Abstract
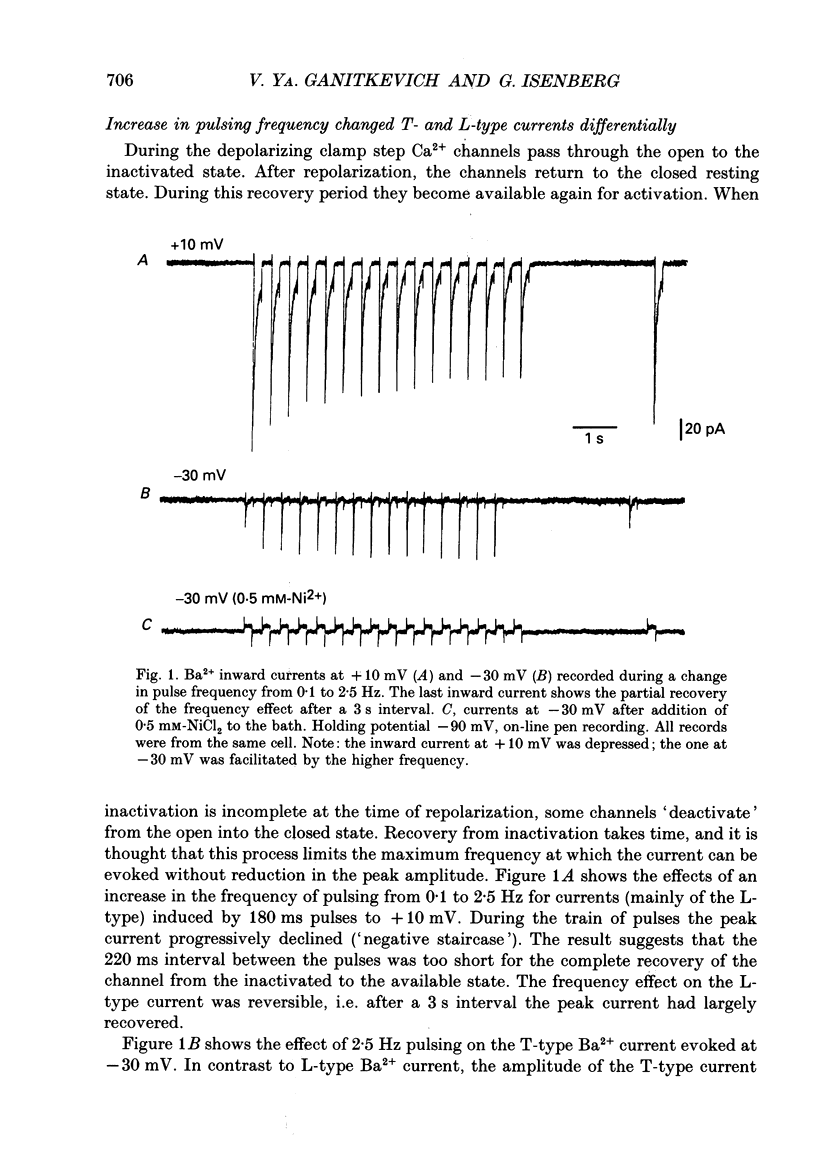
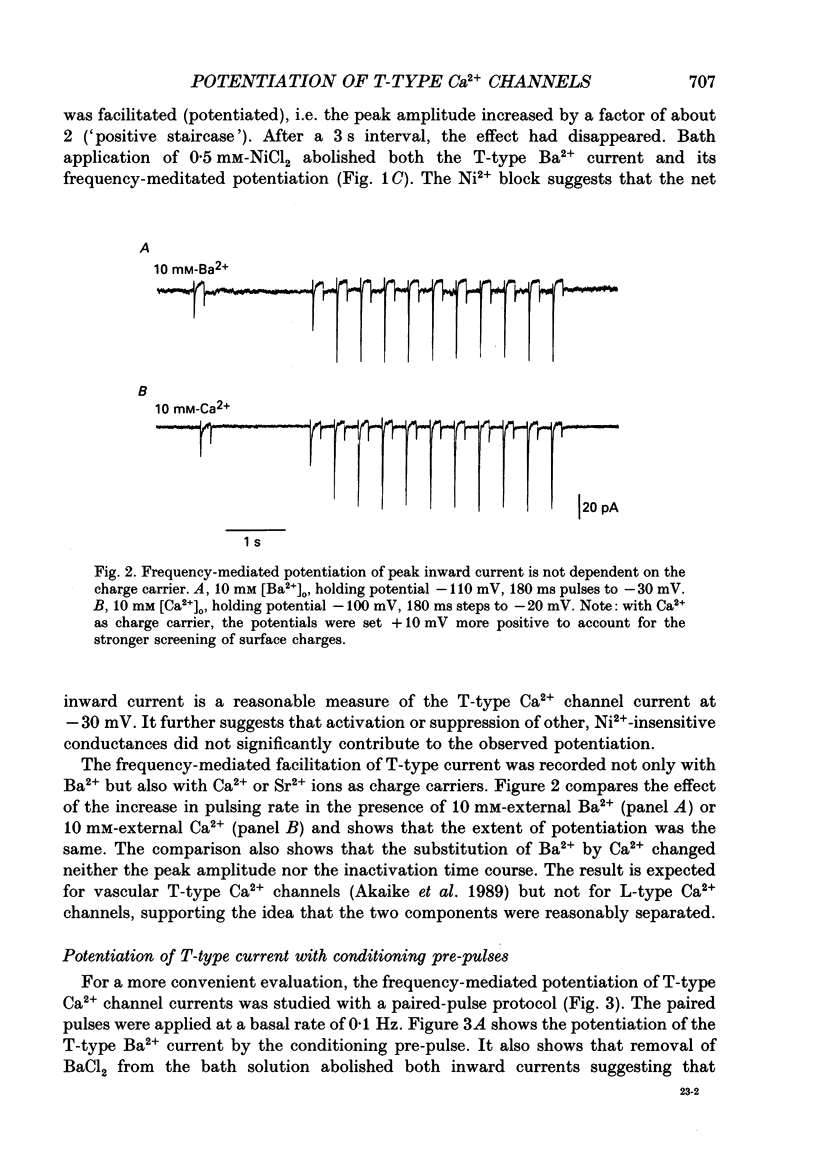
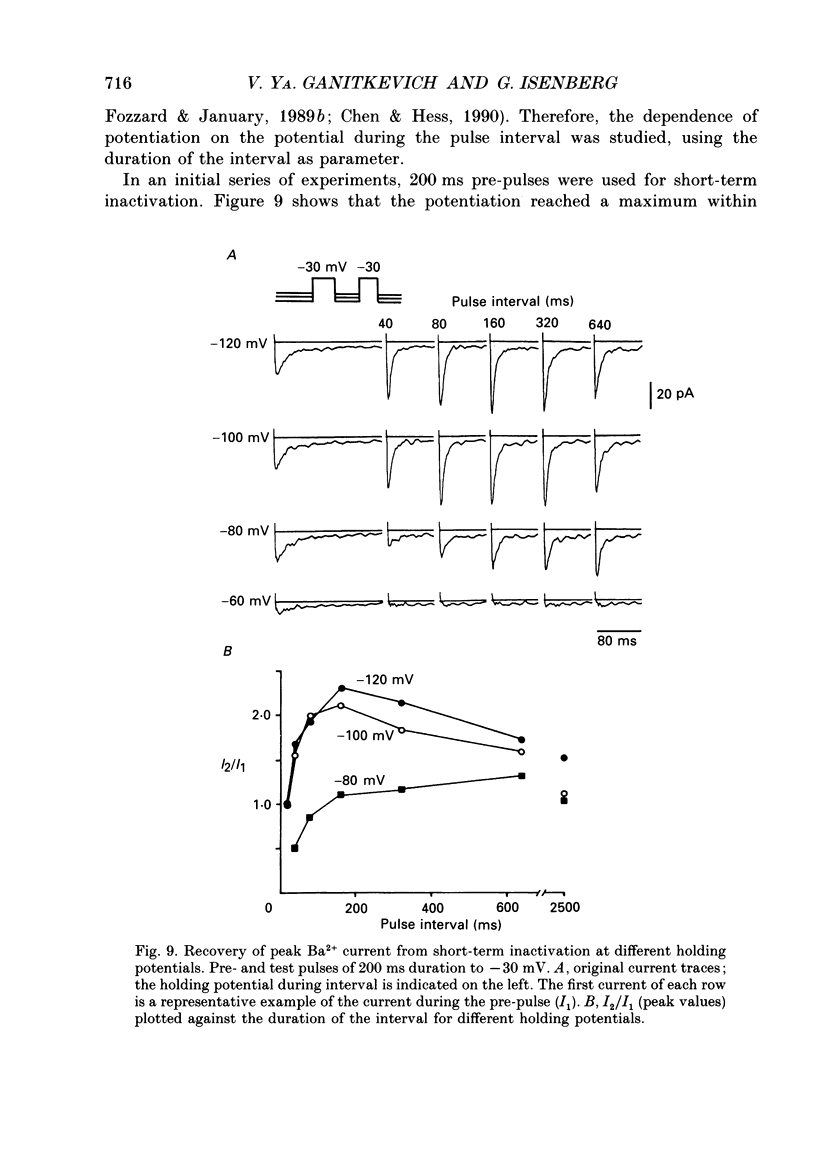
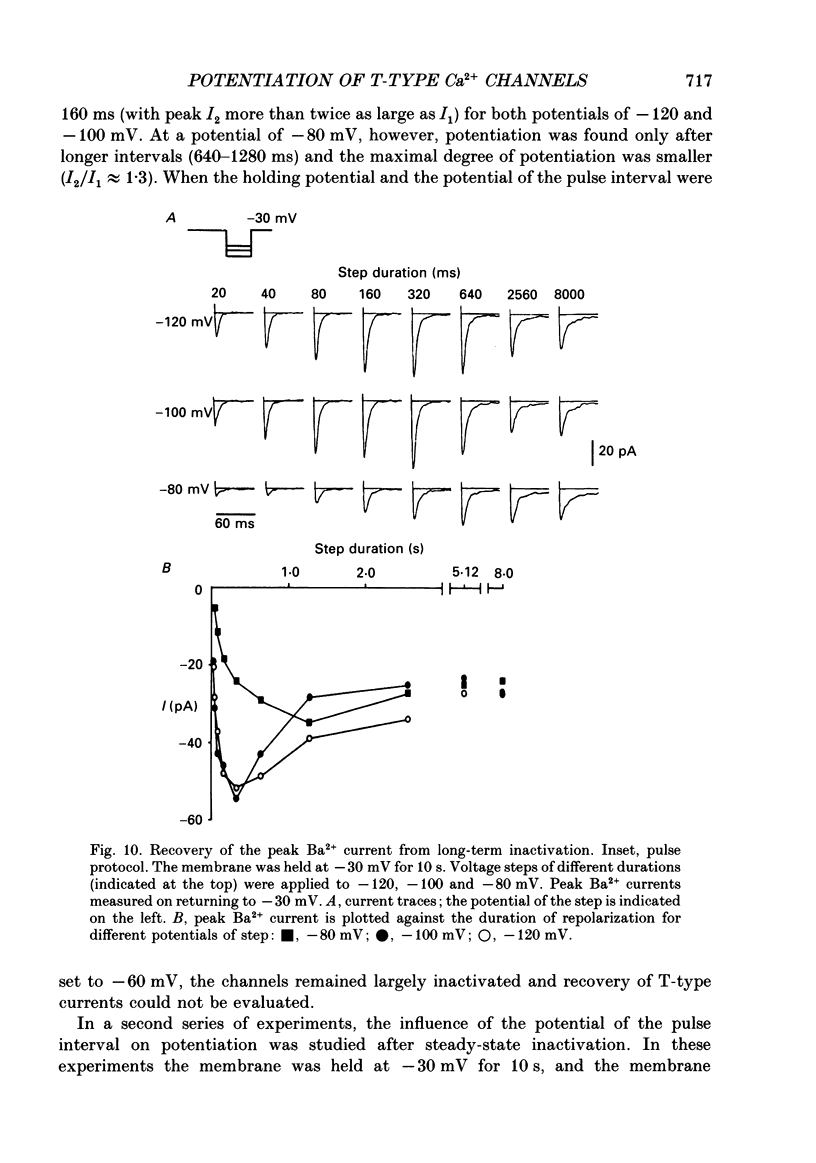
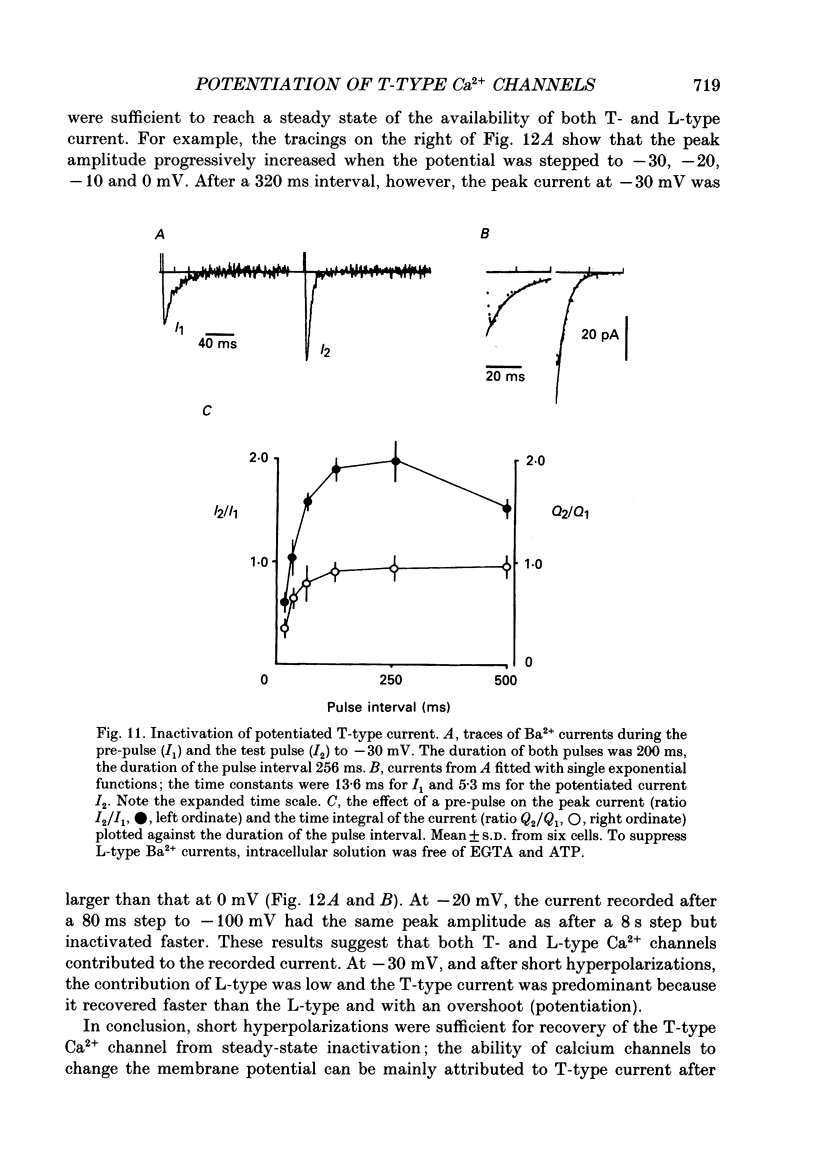
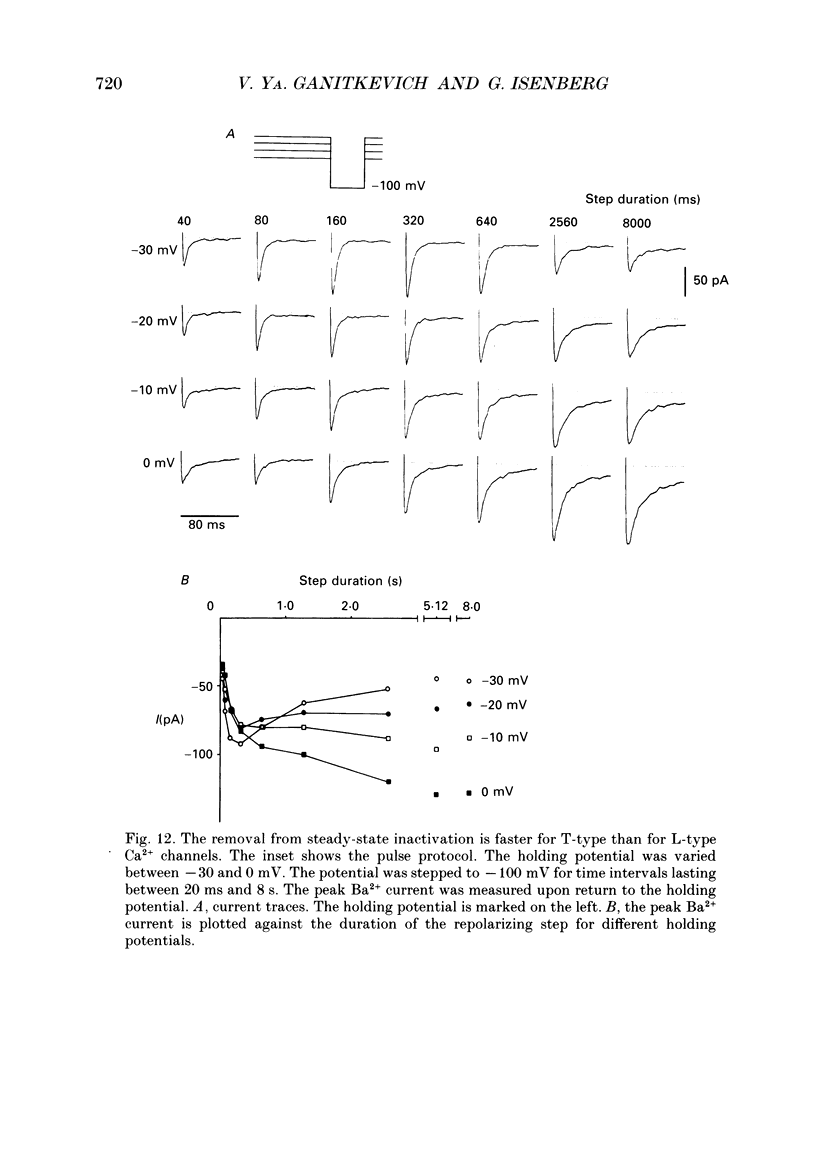
1. Whole-cell Ca2+ channel currents were studied in myocytes isolated from guinea-pig circumflex coronary artery at 36 degrees C and with 10 mM-Ba2+ (or Ca2+) as charge carrier. With 180 ms clamp steps from the holding potential of -100 mV, currents at -30 mV were carried mostly through the T-type calcium channels while at positive potentials currents were mostly of the L-type. 2. The increase in frequency of pulsing from 0.1 to 2.5 Hz resulted in a reduction of peak inward current ('negative staircase') with the 180 ms pulses to + 10 mV, but in a 2-fold potentiation ('positive staircase') with pulses to -30 mV. T-type currents and their frequency-mediated potentiation did not change significantly when Ba2+ was substituted by Ca2+ or Sr2+. 3. Potentiation of T-type currents was further analysed with a paired-pulse protocol: at a basal frequency of 0.1 Hz, a pre-pulse (inducing current I1) was followed by a 200 ms repolarization to -100 mV and a test pulse (inducing current I2). The potentiation could only be recorded using test pulses depolarizing the membrane to potentials between -40 and -10 mV; at more positive test potentials it was masked by the depressant effect of pre-pulses on the L-type current. 4. Potentiation of I2 by 200 ms pre-pulses started at pre-pulse potentials more positive than -60 mV and saturated at -20 mV (I2 potentiated by a factor 2.4). Between -20 and +130 mV the potentiation was not dependent on the pre-pulse potential suggesting that the influx of Ba2+ or Ca2+ is not required for this effect. Potentiation of I2 by a 10 s pre-pulse followed the voltage dependence of the steady-state inactivation curve of the T-type Ca2+ channel; potentiation became visible at potentials more positive than -80 mV and saturated at about -50 mV. 5. When changing the interval between two identical 200 ms pulses, the T-type current was found to recover completely from inactivation within 40 ms at -100 mV; at intervals of 160-320 ms maximal potentiation of I2 occurred. 6. With pre-pulses shorter than 200 ms, potentiation became attenuated when inactivation became less complete. When the potential during the interval between the pulses was -80 instead of -100 mV, maximal potentiation was reduced (I2 potentiated by a factor of 1.3 instead of 2.2) and occurred later (1.28 s). 7. Potentiated T-type currents inactivated faster.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF


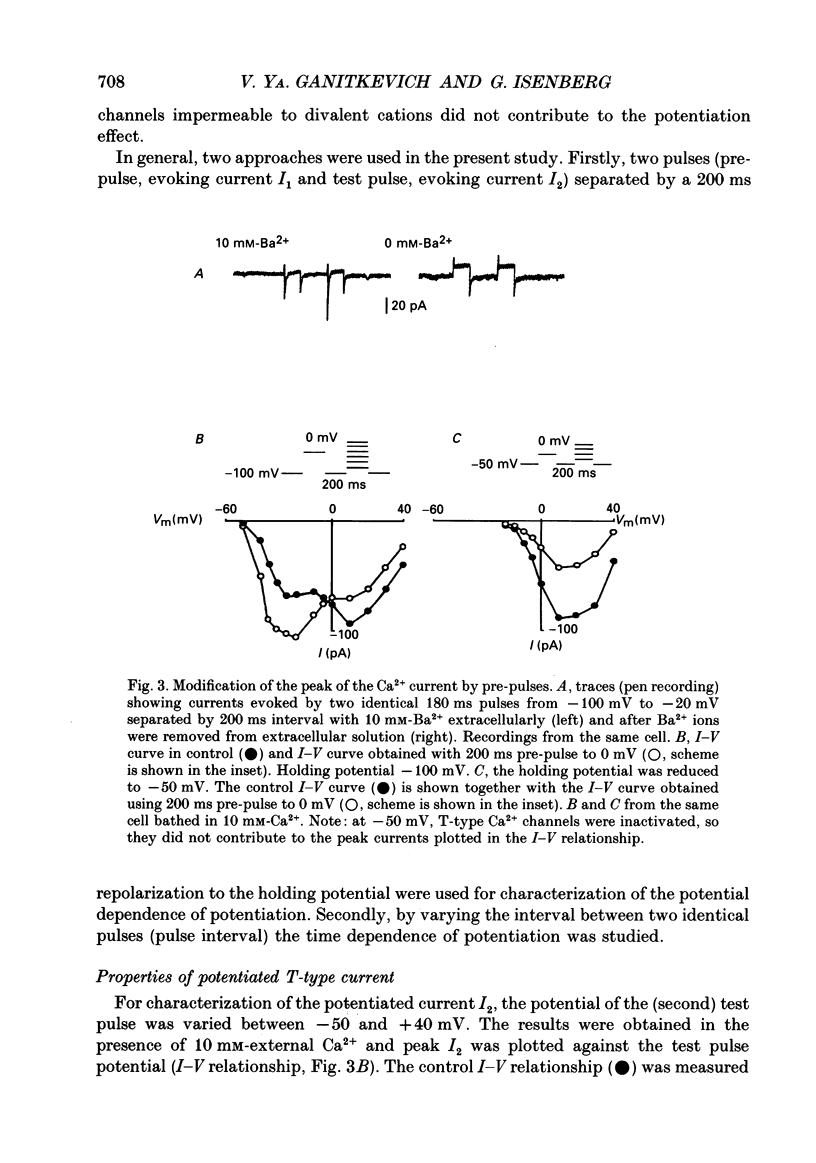
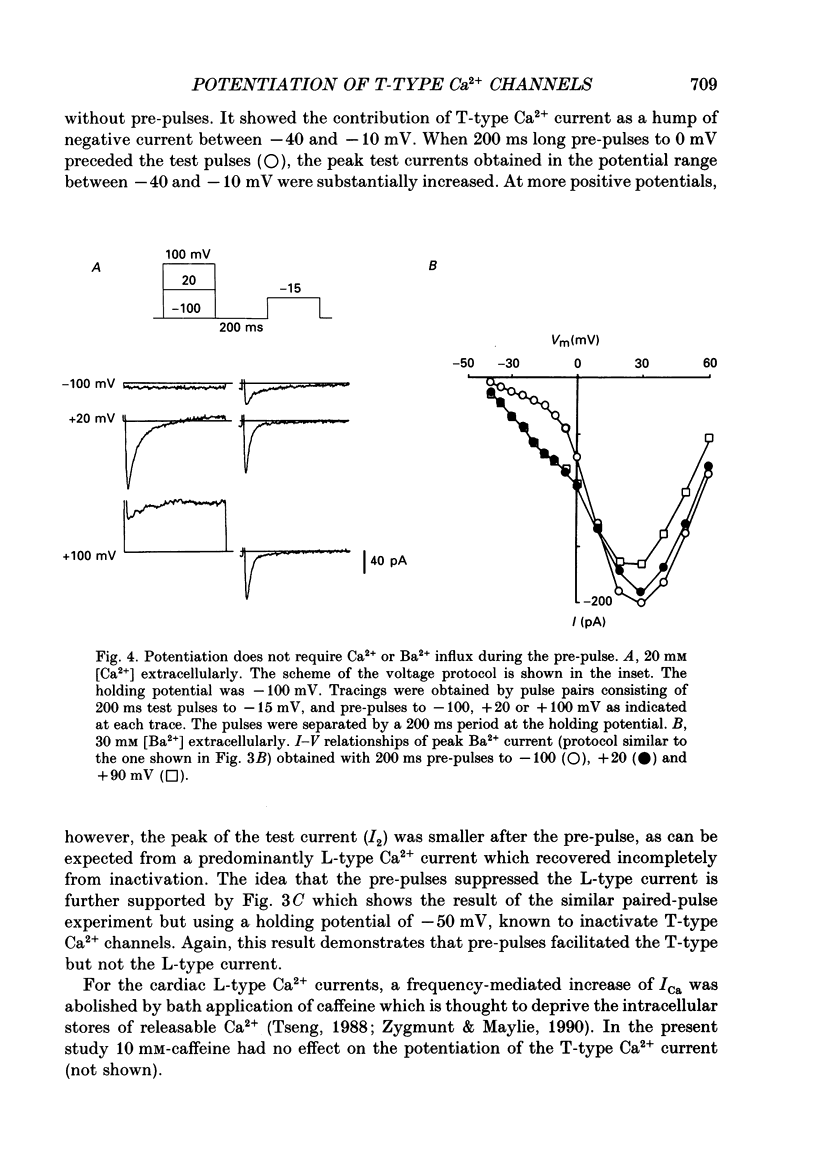

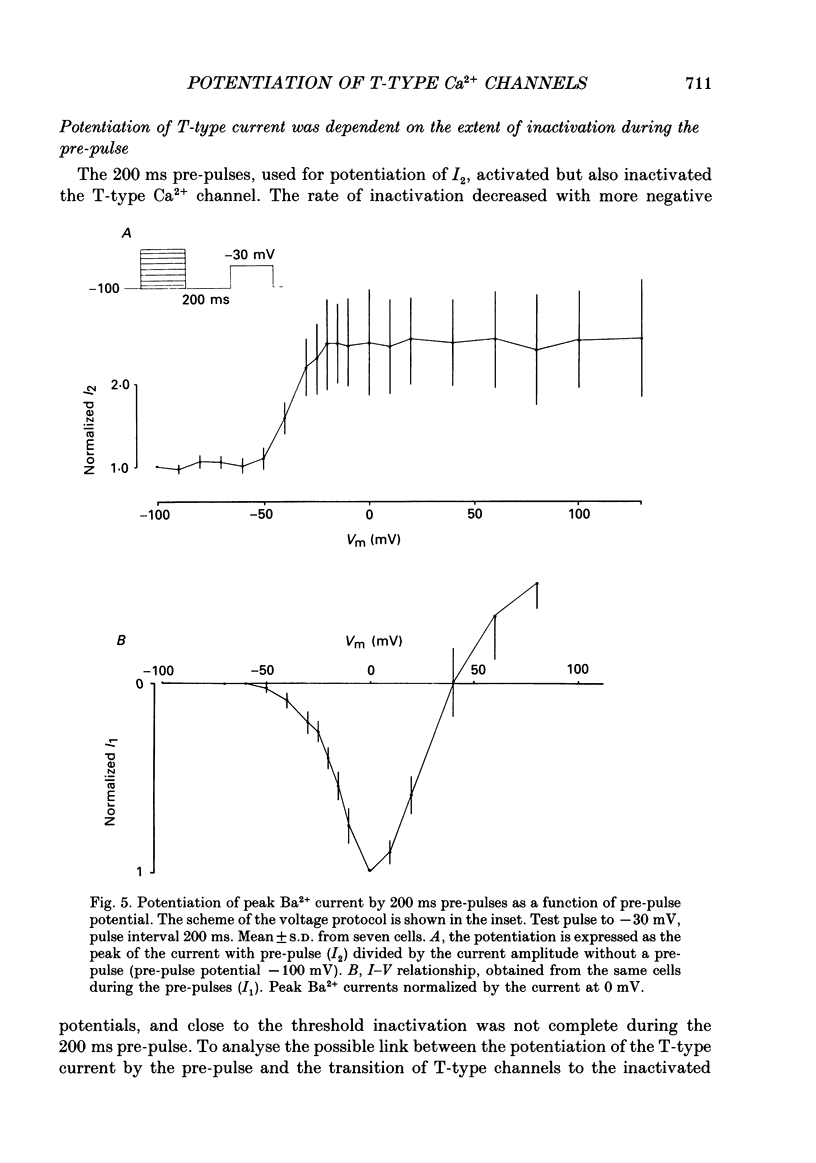
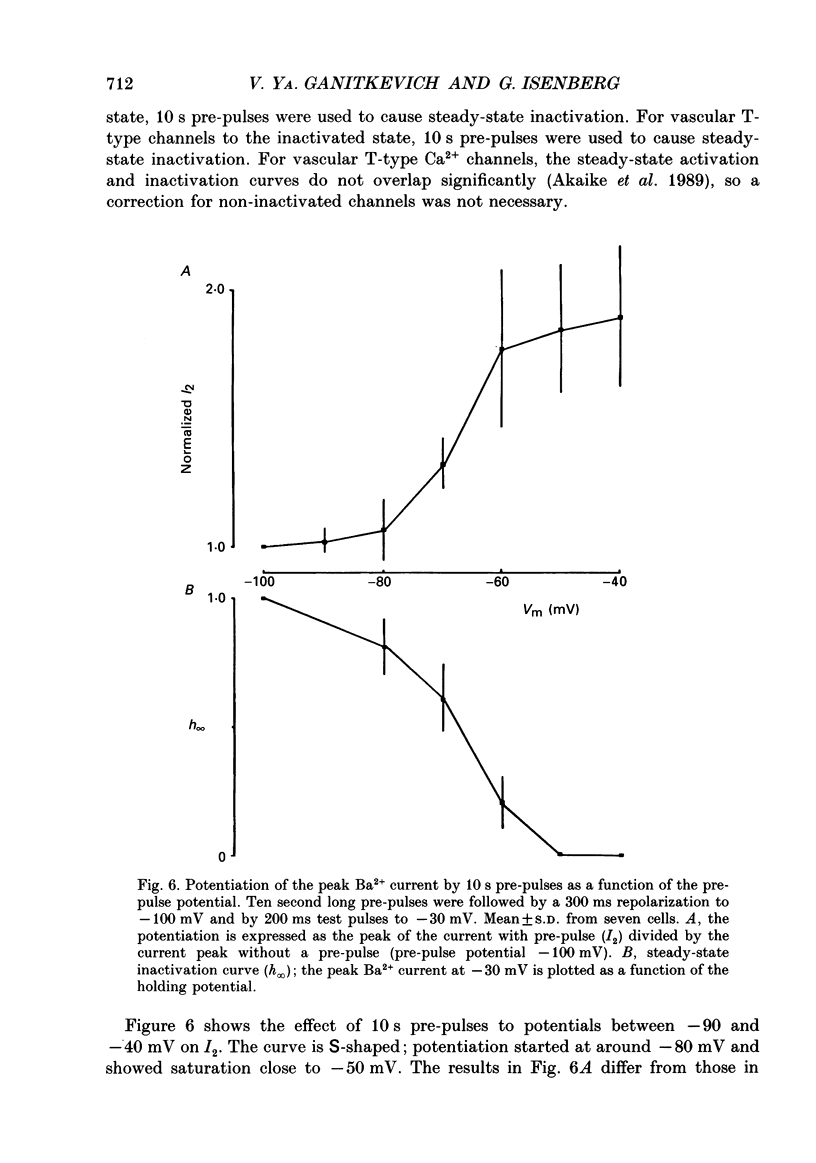







Selected References
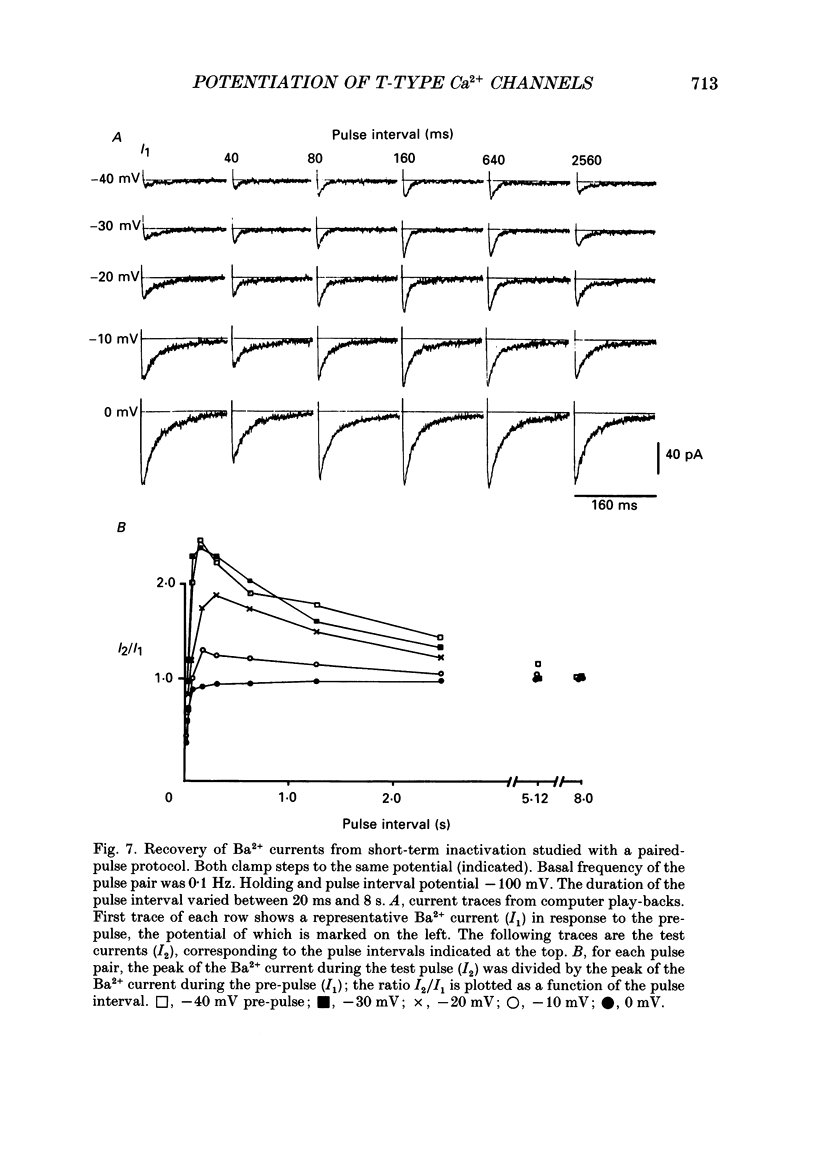
These references are in PubMed. This may not be the complete list of references from this article.
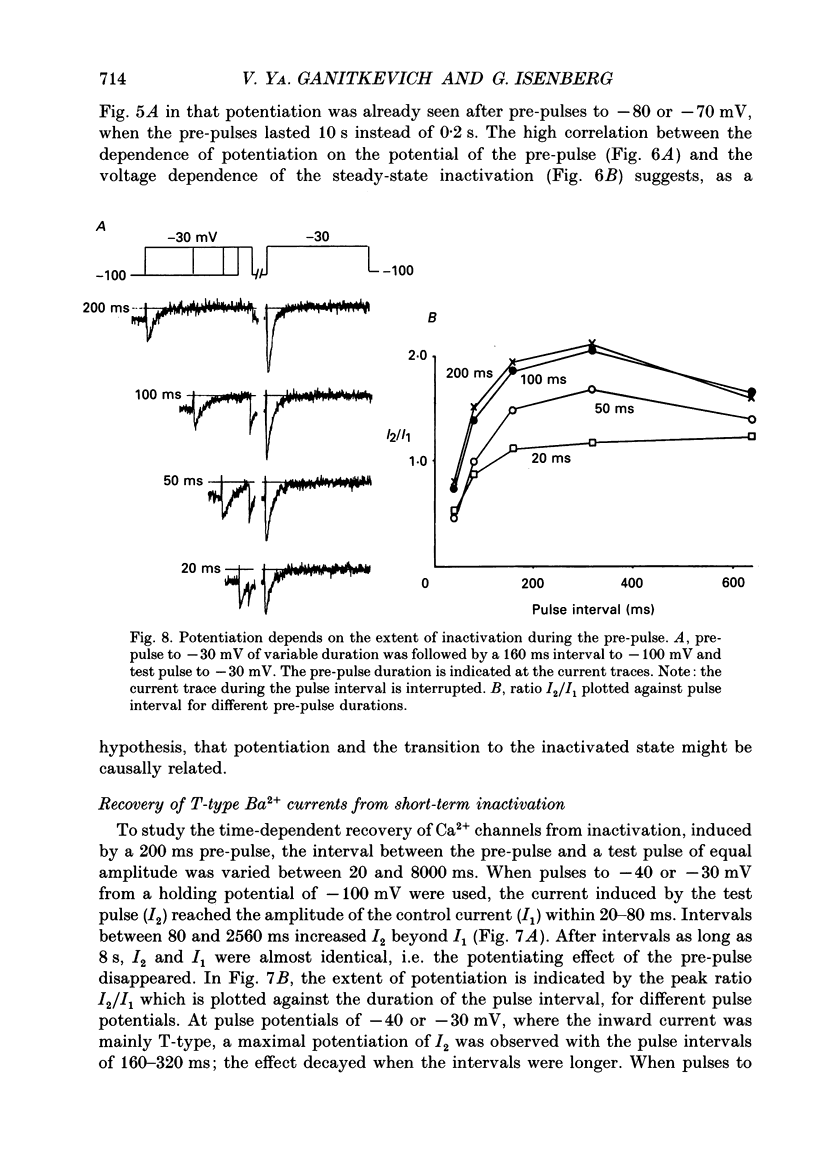
- Akaike N., Kanaide H., Kuga T., Nakamura M., Sadoshima J., Tomoike H. Low-voltage-activated calcium current in rat aorta smooth muscle cells in primary culture. J Physiol. 1989 Sep;416:141–160. doi: 10.1113/jphysiol.1989.sp017754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argibay J. A., Fischmeister R., Hartzell H. C. Inactivation, reactivation and pacing dependence of calcium current in frog cardiocytes: correlation with current density. J Physiol. 1988 Jul;401:201–226. doi: 10.1113/jphysiol.1988.sp017158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beech D. J., Bolton T. B. A voltage-dependent outward current with fast kinetics in single smooth muscle cells isolated from rabbit portal vein. J Physiol. 1989 May;412:397–414. doi: 10.1113/jphysiol.1989.sp017623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbone E., Lux H. D. Kinetics and selectivity of a low-voltage-activated calcium current in chick and rat sensory neurones. J Physiol. 1987 May;386:547–570. doi: 10.1113/jphysiol.1987.sp016551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbone E., Lux H. D. Single low-voltage-activated calcium channels in chick and rat sensory neurones. J Physiol. 1987 May;386:571–601. doi: 10.1113/jphysiol.1987.sp016552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C. F., Hess P. Mechanism of gating of T-type calcium channels. J Gen Physiol. 1990 Sep;96(3):603–630. doi: 10.1085/jgp.96.3.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Droogmans G., Nilius B. Kinetic properties of the cardiac T-type calcium channel in the guinea-pig. J Physiol. 1989 Dec;419:627–650. doi: 10.1113/jphysiol.1989.sp017890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganitkevich VYa, Isenberg G. Contribution of two types of calcium channels to membrane conductance of single myocytes from guinea-pig coronary artery. J Physiol. 1990 Jul;426:19–42. doi: 10.1113/jphysiol.1990.sp018125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara N., Irisawa H., Kameyama M. Contribution of two types of calcium currents to the pacemaker potentials of rabbit sino-atrial node cells. J Physiol. 1988 Jan;395:233–253. doi: 10.1113/jphysiol.1988.sp016916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano Y., Fozzard H. A., January C. T. Characteristics of L- and T-type Ca2+ currents in canine cardiac Purkinje cells. Am J Physiol. 1989 May;256(5 Pt 2):H1478–H1492. doi: 10.1152/ajpheart.1989.256.5.H1478. [DOI] [PubMed] [Google Scholar]
- Hirano Y., Fozzard H. A., January C. T. Inactivation properties of T-type calcium current in canine cardiac Purkinje cells. Biophys J. 1989 Nov;56(5):1007–1016. doi: 10.1016/S0006-3495(89)82745-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst G. D., Edwards F. R. Sympathetic neuroeffector transmission in arteries and arterioles. Physiol Rev. 1989 Apr;69(2):546–604. doi: 10.1152/physrev.1989.69.2.546. [DOI] [PubMed] [Google Scholar]
- Imaizumi Y., Muraki K., Watanabe M. Characteristics of transient outward currents in single smooth muscle cells from the ureter of the guinea-pig. J Physiol. 1990 Aug;427:301–324. doi: 10.1113/jphysiol.1990.sp018173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keef K. D., Bowen S. M. Effect of ACh on electrical and mechanical activity in guinea pig coronary arteries. Am J Physiol. 1989 Oct;257(4 Pt 2):H1096–H1103. doi: 10.1152/ajpheart.1989.257.4.H1096. [DOI] [PubMed] [Google Scholar]
- Lee K. S. Potentiation of the calcium-channel currents of internally perfused mammalian heart cells by repetitive depolarization. Proc Natl Acad Sci U S A. 1987 Jun;84(11):3941–3945. doi: 10.1073/pnas.84.11.3941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loirand G., Mironneau C., Mironneau J., Pacaud P. Two types of calcium currents in single smooth muscle cells from rat portal vein. J Physiol. 1989 May;412:333–349. doi: 10.1113/jphysiol.1989.sp017619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilius B., Hess P., Lansman J. B., Tsien R. W. A novel type of cardiac calcium channel in ventricular cells. Nature. 1985 Aug 1;316(6027):443–446. doi: 10.1038/316443a0. [DOI] [PubMed] [Google Scholar]
- Schouten V. J., Morad M. Regulation of Ca2+ current in frog ventricular myocytes by the holding potential, c-AMP and frequency. Pflugers Arch. 1989 Oct;415(1):1–11. doi: 10.1007/BF00373135. [DOI] [PubMed] [Google Scholar]
- Tseng G. N., Boyden P. A. Multiple types of Ca2+ currents in single canine Purkinje cells. Circ Res. 1989 Dec;65(6):1735–1750. doi: 10.1161/01.res.65.6.1735. [DOI] [PubMed] [Google Scholar]
- Tseng G. N. Calcium current restitution in mammalian ventricular myocytes is modulated by intracellular calcium. Circ Res. 1988 Aug;63(2):468–482. doi: 10.1161/01.res.63.2.468. [DOI] [PubMed] [Google Scholar]
- Wang R., Karpinski E., Pang P. K. Two types of calcium channels in isolated smooth muscle cells from rat tail artery. Am J Physiol. 1989 May;256(5 Pt 2):H1361–H1368. doi: 10.1152/ajpheart.1989.256.5.H1361. [DOI] [PubMed] [Google Scholar]
- Yatani A., Seidel C. L., Allen J., Brown A. M. Whole-cell and single-channel calcium currents of isolated smooth muscle cells from saphenous vein. Circ Res. 1987 Apr;60(4):523–533. doi: 10.1161/01.res.60.4.523. [DOI] [PubMed] [Google Scholar]
- Zygmunt A. C., Maylie J. Stimulation-dependent facilitation of the high threshold calcium current in guinea-pig ventricular myocytes. J Physiol. 1990 Sep;428:653–671. doi: 10.1113/jphysiol.1990.sp018233. [DOI] [PMC free article] [PubMed] [Google Scholar]


