Abstract
Lodging-resistant rice (Oryza sativa) cultivars usually show slow grain filling when nitrogen is applied in large amounts. This study investigated the possibility that a hormonal change may mediate the effect of water deficit that enhances whole plant senescence and speeds up grain filling. Two rice cultivars showing high lodging resistance and slow grain filling were field grown and applied with either normal or high amount nitrogen (HN) at heading. Well-watered and water-stressed (WS) treatments were imposed 9 days post anthesis to maturity. Results showed that WS increased partitioning of fixed 14CO2 into grains, accelerated the grain filling rate but shortened the grain filling period, whereas the HN did the opposite way. Cytokinin (zeatin + zeatin riboside) and indole-3-acetic acid contents in the grains transiently increased at early filling stage and WS treatments hastened their declines at the late grain filling stage. Gibberellins (GAs; GA1 + GA4) in the grains were also high at early grain filling but HN enhanced, whereas WS substantially reduced, its accumulation. Opposite to GAs, abscisic acid (ABA) in the grains was low at early grain filling but WS remarkably enhanced its accumulation. The peak values of ABA were significantly correlated with the maximum grain filling rates (r = 0.92**, P < 0.01) and the partitioning of fixed 14C into grains (r = 0.95**, P < 0.01). Exogenously applied ABA on pot-grown HN rice showed similar results as those by WS. Results suggest that an altered hormonal balance in rice grains by water stress during grain filling, especially a decrease in GAs and an increase in ABA, enhances the remobilization of prestored carbon to the grains and accelerates the grain filling rate.
Plant hormones are considered as key regulators to seed development (Davies, 1987; Brenner and Cheikh, 1995). In cereals, peas (Pisum sp.), and beans (Phaseolus sp.), high levels of cytokinins are generally found in the endosperm of developing seeds, which may be required for the cell division during the early phase of seed setting (Yang et al., 2000a). Morris et al. (1993) reported that zeatin (Z) and zeatin riboside (ZR) in developing rice (Oryza sativa) and wheat (Triticum aestivum) grains showed large transient increases following pollination, which coincided with the period of seed setting and maximum endosperm cell division.
There are many reports that auxins, gibberellins (GAs), and abscisic acid (ABA) are also involved in regulating grain development (Karssen, 1982; Davies, 1987; Kende and Zeevaart, 1997; Hansen and Grossmann, 2000). Eeuwens and Schwabe (1975) stated that GA-like material was highest in liquid endosperm of pea at the time of rapid pod elongation. Relatively high levels of GA1,4,19 existed in the large panicle of rice just before and at anthesis (Kurogochi et al., 1979; Suzuki et al., 1981). Lur and Setter (1993) observed that indole-3-acetic acid (IAA) concentrations abruptly increased in the endosperm of maize (Zea mays) kernels at about 10 d after pollination, which coincided with an increase in DNA content per nucleus. Kato et al. (1993) reported that ABA content in large-size grains was higher than that in small-size grains during rice grain filling. ABA content in wheat grains was positively correlated with grain filling rate at the early grain filling stage (Bai et al., 1989). Wang et al. (1998) and Yang et al. (1999) suggested that the poor grain filling was associated with low IAA and ABA contents in rice grains.
Improving grain filling is vitally important in cases where slow grain filling is a problem, e.g. heavy use of nitrogen (N) fertilizer (Ling et al., 1993; Yang et al., 1996), or adoption of lodging-resistant cultivars of which some stay “green” for too long (Yuan, 1997; Zhu et al., 1997). Our early work showed that water stress imposed during the grain filling of wheat could enhance remobilization of prestored carbon reserves to the grains and grain filling rate (Yang et al., 2000b, 2001). However, little is known about whether and how plant hormones are involved in the processes. The purposes of this study were to investigate the changes in cytokinin, IAA, GA, and ABA contents in the grains of rice subjected to water deficit stress during grain filling, and determine how the hormonal changes were correlated with the grain filling process.
RESULTS
Changes in Leaf Water Potential
Figure 1 illustrates the progression of leaf water potential changes during the first 30 d after withholding water. Both cultivars exhibited a similar trend of leaf water potential changes either at predawn or at midday. When plants were well watered (WW), midday leaf water potential decreased gradually during grain filling, from −0.43 and −0.48 million Pa (MPa) at the beginning to −0.73 and −0.85 MPa at 30 d after withholding water. Water-stressed (WS) treatments substantially reduced midday leaf water potentials from −0.44 and −0.52 MPa initially to −1.25 and −1.84 MPa at 30 d after withholding water. Plants at high amount of N (HN) had a lower midday leaf water potential than those at normal amount of N (NN), even though soil water potential (ψsoil) was kept at the same level, suggesting that plants with better N nutrition lost more water. The differences in predawn leaf water potentials between WW and WS, or between NN and HN treatments, were insignificant, indicating that plants subjected to the water deficit could rehydrate overnight.
Figure 1.
Changes of leaf water potentials of the rice japonica cv Wuyujing 3 (A) and rice indica cvYangdao 4 (B) during the first 30 d after withholding water. The treatments are: NN + WW (●), NN + WS (○), HN + WW (▪), and HN + WS (□). Measurements were made on the flag leaves at predawn (6 am, dashed lines) and at midday (11:30 am, black lines). Vertical bars represent ±se of the mean (n = 6) where these exceed the size of the symbol.
Fixed Carbon Partitioning and Grain Filling Rate
At maturity, 79% to 85% of 14CO2 (14C) fed to the flag leaves was partitioned into the grains under WS-NN, whereas only 55% to 66% went to the grains under WW-NN treatments (Table I), indicating that more fed 14C was remobilized and deposited into the grains by the water stress. In comparison, HN reduced 14C allocation into the grains.
Table I.
Partitioning of fed 14CO2 in rice plants subjected to various N and soil moisture treatments
| Cultivars | Nitrogen Applied | Water Stress Treatment |
14C Partitioning
|
||
|---|---|---|---|---|---|
| Grains | Stems + sheaths | Leaves | |||
| % | |||||
| Wuyujing 3 | NN | WW | 55 ± 4.7 | 23 ± 3.1 | 22 ± 2.5 |
| NN | WS | 79 ± 4.8 | 5.8 ± 1.3 | 15 ± 1.6 | |
| HN | WW | 39 ± 4.1 | 31 ± 2.9 | 30 ± 2.8 | |
| HN | WS | 65 ± 3.9 | 11 ± 2.3 | 24 ± 2.2 | |
| Yangdao 4 | NN | WW | 66 ± 3.8 | 18 ± 2.4 | 16 ± 1.3 |
| NN | WS | 85 ± 5.2 | 3.5 ± 0.7 | 11 ± 1.1 | |
| HN | WW | 51 ± 4.1 | 21 ± 3.1 | 28 ± 3.1 | |
| HN | WS | 71 ± 4.6 | 10 ± 1.2 | 19 ± 1.7 | |
NN and HN indicate normal and high levels of N application at heading time. WW and WS are treatments during the grain filling. Labeling was carried out on the flag leaves at heading. Data are expressed as means ± se of six plants.
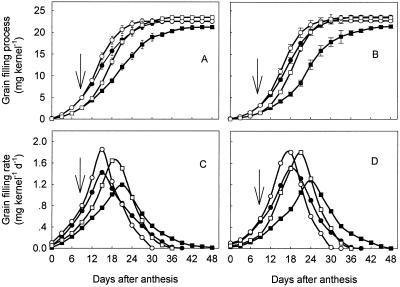
Under WW treatments, HN slowed grain filling (Fig. 2). WS treatments increased the grain filling rate and shortened the grain filling period at both HN and NN. The increased rate and the shortened period were more remarkable at HN than at NN (Fig. 2, C and D).
Figure 2.
Grain filling process (A and B) and grain filling rate (C and D) of the rice japonica cv Wuyujing 3 (A and C) and rice indica cvYangdao 4 (B and D) subjected to various N and soil moisture treatments. The treatments are: NN + WW (●), NN + WS (○), HN + WW (▪), and HN + WS (□). Grain filling rate was calculated according to the Richards (1959) equation. Arrows in the figure indicate the start of withholding water. Vertical bars in the figure A and B represent ±se of the mean (n = 2) where these exceed the size of the symbol.
As showed in Figure 2, A and B, the final grain weight was not significantly different between WW and WS treatments when NN was applied. However, it was significantly increased under WS-HN treatments, implying that the gain from accelerated grain filling rate outweigh the possible loss of photosynthesis as a result of a shortened grain filling period when subjected to water stress during grain filling.
Hormonal Changes in the Grains
The methods used in this study for hormone extraction and purification and for quantification hormones by an ELISA recovered 79.6% of Z, 80.2% of ZR, 70.2% of IAA, 74.6% of GAs (GA1 + GA4), and 83.0% of ABA (Table II). The specificity of the monoclonal antibodies and the other possible nonspecific immunoreactive interference were checked by Wu et al. (1988), Zhang et al. (1991), and He (1993) and proved reliable.
Table II.
Recovery test of ELISA for Z, ZR, IAA, gibberellins (GAs), and abscisic acid (ABA)
| Hormone | Graina | Grain + Standardb | Recovery |
|---|---|---|---|
| ng g fresh wt−1 | % | ||
| Z | 101 ± 13.4 | 499 ± 43.9 | 79.6 ± 6.3 |
| ZR | 128 ± 19.6 | 529 ± 58.7 | 80.2 ± 7.2 |
| IAA | 243 ± 21.3 | 594 ± 58.2 | 70.2 ± 6.8 |
| GAs | 304 ± 26.2 | 677 ± 60.1 | 74.6 ± 6.6 |
| ABA | 146 ± 11.8 | 561 ± 40.8 | 83.0 ± 5.4 |
The test cultivar was Wuyujung 3 (Japonica). Data are expressed as means ± se of four replications.
Grains only.
Grains + synthetic compounds of Z, ZR, IAA, GAs (GA1 + GA4), and ABA, respectively. Five hundred nanograms of each compound was added to 1 g of fresh grains before purification. Z, ZR, IAA, and ABA were from Sigma Chemical Co. (St. Louis) and GAs were provided by China Agricultural University (Beijing, China).
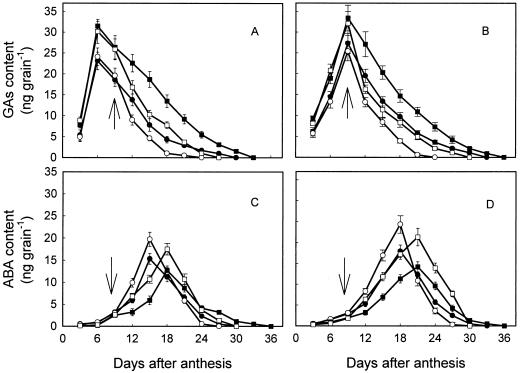
The grains contained 26% to 33% more ZR than Z, and both showed a similar changing pattern during grain filling (data not shown). Z + ZR contents in the grains transiently increased at early grain filling stage, and reached a maximum at 9 to12 DPA at NN and 12 to15 DPA at HN, and decreased thereafter (Fig. 3, A and B). During the first week of withholding water (9–15 DPA), the difference in Z + ZR contents was not significant between WW and WS treatments. The grains under WW contained more Z + ZR than under WS treatments only at the mid- and late-grain filling stages (18 DPA afterward). Z + ZR contents were lower in HN grains than in NN grains at early grain filling stage (3–15 DPA) when ψsoil was kept at the same level. During mid- and late-grain filling periods, the difference was reversed and HN grains had more cytokinins than NN ones.
Figure 3.
Changes of Z + ZR (A and B) and IAA (C and D) in the grains of the rice japonica cv Wuyujing 3 (A and C) and rice indica cv Yangdao 4 (B and D) subjected to various N and soil moisture treatments. The treatments are: NN + WW (●), NN + WS (○), HN + WW (▪), and HN + WS (□). Arrows in the figure indicate the start of withholding water. Vertical bars in the figure represent ±se of the mean (n = 4) where these exceed the size of the symbol.
Very similar to the changing pattern of Z + ZR, IAA content in the grains sharply increased during early grain filling stage, reached a maximum at 9 to 15 DPA, and dropped very quickly thereafter (Fig. 3, C and D). Water stress treatments significantly decreased IAA in the grains during mid- and late-grain filling stages. Compared with the grains with NN treatments, those with HN contained lower IAA before 9 to 12 DPA but higher thereafter when ψsoil was the same.
Increases in Z + ZR and IAA in the grains at early grain filling stage were associated with the increase of grain filling rate (refer to Fig. 2, C and D). The time reaching the maximum for Z + ZR and IAA was just before the time of a maximum grain filling rate, suggesting that cytokinins and IAA may regulate the grain filling of rice at the early stage.
GAs (GA1 + GA4) contents in rice grains were high at early grain filling stage, and reached a maximum at 6 DPA for the japonica and 9 DPA for the rice cv indica under either WW and WS, or NN and HN treatments (Fig. 4, A and B). The HN grains contained more GAs than NN grains when ψsoil was the same. Water stress treatments substantially reduced GAs in the grains at either NN or HN.
Figure 4.
Changes of GAs (GA1 + GA4; A and B) and ABA (C and D) in the grains of the rice japonica cv Wuyujing 3 (A and C) and rice indica cv Yangdao 4 (B and D) subjected to various N and soil moisture treatments. The treatments are: NN + WW (●), NN + WS (○), HN + WW (▪), and HN + WS (□). Arrows in the figure indicate the start of withholding water. Vertical bars in the figure represent ±se of the mean (n = 4) where these exceed the size of the symbol.
Opposite to the appearance of GAs, ABA content in the grains was low and slowly increased at early grain filling stage, and reached a maximum at 15 to18 DPA under NN and 18 to 21 DPA under HN treatments (Fig. 4, C and D). Water stress remarkably enhanced ABA accumulation in the grains at both NN and HN. At early and mid-grain filling stages, the grain with NN contained more ABA than those with HN treatments when the ψsoil was kept at the same level.
The increases and declines of ABA content in the grains coincided with the grain filling rate (refer to Fig. 2, C and D). The time for ABA to reach the peak value was simultaneous with the time of the maximal grain filling rate. ABA accumulation in the grains was also associated with the partitioning of the 14C into the grains (refer to Table I). The peak values of ABA in the grains were positively and significantly correlated with the maximum grain filling rates (r = 0.92**, P < 0.01) and the partitioning of the 14C into grains (r = 0.95**, P < 0.01). The maximal grain filling rate and the partitioning of the C into the grains were not significantly corrected with the peak values of Z + ZR (r = 0.12–0.34, P > 0.05), IAA (r = 0.42–0.46, P > 0.05), and GAs (r = −0.14 to −0.45, P > 0.05).
Effect of Exogenous ABA Application
When ABA was applied to HN and WW pot-grown plants at early grain filling stage (9 DPA), ABA content and the ratio of ABA to GAs in grains were significantly increased (Table III). The active grain filling period was shortened by 6 or 7 d. The grain filling rate, grain weight, and remobilized carbon reserve in the stems were increased by 0.20 to 0.21 mg d−1 kernel−1, 0.8 to 0.9 mg kernel−1, and 44% to 46%, respectively, when compared with the control (Table IV).
Table III.
Effect of exogenously applied ABA on ABA contents in grains
| Cultivar | Treatment | 12 DPA
|
20
DPA
|
||||
|---|---|---|---|---|---|---|---|
| ABA | GAs | ABA/GAs | ABA | GAs | ABA/GAs | ||
| ng grain−1 | |||||||
| Wuyujing 3 | Control | 4.9 | 28.7 | 0.17 | 8.8 | 9.4 | 0.94 |
| 25 × 10−6m ABA | 18.8**a | 25.1* | 0.75** | 15.4** | 5.2* | 2.96** | |
| Yangdao 4 | Control | 7.6 | 24.6 | 0.31 | 10.6 | 9.3 | 1.14 |
| 25 × 10−6m ABA | 21.2** | 18.7* | 1.13** | 17.9** | 7.2b | 2.49** | |
The plants were grown in WW pots with an HN applied. The leaves and panicles were sprayed with 25 × 10−6 m ABA daily for 7 d starting at 9 DPA. Each measurement was five replications. Statistical comparison was within the same column and the same cultivar.
∗ and ∗∗, Significantly different at the P = 0.05 and 0.01 levels of probability, respectively.
Not significantly different.
Table IV.
Effect of exogenously applied ABA on remobilization of carbon, active grain filling period, grain filling rate, and grain wt of the plants grown in WW pots with HN
| Cultivar | Treatment | Active Grain Filling Period | Grain Filling Rate | Grain Wt | Remobilized Carbon Reservea |
|---|---|---|---|---|---|
| d | mg kernel−1 d−1 | mg kernel−1 | % | ||
| Wuyujing 3 | Control | 41 | 0.63 | 25.8 | 35.8 |
| 25 × 10−6m ABA | 34**b | 0.83** | 26.7* | 51.9** | |
| Yangdao 4 | Control | 39 | 0.68 | 26.4 | 39.5 |
| 25 × 10−6m ABA | 33** | 0.89** | 27.6* | 57.4** |
The leaves and panicles were sprayed with 25 × 10−6 m ABA daily for 7 d starting at 9 DPA. Each measurement was five replications. Statistical comparison was within the same column and the same cultivar.
(nonstructural carbohydrate [NSC] in stems at anthesis − NSC in stems at maturity)/NSC in stems at anthesis × 100.
∗ and ∗∗, Significantly different at the P = 0.05 and 0.01 levels of probability, respectively.
DISCUSSION
Our results confirmed early reports (Saha et al., 1986; Morris et al., 1993) that cytokinin contents in rice grains showed a transient increase at early grain filling stage. Our own earlier work (Yang et al., 2000c) with the same rice cultivars observed that maximum cell division rate in endosperm occurred 10 to14 DPA at NN and 12 to 18 DPA at HN. The current study demonstrated that the maximum levels of Z + ZR contents in the grains occurred 9 to12 DPA at NN and 12 to15 DPA at HN, indicating that the maximal level of cytokinins coincides with endosperm cell division in the developing grains.
Davies (1987) stated that auxin also stimulates cell division in combination with cytokinins. High auxin levels in the sink could create an “attractive power,” leading to an increased cytokinin levels in the grains (Seth and Waering, 1967; Singh and Gerung, 1982). In this experiment, the changes in IAA content in the grains were very similar to the changing pattern of Z + ZR (Fig. 3). Both IAA and Z + ZR contents were associated with grain filling rate at the early grain filling stage, and their maximal levels appeared just before the maximal grain filling rate. Results suggest that cytokinin and IAA may regulate grain filling at the early filling stage, probably via manipulating the division of endosperm cells and creating the sink strength.
Water stress imposed during grain filling, especially at the early filling stage, usually results in the reduction in grain weight (Kobata and Takami, 1983; Rahman and Yoshida, 1985; Boonjung and Fukai, 1996; Zhang et al., 1998). The reduction is mainly attributed to the lowered number of endosperm cells, thereby decreased sink size per kernel (Singh and Jenner, 1982; Kobata and Takami, 1983; Rahman and Yoshida, 1985; Michihiro et al., 1994). Our results showed that if a water stress was initiated at 9 DPA and controlled to a degree that plant could rehydrate overnight (Fig. 1), the maximum levels of Z + ZR and IAA in the grains were not affected (Fig. 3). The grain weight was not reduced by the controlled water deficit (Fig. 2). We speculate that the division of endosperm cells was not affected under water stress in this experiment.
Two sharply contrasting patterns were found between GAs (GA1 + GA4) and ABA contents in the grains during grain filling period (Fig. 4). GAs were high at early grain filling stage and had maximal levels at 6 to 9 DPA, which was associated with the rapid enlargement of the embryo (Qin and Tang, 1984), suggesting that GAs may play a role in embryogenesis. ABA content in rice grains was low at the early filling stage. Water stress treatments remarkably increased ABA accumulation, whereas substantially decreased GAs contents in the grains. The maximal grain filling rate and the partitioning of fed 14C into grains were positively and significantly correlated with the peak values of ABA, whereas weakly and negatively correlated with GAs in the grains, indicating that ABA accumulation in grains during grain development could promote grain filling and enhance the remobilization of assimilates to the grains. Similar results were also reported on wheat (Dewdney and McWha, 1979; Bai et al., 1989), barley (Tietz et al., 1981) and soybean (Glycine max L. Merr; Ackerson, 1985).
It is notable that when HN was applied, cytokinins, IAA, and ABA contents in the grains were low at early grain filling stage and high at the late-filling stage, and GAs were kept at high levels during the whole period, much of fed 14C remained in the straws, and slow grain filling and low grain weight happened. When a water deficit stress was imposed during grain filling period, levels of each hormone, especially GAs and ABA contents in the grains changed, and grain filling period was shortened but fed 14C was more remobilized and deposited into the grains and grain filling rate increased.
Very similar to the water deficit stress, exogenous ABA application at early grain filling stage increased ABA and the ratio of ABA to GAs in grains (Table III). The grain filling period was greatly shortened, and remobilized carbon reserve in the stems, grain filling rate, and grain weight were all significantly increased (Table IV). The results support the conclusion that an altered balance of hormones in the grains mediates the effect of water stress that enhances the remobilization of prestored carbon in vegetative tissues to the grains and determines the period and the rate of grain filling in rice.
Therefore, we conclude that if a water deficit during the grain filling of rice is controlled properly so that plant can rehydrate overnight, an altered hormonal balance, especially decreases in GAs and increases in ABA in the grains, may enhance the remobilization of prestored carbon to the grains, shorten the grain filling period, but accelerate grain filling rate. The gain from the accelerated grain filling rate may outweigh the loss of the shortened grain filling period, i.e. the loss of photosynthesis, and increase the grain weight in cases where slow grain filling is a problem with heavy use of N or cultivars that stay green for too long.
MATERIALS AND METHODS
Plant Materials
The experiment was conducted at a farm of Yangzhou University (Jiangsu Province, China; 32o30′N, 119o25′E) during rice (Oryza sativa) growing season (May to October) of 1999, and repeated in 2000. Two lodging-resistant rice cultivars currently used in local rice production, cv Wuyujing 3 (japonica) and cv Yangdao 4 (indica), were grown in the paddy field. Seedlings were raised in the field with the sowing date on May 10 through 11 and transplanted on June 10 through 11 at a hill spacing of 0.20 m × 0.16 m with two seedlings per hill. The soil of the field was sandy loam with 24.5 g kg−1 organic matter and available N-phosphorus-potassium at 105, 33.5, and 66.0 mg kg−1, respectively. N (60 kg ha−1 as urea), phosphorus (30 kg ha−1 as single superphosphate), and potassium (40 kg ha−1 as KCl) were applied and incorporated before transplanting. N as urea was also applied at mid-tillering (40 kg ha−1) and at panicle initiation (25 kg ha−1). Both cultivars (50% of plants) headed on August 20 through 22 and were harvested on October 9 through 10. Except drainage at end-tillering (July 12–14), the field was kept at a 1- to 2-cm water level until 9 DPA, when water deficit treatments were initiated. The temperatures, averaged per 10 d from anthesis (August 21–23) to harvest, were 26.9°C, 26.3°C, 25.2°C, 24.3°C, 23.2°C, and 22.6°C, respectively.
N and Water Stress Treatments
The experiment was a 2 × 2 × 2 (two cultvars, two levels of N, and two levels of soil moisture) factorial design with eight treatment combinations. Each of the treatments had three plots as repetitions in a complete randomized block design. Plot dimension was in 4.2 × 3.2 m and plots were separated by a ridge (40 cm in width) wrapped with plastic film. Two levels of N treatments were applied at initial heading (10% of plants headed on August 18–19). One-half of the plots were top dressed with either 5 g N m−2 (NN) or 10 g N m−2 (HN) as urea. From 9 DPA to maturity, two levels of ψsoil were imposed on the plants of both NN and HN treatments. The WW treatment was kept at 1- to 2-cm water depth (ψsoil = 0) in the field by manually applying tap water every day, and the WS was maintained ψsoil at −0.05 MPa. The ψsoil in the WS treatment was monitored with tension meters buried in the 15- to 20-cm soil depth. Five tension meters were installed in each plot to monitor. Tension meter readings were recorded every 6 h from 6 am to 6 pm. When the reading dropped to the designed value, 100 L of tap water per plot was added manually to the WS treatment. A rain shelter consisting of a steel frame covered with a plastic sheet was used to protect the plot during rains.
Radioactive Labeling
Flag leaves from six main stems from each treatment were labeled with 14CO2 at heading. Labeling was at 9 am to 11 am on a clear day with photosynthetically active radiation at the top of the canopy ranging from 1,000 to 1,100 μmol m−2 s−1. The whole flag leaf was placed into a polyethylene chamber (25-cm length and 4-cm diameter) and sealed with tape and plasticine to get a gas-tight seal. Six milliliters of air in the chamber was drawn out and the same volume of gas was injected into the chamber which contained 0.01 mol L−1 CO2 at specific radioactivity of 14C at 1.48 MBq L−1. The chamber was removed after 0.5 h.
The labeled plants were sampled at maturity. Each plant was divided into leaf blades, culms plus sheaths, and panicles (grains + branches and rachis). Samples were dried at 80°C to constant weight, ground into powder, and then extracted by shaking in 630 g L−1 boiling ethanol. The radioactivity of 14C in the extracted aliquots was counted by a liquid scintillation counter (Beckman Instruments Inc., Fullerton, CA). Radioactivity distribution in each part of the plant was expressed as a percentage of total radioactivity remaining in the aboveground portion of the plant.
Measurement of Leaf Water Potential
Leaf water potential of the flag leaves were measured at predawn (6 am) and midday (11:30 am) on 0, 2, 4, 7, 10, 13, 15, 18, 21, 25, and 30 d after withholding water when the sky was clear. Well-illuminated flag leaves were chosen randomly for such measurements. A pressure chamber (model 3000, Soil Moisture Equipment Corp., Santa Barbara, CA) was used for leaf water potential measurement with six leaves for each treatment.
Sampling
Five-hundred panicles that headed on the same day were chosen and tagged for each treatment. The flowering date and the position of each spikelet on the tagged panicles were recorded. Thirty-six to 45 tagged panicles from each treatment were sampled every 3-d interval from anthesis to maturity. The sampled panicles were divided into four groups (12–15 panicles each) as subsamples. Grains that developed from spikelets that flowered on the same day were removed. Half-sampled grains were frozen in liquid N for 1 min and then stored at −80°C for hormonal assay. Another half-sampled grains were dried at 70°C to constant weight for 72 h, and weighed. The grain-filling process was fitted by Richards' (1959) growth equation as described by Zhu et al. (1988):
 |
1 |
Grain filling rate (G) was calculated as the derivative of Equation 1:
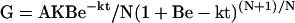 |
2 |
where W is the grain weight (mg), A is the final grain weight (mg), t is the time after anthesis (d), and B, k, and N are coefficients determined by regression. The active grain filling period was defined as that when W was from 5% (t1) to 95% (t2) of A. The average grain filling rate during this period was calculated from t1 to t2.
Hormone Extraction and Purification
The methods for extraction and purification of Z, ZR, IAA, GAs (GA1 + GA4), and (±) ABA were modified from those described by Bollmark et al. (1988) and He (1993). Samples consisting of 50 to 80 dehulled and frozen grains were ground in an ice-cooled mortar in 10 mL 80% (v/v) methanol extraction medium containing 1 mm butylated hydroxytoluence as an antioxidant. The extract was incubated at 4°C for 4 h and centrifuged at 4,000 rpm for 15 min at the same temperature. The supernatant was passed through Chromosep C18 columns (C18 Sep-Park Cartridge, Waters Corp., Millford, MA), prewashed with 10 mL 100% (w/v) and 5 mL 80% (v/v) methanol, respectively. The hormone fractions eluted with 10 mL 100% (v/v) methanol and 10 mL ether from the columns were dried under N2, and dissolved in 2 mL phosphate buffer saline (PBS) containing 0.1% (v/v) Tween 20 and 0.1% (w/v) gelatin (pH 7.5) for analysis by ELISA.
Quantification of Hormones by ELISA
The mouse monoclonal antigens and antibodies against Z, ZR, IAA, GAs (GA1 + GA4), and ABA, and IgG-horseradish peroxidase used in ELISA were produced at the Phytohormones Research Institute (China Agricultural University; see He, 1993). ELISA was performed on a 96-well microtitration plate. Each well on the plate was coated with 100 μL coating buffer (1.5 g L−1 Na2CO3, 2.93 g L−1 NaHCO3, and 0.02 g L−1 NaN3, pH 9.6) containing 0.25 μg mL−1 antigens against the hormones. The coated plates were incubated for 4 h at 37°C for Z, ZR, GAs, and ABA, and overnight at 4°C for IAA, and then kept at room temperature for 30 to 40 min. After washing four times with PBS + Tween 20 (0.1% [v/v]) buffer (pH 7.4), each well was filled with 50 μL of either grain extracts or Z, ZR, IAA, GAs, and ABA standards (0 ˜ 2,000 ng mL−1 dilution range), and 50 μL of 20 μg mL−1 antibodies against Z, ZR, IAA, GAs, and ABA, respectively. The plate was incubated for 3 h at 28°C for Z, ZR, GAs, ABA, and overnight at 4°C for IAA, and then washed as above. One hundred microliters of 1.25 μg mL−1 IgG-horseradish peroxidase substrate was added to each well and incubated for 1 h at 30°C. The plate was rinsed five times with above PBS + Tween 20 buffer, and 100 μL color-appearing solution containing 1.5 mg mL−1 0-phenylenediamine and 0.008% (v/v) H2O2 was added to each well. The reaction progress was stopped by adding of 50 μL 6 n H2SO4 per well when the 2,000 ng mL−1 standard had a pale color, and the 0 ng mL−1 standard had a deep color in the wells. Color development in each well was detected using an ELISA Reader (model EL310, Bio-TEK, Winooski, VT) at optical density A490. Z, ZR, IAA, GAs, and ABA contents were calculated following Weiler et al. (1981). The results are the means ± se of at least four replicates.
Exogenous ABA Application
Plants were grown in the porcelain pots that were placed in a field. Each porcelain pot (30-cm height, 25-cm diameter, and 14.72-L volume) was filled with 18 kg sandy loam soil with the same nutrient contents as the field soil. Thirty-day-old seedlings raised in the field were transplanted on June 11 into the pots with three hills per pot and one seedling per hill. Two grams of N as urea, 0.2 g of phosphorus as single superphosphate, and 0.3 g of potassium as KCl were mixed into the soil in each pot before transplanting. At mid-tillering, panicle initiation, and heading, 0.6, 1.2, and 1 g of N as urea were top dressed into each pot, respectively. The plants were watered daily by hand and the pot was kept at 1- to 2-cm water level during the whole growth period.
Starting at 9 DPA, 25 × 10−6 M ABA (Sigma Chemical Co.) was sprayed on the leaves and panicles daily for 7 consecutive d. The plants sprayed with the same volume of distilled water were taken as a control. Each treatment had 40 pots. ABA in the grains were measured 3 and 11 d after ABA application (12 and 20 DPA), and grain filling rate was measured by weighing grain weight every 5 d from heading to maturity. Measurement methods were the same as described above. Five pots of plants for each treatment were harvested at maturity for examination of the final grain weight and carbon reserve in the stem (culm + sheath). Nonstructural carbohydrate in the stem was determined according to Yoshida et al. (1976). Each measurement had five replicates.
Statistical Analysis
The results were analyzed for variance using the SAS statistical analysis package (version 6.12, SAS Institute, Cary, NC). Data from each sampling date were analyzed separately. Means were tested by lsd at P0.05 level (lsd0.05). Linear regression was used to evaluate the relationship between hormonal contents in the grains with grain filling rate.
Footnotes
This work was supported by the FRG of Hong Kong Baptist University, by the RGC of Hong Kong University Council, by the AOE Research Found of the Chinese University of Hong Kong, by the National Natural Science Foundation of China (project no. 39970424), and by the State Key Basic Research and Development Plan (grant no. G1999011700).
LITERATURE CITED
- Ackerson RC. Invertase activity and abscisic acid in relation to carbohydrate status in developing soybean reproductive structures. Crop Sci. 1985;25:615–618. [Google Scholar]
- Bai XF, Cai YP, Nie F. Relationship between abscisic acid and grain filling of rice and wheat. Physiol Commun (China) 1989;3:40–41. [Google Scholar]
- Bollmark M, Kubat B, Eliasson L. Variations in endogenous cytokinin content during adventitious root formation in pea cuttings. J Plant Physiol. 1988;132:262–265. [Google Scholar]
- Boonjung H, Fukai S. Effects of soil water deficit at different growth stages on rice growth and yield under upland conditions: 2. Phenology, biomass production and yield. Field Crops Res. 1996;48:47–55. [Google Scholar]
- Brenner ML, Cheikh N. The role of hormones in photosynthate partitioning and seed filling. In: Davies PJ, editor. Plant Hormones. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1995. pp. 649–670. [Google Scholar]
- Davies PJ. The plant hormones: their nature, occurrence, and functions. In: Davies PJ, editor. Plant Hormones and Their Role in Plant Growth and Development. Dordrecht, The Netherlands: Martinus Nijhoff Publishers; 1987. pp. 1–11. [Google Scholar]
- Dewdney SJ, McWha JA. Abscisic acid and the movement of photosynthetic assimilates towards developing wheat (Triticum aestivum L.) grains. Z Pflanzenphysiol. 1979;92:186–193. [Google Scholar]
- Eeuwens CJ, Schwabe WW. Seed and pod wall development in Pisum sativum L. in relation to extracted and applied hormones. J Exp Bot. 1975;26:1–14. [Google Scholar]
- Hansen H, Grossmann K. Auxin-induced enthylene triggers abscisic acid biosynthesis and growth inhibition. Plant Physiol. 2000;124:1437–1448. doi: 10.1104/pp.124.3.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Z. Guidance to experiment on chemical control in crop plants. In: He ZP, editor. Guidance to Experiment on Chemical Control in Crop Plants. Beijing Agricultural University Publishers, Beijing. 1993. pp. 60–68. [Google Scholar]
- Karssen CM. The role of endogenous hormones during seed development and the onset of primary dormancy. In: Wareing PF, editor. Plant Growth Substances. London: Academic Press; 1982. pp. 623–632. [Google Scholar]
- Kato T, Sakurai N, Kuraishi S. The changes of endogenous abscisic acid in developing grains of two rice cultivars with different grain size. Jpn J Crop Sci. 1993;62:456–461. [Google Scholar]
- Kende H, Zeevaart JAD. The five “classical” plant hormones. Plant Cell. 1997;9:1197–1210. doi: 10.1105/tpc.9.7.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobata T, Takami S. Grain production and dry matter partitioning in rice (Oryza sativa L.) in response to water deficits during the whole grain filling period. Jpn J Crop Sci. 1983;53:283–290. [Google Scholar]
- Kurogochi S, Murofushi N, Ota Y, Takahashi N. Identification of gibberellins in rice plant and quantitative changes of gibberellin A19 throughout its life circle. Planta. 1979;146:185–191. doi: 10.1007/BF00388230. [DOI] [PubMed] [Google Scholar]
- Ling Q, Zhang H, Cai J, Su Z. Population quality and its approaches for the high-yielding of rice. Sci Agric Sin. 1993;6:1–11. [Google Scholar]
- Lur H-S, Setter TL. Role of auxin in maize endosperm development: timing of nuclear DNA endoreduplication, zein expression, and cytokinins. Plant Physiol. 1993;103:273–280. doi: 10.1104/pp.103.1.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michihiro W, Lui JCB, Garvalho GC. Cultivar difference in leaf photosynthesis and grain yield of wheat under soil water deficit conditions. Jpn J Crop Sci. 1994;63:339–344. [Google Scholar]
- Morris RD, Blevins DG, Dietrich JT, Durly RC, Gelvin SB, Gray J, Hommes NG, Kaminek M, Mathews LJ, Meilan R. Cytokinins in plant pathogenic bacteria and developing cereal grains. Aust J Plant Physiol. 1993;20:621–637. [Google Scholar]
- Qin Z, Tang X. Dynamics of some large bio-molecules during the formation of rice endosperm. China Sci. 1984;12:1103–1110. [Google Scholar]
- Rahman MS, Yoshida S. Effect of water stress on grain filling in rice. Soil Sci Plant Nutr. 1985;31:497–511. [Google Scholar]
- Richards FJ. A flexible growth function for empirical use. J Exp Bot. 1959;10:290–300. [Google Scholar]
- Saha S, Nagar PK, Sircar PK. Cytokinin concentration gradient in the developing grains and upper leaves of rice (Oryza sativa) during grain filling. Can J Bot. 1986;64:2068–2072. [Google Scholar]
- Seth AK, Waering PE. Hormone-directed transport of metabolites and its possible role in plant senescence. J Exp Bot. 1967;18:65–77. [Google Scholar]
- Singh BM, Jenner CF. A modified method for the determination of cell number in wheat endosperm. Plant Sci Lett. 1982;26:273–278. [Google Scholar]
- Singh G, Gerung SB. Hormonal role in the problem of sterility in Oryza sativa. Plant Physiol Biochem. 1982;9:22–23. [Google Scholar]
- Suzuki Y, Kurogochi S, Murofushi N, Ota Y, Takahashi N. Seasonal changes of GA1, GA19 and abscisic acid in three rice cultivars. Plant Cell Physiol. 1981;22:1085–1093. [Google Scholar]
- Tietz A, Ludwig M, Dingkuhn M, Dorffling K. Effect of abscisic acid on the transport of assimilates in barley. Plant. 1981;152:557–561. doi: 10.1007/BF00380827. [DOI] [PubMed] [Google Scholar]
- Wang Z, Yang J, Zhu Q, Zhang Z, Lang Y, Wang X. Reasons for poor grain filling in intersubspecific hybrid rice. Acta Agron Sin. 1998;24:782–787. [Google Scholar]
- Weiler EW, Jordan PS, Conrad W. Levels of indole-3-acetic acid in intact and decapitated coleoptiles as determined by a specific and highly sensitive solid-phase enzyme immunoassay. Planta. 1981;153:561–571. doi: 10.1007/BF00385542. [DOI] [PubMed] [Google Scholar]
- Wu S, Chen W, Zhou X. Enzyme linked immunosorbent assay for endogenous plant hormones. Plant Physiol Commun (China) 1988;5:53–57. [Google Scholar]
- Yang J, Liu L, Wang Z, Zhu Q. Effects of flowering time of spikelets on endosperm development in rice and its physiological mechanism. Chinese Agric Sci. 2000c;2:70–79. [Google Scholar]
- Yang J, Peng S, Visperas RM, Sanico AL, Zhu Q, Gu S. Grain filling pattern and cytokinin content in the grains and roots of rice plants. Plant Growth Regul. 2000a;30:261–270. [Google Scholar]
- Yang J, Wang Z, Zhu Q. Effect of nitrogen nutrition on grain yield of rice and its physiological mechanism under different soil moisture. Sci Agric Sin. 1996;4:7–14. [Google Scholar]
- Yang J, Wang Z, Zhu Q, Lang Y. Regulation of ABA and GA to rice grain filling. Acta Agron Sin. 1999;25:341–348. [Google Scholar]
- Yang J, Zhang J, Huang Z, Zhu Q, Wang L. Remobilization of carbon reserves is improved by controlled soil-drying during grain filling of wheat. Crop Sci. 2000b;40:1645–1655. [Google Scholar]
- Yang J, Zhang J, Wang Z, Zhu Q, Liu L. Water-deficit induced senescence and its relationship to the remobilization of pre-stored carbon in wheat during grain filling. Agron J. 2001;93:196–206. [Google Scholar]
- Yoshida S, Forno D, Cock J, Comez K. Determination of sugar and starch in plant tissue. In: Yoshida S, editor. Laboratory Manual for Physiological Studies of Rice. The International Rice Research Institute, The International Rice Research Institute, Los Banos, The Philippines. 1976. pp. 46–49. [Google Scholar]
- Yuan LP. Hybrid rice breeding for super high yield. Hybrid Rice. 1997;12:1–6. [Google Scholar]
- Zhang J, He Z, Wu Y. Establishment of an indirect enzyme-linked immunosorbent asssay for zeatin and zeatin riboside. J Beijing Agric Univ (China) Suppl. 1991;17:145–151. [Google Scholar]
- Zhang J, Sui X, Li B, Su B, Li J, Zhou D. An improved water-use efficiency for winter wheat grown under reduced irrigation. Field Crops Res. 1998;59:91–98. [Google Scholar]
- Zhu Q, Cao X, Luo Y. Growth analysis in the process of grain-filling in rice. Acta Agron Sin. 1988;14:182–192. [Google Scholar]
- Zhu Q, Zhang Z, Yang J, Cao X, Lang Y, Wang Z. Source-sink characteristics related to the yield in intersubspecific hybrid rice. Sci Agric Sin. 1997;4:52–59. [Google Scholar]