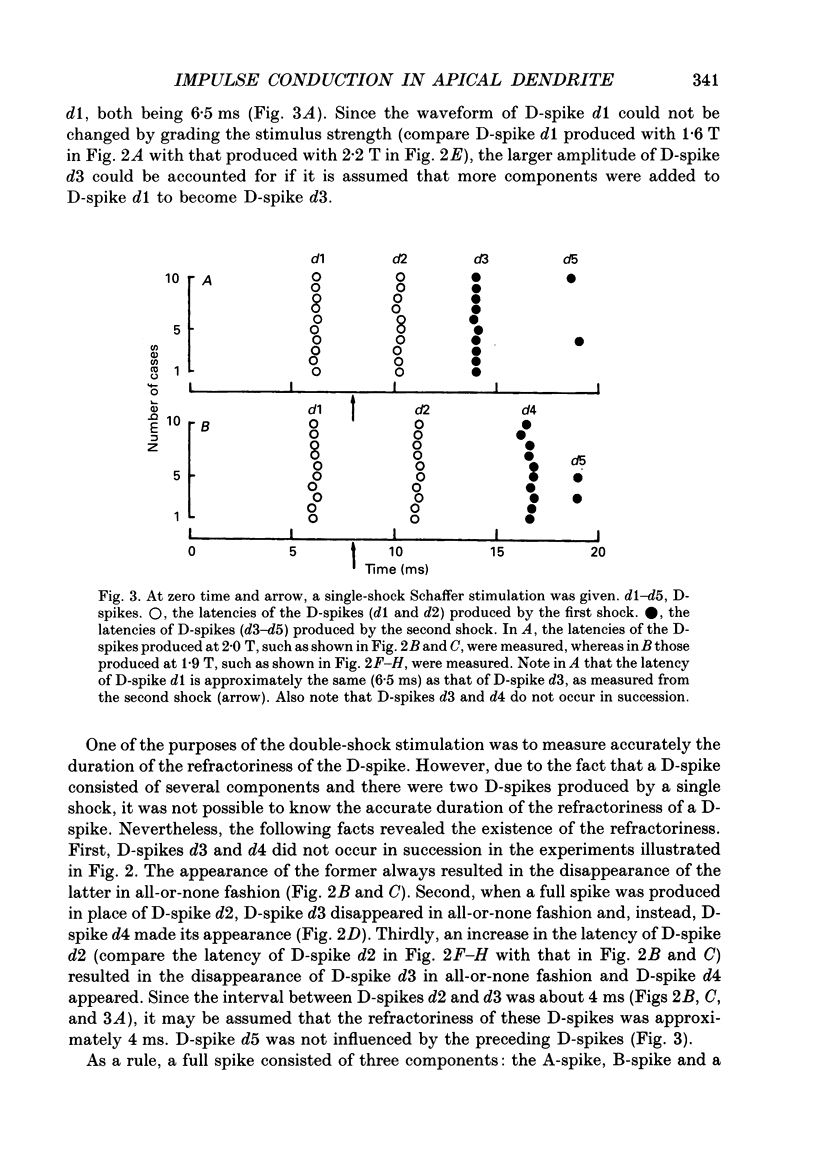
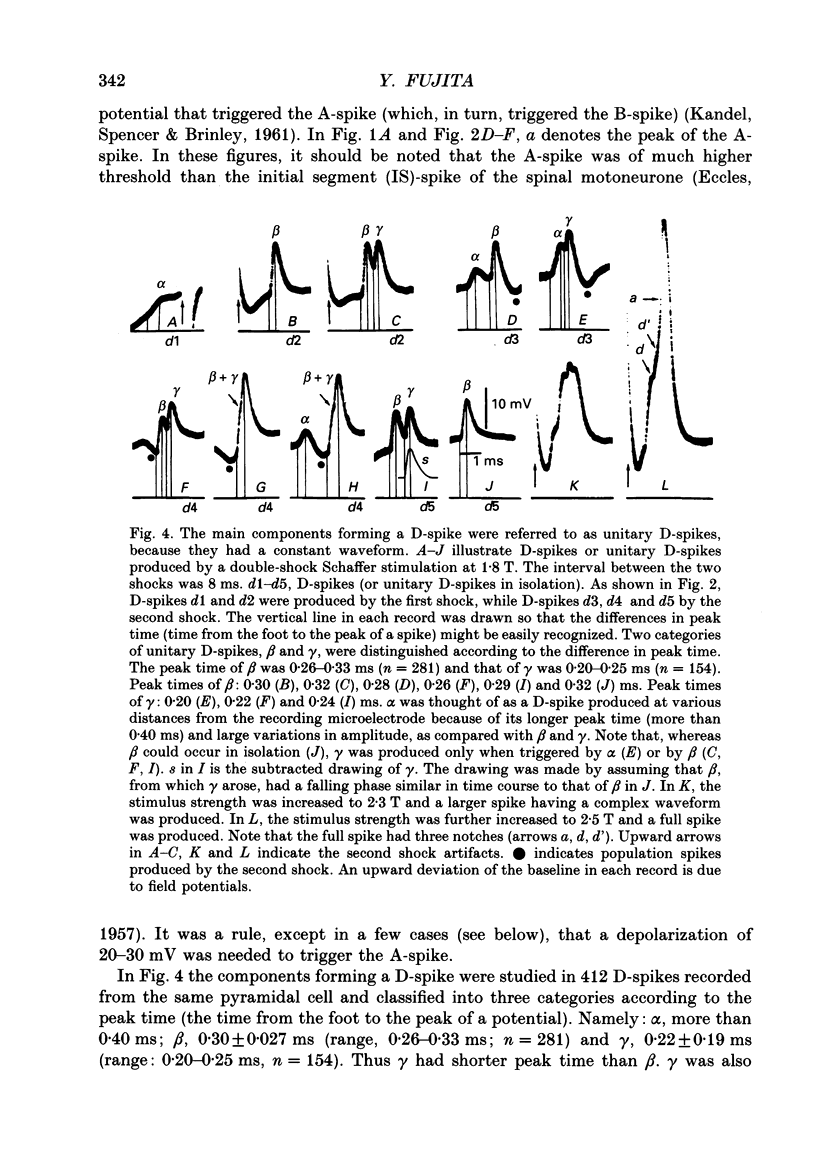
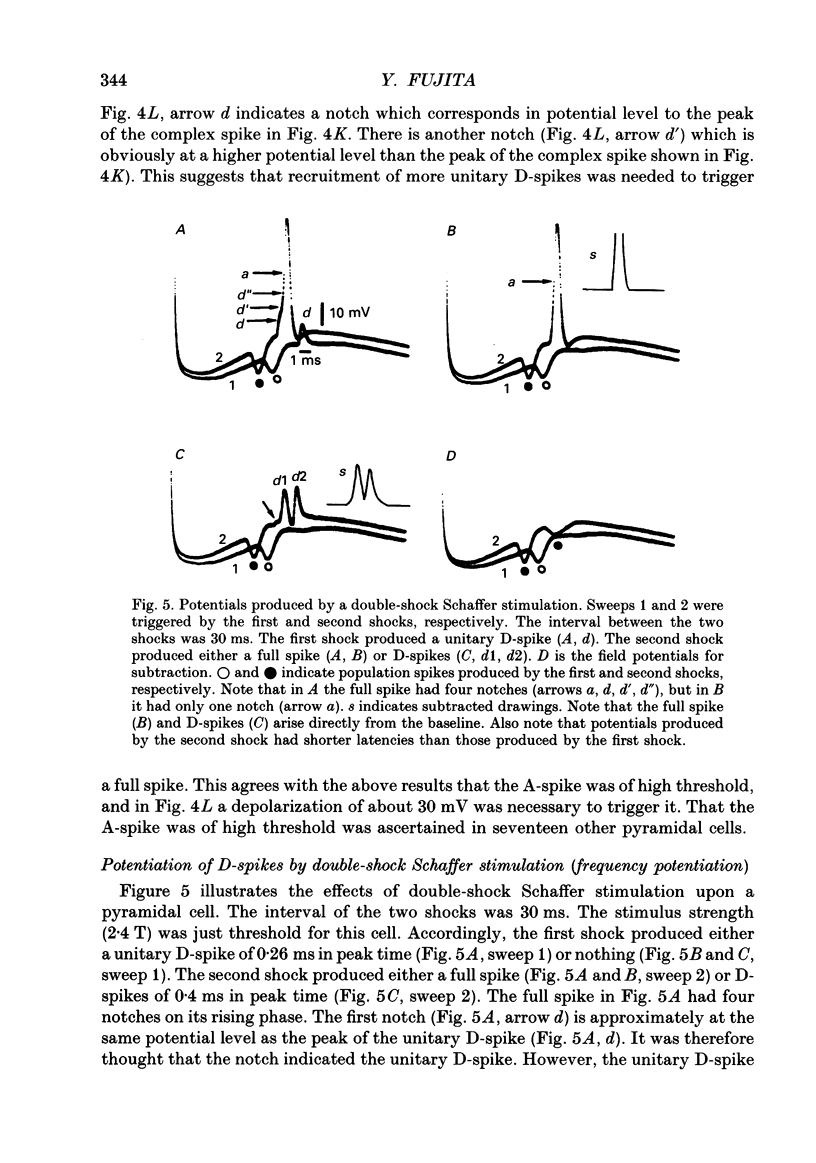
1. Impulse conduction in CA1 apical dendrites was studied by stimulating afferent fibres to the distal portion of the dendrites (Schaffer stimulation) and recording intracellularly from the pyramidal cell body in the hippocampus of the rabbit anaesthetized with sodium pentobarbitone and immobilized with d-tubocurarine. 2. The stimulation, when strong enough, produced a full spike in all the pyramidal cells (n = 48) which were capable of producing spikes of 51-67 mV in amplitude. A single-shock Schaffer stimulation produced a single spike in forty-six cells and a two-spike burst in two cells. All the single spikes and the first spike of the two-spike burst arose directly from the baseline. 3. By reducing the stimulus strength, three categories of small spikes (alpha, beta and gamma) could be distinguished in twenty-two pyramidal cells. alpha was of the lowest threshold with an amplitude of less than 7.5 mV and the time from the foot of the spike to its peak (peak time) was more than 0.40 ms. beta was of the next lowest threshold with an amplitude of 8.7-13.5 mV and had a peak time of 0.26-0.36 ms (n = 3242). gamma was of the highest threshold with an amplitude of 6.6-9.7 mV and had a peak time of 0.20-0.27 ms (n = 1783). The duration of alpha was 1.5-4.0 ms, whereas that of beta and gamma was 1.2-1.5 ms. 4. Within a given pyramidal cell, the waveform of beta and gamma was remarkably constant, being independent of stimulus strength. They were therefore regarded as units and referred to as unitary D-spikes. The unitary D-spikes tended to summate forming a larger, longer-lasting potential which was referred to as the D-spike. alpha was probably a D-spike produced at a greater distance away from the recording microelectrode, as compared with beta and gamma. 5. Within a given pyramidal cell unitary D-spikes beta and gamma could be further subdivided into two subclasses, respectively, according to the differences in amplitude. Furthermore alpha contained at least one unitary D-spike. Thus, at least five different unitary D-spikes could be distinguished in the same cell. They were thought to be dendritic in origin, because only the dendrites could possibly give rise to so many small spikes which could be seen with the intrasomatically placed microelectrode. 6. In most cases a full spike consisted of the A-, B- and D-spikes.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF














Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDERSEN P., ECCLES J. C., LOYNING Y. LOCATION OF POSTSYNAPTIC INHIBITORY SYNAPSES ON HIPPOCAMPAL PYRAMIDS. J Neurophysiol. 1964 Jul;27:592–607. doi: 10.1152/jn.1964.27.4.592. [DOI] [PubMed] [Google Scholar]
- ANDERSEN P., ECCLES J. C., LOYNING Y. PATHWAY OF POSTSYNAPTIC INHIBITION IN THE HIPPOCAMPUS. J Neurophysiol. 1964 Jul;27:608–619. doi: 10.1152/jn.1964.27.4.608. [DOI] [PubMed] [Google Scholar]
- ANDERSEN P. Interhippocampal impulses. II. Apical dendritic activation of CAI neurons. Acta Physiol Scand. 1960 Mar 18;48:178–208. doi: 10.1111/j.1748-1716.1960.tb01858.x. [DOI] [PubMed] [Google Scholar]
- Alger B. E., Nicoll R. A. Feed-forward dendritic inhibition in rat hippocampal pyramidal cells studied in vitro. J Physiol. 1982 Jul;328:105–123. doi: 10.1113/jphysiol.1982.sp014255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson P., Lomo T. Mode of activation of hippocampal pyramidal cells by excitatory synapses on dendrites. Exp Brain Res. 1966;2(3):247–260. [PubMed] [Google Scholar]
- Babb T. L., Pretorius J. K., Kupfer W. R., Crandall P. H. Glutamate decarboxylase-immunoreactive neurons are preserved in human epileptic hippocampus. J Neurosci. 1989 Jul;9(7):2562–2574. doi: 10.1523/JNEUROSCI.09-07-02562.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss T. V., Lomo T. Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J Physiol. 1973 Jul;232(2):331–356. doi: 10.1113/jphysiol.1973.sp010273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CRAGG B. G., HAMLYN L. H. Action potentials of the pyramidal neurones in the hippocampus of the rabbit. J Physiol. 1955 Sep 28;129(3):608–627. doi: 10.1113/jphysiol.1955.sp005382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FUJITA Y., NAKAMURA Y. Effect of fornical stimulation upon the CA1 and CA2 apical dendrite of rabbit's hippocampus. Jpn J Physiol. 1961 Aug 15;11:357–368. doi: 10.2170/jjphysiol.11.357. [DOI] [PubMed] [Google Scholar]
- FUJITA Y., SAKATA H. Electrophysiological properties of CA1 and CA2 apical dendrites of rabbit hippocampus. J Neurophysiol. 1962 Mar;25:209–222. doi: 10.1152/jn.1962.25.2.209. [DOI] [PubMed] [Google Scholar]
- FUJITA Y., SATO T. INTRACELLULAR RECORDS FROM HIPPOCAMPAL PYRAMIDAL CELLS IN RABBIT DURING THETA RHYTHM ACTIVITY. J Neurophysiol. 1964 Nov;27:1012–1025. doi: 10.1152/jn.1964.27.6.1011. [DOI] [PubMed] [Google Scholar]
- Fujita Y. Activity of dendrites of single Purkinje cells and its relationship to so-called inactivation response in rabbit cerebellum. J Neurophysiol. 1968 Mar;31(2):131–141. doi: 10.1152/jn.1968.31.2.131. [DOI] [PubMed] [Google Scholar]
- Fujita Y. Evidence for the existence of inhibitory postsynaptic potentials in dendrites and their functional significance in hippocampal pyramidal cells of adult rabbits. Brain Res. 1979 Oct 12;175(1):59–69. doi: 10.1016/0006-8993(79)90514-6. [DOI] [PubMed] [Google Scholar]
- Fujita Y., Harada H., Kitamura T., Minami S., Sato T. Dendritic activities of spinal motoneurones in pigs and rabbits enhanced through chronic stimulation of a dorsal root. J Physiol. 1987 Feb;383:171–190. doi: 10.1113/jphysiol.1987.sp016403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita Y., Harada H., Takeuchi T., Sato H., Minami S. Enhancement of EEG spikes and hyperpolarizations of pyramidal cells in the kindled hippocampus of the rabbit. Jpn J Physiol. 1983;33(2):227–238. doi: 10.2170/jjphysiol.33.227. [DOI] [PubMed] [Google Scholar]
- Fujita Y., Iwasa H. Electrophysiological properties of so-called inactivation response and their relationship to dendritic activity in hippocampal pyramidal cells of rabbits. Brain Res. 1977 Jul 8;130(1):89–99. doi: 10.1016/0006-8993(77)90844-7. [DOI] [PubMed] [Google Scholar]
- Fujita Y., Sakuranaga M. Spontaneous hyperpolarizations in pyramidal cells of chronically stimulated rabbit hippocampus. Jpn J Physiol. 1981;31(6):879–889. doi: 10.2170/jjphysiol.31.879. [DOI] [PubMed] [Google Scholar]
- Fujita Y., Sato H., Takeuchi T., Minami S. Median raphe- and contralateral hippocampus-elicited EEG spikes which correspond to hyperpolarizations of pyramidal cells in the kindled hippocampus of the rabbit. Brain Res. 1983 Nov 14;278(1-2):313–317. doi: 10.1016/0006-8993(83)90262-7. [DOI] [PubMed] [Google Scholar]
- Fujita Y. Two types of depolarizing after-potentials in hippocampal pyramidal cells of rabbits. Brain Res. 1975 Sep 5;94(3):435–446. doi: 10.1016/0006-8993(75)90227-9. [DOI] [PubMed] [Google Scholar]
- GRANIT R., PHILLIPS C. G. Excitatory and inhibitory processes acting upon individual Purkinje cells of the cerebellum in cats. J Physiol. 1956 Sep 27;133(3):520–547. doi: 10.1113/jphysiol.1956.sp005606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREEN J. D., ADEY W. R. Electrophysiological studies of hippocampal connections and excitability. Electroencephalogr Clin Neurophysiol. 1956 May;8(2):245–263. doi: 10.1016/0013-4694(56)90117-1. [DOI] [PubMed] [Google Scholar]
- KANDEL E. R., SPENCER W. A., BRINLEY F. J., Jr Electrophysiology of hippocampal neurons. I. Sequential invasion and synaptic organization. J Neurophysiol. 1961 May;24:225–242. doi: 10.1152/jn.1961.24.3.225. [DOI] [PubMed] [Google Scholar]
- KANDEL E. R., SPENCER W. A. Electrophysiology of hippocampal neurons. II. After-potentials and repetitive firing. J Neurophysiol. 1961 May;24:243–259. doi: 10.1152/jn.1961.24.3.243. [DOI] [PubMed] [Google Scholar]
- Kuno M., Llinás R. Enhancement of synaptic transmission by dendritic potentials in chromatolysed motoneurones of the cat. J Physiol. 1970 Nov;210(4):807–821. doi: 10.1113/jphysiol.1970.sp009243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyakawa H., Kato H. Active properties of dendritic membrane examined by current source density analysis in hippocampal CA1 pyramidal neurons. Brain Res. 1986 Dec 10;399(2):303–309. doi: 10.1016/0006-8993(86)91520-9. [DOI] [PubMed] [Google Scholar]
- Purpura D. P., McMurtry J. G., Leonard C. F., Malliani A. Evidence for dendritic origin of spikes without depolarizing prepotentials in hippocampal neurons during and after seizure. J Neurophysiol. 1966 Sep;29(5):954–979. doi: 10.1152/jn.1966.29.5.954. [DOI] [PubMed] [Google Scholar]
- Purpura D. P., Prelevic S., Santini M. Postsynaptic potentials and spike variations in the feline hippocampus during postnatal ontogenesis. Exp Neurol. 1968 Nov;22(3):408–422. doi: 10.1016/0014-4886(68)90006-x. [DOI] [PubMed] [Google Scholar]
- Schwartzkroin P. A. Characteristics of CA1 neurons recorded intracellularly in the hippocampal in vitro slice preparation. Brain Res. 1975 Mar 7;85(3):423–436. doi: 10.1016/0006-8993(75)90817-3. [DOI] [PubMed] [Google Scholar]
- Taube J. S., Schwartzkroin P. A. Mechanisms of long-term potentiation: EPSP/spike dissociation, intradendritic recordings, and glutamate sensitivity. J Neurosci. 1988 May;8(5):1632–1644. doi: 10.1523/JNEUROSCI.08-05-01632.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taube J. S., Schwartzkroin P. A. Mechanisms of long-term potentiation: a current-source density analysis. J Neurosci. 1988 May;8(5):1645–1655. doi: 10.1523/JNEUROSCI.08-05-01645.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong R. K., Prince D. A., Basbaum A. I. Intradendritic recordings from hippocampal neurons. Proc Natl Acad Sci U S A. 1979 Feb;76(2):986–990. doi: 10.1073/pnas.76.2.986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zola-Morgan S., Squire L. R., Amaral D. G. Human amnesia and the medial temporal region: enduring memory impairment following a bilateral lesion limited to field CA1 of the hippocampus. J Neurosci. 1986 Oct;6(10):2950–2967. doi: 10.1523/JNEUROSCI.06-10-02950.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]


