Abstract
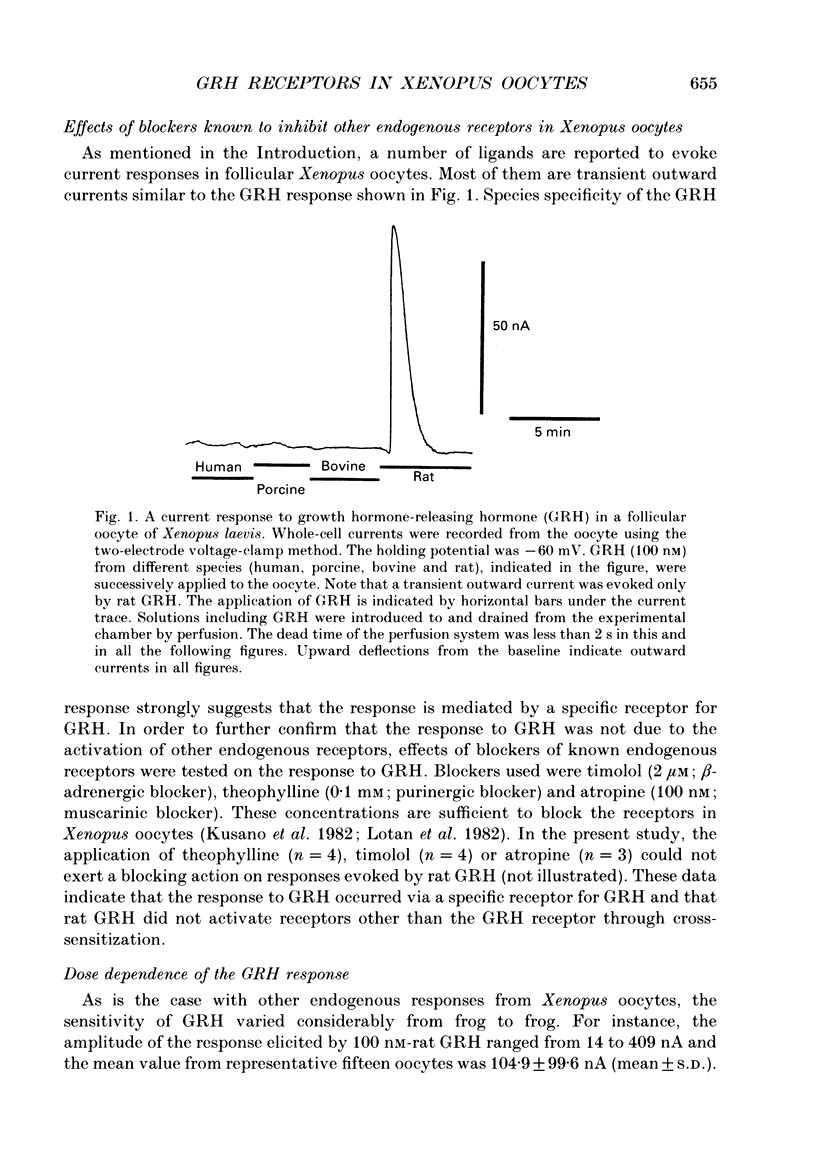
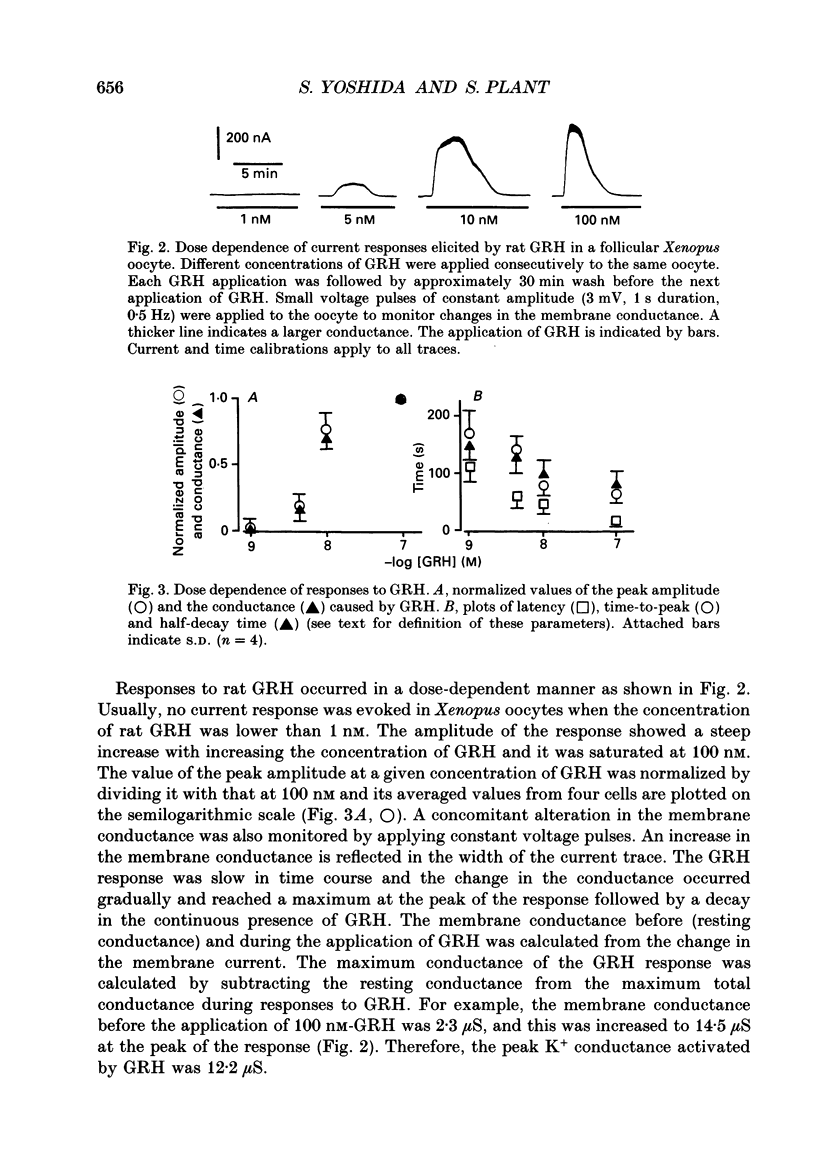
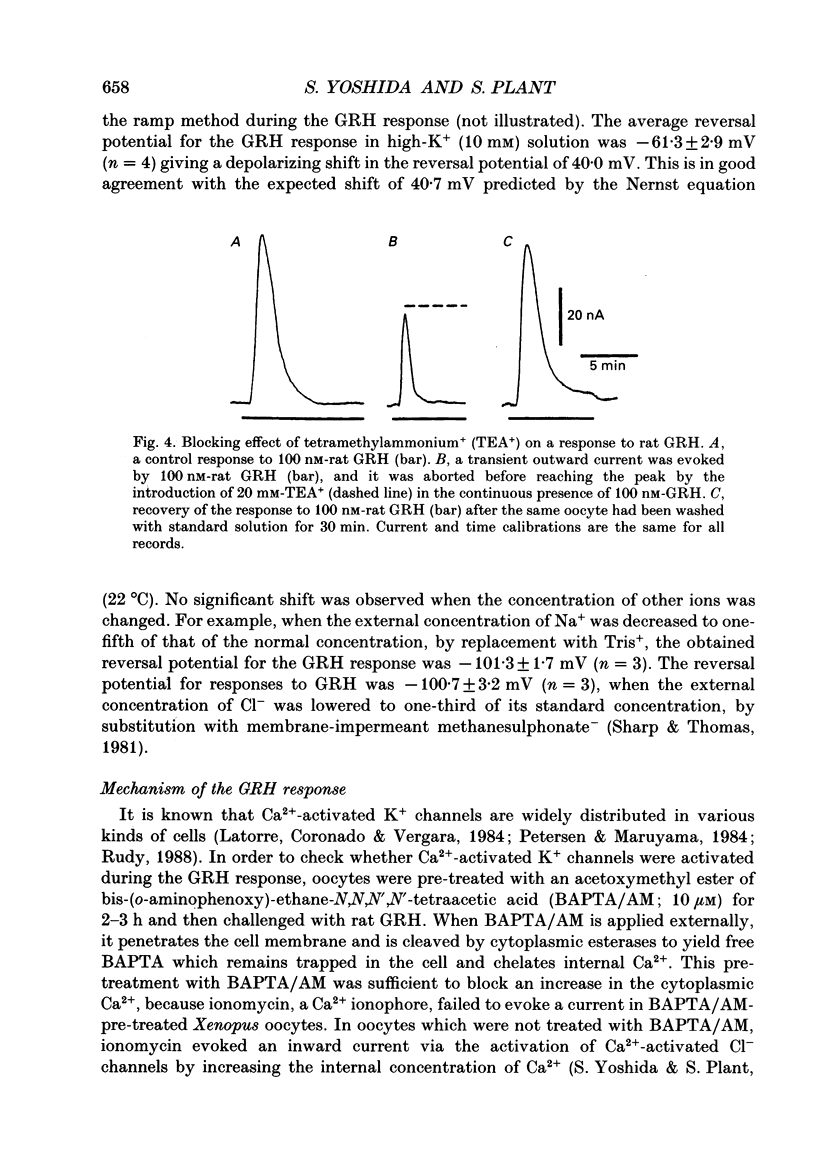
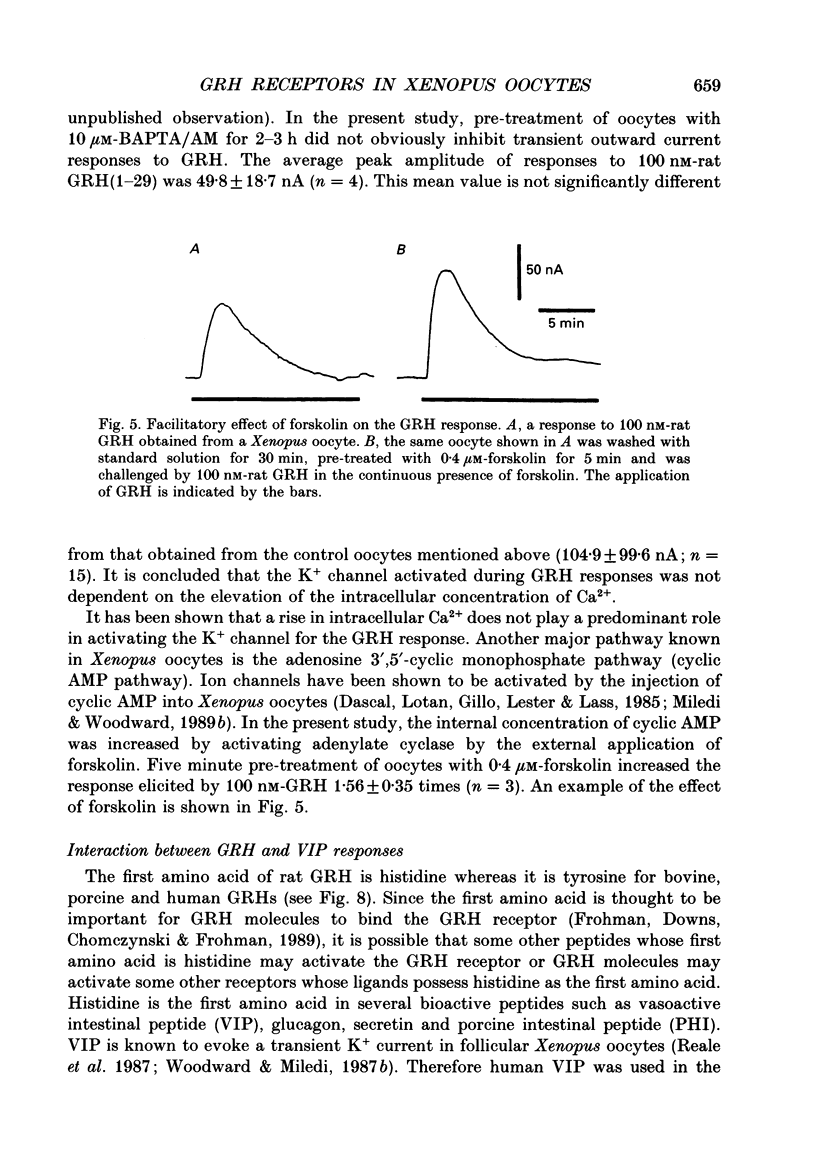
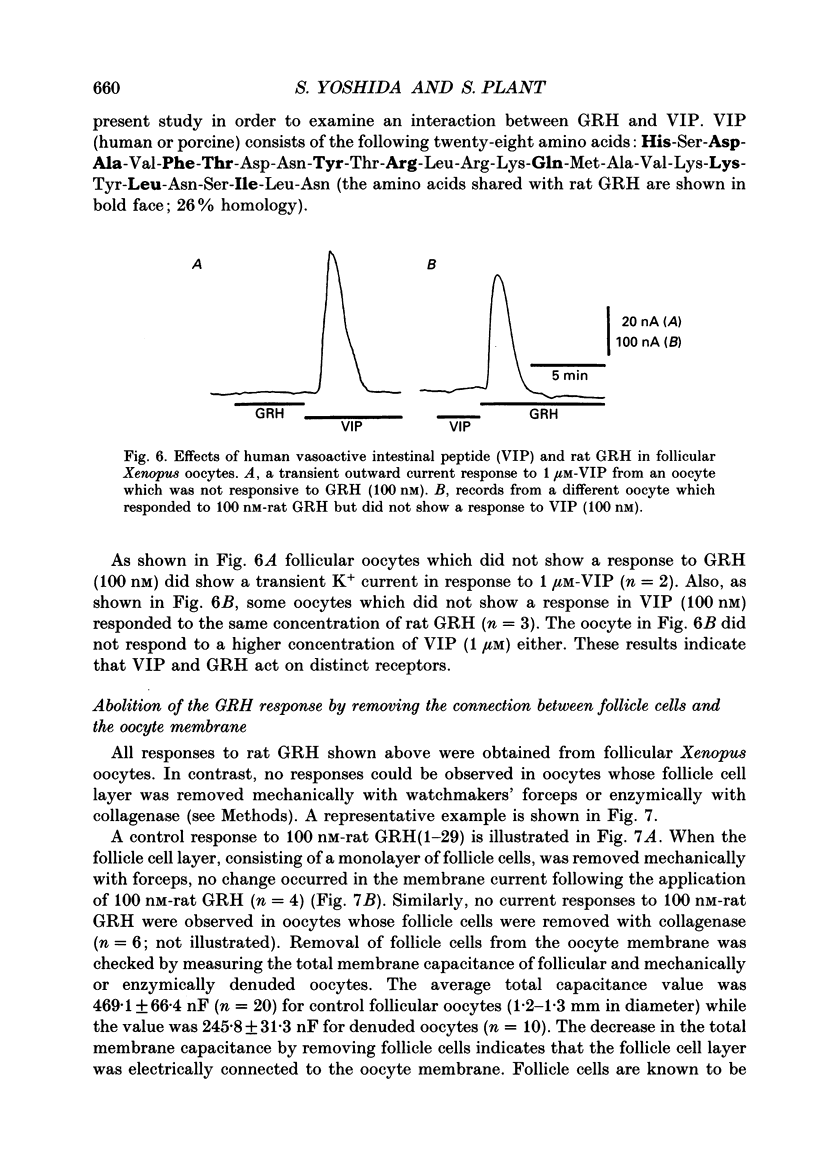
1. Electrophysiological properties of the growth hormone-releasing hormone (GRH) receptor were studied in Xenopus oocytes with an intact follicle cell layer (i.e. follicular oocytes) by measuring whole-cell current using the two-electrode voltage-clamp method. 2. A slow transient outward current was elicited in oocytes, clamped at -60 mV, by the application of rat GRH but not bovine, porcine, or human GRH. 3. The response to GRH was not suppressed by blockers known to inhibit other endogenous receptors present in follicular Xenopus oocytes; blockers used were timolol (2 microM; beta-adrenergic blocker), theophylline (0.1 mM; purinergic blocker) and atropine (100 nM; muscarinic blocker). 4. The current response evoked by rat GRH occurred in a dose-dependent manner. The concentrations of GRH for threshold and maximum responses were 1 and 100 nM respectively and the estimated EC50 (half-maximal effective concentration) was approximately 7 nM. The amplitude and conductance of the response became larger and the latency, time-to-peak and half-decay time were shortened when the concentration of GRH was increased. 5. The GRH response was reversibly inhibited by a K+ channel blocker, tetraethylammonium+ (TEA+; 20 mM). The reversal potential for the GRH response was around -100 mV and was compatible with the reported value for a K+ current in Xenopus oocytes. Furthermore, a depolarizing shift of 40 mV in the reversal potential was observed when the external K+ concentration was increased from 2 to 10 mM, agreeing with the Nernst equation. In contrast, no significant shift in the reversal potential was observed by changing the external concentration of Na+ or Cl-. 6. The GRH response was not suppressed in oocytes treated with an acetoxy-methyl ester of bis-(o-aminophenoxy)-ethane-N,N,N',N'-tetraacetic acid (BAPTA/AM; 10 microM) which penetrates the cell membrane and chelates internal Ca2+. 7. The GRH response was potentiated by pre-treatment with forskolin (0.4 microM; 5 min), which stimulates adenylate cyclase and increases the internal concentration of adenosine 3',5'-cyclic monophosphate (cyclic AMP). 8. The GRH response was not obtainable when follicle cells surrounding oocytes were removed mechanically with forceps or enzymically with collagenase (i.e. denuded oocytes). The response was also suppressed when gap junctions, which electrically couple follicle cells and the oocyte, were blocked by 1-octanol (1 mM). 9. The first amino acid is considered to be important for the binding of peptide ligands to their receptors.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF




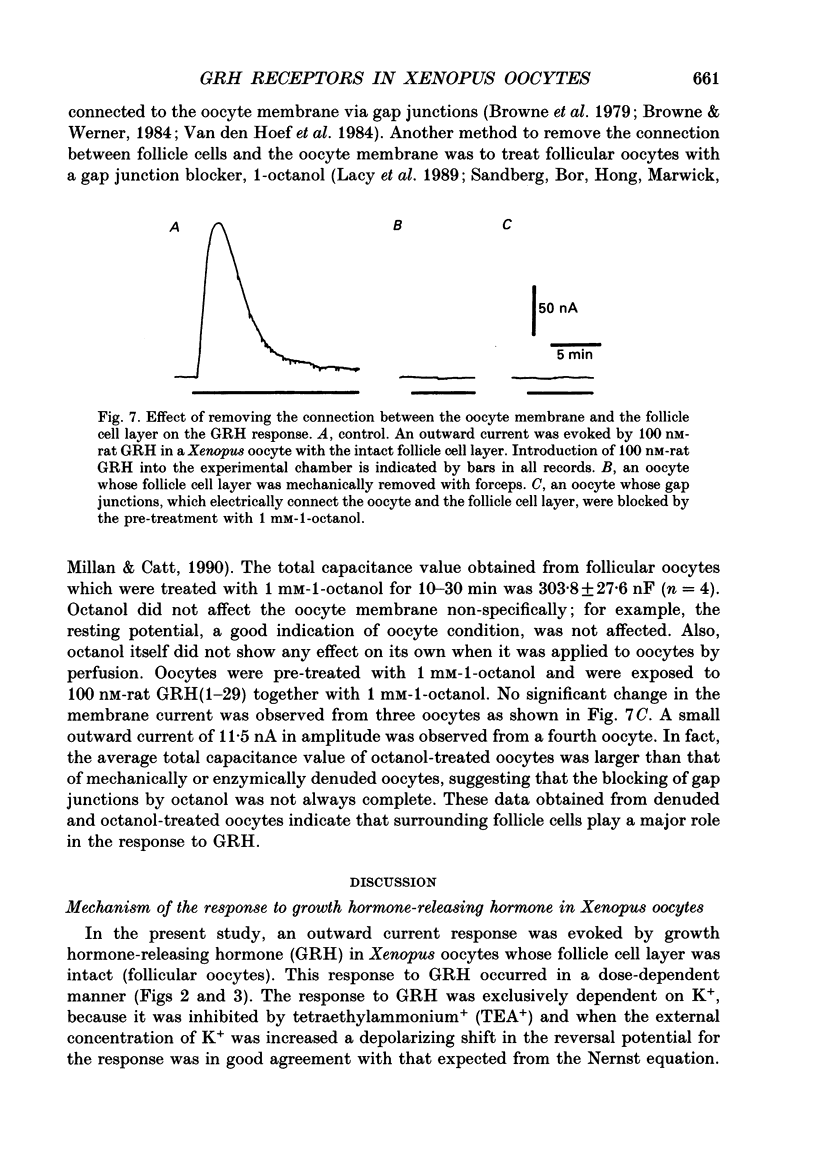






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARTH L. G., BARTH L. J. Differentiation of cells of the Rana pipiens gastrula in unconditioned medium. J Embryol Exp Morphol. 1959 Jun;7:210–222. [PubMed] [Google Scholar]
- Barish M. E. A transient calcium-dependent chloride current in the immature Xenopus oocyte. J Physiol. 1983 Sep;342:309–325. doi: 10.1113/jphysiol.1983.sp014852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilezikjian L. M., Vale W. W. Stimulation of adenosine 3',5'-monophosphate production by growth hormone-releasing factor and its inhibition by somatostatin in anterior pituitary cells in vitro. Endocrinology. 1983 Nov;113(5):1726–1731. doi: 10.1210/endo-113-5-1726. [DOI] [PubMed] [Google Scholar]
- Boton R., Dascal N., Gillo B., Lass Y. Two calcium-activated chloride conductances in Xenopus laevis oocytes permeabilized with the ionophore A23187. J Physiol. 1989 Jan;408:511–534. doi: 10.1113/jphysiol.1989.sp017473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brazeau P., Ling N., Esch F., Böhlen P., Mougin C., Guillemin R. Somatocrinin (growth hormone releasing factor) in vitro bioactivity; Ca++ involvement, cAMP mediated action and additivity of effect with PGE2. Biochem Biophys Res Commun. 1982 Nov 30;109(2):588–594. doi: 10.1016/0006-291x(82)91762-4. [DOI] [PubMed] [Google Scholar]
- Browne C. L., Werner W. Intercellular junctions between the follicle cells and oocytes of Xenopus laevis. J Exp Zool. 1984 Apr;230(1):105–113. doi: 10.1002/jez.1402300114. [DOI] [PubMed] [Google Scholar]
- Browne C. L., Wiley H. S., Dumont J. N. Oocyte-follicle cell gap junctions in Xenopus laevis and the effects of gonadotropin on their permeability. Science. 1979 Jan 12;203(4376):182–183. doi: 10.1126/science.569364. [DOI] [PubMed] [Google Scholar]
- Chen C., Israel J. M., Vincent J. D. Electrophysiological responses of rat pituitary cells in somatotroph-enriched primary culture to human growth-hormone releasing factor. Neuroendocrinology. 1989 Dec;50(6):679–687. doi: 10.1159/000125299. [DOI] [PubMed] [Google Scholar]
- Dascal N., Lotan I., Gillo B., Lester H. A., Lass Y. Acetylcholine and phorbol esters inhibit potassium currents evoked by adenosine and cAMP in Xenopus oocytes. Proc Natl Acad Sci U S A. 1985 Sep;82(17):6001–6005. doi: 10.1073/pnas.82.17.6001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dascal N. The use of Xenopus oocytes for the study of ion channels. CRC Crit Rev Biochem. 1987;22(4):317–387. doi: 10.3109/10409238709086960. [DOI] [PubMed] [Google Scholar]
- Dumont J. N. Oogenesis in Xenopus laevis (Daudin). I. Stages of oocyte development in laboratory maintained animals. J Morphol. 1972 Feb;136(2):153–179. doi: 10.1002/jmor.1051360203. [DOI] [PubMed] [Google Scholar]
- Frohman M. A., Downs T. R., Chomczynski P., Frohman L. A. Cloning and characterization of mouse growth hormone-releasing hormone (GRH) complementary DNA: increased GRH messenger RNA levels in the growth hormone-deficient lit/lit mouse. Mol Endocrinol. 1989 Oct;3(10):1529–1536. doi: 10.1210/mend-3-10-1529. [DOI] [PubMed] [Google Scholar]
- Gubler U., Monahan J. J., Lomedico P. T., Bhatt R. S., Collier K. J., Hoffman B. J., Böhlen P., Esch F., Ling N., Zeytin F. Cloning and sequence analysis of cDNA for the precursor of human growth hormone-releasing factor, somatocrinin. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4311–4314. doi: 10.1073/pnas.80.14.4311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillemin R., Brazeau P., Böhlen P., Esch F., Ling N., Wehrenberg W. B. Growth hormone-releasing factor from a human pancreatic tumor that caused acromegaly. Science. 1982 Nov 5;218(4572):585–587. doi: 10.1126/science.6812220. [DOI] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952 Aug;117(4):500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart G. R., Ray K. P., Wallis M. Mechanisms involved in the effects of TRH on GHRH-stimulated growth hormone release from ovine and bovine pituitary cells. Mol Cell Endocrinol. 1988 Mar;56(1-2):53–61. doi: 10.1016/0303-7207(88)90008-1. [DOI] [PubMed] [Google Scholar]
- Kusano K., Miledi R., Stinnakre J. Acetylcholine receptors in the oocyte membrane. Nature. 1977 Dec 22;270(5639):739–741. doi: 10.1038/270739a0. [DOI] [PubMed] [Google Scholar]
- Kusano K., Miledi R., Stinnakre J. Cholinergic and catecholaminergic receptors in the Xenopus oocyte membrane. J Physiol. 1982 Jul;328:143–170. doi: 10.1113/jphysiol.1982.sp014257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labrie F., Gagné B., Lefèvre G. Growth hormone-releasing factor stimulates adenylate cyclase activity in the anterior pituitary gland. Life Sci. 1983 Nov 28;33(22):2229–2233. doi: 10.1016/0024-3205(83)90295-3. [DOI] [PubMed] [Google Scholar]
- Lacy M. P., McIntosh R. P., McIntosh J. E. Angiotensin II stimulates an endogenous response in Xenopus laevis ovarian follicles. Biochem Biophys Res Commun. 1989 Mar 15;159(2):658–663. doi: 10.1016/0006-291x(89)90045-4. [DOI] [PubMed] [Google Scholar]
- Lamb T. D. An inexpensive digital tape recorder suitable for neurophysiological signals. J Neurosci Methods. 1985 Oct;15(1):1–13. doi: 10.1016/0165-0270(85)90057-3. [DOI] [PubMed] [Google Scholar]
- Latorre R., Coronado R., Vergara C. K+ channels gated by voltage and ions. Annu Rev Physiol. 1984;46:485–495. doi: 10.1146/annurev.ph.46.030184.002413. [DOI] [PubMed] [Google Scholar]
- Law G. J., Ray K. P., Wallis M. Effects of growth hormone-releasing factor, somatostatin and dopamine on growth hormone and prolactin secretion from cultured ovine pituitary cells. FEBS Lett. 1984 Jan 23;166(1):189–193. doi: 10.1016/0014-5793(84)80070-8. [DOI] [PubMed] [Google Scholar]
- Lawrence T. S., Beers W. H., Gilula N. B. Transmission of hormonal stimulation by cell-to-cell communication. Nature. 1978 Apr 6;272(5653):501–506. doi: 10.1038/272501a0. [DOI] [PubMed] [Google Scholar]
- Lotan I., Dascal N., Cohen S., Lass Y. Adenosine-induced slow ionic currents in the Xenopus oocyte. Nature. 1982 Aug 5;298(5874):572–574. doi: 10.1038/298572a0. [DOI] [PubMed] [Google Scholar]
- Lotan I., Dascal N., Oron Y., Cohen S., Lass Y. Adenosine-induced K+ current in Xenopus oocyte and the role of adenosine 3',5'-monophosphate. Mol Pharmacol. 1985 Aug;28(2):170–177. [PubMed] [Google Scholar]
- Maller J. L., Krebs E. G. Regulation of oocyte maturation. Curr Top Cell Regul. 1980;16:271–311. doi: 10.1016/b978-0-12-152816-4.50012-1. [DOI] [PubMed] [Google Scholar]
- Mayo K. E., Cerelli G. M., Lebo R. V., Bruce B. D., Rosenfeld M. G., Evans R. M. Gene encoding human growth hormone-releasing factor precursor: structure, sequence, and chromosomal assignment. Proc Natl Acad Sci U S A. 1985 Jan;82(1):63–67. doi: 10.1073/pnas.82.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayo K. E., Cerelli G. M., Rosenfeld M. G., Evans R. M. Characterization of cDNA and genomic clones encoding the precursor to rat hypothalamic growth hormone-releasing factor. Nature. 1985 Apr 4;314(6010):464–467. doi: 10.1038/314464a0. [DOI] [PubMed] [Google Scholar]
- McCutcheon S. N., Bauman D. E., Murphy W. A., Lance V. A., Coy D. H. Effect of synthetic human pancreatic growth hormone-releasing factors on plasma growth hormone concentrations in lactating cows. J Dairy Sci. 1984 Dec;67(12):2881–2886. doi: 10.3168/jds.S0022-0302(84)81650-1. [DOI] [PubMed] [Google Scholar]
- Miledi R., Woodward R. M. Effects of defolliculation on membrane current responses of Xenopus oocytes. J Physiol. 1989 Sep;416:601–621. doi: 10.1113/jphysiol.1989.sp017780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miledi R., Woodward R. M. Membrane currents elicited by prostaglandins, atrial natriuretic factor and oxytocin in follicle-enclosed Xenopus oocytes. J Physiol. 1989 Sep;416:623–643. doi: 10.1113/jphysiol.1989.sp017781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriarty T. M., Gillo B., Sealfon S., Landau E. M. Activation of ionic currents in Xenopus oocytes by corticotropin-releasing peptides. Brain Res. 1988 Nov;464(3):201–205. doi: 10.1016/0169-328x(88)90026-5. [DOI] [PubMed] [Google Scholar]
- Nussinovitch I. Growth hormone releasing factor evokes rhythmic hyperpolarizing currents in rat anterior pituitary cells. J Physiol. 1988 Jan;395:303–318. doi: 10.1113/jphysiol.1988.sp016920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen O. H., Maruyama Y. Calcium-activated potassium channels and their role in secretion. Nature. 1984 Feb 23;307(5953):693–696. doi: 10.1038/307693a0. [DOI] [PubMed] [Google Scholar]
- Pitts J. D., Simms J. W. Permeability of junctions between animal cells. Intercellular transfer of nucleotides but not of macromolecules. Exp Cell Res. 1977 Jan;104(1):153–163. doi: 10.1016/0014-4827(77)90078-7. [DOI] [PubMed] [Google Scholar]
- Reale V., Ashford M. L., Barnard E. A. Vasoactive intestinal peptide (VIP) induces a K+ current in the Xenopus oocyte membrane. Neurosci Lett. 1987 Dec 16;83(1-2):89–94. doi: 10.1016/0304-3940(87)90221-7. [DOI] [PubMed] [Google Scholar]
- Rudy B. Diversity and ubiquity of K channels. Neuroscience. 1988 Jun;25(3):729–749. doi: 10.1016/0306-4522(88)90033-4. [DOI] [PubMed] [Google Scholar]
- Sandberg K., Bor M., Ji H., Markwick A., Millan M. A., Catt K. J. Angiotensin II-induced calcium mobilization in oocytes by signal transfer through gap junctions. Science. 1990 Jul 20;249(4966):298–301. doi: 10.1126/science.2374929. [DOI] [PubMed] [Google Scholar]
- Schettini G., Cronin M. J., Hewlett E. L., Thorner M. O., MacLeod R. M. Human pancreatic tumor growth hormone-releasing factor stimulates anterior pituitary adenylate cyclase activity, adenosine 3',5'-monophosphate accumulation, and growth hormone release in a calmodulin-dependent manner. Endocrinology. 1984 Oct;115(4):1308–1314. doi: 10.1210/endo-115-4-1308. [DOI] [PubMed] [Google Scholar]
- Sharp A. P., Thomas R. C. The effects of chloride substitution on intracellular pH in crab muscle. J Physiol. 1981 Mar;312:71–80. doi: 10.1113/jphysiol.1981.sp013616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A. A., Brooker T., Brooker G. Expression of rat mRNA coding for hormone-stimulated adenylate cyclase in Xenopus oocytes. FASEB J. 1987 Nov;1(5):380–387. doi: 10.1096/fasebj.1.5.2824269. [DOI] [PubMed] [Google Scholar]
- Snutch T. P. The use of Xenopus oocytes to probe synaptic communication. Trends Neurosci. 1988 Jun;11(6):250–256. doi: 10.1016/0166-2236(88)90102-6. [DOI] [PubMed] [Google Scholar]
- Soreq H. The biosynthesis of biologically active proteins in mRNA-microinjected Xenopus oocytes. CRC Crit Rev Biochem. 1985;18(3):199–238. doi: 10.3109/10409238509085134. [DOI] [PubMed] [Google Scholar]
- Spiess J., Rivier J., Vale W. Characterization of rat hypothalamic growth hormone-releasing factor. Nature. 1983 Jun 9;303(5917):532–535. doi: 10.1038/303532a0. [DOI] [PubMed] [Google Scholar]
- Stinnakre J., Van Renterghem C. Cyclic adenosine monophosphate, calcium, acetylcholine and the current induced by adenosine in the Xenopus oocyte. J Physiol. 1986 May;374:551–569. doi: 10.1113/jphysiol.1986.sp016097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumikawa K., Parker I., Miledi R. Messenger RNA from rat brain induces noradrenaline and dopamine receptors in Xenopus oocytes. Proc R Soc Lond B Biol Sci. 1984 Dec 22;223(1231):255–260. doi: 10.1098/rspb.1984.0093. [DOI] [PubMed] [Google Scholar]
- Sáez J. C., Connor J. A., Spray D. C., Bennett M. V. Hepatocyte gap junctions are permeable to the second messenger, inositol 1,4,5-trisphosphate, and to calcium ions. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2708–2712. doi: 10.1073/pnas.86.8.2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T., Neher E., Sakmann B. Rat brain serotonin receptors in Xenopus oocytes are coupled by intracellular calcium to endogenous channels. Proc Natl Acad Sci U S A. 1987 Jul;84(14):5063–5067. doi: 10.1073/pnas.84.14.5063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Renterghem C., Penit-Soria J., Stinnakre J. Beta-adrenergic induced potassium current in Xenopus oocyte: involvement of cyclic AMP. Biochimie. 1984 Feb;66(2):135–138. doi: 10.1016/0300-9084(84)90202-5. [DOI] [PubMed] [Google Scholar]
- Van Renterghem C., Renit-Soria J., Stinnakre J. beta-Adrenergic induced K+ current in Xenopus oocytes: role of cAMP, inhibition by muscarinic agents. Proc R Soc Lond B Biol Sci. 1985 Jan 22;223(1232):389–402. doi: 10.1098/rspb.1985.0008. [DOI] [PubMed] [Google Scholar]
- Woodward R. M., Miledi R. Hormonal activation of ionic currents in follicle-enclosed Xenopus oocytes. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4135–4139. doi: 10.1073/pnas.84.12.4135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward R. M., Miledi R. Membrane currents elicited by porcine vasoactive intestinal peptide (VIP) in follicle-enclosed Xenopus oocytes. Proc R Soc Lond B Biol Sci. 1987 Sep 22;231(1265):489–497. doi: 10.1098/rspb.1987.0057. [DOI] [PubMed] [Google Scholar]
- Yamashita N. Enhancement of ionic currents through voltage-gated channels in the mouse oocyte after fertilization. J Physiol. 1982 Aug;329:263–280. doi: 10.1113/jphysiol.1982.sp014302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida S., Plant S., Taylor P. L., Eidne K. A. Chloride channels mediate the response to gonadotropin-releasing hormone (GnRH) in Xenopus oocytes injected with rat anterior pituitary mRNA. Mol Endocrinol. 1989 Dec;3(12):1953–1960. doi: 10.1210/mend-3-12-1953. [DOI] [PubMed] [Google Scholar]
- van den Hoef M. H., Dictus W. J., Hage W. J., Bluemink J. G. The ultrastructural organization of gap junctions between follicle cells and the oocyte in Xenopus laevis. Eur J Cell Biol. 1984 Mar;33(2):242–247. [PubMed] [Google Scholar]


