Abstract
The interaction of light perception with development is the subject of intensive genetic analysis in the model plant Arabidopsis. We performed genetic screens in low white light—a threshold condition in which photomorphogenetic signaling pathways are only partially active—for ethyl methane sulfonate-generated mutants with altered developmental phenotypes. Recessive mutants with exaggerated developmental responses were obtained in eight complementation groups designated shl for seedlings hyperresponsive to light. shl1, shl2, shl5, and shl3 shl4 (double mutant) seedlings showed limited or no phenotypic effects in darkness, but showed significantly enhanced inhibition of hypocotyl elongation in low-white, red, far-red, blue, and green light across a range of fluences. These results reflect developmental hyper-responsiveness to signals generated by both phytochrome and cryptochrome photoreceptors. The shl11 mutant retained significant phenotypic effects on hypocotyl length in both the phyA mutant and phyB mutant backgrounds but may be dependent on CRY1 for phenotypic expression in blue light. The shl2 phenotype was partially dependent on PHYB, PHYA, and CRY1 in red, far-red, and blue light, respectively. shl2 and, in particular, shl1 were partially dependent on HY5 activity for their light-hyperresponsive phenotypes. The SHL genes act (genetically) as light-dependent negative regulators of photomorphogenesis, possibly in a downstream signaling or developmental pathway that is shared by CRY1, PHYA, and PHYB and other photoreceptors (CRY2, PHYC, PHYD, and PHYE).
Light is a critical environmental signal that effects nearly every aspect of plant development, including seed germination, seedling morphogenesis, and floral initiation. A complex network of photoreceptors and signaling pathways have evolved to regulate developmental responses to light quantity, quality, and duration. The photoreceptors include the red (R)- and far-red (FR)-responsive phytochromes and several blue (B) and UV receptors, including the cryptocromes. Light perception has been the subject of intensive genetic analysis, primarily in Arabidopsis (Deng and Quail, 1999; Neff et al., 2000), and has become a model for interactions of environment with development (Smith, 2000, and references therein). Phytochrome apoproteins are encoded by five genes in Arabidopsis (PHYA–PHYE), and cryptochromes are encoded by two genes, CRY1 and CRY2. Pioneering genetic screens identified the long hypocotyl (hy) mutants in white light (Koorneef et al., 1980), which were defective in PHYB, CRY1, and HY5, a transcriptional regulator, as well as several gene products involved in the biosynthesis of the phytochrome chromophore (Ahmad and Cashmore, 1993; Reed et al., 1993; Somers et al., 1993; Oyama et al., 1997). Screens for mutants with a light-grown or “de-etiolated” phenotype in darkness (Chory et al., 1989; Deng et al., 1991) have identified several nuclear genes that act as negative regulators of photomorphogenesis (Deng et al., 1992; Pepper et al., 1994; Wei et al., 1994a). “Second generation” genetic screens included specific, physiology-based strategies, such as the search for phyA mutants in FR light (Dehesh et al., 1993; Nagatani et al., 1993; Parks and Quail, 1993; Whitelam et al., 1993), as well as screens for extragenic suppressors of “first generation” mutants such as hy2 (Kim et al., 1996), det1 (Pepper and Chory, 1997), phyB (Reed et al., 1998), and phyA (Hoecker et al., 1998). Other screens made use of floral initiation rather than seedling morphology in primary mutant screens (Ahmad and Cashmore, 1996). Some recent screens have exploited a light-inducible CAB2-LUC promoter-reporter transgene (Genoud et al., 1998) or have identified extragenic suppressors of a PHYB overexpressing transgene (Huq et al., 2000). Finally, important photoregulatory genes have also recently been identified by protein-protein interactions (Ni et al., 1998; Choi et al., 1999; Fankhauser et al., 1999).
In the present study, we screened for mutants with phenotypic effects in low light—a threshold condition in which the normal photoperception pathways are only partially active, leading to limited de-etiolation responses in wild-type (WT) seedlings. Using screens performed in low light, we obtained two classes of mutants: 1) those which had completely etiolated phenotypes, and 2) those which had completely de-etiolated phenotypes. Whereas some of the mutations in the former class mapped to known genetic loci (PHYB, CRY1), others appeared to be novel genetic loci (characterization of these will be presented elsewhere). Here, we present our initial analysis of several mutants with exaggerated developmental responses to available light.
RESULTS
Identification of shl Mutants
To identify novel regulatory components at the interface of light signaling and development, we screened M2 seed pools from ±28,000 individual ethyl methane sulfonate mutagenized M1 plants. Aliquots from 16,420 seed pools were divided and screened simultaneously in low-intensity white light (4 μmol m−2 s−1) and in darkness. An additional 9,540 seed pools were screened in darkness and under a yellow-green filter (24 μmol m−2 s−1) that depleted much of the photomorphogenetically active B, R, and FR regions of the spectrum (the yellow-green filter was technically advantageous in that WT seedlings showed less phenotypic variance than that observed in low white light). Under each of these conditions, WT seedlings displayed a long hypocotyl and unfolded but poorly developed cotyledons. We identified 380 M2 families that segregated multiple individuals with short hypocotyls and expanded cotyledons in low light. In darkness, 202 of the 380 M2 families segregated individuals with de-etiolated phenotypes, and an additional 99 families segregated individuals with severe developmental abnormalities (e.g. no root, fused cotyledons, and fasciated). The remaining 79 M2 families had normal etiolated phenotypes in darkness. In the M3 generation, 15 of these families (±19%) exhibited heritable light-hyperresponsive phenotypes. The candidate mutants obtained from these families were designated shl for seedlings hyperresponsive to light.
Genetic Characterization of shl Mutants
All 15 shl mutants were recessive in back-crosses to WT Columbia ecotype (Col-0). Mutant lines were assigned to complementation groups by F1 complementation analysis. Three complementation groups, designated shl1, shl2, and shl5 contained multiple alleles (with five, four, and two alleles, respectively). Various alleles of shl1 and shl2 were obtained from both the yellow-green light and the low-intensity white light conditions, indicating that the two light regimes were effectively similar. The remaining four mutant lines fell into mono-allelic complementation groups, indicating that our screens were far from exhaustive or “saturating.”
Phenotypic analysis of the F2 progeny from back-crosses to Columbia (Col-0 or Col-0 seeds carrying the glabrous mutation [Col-gl1]) indicated that in 14 of 15 mutant lines, the light-hypersensitive trait was conditioned by a single gene (a subset of these data is presented in Table I). In the remaining line, mutant progeny were observed segregating in a ratio near 1:15 (P > 0.70), suggesting that the mutant phenotype in this line was due to recessive alleles at two unlinked loci. F3 seeds were obtained by selfing of 20 of these F2 progeny. Ten of the F3 families segregated shl mutant individuals. This result closely fits (P > 0.4) the expectation for an F2 population segregating two unlinked recessive loci, in which 7/16 of the individuals with WT phenotypes would be expected to carry at least one mutant allele at both loci. Furthermore, mutant to WT ratios near 1:15 were consistently obtained in subsequent back-crosses to Col-0 and in out-crosses to Landsberg erecta. The putative double-mutant line complemented all other lines, and the loci were tentatively designated shl3 and shl4. Neither shl3 nor shl4 had an obvious morphological phenotype in the single-mutant homozygous state, although one of these loci had a subtle quantitative effect on hypocotyl length in high-irradiance FR light.
Table I.
Segregation analysis of shl mutants
| Cross | WT | shl− | Ratio | n.h. | χ2 | P |
|---|---|---|---|---|---|---|
| shl1-1 × WT Col-0 | 624 | 200 | 3.12:1 | 3:1 | 0.235 | >0.70 |
| shl2-2 × WT Col-0 | 471 | 163 | 2.89:1 | 3:1 | 0.161 | >0.70 |
| shl3 shl4 × WT Col-0 | 521 | 32 | 16.28:1 | 3:1 | 108.9 | <0.01 (r) |
| 15:1 | 0.211 | >0.70 | ||||
| shl5-1 × WT Col-0 | 425 | 123 | 3.46:1 | 3:1 | 1.9 | >0.15 |
Mutants were back-crossed to WT Col-0 ecotype, and F2 progeny were scored in low light for WT or light hyperresponsive (shl−) phenotypes. Chi-squared (χ2) analysis was applied using the null hypotheses (n.h.). Hypotheses indicated by (r) were rejected.
After two back-crosses to Col-0, representative alleles of the shl1, shl2, shl5 complementation groups, as well as the putative shl3 shl4 double mutant, were out-crossed to Landsberg erecta to create F2 mapping populations. Molecular genotyping of 94 mutant F2 individuals using PCR-based markers localized shl1 to the top of chromosome 1, showing complete cosegregation with single sequence length polymorphism (SSLP) marker nga59. A mapping population of 94 mutant F2 individuals was used to map shl2 to a location on chromosome 2, ±7.0 cM telomeric to PHYB. Genetic mapping of shl3 and shl4 were limited by the relatively small number of mutant individuals in the F2 generation. However, we found convincing linkage of one of these loci to chromosome 1, between SSLP marker nga63 (11.48 cM) and cleaved amplified polymorphic sequence (CAPS) marker CAT3 (29.91 cM). A smaller mapping population (38 mutant individuals) was used to locate shl5 to chromosome 5, in close proximity to SSLP marker nga225 (±1.3 cM).
shl Mutant Phenotypes
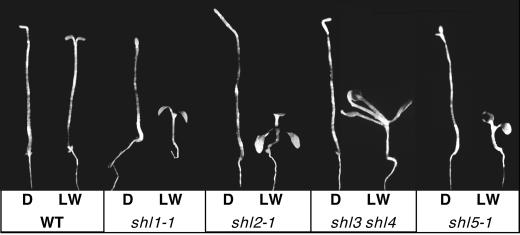
After 7 d in low white light, shl1, shl2, shl5, and the shl3 shl4 double mutant had comparatively short hypocotyls and expanded cotyledons relative to WT (Fig. 1). Precocious development of the first set of true leaves was readily apparent in shl2 and shl3 shl4 and was also evident in shl1 and shl5—particularly after 8 to 9 d in low light. All four mutant lines had a normal etiolated morphology in darkness (Fig. 1). A minority of shl5-1 seedlings had partially open, but not expanded, cotyledons (as shown). The frequency of such seedlings was not reproducible from experiment to experiment.
Figure 1.
Morphologies of WT and shl mutant seedlings. Seedlings were grown for 7 d on Murashige and Skoog/phytagar/2% (w/v) Suc media in darkness (D) or in low white light (LW) at a fluence of 4 μmol m−2 s−1.
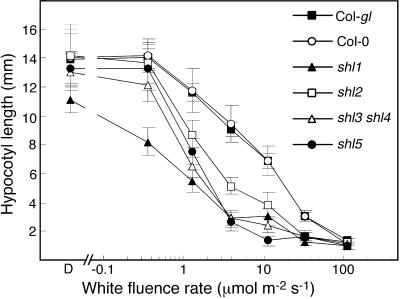
Hypocotyl length was used as simple quantitative measure of seedling developmental sensitivity to light (Fig. 2). In darkness, the strongest allele of shl1 had a slightly shorter hypocotyl than WT. The shl3 shl4 double mutant, and the strongest alleles of the shl2 and shl5 complementation groups had dark-grown hypocotyl lengths that were indistinguishable from WT. However, each of the mutants showed enhanced sensitivity to white light over a wide range of white light fluence conditions. For example, shl1-1 showed 26% inhibition of hypocotyl growth at 0.37 μmol m−2 s−1—a condition that had no effect on WT hypocotyl length. All of the shl mutants showed significantly enhanced inhibition of hypocotyl growth in the range of 1 to 30 μmol m−2 s−1. At an intensity of 110 μmol m−2 s−1, growth of WT and shl mutant hypocotyls was similarly inhibited.
Figure 2.
Hypocotyl length responses to white light of varying intensity in WT and shl mutant seedlings. Hypocotyls were measured in seedlings grown for 6 d. Error bars = sd.
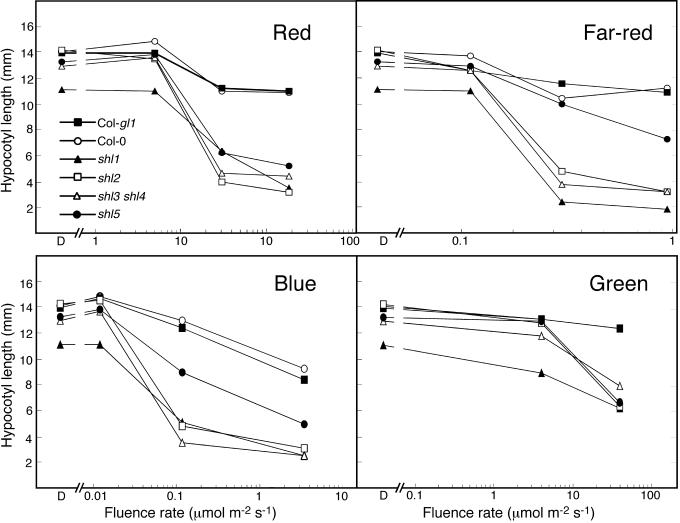
To determine the spectral dependence of expression of the shl phenotypes, shl1-1, shl2-1, shl3 shl4, and shl5-1 were examined in narrow-spectrum R, FR, B, and green (G) light (Fig. 3). Each mutant displayed enhanced responsiveness to light of each of these spectral conditions. The shl5 mutant showed comparatively less responsiveness to FR and (to a lesser extent) B than the other shl mutants, which showed similar patterns of responsiveness in the light conditions tested. Additional alleles of shl1 (shl1-2) and shl2 (shl2-2, shl2-3, shl2-4) showed qualitatively similar responses to those of the reference alleles shown in Figure 3. In all cases, the shorter hypocotyl length of the shl mutant was accompanied by increased expansion of the cotyledons relative to the WT controls.
Figure 3.
Hypocotyl length responses to various spectral conditions in WT and shl mutant seedlings. Hypocotyls were measured in seedlings grown for 6 d in R, FR, B, and G narrow-spectrum light sources at the range of fluences indicated. D, Dark condition.
Phenotypes of shl mutants were also examined in mature plants. All of the shl mutants displayed shorter petioles and a more compact rosette than WT. Plants carrying the most severe mutant allele of shl1 showed a dramatic reduction in fertility and a moderate decrease in apical dominance (Table II). Whereas the shl2-1 mutation and the shl3 shl4 double mutation resulted in modest increases (±2-fold) in the accumulation of anthocyanin, the shl5 mutation resulted in more dramatic increases (±10-fold). Finally, severe shl2 alleles showed a moderate late-flowering phenotype.
Table II.
Phenotypic analysis of shl mutant plants
| Genotype | Anthocyanin | Inflorescence Axes | Leaf No. |
|---|---|---|---|
| Col-0 | 1.20 ± 0.47 | 1.0 ± 0.00 | 8.38 ± 0.52 |
| Col-gl1 | 1.18 ± 0.34 | 1.25 ± 0.46 | 8.50 ± 0.76 |
| shl1-1 | 1.06 ± 0.31 | 2.86 ± 1.67 | 10.86 ± 2.27 |
| shl2-1 | 2.46 ± 0.34 | 1.42 ± 0.49 | 14.10 ± 1.55 |
| shl3 shl4 | 3.06 ± 0.45 | 1.34 ± 0.66 | 9.49 ± 0.96 |
| shl5-1 | 13.67 ± 3.29 | 1.0 ± 0.00 | 8.42 ± 0.53 |
Plants were examined at the flowering stage, after ±35 d growth in long-day (16-h) conditions. A minimum of eight plants was examined for each determination. Anthocyanin content was measured as a ratio of (A530 − A657)/g fresh wt. The number of elongated inflorescence axes was used as an indicator of apical dominance. Total leaf number was used as a measure of flowering time.
Genetic Interactions with Photoreceptors PHYA, PHYB, and CRY1
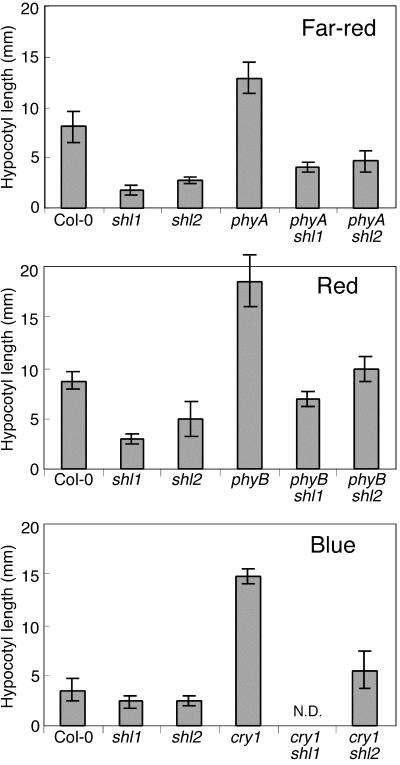
The photoreceptors PHYA, PHYB, and CRY1 play predominant—but not exclusive—roles in seedling photomorphogenetic responses to FR, R, and B, respectively (Whitelam et al., 1993; Reed et al., 1994; Ahmad and Cashmore, 1997; Neff and Chory, 1998; Casal and Mazzella, 1998). To test for functional dependence of the shl phenotypes on each of these photoreceptors, shl1-1 and shl2-2 were placed in double-mutant combinations with phyA-211, phyB-9, and cry1-B36 (in the Col-0 genetic background). Hypocotyl phenotypes of shl phyA, shl phyB, and shl cry1 double mutants were determined in FR, R, and B, respectively.
As shown in Figure 4, the shl1 mutant retained significant phenotypic effects on hypocotyl length in both the phyA mutant and phyB mutant backgrounds. In the cross of shl1 to cry1, five homozygous cry1 mutant individuals were identified in the F2 generation by PCR; all had a long hypocotyl phenotype in B, similar to the cry1 control. However, in the F3 progeny from these five F2 individuals, no novel phenotypes were observed. Thus, we could not definitively identify a phenotype for the shl1 cry1 double mutant. Given that shl1 is not linked to cry1, we would have expected that two-thirds of the five F2 individuals would have been heterozygous for shl1. The probability that at least one of the five F2 individuals was heterozygous for shl1 is approximately 99.6%. Thus, there is a strong possibility that the phenotype of shl1 in B light is strictly dependent on CRY1 activity. This hypothesis is supported by the fact that there were no homozygous cry1 individuals with a hypocotyl phenotype that was shorter that the cry1 mutant control. However, it remains remotely possible that shl1 does indeed exert an effect in the cry1 mutant background and that none of the homozygous cry1 F2 individuals were heterozygous or homozygous for shl1.
Figure 4.
Genetic interactions between shl-1 and shl2-2, and the photoreceptor mutants phyA-211, phyB-9, and cry1-B36. Hypocotyls were measured in seedlings grown for 7 d in R (64.4 μmol m−2 s−1), FR (7.8 μmol m−2 s−1), and B (2.78 μmol m−2 s−1). These intensities were selected to provide effective phenotypic discrimination between WT and photoreceptor mutants. N.D., Not determined. Error bars = sd.
The light-hyperresponsive phenotype of the shl2 mutant was only partially dependent on PHYB, PHYA, and CRY1 in R, FR, and B, respectively (Fig. 4). For example, although the shl2 phyB had a slightly longer hypocotyl than WT, it was still significantly inhibited compared to the phyB single mutant.
Genetic Interactions with HY5
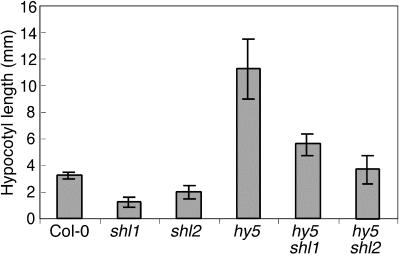
HY5 is a basic-Leu zipper transcription factor that positively regulates seedling de-etiolation and in the process actively promotes the inhibition of hypocotyl elongation (Koorneef et al., 1980; Oyama et al., 1997). The hypocotyl phenotypes of shl1 hy5 and shl2 hy5 double mutants were examined in moderate white light (Fig. 5). The phenotypes of the double mutants were additive, with both shl mutants showing a partial dependence on HY5 activity for expression of their light-hyperresponsive phenotypes. Interestingly, shl1, which as a single mutant showed the greater inhibition of hypocotyl length in this light condition, also showed the greater degree of dependence on HY5 activity.
Figure 5.
Genetic interactions between shl-1 and shl2-2 and the hy5-5C mutant. Hypocotyls were measured in seedlings grown for 7 d in white light at an intensity of 45 μmol m−2 s−1. Error bars = sd.
DISCUSSION
To identify mutants in genes acting at the interface of light perception and developmental pathways—“downstream” from the photoreceptors and photoreceptor-specific signaling elements—we employed broad-spectrum white light to cast a “wide net” for mutants that were light hyposensitive or hypersensitive to a wide range of spectral conditions. At the onset, mutant seed pools were “counter-screened” in darkness to eliminate mutants in the det/cop/fus class and those with severe pleiotropic developmental defects. In pilot experiments, we found that under low-light conditions, even unmutagenized WT seed stocks gave rise to abnormal seedlings with a relatively short hypocotyl and well-developed cotyledons at a low, but potentially problematic, frequency. This frequency appeared to increase with the age of the seeds, and with the length of time that the seeds are stored in an imbibed state. We concluded that a typical en masse screen of M2 seedlings for mutants with exaggerated de-etiolation responses would yield an overwhelming number of seedlings with phenotypes that were not due to heritable mutation. To avoid this source of false mutants, we screened M2 families derived from single M1 plants and identified pools that segregated multiple individuals with light-hyperresponsive phenotypes. By this strategy, we isolated recessive light-hyperresponsive mutants in eight genetic loci.
On the basis of their recessive nature, we expect that the SHL genes act as negative regulators of photomorphogenesis. However, they are functionally distinct from mutants in det/cop/fus class in that they give rise to phenotypes that are hyperresponsive to available light, rather than light independent.
There is a formal possibility that shl mutants are extremely weak alleles of mutants in det/cop/fus class that express overt phenotypes only in the light. However, the overwhelming majority of mutants in the det/cop/fus class have been mapped (Chory et al., 1989; Chory et al., 1991; Deng et al., 1991; Wei and Deng, 1992; Miserá et al., 1994; Wei et al., 1994b; Franzmann et al., 1995). shl1 and shl5 do not appear to be closely linked to any of these mapped loci. shl2 mapped to within 10 cM of the published map position of fus12 (also known as cop12) on chromosome 2 (Miserá et al., 1994), but a complementation test demonstrated that shl2 is not an allele of fus12. Thus shl1, shl2, and shl5 do not appear to be new alleles of mapped det, cop, or fus loci.
The shl1, shl2, shl5 mutants and the postulated shl3 shl4 double mutant are phenotypically distinct from other recently identified light-hypersensitive mutants. The spa1 (Hoecker et al., 1998) and eid1 (Buche et al., 2000) mutants appear to be FR-specific in their phenotypic expression. psi2 (Genoud et al., 1998) displays hypersensitivity to both R and FR light, but is dependent on PHYB and PHYA, respectively, for these effects and did not show a significant phenotype when tested in a range of B light intensities. Mutations in SUB1, a Ca2+ binding protein, show enhanced responsiveness to B and FR, but not to R (Guo et al., 2001). Finally, shy1 (Kim et al., 1996) and srl1 (Huq et al., 2000) have R-light-dependent phenotypes. srl1 was located on chromosome 2 near the mapped location of shl2, but its phenotypic expression is strictly dependent on PHYB. In contrast, shl2 was not strictly dependent on PHYB even for its R-light hypersensitivity. Furthermore, all four alleles of shl2 showed clear hyperresponsive phenotypes in R, FR, B, and G light. Finally, although one of the shl3 or shl4 loci had a subtle hyperresponsive phenotype in FR (as a single mutant), we did not detect any linkage of either loci to nga168, which is linked to SPA1 on chromosome 2 (Hoecker et al., 1998), or to nga8, which is linked to EID1 on chromosome 4 (Buche et al., 2000).
shl1, shl2, the shl3 shl4 double mutant, and (to a lesser extent) shl5 exhibit hyperresponsive phenotypes in FR, R, B, and G. One interpretation of this finding is that the SHL genes are acting in a downstream signaling pathway that is shared by CRY1, PHYA, and PHYB and possibly other photoreceptors (CRY2, PHYC, PHYD, PHYE). This downstream placement of the SHL genes would place them at or near the interface where light signal transduction elements are interacting with developmental regulators. The phenotypes of the shl mutants may be due to mutations in signaling molecules or other regulators that result in an increase in the sensitivity of a particular signaling process or amplify the developmental responses. In this respect, SHL3 and SHL4 appear to have at least partially overlapping functions. Several of the mutants also had light-related phenotypes as adult plants, displaying short petioles, elevated anthocyanin (shl2, shl3 shl4, and shl5), and in the case of shl2, a moderate late-flowering phenotype similar to that seen in plants overexpressing CRY1 (Lin et al., 1996).
The photoreceptors PHYA, PHYB, and CRY1 play the dominant roles in seedling photomorphogenetic responses to FR, R, and B, respectively (Reed et al., 1994; Ahmad and Cashmore, 1997; Casal and Mazzella, 1998; Neff and Chory, 1998). It is interesting to note that the FR, R, and B phenotypes of shl1 and shl2 were only partially dependent on PHYA, PHYB, and CRY1, respectively. However, the roles played by these major photoreceptors are not exclusive. For example, both Pr and Pfr absorb in the B region of the spectrum (Smith, 1986). PHYA plays a subsidiary role in B inhibition of hypocotyl elongation (Whitelam et al., 1993; Ahmad and Cashmore, 1997; Casal and Mazzella, 1998; Neff and Chory, 1998). CRY2 plays a significant role in B-dependent inhibition of hypocotyl elongation at low fluence levels (<10 μmol m−2 s−1), similar to those used in our phenotypic analyses. Finally, PHYB plays a minor role in FR-stimulated opening of the apical hook (Neff and Chory, 1998), and other phytochromes (PHYC, PHYD, and PHYE) are either known to, or presumed to, play subsidiary roles in various photomorphogenetic responses to R and FR (Aukerman et al., 1997; Poppe and Schäfer, 1997; Devlin et al., 1999). Thus, the phenotypes of the shl mutants in R, FR, and B may be dependent on signals generated by a larger set of photoreceptors with partially overlapping, and often synergistic, activities that may include PHYA through PHYE and both CRY1 and CRY2.
Although PHYA and PHYB are required for full activity of CRY1 (Ahmad and Cashmore, 1997; Casal and Mazzella, 1998; Neff and Chory, 1998), CRY1 can also act independently of PHYA and PHYB (Casal and Mazzella, 1998; Neff and Chory, 1998). All of the shl mutants showed substantial hyperresponsiveness to G light. Both Pr and Pfr have absorption minima in the green region of the spectrum (Smith, 1986), and hypersensitivity to G light has previously only been observed in transgenic plants overexpressing CRY1 (Lin et al., 1996). This result strongly indicates that the shl mutations affect pathways that are downstream from cryptochrome(s), as well as the phytochromes. In this respect, it is interesting to note the possible dependence of shl1 on CRY1 for expression of its B-hyperresponsive phenotype. This finding would suggest a direct interaction between SHL1 and CRY1 in B signaling.
Unlike sub1, which is entirely dependent on the activity of HY5 for the expression of its B and FR hyper-responsive phenotype (Guo et al., 2001), both the shl1 and shl2 mutant phenotypes were only partially independent of HY5. Since we do not know for certain that shl1-1 and shl2-2 are null alleles, all we can conclude is that signals generated by these mutations do act through HY5, but also act through alternate pathways. shl1-1 was more dependent on HY5 for its phenotypic effect than was shl2-2, suggesting that a significant portion of the photomorphogenetic signaling generated in the shl1 mutant exerts its effect through HY5 and that SHL1 may act in a pathway that is upstream from HY5 and other regulators.
The shl mutants may also be defective in elements of phytohormone signaling and perception pathways—a finding that is not mutually exclusive with their involvement in light perception. Cytokinin, giberellins, and brassinolides have all been implicated in the regulation of seedling development by light (Chory et al., 1994; Chin-Atkins et al., 1996; Chory and Li, 1997). Recently, several lines of evidence have directed attention towards the interplay of light and auxin signaling. shy2, an extragenic suppressor of phyB and hy2, is mutated in an auxin regulatory gene IAA3 (Tian and Reed, 1999; Soh et al., 1999). Expression of FIN219, a phytochrome A signaling molecule, is also regulated by auxin (Hsieh et al., 2000). It is of particular interest to note that napthylphthalamic acid, an inhibitor of auxin transport, is also a potent inhibitor of hypocotyl elongation in light-grown, but not dark-grown, seedlings (Jenson et al., 1998). This effect was stimulated by R, FR, and B, and was fluence dependent. This finding suggests that shl phenotypes could be generated through mutations that perturb auxin transport or signaling.
shl1 in particular displays several of the phenotypic hallmarks of a phytohormone signaling defect (Chory and Li, 1997; Leyser, 1998), including reduced fertility, reduced leaf elongation, and reduced apical dominance. Although shl1 maps to the top of chromosome 1, where AXR1 and AXR3 are located, it complements a recessive allele of axr1, and was separated from the location of the cloned AXR1 gene (Leyser et al., 1993) and the mapped position of axr3 by multiple recombination breakpoints. It is interesting to note that shl1 did not show elevated levels of anthocyanin, indicating that only a subset of light-dependent responses are effected. Thus, SHL1 may play a role in the regulation of only a subset of developmental responses affecting growth and morphology.
MATERIALS AND METHODS
Plant Materials, Growth Conditions, and Mutant Isolation
Arabidopsis ecotype Col-gl1 seeds were obtained from Lehle Seeds (Round Rock, TX). Null mutants phyA-211, phyB-9, and cry1-B36 (in the Col-0 ecotypic background) were obtained from Jason Reed (Reed et al., 1994). The hy5-5C null allele was isolated in the Col-0 background as a suppressor of det1-1 (Pepper and Chory, 1997). This hy5 allele was back-crossed twice to WT Col-0 and was homozygous for the WT DET1 allele. Col-gl1 seeds were mutagenized by imbibation in 0.3% (v/v) ethyl methane sulfonate for 12 h, followed by extensive washing with H20. M1 seeds were sown on soil to achieve a final density of 0.25 plants cm−2, grown under an 8-h day-length regime for 40 d, then transferred to a 16-h day length in order to stimulate flowering. This protocol produced mature plants with a stout, erect inflorescence, thus preventing entanglement and greatly facilitating the harvest of independent M2 seed pools from individual M1 plants. Aliquots of ±80 seeds from each M2 seed pool were surface sterilized (Chory et al., 1989), resuspended in sterile 0.1% (w/v) phytagar, then cold treated at 4°C for 40 h. Seed pools were then dispersed onto duplicate plates containing Murashige and Skoog/phytagar/2% (w/v) Suc media. Seeds were illuminated for 4 h with white light (100 μmol m−2 s−1) to ensure optimal germination, then screened simultaneously in darkness and in either low-intensity white light (4 μmol m−2 s−1) or under a yellow-green acrylic filter (24 μmol m−2 s−1). Mutants were identified after 7 to 8 d. Unless stated otherwise, experiments were performed at 23°C ± 0.5°C under a 16-h day-length regime.
Genetic Analysis
The genetic methods employed have been described previously (Chory et al., 1989; Pepper and Chory, 1997). Routine phenotyping for complementation, segregation, and mapping experiments was performed under low white light or under a yellow-green acrylic filter. Genomic DNAs were isolated using the micropreparation method described by Pepper and Chory (1997). Mapping of shl mutants was performed using PCR-based CAPS (Konieczny and Ausubel, 1993) and SSLP (Bell and Ecker, 1994; Lukowitz et al., 2000) markers. Mutants were back-crossed to WT Col-0 or Col-gl1 at least three times prior to comprehensive phenotypic analysis.
Our strategy for the identification of shl phyA, shl phyB, shl cry1, and shl hy5 double mutants was partially dependent on an assumption that the shl mutations acted in a fully recessive manner. Alleles shl1–1 and shl2–2 were crossed with phyA-211, phyB-9, cry1-B36, and hy5-5C. We phenotyped the F2 generation under conditions that gave excellent discrimination between the WT and phyA (7.8 μmol m−2 s−1 FR), phyB (64.4 μmol m−2 s−1 R), cry1 (2.78 μmol m−2 s−1 B), and hy5 (45 μmol m−2 s−1 white light) and identified individuals with phenotypes that were similar to phyA-211, phyB-9, cry1-B36, and hy5-5C controls. These F2 individuals, assumed to be homozygous for their respective photoperception-deficient alleles, were then examined in the F3 generation for the appearance of distinct short hypocotyl progeny at a frequency consistent with the segregation of the recessive shl mutant (1, short; 3, long). In the absence of such progeny, the phenotype of the double mutant could not be conclusively determined. F2 and F3 individuals homozygous for the cry1-B36 mutant allele were identified by a PCR-based assay: oligonucleotide primers CRY1-F2 (5′-GATCAAACAGGTCGCGTGG-3′) and CRY1-R2 (5′-TTTCATGCCACTTGGTTAGACC-3′) failed to produce an amplification product in the homozygous cry1-B36 mutant.
Analytical Methods
Occasional seedlings with obvious severe developmental defects were omitted from any phenotypic analyses. For measurements of hypocotyl length, 30 seeds of each genotype were evenly dispersed onto Murashige and Skoog/phytager/2% (w/v) Suc media in a 7-mm grid pattern. All seeds were subjected to 4 h of white light (100 μmol m−2 s−1) prior to placement in the dark or in various light regimes for 6 d. Hypocotyls were straightened using forceps if necessary, then measured under a stereo dissecting microscope using a 0.5-mm ruler. Hypocotyls of seedlings growing appressed to the agar media were not measured. Analyses of anthocyanin content (by an acid-methanol extraction), flowering time, and apical dominance were performed as described in Pepper and Chory (1997).
Light Sources
Narrow-spectrum R and FR light were supplied by light-emitting diode arrays (models SL515-670 [670-nm maximum] and SL515-735 [735-nm maximum], respectively; Quantum Devices, Inc., Barneveld, WI). Narrow-spectrum B light (420-nm maximum) was supplied by Coralife Actinic 03 fluorescent aquarium bulbs (Energy Savers Unlimited, Inc., Carson, CA) filtered through a Kopp 5-57 blue glass filter (Kopp Glass, Inc., Swissvale, PA). White light was supplied by an equal mixture of cool-white and Grow-lux wide-spectrum fluorescent bulbs (Sylvania, Danvers, MA). A 2472 yellow-green acrylic filter (Polycast Technology, Stamford, CT) with a transmission maximum of ±550 produced light that was partially depleted in the photomorphogenetically active UV, B, R, and FR regions of the spectrum. Narrow-spectrum G light (±520-nm maximum) was produced by a 2092 green acrylic filter (Polycast Technology), as described previously (Ahmad and Cashmore, 1993; Lin et al., 1996). Dark experiments were performed in a passively ventilated dark box. Fluence rates of white, R, B, yellow, and G light were measured with a quantum photometer (model LI-189, LI-COR, Lincoln, NE). Fluence rates of FR light were measured using a radiometer (model IL1400, International Light, Newburyport, MA) with FR probe (model SEL033, International Light).
ACKNOWLEDGMENTS
We thank Terry Thomas and members of his laboratory for assistance with the propagation and harvesting of the M2 seed pools. We would also like to thank Robert Corbett for valuable advice and assistance throughout the project. Heather Herrick and Andrew Strittmatter also provided technical assistance.
Footnotes
This work was supported by a research enhancement grant from the College of Science, Texas A&M University. Major support was provided by the National Science Foundation (grant no. IBN–9874531).
LITERATURE CITED
- Ahmad M, Cashmore A. HY4 gene of Arabidopsis thaliana encodes a protein with characteristics of a blue-light photoreceptor. Nature. 1993;366:162–166. doi: 10.1038/366162a0. [DOI] [PubMed] [Google Scholar]
- Ahmad M, Cashmore AR. The pef mutants of Arabidopsis thaliana define lesions early in the phytochrome signalling pathway. Plant J. 1996;10:1103–1110. doi: 10.1046/j.1365-313x.1996.10061103.x. [DOI] [PubMed] [Google Scholar]
- Ahmad M, Cashmore AR. The blue-light receptor cryptochrome 1 shows functional dependence on phytochrome A or phytochrome B in Arabidopsis thaliana. Plant J. 1997;11:421–427. doi: 10.1046/j.1365-313x.1997.11030421.x. [DOI] [PubMed] [Google Scholar]
- Aukerman MJ, Hirschfeld M, Wester L, Weaver M, Clack T, Amasino RM, Sharrock RA. A deletion in the PHYD gene of the Arabidopsis Wassilewskija ecotype defines a role for phytochrome D in red/far-red light sensing. Plant Cell. 1997;9:1317–1326. doi: 10.1105/tpc.9.8.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batschauer A, Rocholl M, Kaiser T, Nagatani A, Furuya M, Schaefer E. Blue and UV-A light-regulated CHS expression in Arabidopsis independent of phytochrome A and phytochrome B. Plant J. 1996;9:63–69. [Google Scholar]
- Bell CJ, Ecker JR. Assignment of 30 microsatellite loci to the linkage map of Arabidopsis. Genomics. 1994;19:137–144. doi: 10.1006/geno.1994.1023. [DOI] [PubMed] [Google Scholar]
- Buche C, Poppe C, Schafer E, Kretsch T. eid1: a new Arabidopsis mutant hypersensitive in phytochrome A-dependent high irradiance responses. Plant Cell. 2000;12:547–558. [PMC free article] [PubMed] [Google Scholar]
- Casal JJ, Mazzella MA. Conditional synergism between cryptochrome 1 and phytochrome B is shown by the analysis of phyA, phyB, and hy4 simple, double, and triple mutants in Arabidopsis. Plant Physiol. 1998;118:19–25. doi: 10.1104/pp.118.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin-Atkins AN, Craig S, Hocart CH, Dennis ES, Chaudhury AM. Increased endogenous cytokinin in the Arabidopsis amp1 mutant corresponds with de-etiolation responses. Planta. 1996;198:549–556. doi: 10.1007/BF00262641. [DOI] [PubMed] [Google Scholar]
- Choi G, Yi H, Lee J, Kwon Y-K, Soh MS, Shin B, Luka Z, Hahn T-R, Song P-S. Phytochrome signalling is mediated through nucleoside diphosphate kinase 2. Nature. 1999;401:610–613. doi: 10.1038/44176. [DOI] [PubMed] [Google Scholar]
- Chory J, Li J. Gibberellins, brassinosteroids and light-regulated development. Plant Cell Environ. 1997;20:801–806. [Google Scholar]
- Chory J, Nagpal P, Peto CA. Phenotypic and genetic analysis of det2, a new mutant that affects light-regulated seedling development in Arabidopsis. Plant Cell. 1991;3:445–459. doi: 10.1105/tpc.3.5.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chory J, Peto C, Feinbaum R, Pratt L, Ausubel F. Arabidopsis thaliana mutant that develops as a light-grown plant in the absence of light. Cell. 1989;58:991–999. doi: 10.1016/0092-8674(89)90950-1. [DOI] [PubMed] [Google Scholar]
- Chory J, Reinecke D, Sim S, Washburn T, Brenner M. A role for cytokinins in de-etiolation in Arabidopsis. Plant Physiol. 1994;104:339–347. doi: 10.1104/pp.104.2.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehesh K, Franci C, Parks BM, Seeley KA, Short TW, Tepperman JM, Quail PH. Arabidopsis hy8 locus encodes phytochrome A. Plant Cell. 1993;5:1081–1088. doi: 10.1105/tpc.5.9.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng X-W, Caspar T, Quail PH. COP1: a regulatory locus involved in light-controlled development and gene expression in Arabidopsis. Genes Dev. 1991;5:1172–1182. doi: 10.1101/gad.5.7.1172. [DOI] [PubMed] [Google Scholar]
- Deng X-W, Matsui M, Wei N, Wagner D, Chu AM, Feldman KA, Quail PH. COP1, an Arabidopsis phoromorphogenic regulatory gene, encodes a protein with both a Zn-binding motif and a G-β homologous domain. Cell. 1992;71:791–801. doi: 10.1016/0092-8674(92)90555-q. [DOI] [PubMed] [Google Scholar]
- Deng X-W, Quail PH. Signalling in light-controlled development. Semin Cell Dev Biol. 1999;10:121–129. doi: 10.1006/scdb.1999.0287. [DOI] [PubMed] [Google Scholar]
- Devlin PF, Robson PRH, Patel SR, Goosey L, Sharrock RA, Whitelam GC. Phytochrome D acts in the shade-avoidance syndrome in Arabidopsis by controlling elongation growth and flowering time. Plant Physiol. 1999;119:909–915. doi: 10.1104/pp.119.3.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fankhauser C, Yeh K-C, Lagarias JC, Zhang H, Elich TD, Chory J. PKS1, a substrate phosphorylated by phytochrome that modulates light signaling in Arabidopsis. Science. 1999;284:1539–1541. doi: 10.1126/science.284.5419.1539. [DOI] [PubMed] [Google Scholar]
- Franzmann LH, Yoon ES, Meinke D. Saturating the genetic map of Arabidopsis thaliana with embryonic mutations. Plant J. 1995;7:341–350. [Google Scholar]
- Genoud T, Millar AJ, Nishizawa N, Kay SA, Schäfer E, Nagatani A, Chua N-H. An Arabidopsis mutant hypersensitive to red and far-red signals. Plant Cell. 1998;10:889–904. doi: 10.1105/tpc.10.6.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo H, Mockler T, Duong H, Lin C. SUB1, an Arabdidopsis Ca2+-binding protein involved in cryptochrome and phytochrome coaction. Science. 2001;291:487–490. doi: 10.1126/science.291.5503.487. [DOI] [PubMed] [Google Scholar]
- Hoecker U, Xu Y, Quail PH. SPA1: a new genetic locus involved in phytochrome A-specific signal transduction. Plant Cell. 1998;10:19–33. doi: 10.1105/tpc.10.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh H-L, Okamoto H, Wang M, Ang L-H, Matsui M, Goodman H, Deng X-W. FIN219, an auxin-regulated gene, defines a link between phytochrome A and the downstream regulator COP1 in light control of Arabidopsis development. Genes Dev. 2000;14:1958–1970. [PMC free article] [PubMed] [Google Scholar]
- Huq E, Kang Y, Halliday KJ, Qin M, Quail PH. SRL1: a new locus specific to the phyB-signaling pathway in Arabidopsis. Plant J. 2000;23:461–470. doi: 10.1046/j.1365-313x.2000.00810.x. [DOI] [PubMed] [Google Scholar]
- Jenson PJ, Hangarter RP, Estelle M. Auxin transport is required for hypocotyl elongation in light-grown but not dark-grown Arabidopsis. Plant Physiol. 1998;116:445–462. doi: 10.1104/pp.116.2.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim BC, Soh MS, Kang BJ, Furuya M, Nam HG. Two dominant photomorphogenic mutations of Arabidopsis thaliana identified as suppressor mutations of hy2. Plant J. 1996;9:441–456. doi: 10.1046/j.1365-313x.1996.09040441.x. [DOI] [PubMed] [Google Scholar]
- Konieczny A, Ausubel F. A procedure for quick mapping of Arabidopsis mutants using ecotype specific markers. Plant J. 1993;4:403–410. doi: 10.1046/j.1365-313x.1993.04020403.x. [DOI] [PubMed] [Google Scholar]
- Koorneef M, Rolff E, Spruitt CJP. Genetic control of light inhibited hypocotyl elongation in Arabidopsis thaliana (L.) Heynh. Z Pflanzenphysiol. 1980;100:147–160. [Google Scholar]
- Leyser HMO. Primer: plant hormones. Curr Biol. 1998;8:R5–R7. doi: 10.1016/s0960-9822(98)70006-5. [DOI] [PubMed] [Google Scholar]
- Leyser HMO, Lincoln CA, Timpte C, Lammer D, Turner J, Estelle M. Arabidopsis auxin-resistance gene AXR1 encodes a protein related to ubiquitin-activating enzyme E1. Nature. 1993;364:161–164. doi: 10.1038/364161a0. [DOI] [PubMed] [Google Scholar]
- Lin C, Ahmad M, Cashmore AR. Arabidopsis cyptochrome 1 is a soluable protein mediating blue light-dependent regulation of plant growth and development. Plant J. 1996;10:893–902. doi: 10.1046/j.1365-313x.1996.10050893.x. [DOI] [PubMed] [Google Scholar]
- Lukowitz W, Gillmor CS, Scheible WR. Positional cloning in Arabidopsis: why it feels good to have a genome initiative working for you. Plant Physiol. 2000;123:795–806. doi: 10.1104/pp.123.3.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miserá S, Mueller AJ, Weiland-Heidecker U, Jurgens G. The FUSCA genes of Arabidopsis: negative regulators of light responses. Mol Gen Genet. 1994;244:242–252. doi: 10.1007/BF00285451. [DOI] [PubMed] [Google Scholar]
- Nagatani A, Reed JW, Chory J. Isolation and initial characterization of Arabidopsis mutants that are deficient in phytochrome A. Plant Physiol. 1993;102:269–277. doi: 10.1104/pp.102.1.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neff M, Chory J. Genetic interactions between phytochrome A, phytochrome B, and cryptochrome 1 during Arabidopsis development. Plant Physiol. 1998;118:27–36. doi: 10.1104/pp.118.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neff MM, Fankhauser C, Chory J. Light: an indicator of time and place. Genes Dev. 2000;14:257–271. [PubMed] [Google Scholar]
- Ni M, Tepperman JM, Quail PH. PIF3, a phytochrome-interacting factor necessary for normal photoinduced signal transduction, is a novel basic helix-loop-helix protein. Cell. 1998;95:657–667. doi: 10.1016/s0092-8674(00)81636-0. [DOI] [PubMed] [Google Scholar]
- Oyama T, Shimura Y, Okada K. The Arabidopsis HY5 gene encodes a bZIP protein that regulates stimulus-induced development of root and hypocotyl. Genes Dev. 1997;11:2983–2995. doi: 10.1101/gad.11.22.2983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks BM, Quail PH. hy8, a new class of Arabidopsis long hypocotyl mutants deficient in functional phytochrome A. Plant Cell. 1993;5:39–48. doi: 10.1105/tpc.5.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepper A, Chory J. Extragenic suppressors of the Arabidopsis det1 mutant identify elements of flowering-time and light-response regulatory pathways. Genetics. 1997;145:1125–1137. doi: 10.1093/genetics/145.4.1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepper A, Delaney T, Washburn T, Poole D, Chory J. DET1, a negative regulator of light-mediated development and gene expression in Arabidopsis, encodes a novel nuclear localized protein. Cell. 1994;78:109–116. doi: 10.1016/0092-8674(94)90577-0. [DOI] [PubMed] [Google Scholar]
- Poppe C, Schäfer E. Seed germination of Arabidopsis thaliana phyA/phyB double mutants is under phytochrome control. Plant Physiol. 1997;114:1487–1492. doi: 10.1104/pp.114.4.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed JW, Elumalai RP, Chory J. Suppressors of an Arabidopsis thaliana phyB mutation identify genes that control light signaling and hypocotyl elongation. Genetics. 1998;148:1295–1310. doi: 10.1093/genetics/148.3.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed JW, Nagatani A, Elich TD, Fagan M, Chory J. Phytochrome A and phytochrome B have overlapping but distinct functions in Arabidopsis development. Plant Physiol. 1994;104:1139–1149. doi: 10.1104/pp.104.4.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed JW, Nagpal P, Poole DS, Furuya M, Chory J. Mutations in the gene for the red/far-red light receptor phytochrome B alter cell elongation and physiological responses throughout Arabidopsis development. Plant Cell. 1993;5:147–157. doi: 10.1105/tpc.5.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H. Perception of light quality. In: Kendrick RE, Kronenberg GHM, editors. Photomorphogenesis in Plants. Dordrecht, The Netherlands: Martinus Nijhoff; 1986. pp. 547–563. [Google Scholar]
- Smith H. Phytochromes and light signal perception by plants: an emerging synthesis. Nature. 2000;407:585–591. doi: 10.1038/35036500. [DOI] [PubMed] [Google Scholar]
- Soh M, Hong SH, Kim BC, Vizir I, Park DH, Choi G, Hong MY, Chung Y-Y, Furuya M, Nam H-G. Regulation of both light- and auxin-mediated development by the Arabidopsis IAA3/SHY2 gene. J Plant Biol. 1999;42:239–246. [Google Scholar]
- Somers DE, Sharrock RA, Tepperman JM, Quail PH. The hy3 long hypocotyl mutant of Arabidopsis is deficient in phytochrome B. Plant Cell. 1993;3:1263–1274. doi: 10.1105/tpc.3.12.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian Q, Reed JW. Control of auxin-regulated root development by the Arabidopsis thaliana SHY2/IAA3 gene. Development. 1999;126:711–721. doi: 10.1242/dev.126.4.711. [DOI] [PubMed] [Google Scholar]
- Wei N, Chamovitz DA, Deng X-W. Arabidopsis COP9 is a component of a novel signaling complex mediating light control of development. Cell. 1994a;78:117–124. doi: 10.1016/0092-8674(94)90578-9. [DOI] [PubMed] [Google Scholar]
- Wei N, Deng XW. COP9: a new genetic locus involved in light-regulated development and gene expression in Arabidopsis. Plant Cell. 1992;4:1507–1518. doi: 10.1105/tpc.4.12.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei N, Kwok SF, Von Arnim AG, Lee A, McNellis TW, Piekos B, Deng X-W. Arabidopsis COP8, COP10, and COP11 genes are involved in repression of photomorphogenic development in darkness. Plant Cell. 1994b;6:629–643. doi: 10.1105/tpc.6.5.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitelam GC, Johnson E, Peng J, Carol P, Anderson ML, Cowl JS, Harberd NP. Phytochrome A null mutants of Arabidopsis display a wild-type phenotype in white light. Plant Cell. 1993;5:757–768. doi: 10.1105/tpc.5.7.757. [DOI] [PMC free article] [PubMed] [Google Scholar]