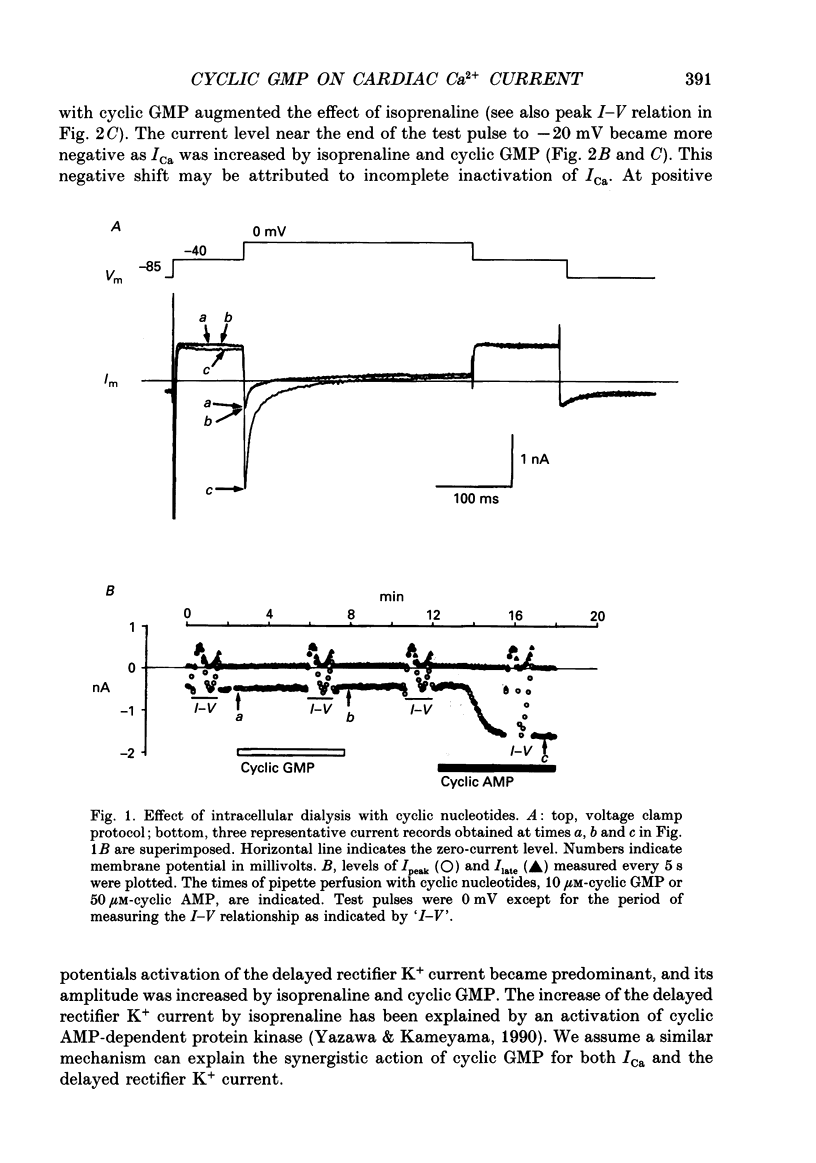
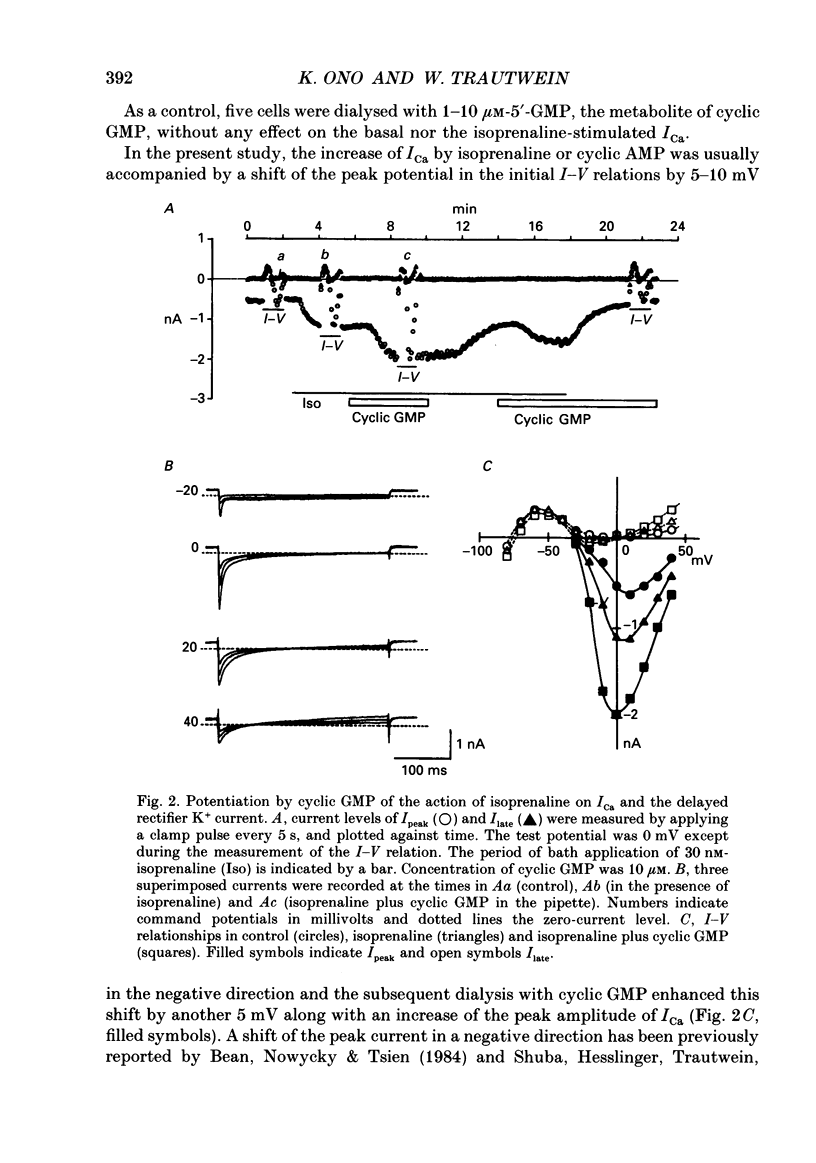
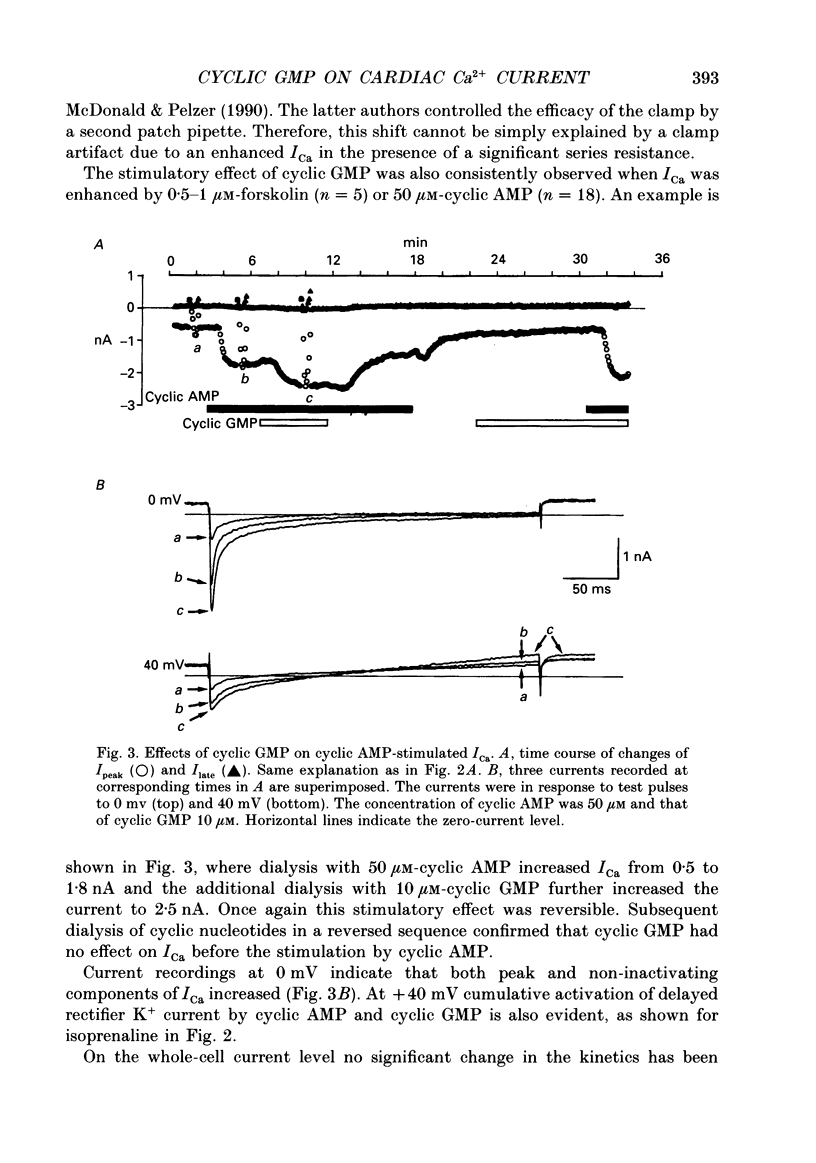
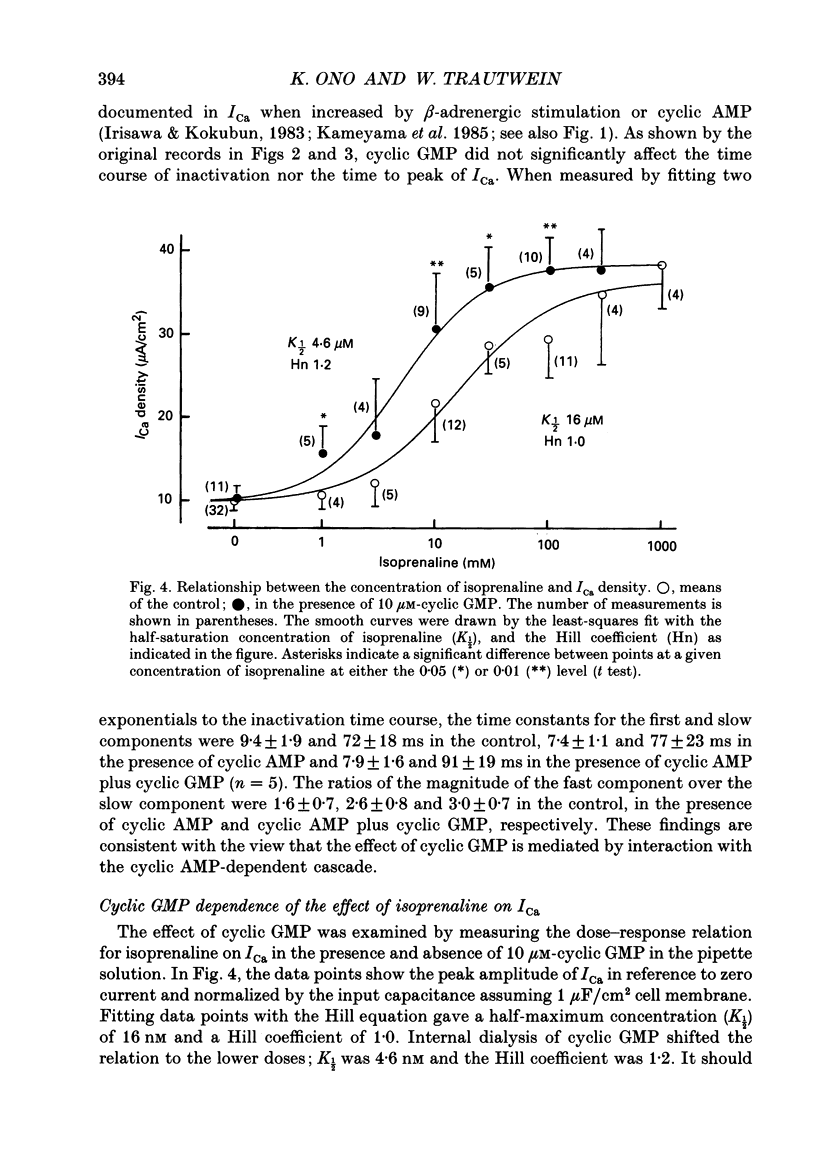
Abstract
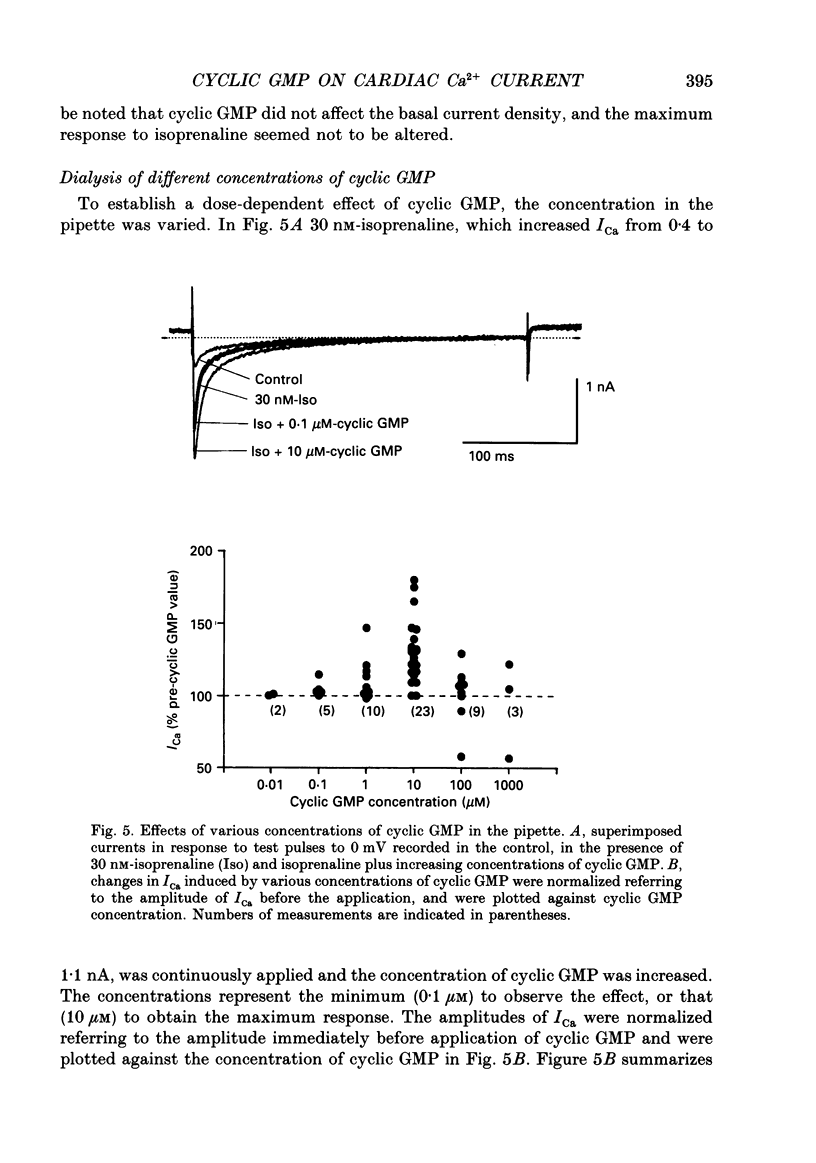
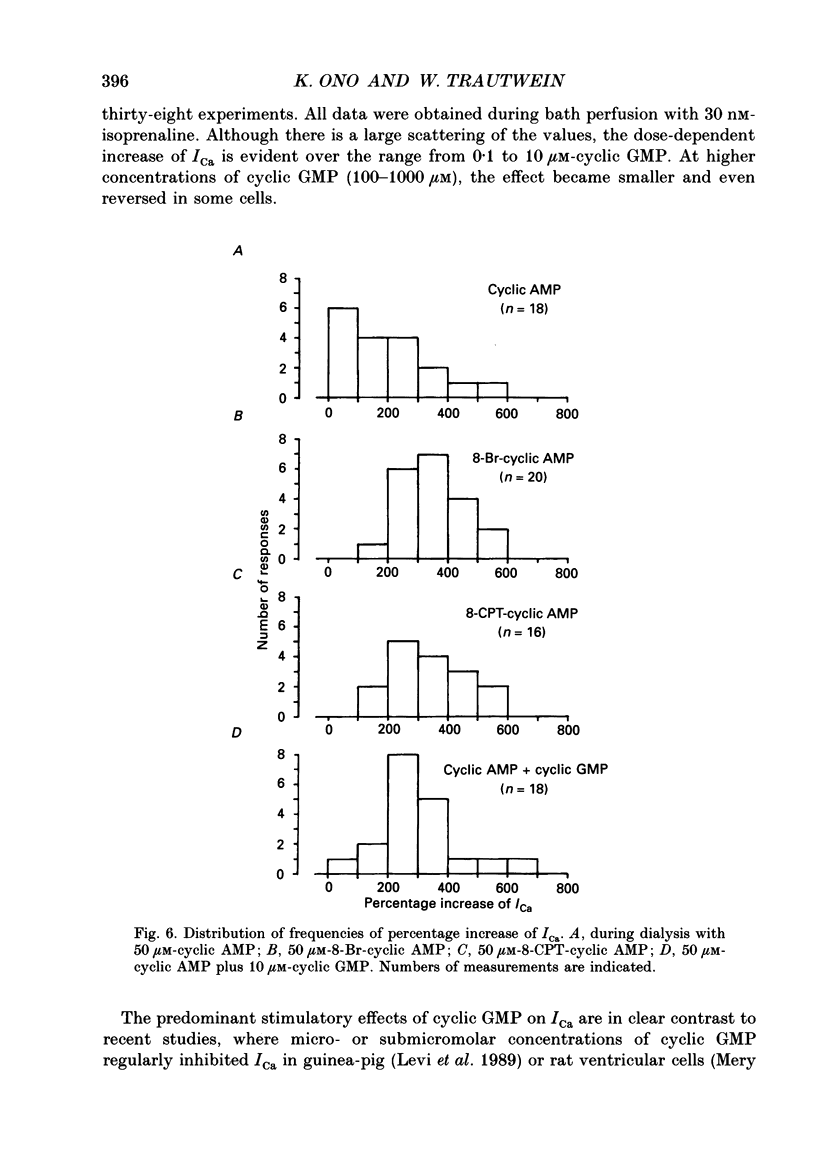
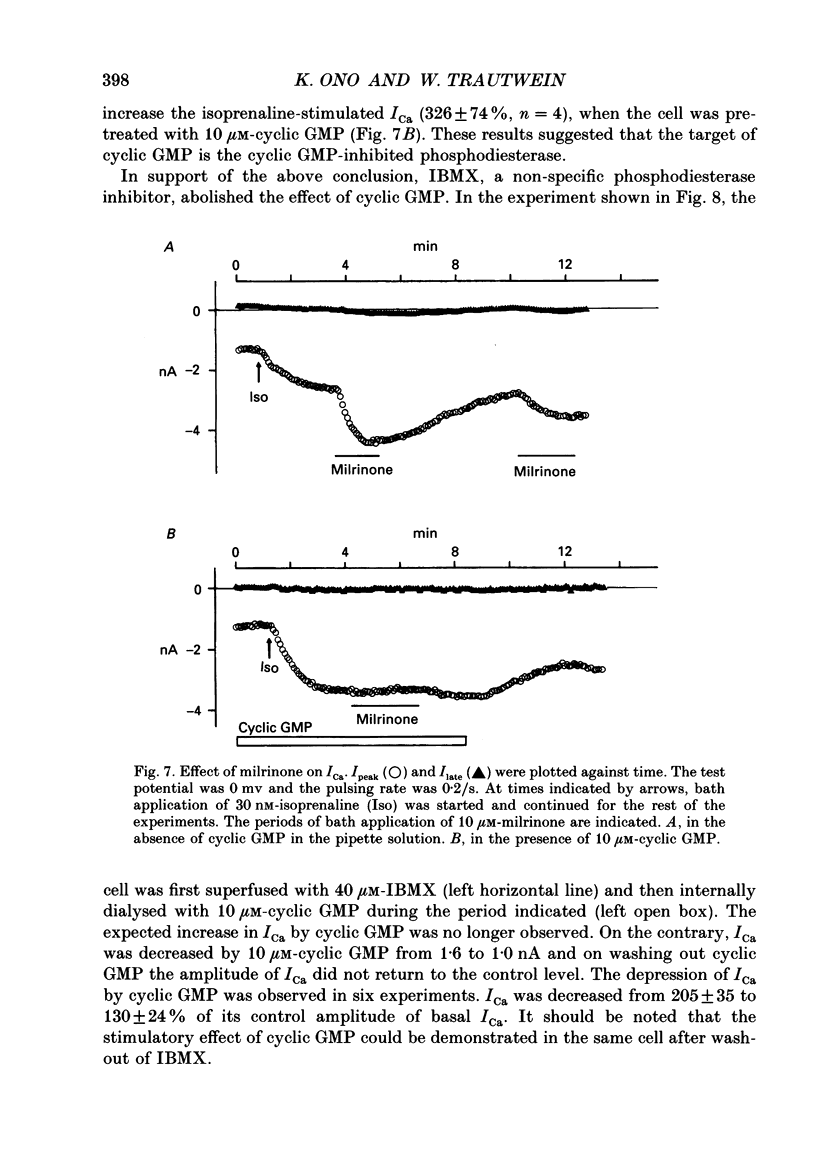
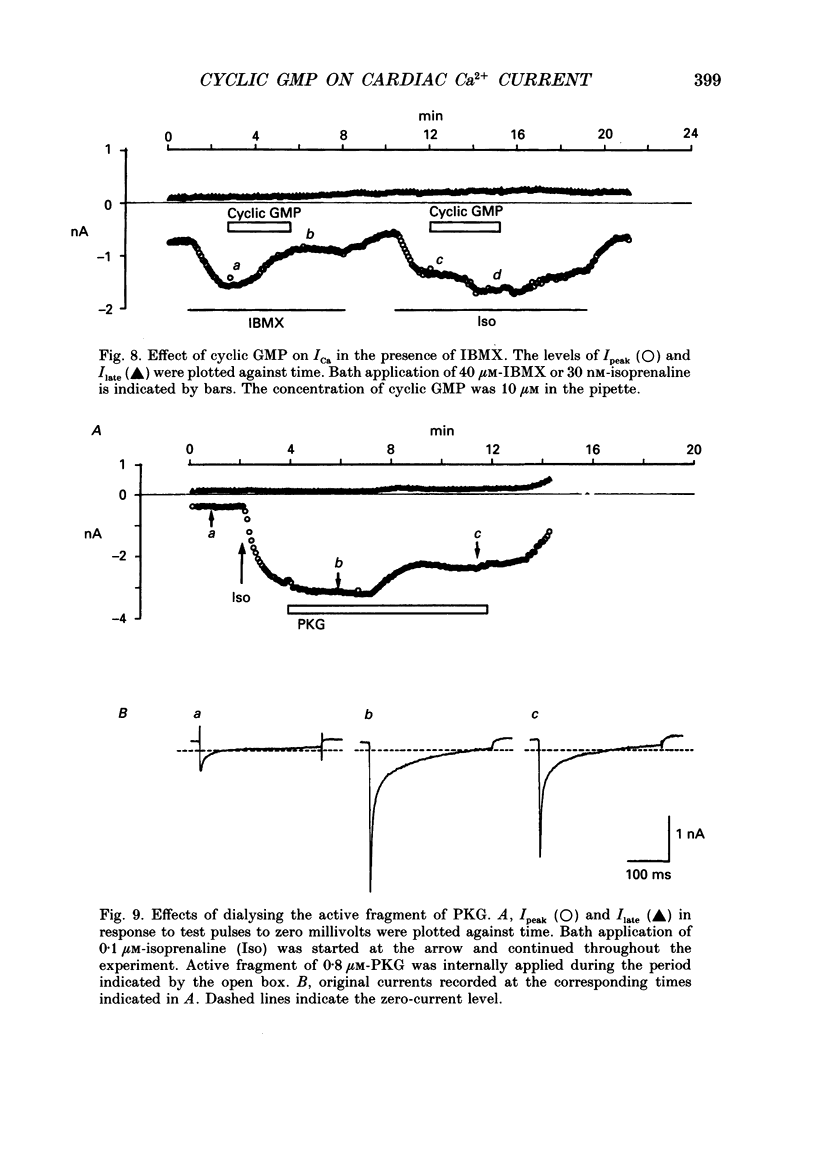
1. Effects of cyclic GMP on L-type Ca2+ current (ICa) were investigated in myocytes isolated from guinea-pig ventricles using the patch clamp method in the whole-cell configuration combined with intracellular perfusion. 2. When ICa was increased by bath application of isoprenaline (0.001-0.1 microM) or forskolin (0.5-1 microM), or by intracellular dialysis with cyclic AMP (50-100 microM), dialysis with 10 microM-cyclic GMP resulted in an additional stimulation of ICa. Without these pre-treatments, cyclic GMP (1-100 microM) had no effect on the basal ICa. 5'-GMP was without effect. 3. The stimulatory effect of cyclic GMP was observed at concentrations higher than 0.1 microM with a maximum at around 10 microM in the pipette. The dose-response relation between isoprenaline and ICa was shifted to the left by (10 microM) cyclic GMP; the half-maximum isoprenaline concentration shifted from 16 to 4.6 nM. 4. The increase of ICa on dialysing 50 microM-cyclic AMP varied from cell to cell, probably due to a difference in phosphodiesterase activity. The cells responding weakly to cyclic AMP showed a greater response to cyclic GMP, and vice versa. In cells dialysed with hydrolysis-resistant derivatives (10-50 microM-8-(4-chlorophenylthio)-cyclic AMP or 50 microM-8-bromo-cyclic AMP), additional dialysis with cyclic GMP failed to modify ICa. Dialysis with cyclic GMP abolished the stimulatory effect of milrinone, a specific inhibitor of cyclic GMP-inhibited phosphodiesterase. These findings suggested that inhibition of cyclic GMP-sensitive phosphodiesterase was responsible for the stimulatory effect of cyclic GMP. 5. In the presence of isoprenaline, direct application of an active fragment of cyclic GMP-dependent protein kinase (PKG) failed to modify ICa in most cells. Activation of native PKG by intracellular dialysis with 8-bromo-cyclic GMP, or higher concentrations of cyclic GMP (100-1000 microM), depressed ICa in about 25% of the cells. Furthermore, dialysis of cyclic GMP reversed the increase of ICa by the non-specific phosphodiesterase inhibitor, 3-isobutyl-1-methyl-xanthine (IBMX). These findings suggested the presence of antagonistic mechanisms of cyclic GMP, which are independent from the above synergistic action. PKG may be involved in this antagonistic effect.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bean B. P., Nowycky M. C., Tsien R. W. Beta-adrenergic modulation of calcium channels in frog ventricular heart cells. 1984 Jan 26-Feb 1Nature. 307(5949):371–375. doi: 10.1038/307371a0. [DOI] [PubMed] [Google Scholar]
- Beavo J. A. Multiple isozymes of cyclic nucleotide phosphodiesterase. Adv Second Messenger Phosphoprotein Res. 1988;22:1–38. [PubMed] [Google Scholar]
- Corbin J. D., Ogreid D., Miller J. P., Suva R. H., Jastorff B., Døskeland S. O. Studies of cGMP analog specificity and function of the two intrasubunit binding sites of cGMP-dependent protein kinase. J Biol Chem. 1986 Jan 25;261(3):1208–1214. [PubMed] [Google Scholar]
- Fesenko E. E., Kolesnikov S. S., Lyubarsky A. L. Induction by cyclic GMP of cationic conductance in plasma membrane of retinal rod outer segment. Nature. 1985 Jan 24;313(6000):310–313. doi: 10.1038/313310a0. [DOI] [PubMed] [Google Scholar]
- Fischmeister R., Hartzell H. C. Cyclic guanosine 3',5'-monophosphate regulates the calcium current in single cells from frog ventricle. J Physiol. 1987 Jun;387:453–472. doi: 10.1113/jphysiol.1987.sp016584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganitkevich VYa, Isenberg G. Contribution of two types of calcium channels to membrane conductance of single myocytes from guinea-pig coronary artery. J Physiol. 1990 Jul;426:19–42. doi: 10.1113/jphysiol.1990.sp018125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Harrison S. A., Reifsnyder D. H., Gallis B., Cadd G. G., Beavo J. A. Isolation and characterization of bovine cardiac muscle cGMP-inhibited phosphodiesterase: a receptor for new cardiotonic drugs. Mol Pharmacol. 1986 May;29(5):506–514. [PubMed] [Google Scholar]
- Hartzell H. C., Fischmeister R. Opposite effects of cyclic GMP and cyclic AMP on Ca2+ current in single heart cells. Nature. 1986 Sep 18;323(6085):273–275. doi: 10.1038/323273a0. [DOI] [PubMed] [Google Scholar]
- Hartzell H. C. Regulation of cardiac ion channels by catecholamines, acetylcholine and second messenger systems. Prog Biophys Mol Biol. 1988;52(3):165–247. doi: 10.1016/0079-6107(88)90014-4. [DOI] [PubMed] [Google Scholar]
- Heil W. G., Landgraf W., Hofmann F. A catalytically active fragment of cGMP-dependent protein kinase. Occupation of its cGMP-binding sites does not affect its phosphotransferase activity. Eur J Biochem. 1987 Oct 1;168(1):117–121. doi: 10.1111/j.1432-1033.1987.tb13395.x. [DOI] [PubMed] [Google Scholar]
- Irisawa H., Kokubun S. Modulation by intracellular ATP and cyclic AMP of the slow inward current in isolated single ventricular cells of the guinea-pig. J Physiol. 1983 May;338:321–337. doi: 10.1113/jphysiol.1983.sp014675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kameyama M., Hescheler J., Hofmann F., Trautwein W. Modulation of Ca current during the phosphorylation cycle in the guinea pig heart. Pflugers Arch. 1986 Aug;407(2):123–128. doi: 10.1007/BF00580662. [DOI] [PubMed] [Google Scholar]
- Kameyama M., Hofmann F., Trautwein W. On the mechanism of beta-adrenergic regulation of the Ca channel in the guinea-pig heart. Pflugers Arch. 1985 Oct;405(3):285–293. doi: 10.1007/BF00582573. [DOI] [PubMed] [Google Scholar]
- Levi R. C., Alloatti G., Fischmeister R. Cyclic GMP regulates the Ca-channel current in guinea pig ventricular myocytes. Pflugers Arch. 1989 Apr;413(6):685–687. doi: 10.1007/BF00581823. [DOI] [PubMed] [Google Scholar]
- Maurice D. H., Haslam R. J. Molecular basis of the synergistic inhibition of platelet function by nitrovasodilators and activators of adenylate cyclase: inhibition of cyclic AMP breakdown by cyclic GMP. Mol Pharmacol. 1990 May;37(5):671–681. [PubMed] [Google Scholar]
- Shuba Y. M., Hesslinger B., Trautwein W., McDonald T. F., Pelzer D. Whole-cell calcium current in guinea-pig ventricular myocytes dialysed with guanine nucleotides. J Physiol. 1990 May;424:205–228. doi: 10.1113/jphysiol.1990.sp018063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soejima M., Noma A. Mode of regulation of the ACh-sensitive K-channel by the muscarinic receptor in rabbit atrial cells. Pflugers Arch. 1984 Apr;400(4):424–431. doi: 10.1007/BF00587544. [DOI] [PubMed] [Google Scholar]
- Sperelakis N. Regulation of calcium slow channels of cardiac muscle by cyclic nucleotides and phosphorylation. J Mol Cell Cardiol. 1988 Mar;20 (Suppl 2):75–105. doi: 10.1016/0022-2828(88)90334-3. [DOI] [PubMed] [Google Scholar]
- Waldman S. A., Murad F. Cyclic GMP synthesis and function. Pharmacol Rev. 1987 Sep;39(3):163–196. [PubMed] [Google Scholar]
- Walter U. Physiological role of cGMP and cGMP-dependent protein kinase in the cardiovascular system. Rev Physiol Biochem Pharmacol. 1989;113:41–88. doi: 10.1007/BFb0032675. [DOI] [PubMed] [Google Scholar]
- Weishaar R. E., Burrows S. D., Kobylarz D. C., Quade M. M., Evans D. B. Multiple molecular forms of cyclic nucleotide phosphodiesterase in cardiac and smooth muscle and in platelets. Isolation, characterization, and effects of various reference phosphodiesterase inhibitors and cardiotonic agents. Biochem Pharmacol. 1986 Mar 1;35(5):787–800. doi: 10.1016/0006-2952(86)90247-9. [DOI] [PubMed] [Google Scholar]
- Weishaar R. E., Kobylarz-Singer D. C., Kaplan H. R. Subclasses of cyclic AMP phosphodiesterase in cardiac muscle. J Mol Cell Cardiol. 1987 Oct;19(10):1025–1036. doi: 10.1016/s0022-2828(87)80574-6. [DOI] [PubMed] [Google Scholar]
- Wetzel B., Hauel N. New cardiotonic agents--a promising approach for treatment of heart failure. Trends Pharmacol Sci. 1988 May;9(5):166–170. doi: 10.1016/0165-6147(88)90031-4. [DOI] [PubMed] [Google Scholar]
- Yazawa K., Kameyama M. Mechanism of receptor-mediated modulation of the delayed outward potassium current in guinea-pig ventricular myocytes. J Physiol. 1990 Feb;421:135–150. doi: 10.1113/jphysiol.1990.sp017937. [DOI] [PMC free article] [PubMed] [Google Scholar]


