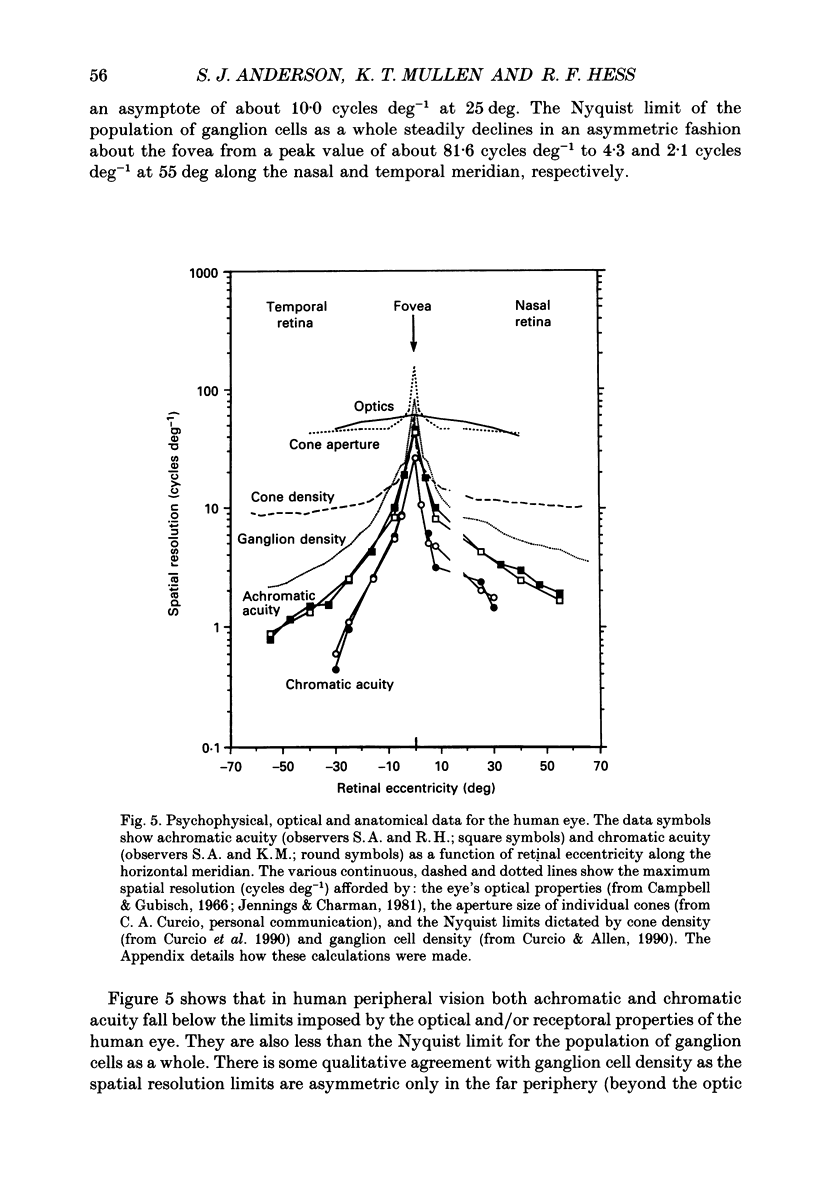
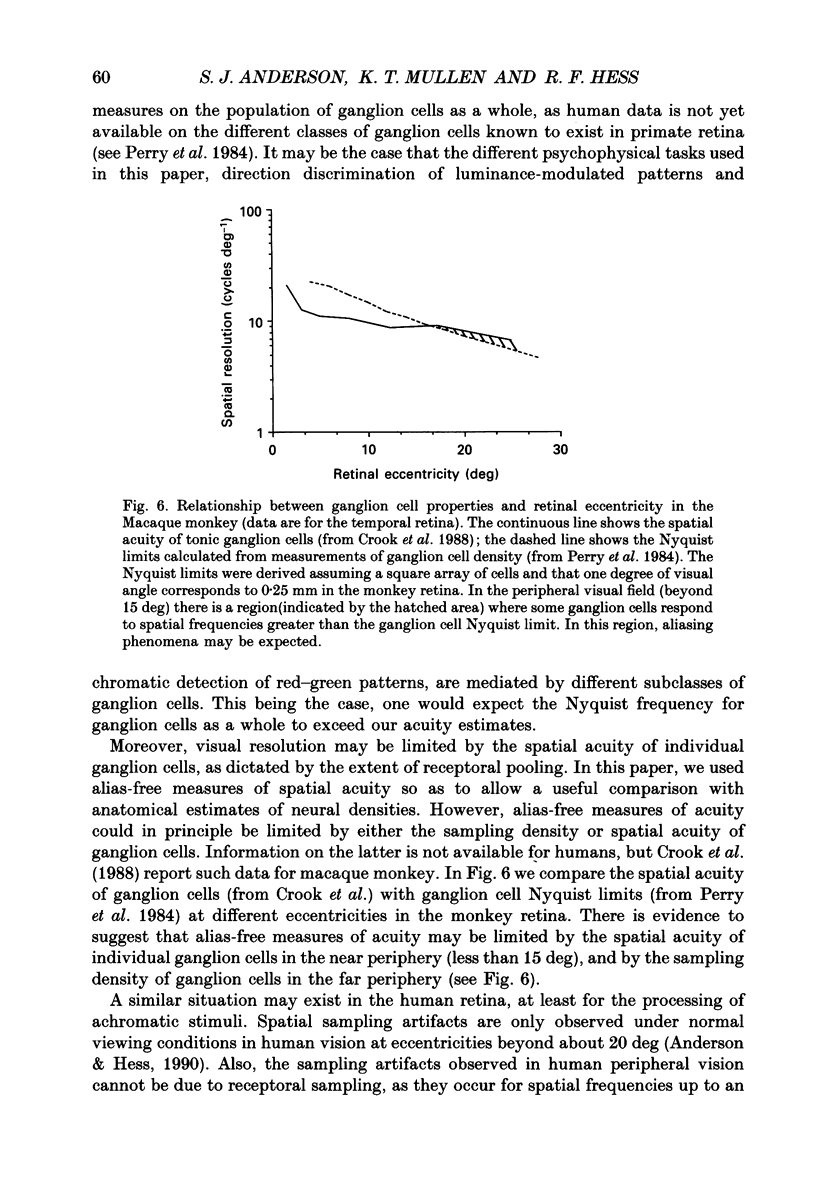
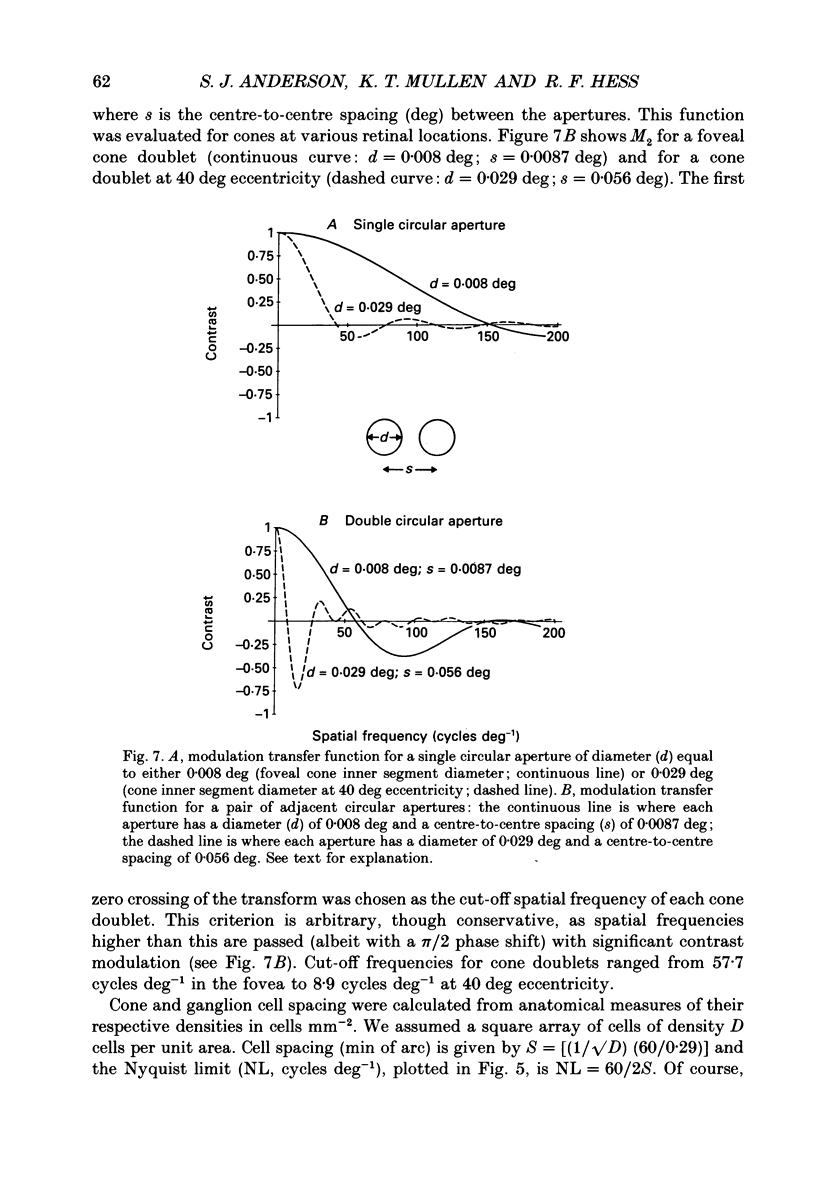
Abstract
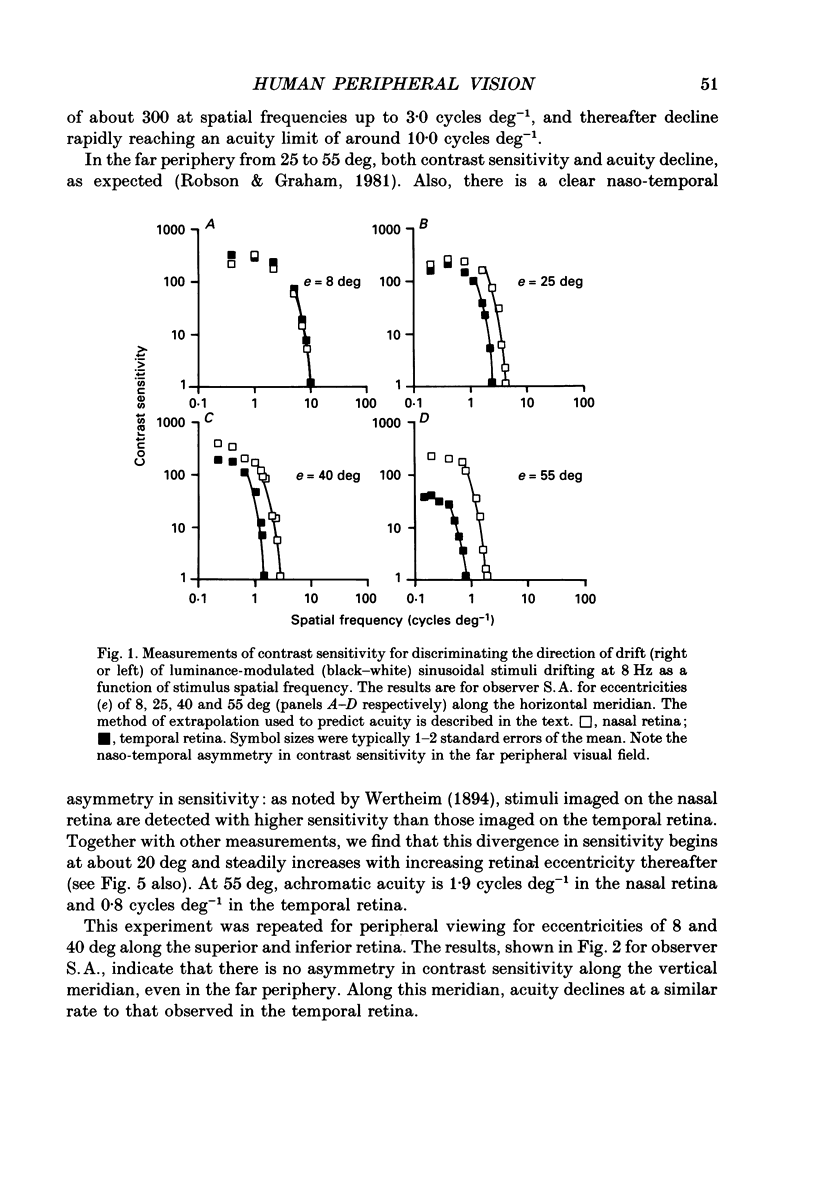
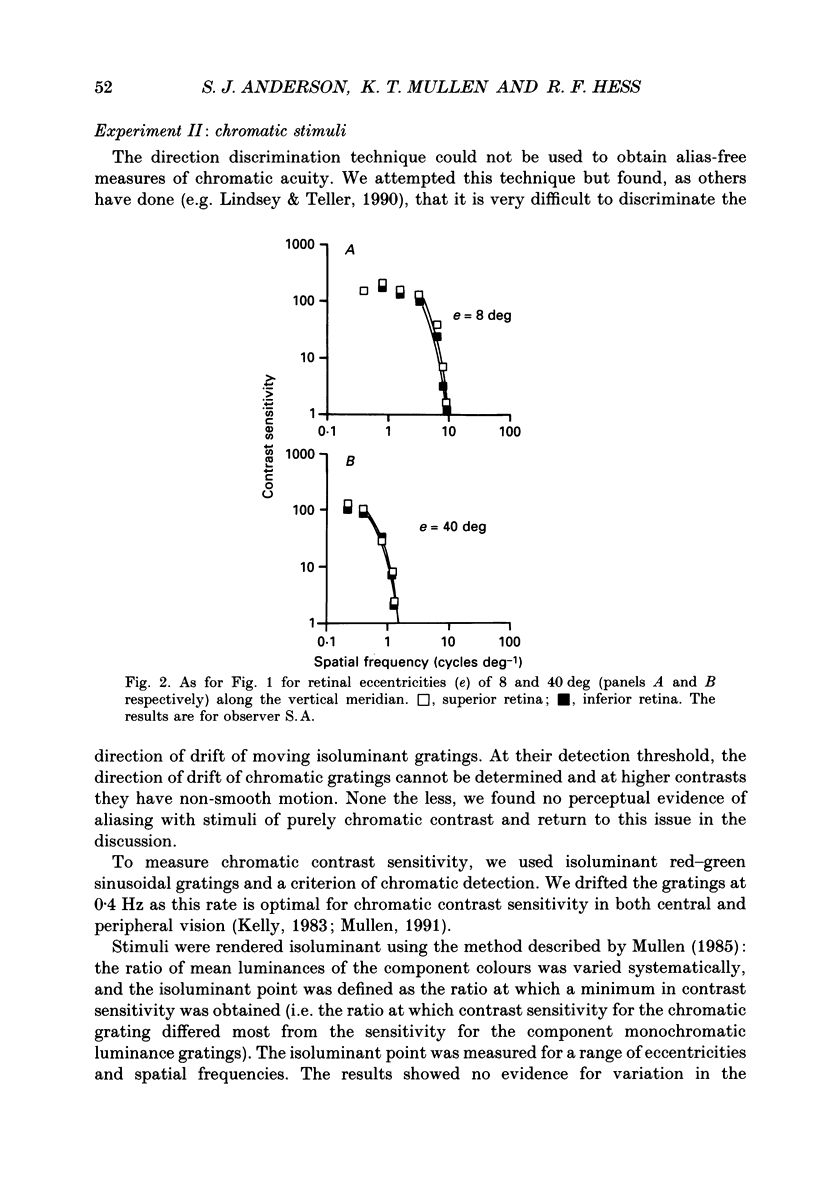
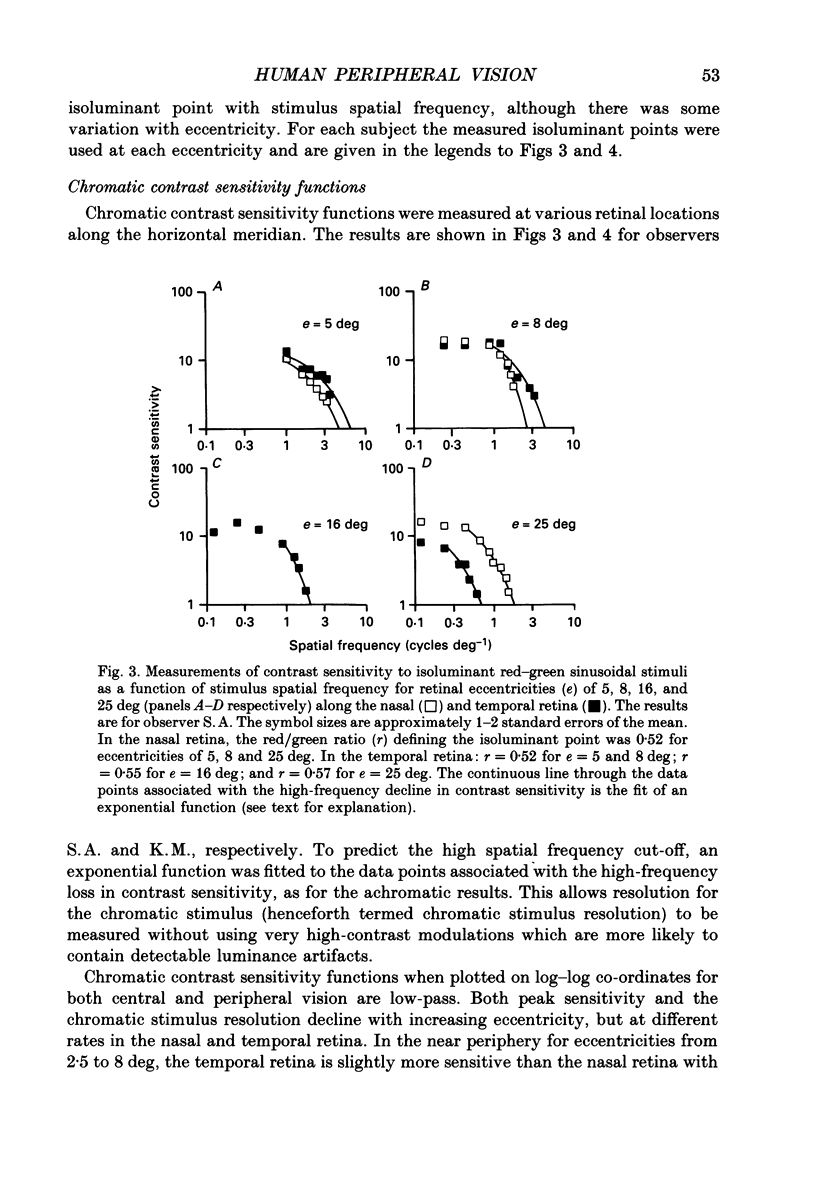
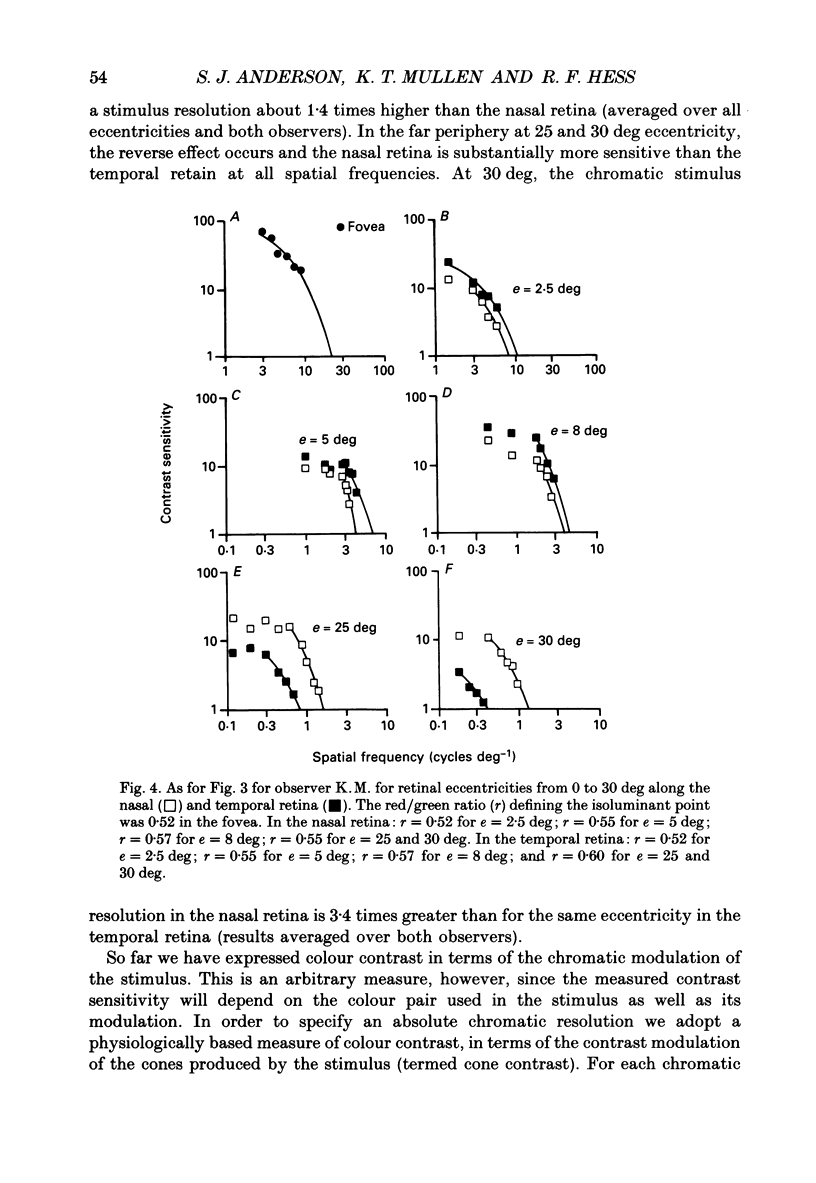
1. The aim of this study was to determine whether optical, receptoral or higher-order neural properties limit spatial resolution (acuity) in human vision, especially in the peripheral regions of the visual field. 2. Both achromatic and chromatic stimuli were used, and measures were taken to ensure that the resolution estimates were not contaminated by the detection of spatial sampling artifacts. Spatial contrast sensitivity functions were measured at retinal locations from 0 to 55 deg along the naso-temporal meridian for: (i) discriminating the direction of drift of luminance-modulated (black-white) sinusoidal stimuli drifting at 8 Hz (achromatic task); and (ii) for detecting isoluminant red-green sinusoidal stimuli drifting at 0.4 Hz (chromatic task). Achromatic contrast sensitivity functions were also measured along the vertical meridian for eccentricities of 8 and 40 deg. Each achromatic function was extrapolated to a contrast sensitivity of one (100% contrast) to estimate achromatic acuity. Chromatic acuities were obtained by expressing chromatic contrast in terms of cone contrasts and using the same method of extrapolation. We compared the results with recent data on human optical properties and retinal anatomy. 3. Both achromatic and chromatic acuity decline with distance from the fovea, but at a faster rate than that dictated by the known optical and/or receptoral properties of the human eye. We conclude that, for stimuli of either achromatic or chromatic contrast, peripheral spatial resolution is limited by post-receptoral mechanisms. Also, chromatic acuity declines more steeply than luminance acuity with eccentricity suggesting that there are additional post-receptoral limitations on colour resolution in the periphery. 4. A clear naso-temporal asymmetry is seen in the resolution whose dependence is qualitatively, but not quantitatively, similar to the Nyquist limits imposed by the asymmetric density of human retinal ganglion cells. We discuss the possibility that in peripheral vision (beyond the optic nerve head) the spacing of ganglion cells may pose a fundamental limit on the resolution of achromatic stimuli, but not chromatic stimuli.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson S. J., Burr D. C. Receptive field size of human motion detection units. Vision Res. 1987;27(4):621–635. doi: 10.1016/0042-6989(87)90047-2. [DOI] [PubMed] [Google Scholar]
- Anderson S. J., Hess R. F. Post-receptoral undersampling in normal human peripheral vision. Vision Res. 1990;30(10):1507–1515. doi: 10.1016/0042-6989(90)90031-f. [DOI] [PubMed] [Google Scholar]
- Banks M. S., Geisler W. S., Bennett P. J. The physical limits of grating visibility. Vision Res. 1987;27(11):1915–1924. doi: 10.1016/0042-6989(87)90057-5. [DOI] [PubMed] [Google Scholar]
- Burr D. C., Ross J. Contrast sensitivity at high velocities. Vision Res. 1982;22(4):479–484. doi: 10.1016/0042-6989(82)90196-1. [DOI] [PubMed] [Google Scholar]
- Campbell F. W., Gubisch R. W. Optical quality of the human eye. J Physiol. 1966 Oct;186(3):558–578. doi: 10.1113/jphysiol.1966.sp008056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coletta N. J., Williams D. R., Tiana C. L. Consequences of spatial sampling for human motion perception. Vision Res. 1990;30(11):1631–1648. doi: 10.1016/0042-6989(90)90149-f. [DOI] [PubMed] [Google Scholar]
- Crook J. M., Lange-Malecki B., Lee B. B., Valberg A. Visual resolution of macaque retinal ganglion cells. J Physiol. 1988 Feb;396:205–224. doi: 10.1113/jphysiol.1988.sp016959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curcio C. A., Allen K. A. Topography of ganglion cells in human retina. J Comp Neurol. 1990 Oct 1;300(1):5–25. doi: 10.1002/cne.903000103. [DOI] [PubMed] [Google Scholar]
- Curcio C. A., Sloan K. R., Kalina R. E., Hendrickson A. E. Human photoreceptor topography. J Comp Neurol. 1990 Feb 22;292(4):497–523. doi: 10.1002/cne.902920402. [DOI] [PubMed] [Google Scholar]
- ENOCH J. M. Nature of the transmission of energy in the retinal receptors. J Opt Soc Am. 1961 Oct;51:1122–1126. doi: 10.1364/josa.51.001122. [DOI] [PubMed] [Google Scholar]
- Fahle M. Naso-temporal asymmetry of binocular inhibition. Invest Ophthalmol Vis Sci. 1987 Jun;28(6):1016–1017. [PubMed] [Google Scholar]
- Fahle M., Schmid M. Naso-temporal asymmetry of visual perception and of the visual cortex. Vision Res. 1988;28(2):293–300. doi: 10.1016/0042-6989(88)90157-5. [DOI] [PubMed] [Google Scholar]
- French A. S., Snyder A. W., Stavenga D. G. Image degradation by an irregular retinal mosaic. Biol Cybern. 1977 Oct 14;27(4):229–233. doi: 10.1007/BF00344144. [DOI] [PubMed] [Google Scholar]
- Götz K. G. Optomotorische Untersuchung des visuellen Systems einiger Augenmutanten der Fruchtfliege Drosophila. Kybernetik. 1964 Jun;2(2):77–92. doi: 10.1007/BF00288561. [DOI] [PubMed] [Google Scholar]
- Hess R. F., Mullen K. T., Zrenner E. Human photopic vision with only short wavelength cones: post-receptoral properties. J Physiol. 1989 Oct;417:151–172. doi: 10.1113/jphysiol.1989.sp017795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings J. A., Charman W. N. Off-axis image quality in the human eye. Vision Res. 1981;21(4):445–455. doi: 10.1016/0042-6989(81)90091-2. [DOI] [PubMed] [Google Scholar]
- Kelly D. H. Spatiotemporal variation of chromatic and achromatic contrast thresholds. J Opt Soc Am. 1983 Jun;73(6):742–750. doi: 10.1364/josa.73.000742. [DOI] [PubMed] [Google Scholar]
- LOW F. N. Peripheral visual acuity. AMA Arch Ophthalmol. 1951 Jan;45(1):80–99. doi: 10.1001/archopht.1951.01700010083011. [DOI] [PubMed] [Google Scholar]
- Lennie P., D'Zmura M. Mechanisms of color vision. Crit Rev Neurobiol. 1988;3(4):333–400. [PubMed] [Google Scholar]
- Lindsey D. T., Teller D. Y. Motion at isoluminance: discrimination/detection ratios for moving isoluminant gratings. Vision Res. 1990;30(11):1751–1761. doi: 10.1016/0042-6989(90)90157-g. [DOI] [PubMed] [Google Scholar]
- Merigan W. H., Katz L. M. Spatial resolution across the macaque retina. Vision Res. 1990;30(7):985–991. doi: 10.1016/0042-6989(90)90107-v. [DOI] [PubMed] [Google Scholar]
- Miller W. H., Bernard G. D. Averaging over the foveal receptor aperture curtails aliasing. Vision Res. 1983;23(12):1365–1369. doi: 10.1016/0042-6989(83)90147-5. [DOI] [PubMed] [Google Scholar]
- Mullen K. T. Colour vision as a post-receptoral specialization of the central visual field. Vision Res. 1991;31(1):119–130. doi: 10.1016/0042-6989(91)90079-k. [DOI] [PubMed] [Google Scholar]
- Mullen K. T. The contrast sensitivity of human colour vision to red-green and blue-yellow chromatic gratings. J Physiol. 1985 Feb;359:381–400. doi: 10.1113/jphysiol.1985.sp015591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noorlander C., Koenderink J. J., den Ouden R. J., Edens B. W. Sensitivity to spatiotemporal colour contrast in the peripheral visual field. Vision Res. 1983;23(1):1–11. doi: 10.1016/0042-6989(83)90035-4. [DOI] [PubMed] [Google Scholar]
- Paradiso M. A., Carney T. Orientation discrimination as a function of stimulus eccentricity and size: nasal/temporal retinal asymmetry. Vision Res. 1988;28(8):867–874. doi: 10.1016/0042-6989(88)90096-x. [DOI] [PubMed] [Google Scholar]
- Perry V. H., Oehler R., Cowey A. Retinal ganglion cells that project to the dorsal lateral geniculate nucleus in the macaque monkey. Neuroscience. 1984 Aug;12(4):1101–1123. doi: 10.1016/0306-4522(84)90006-x. [DOI] [PubMed] [Google Scholar]
- Robson J. G., Graham N. Probability summation and regional variation in contrast sensitivity across the visual field. Vision Res. 1981;21(3):409–418. doi: 10.1016/0042-6989(81)90169-3. [DOI] [PubMed] [Google Scholar]
- Rovamo J., Virsu V. An estimation and application of the human cortical magnification factor. Exp Brain Res. 1979;37(3):495–510. doi: 10.1007/BF00236819. [DOI] [PubMed] [Google Scholar]
- Smith R. A., Cass P. F. Aliasing in the parafovea with incoherent light. J Opt Soc Am A. 1987 Aug;4(8):1530–1534. doi: 10.1364/josaa.4.001530. [DOI] [PubMed] [Google Scholar]
- Snyder A. W., Miller W. H. Photoreceptor diameter and spacing for highest resolving power. J Opt Soc Am. 1977 May;67(5):696–698. doi: 10.1364/josa.67.000696. [DOI] [PubMed] [Google Scholar]
- Thibos L. N., Cheney F. E., Walsh D. J. Retinal limits to the detection and resolution of gratings. J Opt Soc Am A. 1987 Aug;4(8):1524–1529. doi: 10.1364/josaa.4.001524. [DOI] [PubMed] [Google Scholar]
- Thibos L. N., Walsh D. J., Cheney F. E. Vision beyond the resolution limit: aliasing in the periphery. Vision Res. 1987;27(12):2193–2197. doi: 10.1016/0042-6989(87)90134-9. [DOI] [PubMed] [Google Scholar]
- Williams D. R. Aliasing in human foveal vision. Vision Res. 1985;25(2):195–205. doi: 10.1016/0042-6989(85)90113-0. [DOI] [PubMed] [Google Scholar]
- Williams D. R., Coletta N. J. Cone spacing and the visual resolution limit. J Opt Soc Am A. 1987 Aug;4(8):1514–1523. doi: 10.1364/josaa.4.001514. [DOI] [PubMed] [Google Scholar]
- Wässle H., Grünert U., Röhrenbeck J., Boycott B. B. Cortical magnification factor and the ganglion cell density of the primate retina. Nature. 1989 Oct 19;341(6243):643–646. doi: 10.1038/341643a0. [DOI] [PubMed] [Google Scholar]
- Yellott J. I., Jr Spectral analysis of spatial sampling by photoreceptors: topological disorder prevents aliasing. Vision Res. 1982;22(9):1205–1210. doi: 10.1016/0042-6989(82)90086-4. [DOI] [PubMed] [Google Scholar]
- Zeki S. M. Uniformity and diversity of structure and function in rhesus monkey prestriate visual cortex. J Physiol. 1978 Apr;277:273–290. doi: 10.1113/jphysiol.1978.sp012272. [DOI] [PMC free article] [PubMed] [Google Scholar]


